SUMMARY
Genetic programs, environmental factors and electrical activity interact to drive the maturation of the brain. Although the cascade of transcription factors that leads to specification of the serotonergic phenotype has been well characterized, its interactions with electrical activity are not known. Here we show that spontaneous calcium spike activity in the hindbrain of developing X. laevis larvae modulates the specification of serotonergic neurons via regulation of expression of the Lmx1b transcription factor. Activity acts downstream of Nkx2.2 but upstream of Lmx1b, leading to regulation of the serotonergic phenotype. Using global manipulation of activity and targeted alteration of Lmx1b expression, we also demonstrate that changes in the number of serotonergic neurons change larval swimming behavior. The results link activity-dependent regulation of a transcription factor to transmitter specification and altered behavior.
Keywords: transmitter specification, activity-dependence, serotonin, raphe nucleus, Lmx1b, swimming behavior, Xenopus laevis
INTRODUCTION
Serotonin (5-HT) regulates many aspects of central nervous system (CNS) development, including neuronal proliferation, migration, differentiation and synaptogenesis (Gaspar et al., 2003; Lauder, 1993; Whitaker-Azmitia et al., 1996). Changes in serotonergic function have been implicated in neurological disorders such as depression, anxiety, drug addiction and autism spectrum disorders (Gingrich and Hen, 2001; Whitaker-Azmitia, 2001). More recently, transient developmental alterations of the serotonergic system in mice have been shown to lead to chronic phenotypes that persist in adulthood (Alexandre et al., 2006; Gross et al., 2002). These results highlight the importance of a better understanding of the genetic and epigenetic pathways involved in the maturation of the serotonergic system, and the interactions between them.
The molecular regulation of 5-HT specification has been well described and involves conserved transcription factors among vertebrates (Alenina et al., 2006; Cordes, 2005; Lillesaar et al., 2007; Scott and Deneris, 2005). We focused on two key regulators of serotonergic differentiation: Nkx2.2 and Lmx1b. Nkx2.2 is a transcription factor transiently expressed in serotonergic precursors and knockout mice lack most serotonergic neurons (Briscoe et al., 1999; Cheng et al., 2003); (Ding et al., 2003). Lmx1b is expressed later, persistently, and is required for the maintenance of 5-HT expression in the CNS. Loss-of-function experiments lead to complete absence of 5-HT neurons, while Lmx1b gain-of-function, in combination with Nkx2.2 and Pet1, is required to induce the development of ectopic serotonergic neurons in the chick neural tube (Cheng et al., 2003; Ding et al., 2003; Zhao et al., 2006). The specification of 5-HT is also plastic and modulated by epigenetic factors. In vitro, application of CNTF induces a switch of RN46A cells from a serotonergic to a cholinergic phenotype while application of BDNF or dibutyryl cAMP to primary cultures of E14 rat embryonic rostral raphe upregulates the serotonergic phenotype (Rudge et al., 1996; Rumajogee et al., 2002).
Transmitter specification is homeostatically regulated by calcium (Ca) spikes in the spinal cord and hypothalamus of Xenopus embryos and larvae (Borodinsky et al., 2004; Dulcis and Spitzer, 2008). Ca spike-dependent transcription factor phosphorylation plays a key role in this process (Marek et al., in press). Taking advantage of the conserved transcription factor cascade for 5-HT specification and the modulation of fictive swimming by 5-HT in X. laevis (Wedderburn and Sillar, 1994), we provide a missing link in the analysis of activity-dependent specification of neurotransmitters, showing for the first time that activity affects expression of a transcription factor regulating transmitter synthesis, with behavioral consequences. The serotonergic phenotype is regulated by an interaction of Ca spikes and transcription factor expression in the CNS of Xenopus embryos. The subsequent modulation of the 5-HT expression alters larval swimming behavior. In view of the critical role of 5-HT during physiological and pathological development, better understanding of activity-dependent control of the serotonergic phenotype may provide novel insights into mechanisms of neuronal plasticity and suggest new ways in which to tackle neurological disorders involving dysregulation of 5-HT metabolism.
RESULTS
Presumptive serotonergic neurons generate spontaneous Ca spikes in the developing raphe
Serotonergic raphe neurons were identified by their ventral midline location in the hindbrain and their immunoreactivity for tryptophan hydroxylase (TPH), the rate limiting serotonin-synthetic enzyme (Figure S1, available online). We used TPH as a cell identity marker for serotonergic neurons since some cells recapture and accumulate 5-HT without producing it (Hansson et al., 1998; Lebrand et al., 1996; Zhou et al., 2000). TPH-immunoreactive (TPH-IR) neurons were first detected at stage 25, an early tailbud stage, and their number increased through stage 41, an early larval stage (Figure S2), as previously described using an antibody raised against 5-HT (van Mier et al., 1986). Additional TPH-IR neurons are present more rostrally at the level of the infundibulum.
To determine the spatial and temporal patterns of Ca spiking during the period of 5-HT specification, we imaged spontaneous Ca spikes in the ventral rhombencephalon at embryonic and larval stages of development (Figure 1A-C). We focused our analysis on embryonic stage 28, a late tailbud stage, when only a few 5-HT neurons are observed and more are differentiating, and post-embryonic larval stage 35/36, around hatching, when some 5-HT neurons have already sent axons to distal regions while others are still acquiring 5-HT (Figure S2) (van Mier et al., 1986). Spontaneous Ca spike activity was observed at both stages. The durations and amplitudes of individual spikes were similar to those of spikes generated in the spinal cord (Figure 1B) (Borodinsky et al., 2004). At stage 28, when spontaneous Ca spikes are still observed in the spinal cord, spontaneous Ca spikes occurred in the hindbrain with an incidence of 23% during 1 hr periods of imaging and a frequency of 4.6 h−1 in active cells (n=8; Figure 1C and Movie S1, available online). At stage 35/36, when the critical period for Ca spiking in the spinal cord has passed, the incidence was 15% with a frequency of 2.9 h−1 in active cells (n=5; Figure 1C). The incidence and frequency of Ca spiking in the hindbrain are similar to those in the spinal cord, but spiking extends to later stages and is expressed over a longer developmental period (Figure S3A) (Borodinsky et al., 2004; Root et al., 2008). Because the incidence of spiking increases with the duration of the period of image acquisition (Gu et al., 1994; Gu and Spitzer, 1995), it is likely that most cells generate Ca spikes during these several days of development.
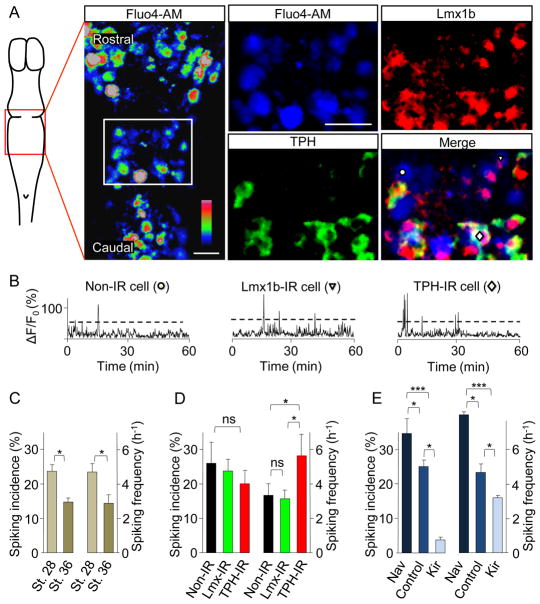
Figure 1. Neurons in the presumptive raphe generate spontaneous calcium spikes during the period of 5-HT specification.
(A) Confocal images of the ventral surface of a Fluo-4-loaded stage 28 rhombencephalon are shown as a maximum projection in the time dimension to visualize neurons that generated Ca spikes during the 1 hr recording period (left panel and Movie S1). Imaging was followed by fixation and immunostaining for Lmx1b and TPH. Merge of the projected z-stack images for Fluo-4 (blue), Lmx1b (red) and TPH (green) (right panels, enlargements of the boxed region at left) enabled analysis of the Ca activity profile in three identified cell populations: cells not stained for either marker (non-IR cells, ●), putatively differentiating serotonergic neurons stained for Lmx1b only (Lmx1b cells, ▼) and serotonergic neurons stained for TPH and Lmx1b (TPH cells, ◆). Color bar: blue, low Ca; red, high Ca. Scale bars, 40 μm.
(B) Spike activity in the cells indicated by symbols in (A). Spikes are events with rise times ≤5 seconds (1 acquisition interval) and amplitudes greater than three times the standard deviation of the noise (dashed line).
(C-E) Mean incidence (left) and frequency (right) of spiking at different stages and in different conditions.
(C) Neurons at embryonic stage 28 (light brown bars) exhibit a higher overall incidence and frequency of spiking than post-embryonic cells at stage 35/36 (dark brown bars).
(D) At stage 28 there are no significant differences in spike incidence between non-IR neurons (black), Lmx1b-IR neurons (green) and TPH-IR neurons (red), while the spiking frequency is higher in TPH-IR neurons than in the other 2 classes of neurons.
(E) At stage 28 spike incidence and frequency are higher in embryos overexpressing Nav mRNA (dark blue bars) and are lower in embryos overexpressing Kir mRNA (light blue bars) compared to control. n≥6; ns, no significant difference, * p<0.05, ***p<0.001. Mann-Whitney (U) test (C); Kruskal-Wallis test with Conover post-hoc analysis (D and E).
To determine more precisely whether spontaneous Ca spikes are generated in serotonergic neurons and in neurons that will become serotonergic in the raphe, we immunostained preparations following Ca imaging at stage 28 using antibodies to TPH and Lmx1b (n=7; Figure 1A). Differentiated serotonergic neurons were identified based on their immunoreactivity for TPH. Presumptive serotonergic neurons were identified based on their staining for Lmx1b, absence of staining for TPH and their ventral location along the midline of the developing raphe. TPH-IR neurons were invariably Lmx1b-IR whereas a subpopulation of ventral Lmx1b-IR neurons was not TPH-IR. The proportion of this Lmx1b-IR/non-TPH-IR population decreased as the embryos grew older, suggesting that it is a pool of differentiating neurons about to become TPH-IR, consistent with the role of Lmx1b in 5-HT expression described for other species (Figure S2, Movie S2) (Cheng et al., 2003; Ding et al., 2003).
At stage 28, 12% of spiking cells were Lmx1b-IR but not TPH-IR suggesting that neurons about to become serotonergic generate spontaneous Ca spikes. 18% of spiking cells were both TPH-IR and Lmx1b-IR, indicating that neurons that have already acquired 5-HT continue to be spontaneously active. 70% of spiking cells expressed neither marker. Spike incidence was similar across these three populations (non-IR, 26%; Lmx1b-IR, 24%; TPH-IR, 20%). However, spike frequency in TPH-IR neurons was significantly higher than in non-IR neurons and in neurons expressing Lmx1b-IR without TPH-IR (non-IR, 3.4 h−1; Lmx1b-IR, 3.1 h−1; TPH-IR, 5.7 h−1; Figure 1D). This result raises the possibility that low frequencies of spiking are necessary to enable serotonergic differentiation and suggests that the generation of Ca spike activity and the cellular response to this activity are developmentally regulated and change following specification of 5-HT.
To find out whether overexpression of ion channels affects the pattern of spontaneous Ca spikes we injected ion channel mRNA into both blastomeres of 2-cell stage embryos and assessed the spontaneous Ca activity in stage 28 embryos. Injection of mRNA encoding human inward rectifier 2.1 potassium channels (Kir) decreased the incidence and frequency of Ca spiking in the hindbrain while injection of mRNA encoding rat 1.2 sodium channels (Nav) increased the incidence and frequency of Ca spikes (incidence: control, 25%; Kir, 4%; Nav, 35%; frequency: control, 4.6 h−1; Kir, 3.3 h−1; Nav, 7.8 h−1; n=6; Figure 1E). These results demonstrate the efficacy of ion channel overexpression in modulating the incidence and frequency of Ca spikes in the raphe of Xenopus embryos at the time of 5-HT specification, as seen in spinal cord and hypothalamus (Borodinsky et al., 2004; Dulcis and Spitzer, 2008).
Expression of Lmx1b and TPH but not Nkx2.2 is regulated by activity
To investigate the role of spontaneous Ca spike activity in the specification of 5-HT, we analyzed the incidence of Nkx2.2-IR, Lmx1b-IR and TPH-IR neurons in the raphe following perturbations of activity. Altering Ca spike activity by ion channel misexpression changed the number of Lmx1b-IR and TPH-IR neurons, but had no effect on the number of Nkx2.2-IR neurons assayed at stage 41 (Figure 2A-G). Increasing Ca spike frequency and incidence, by overexpression of Nav channels, produced a 28% decrease in the number of Lmx1b-IR plus TPH-IR neurons (control, 127±7; Nav, 91±8, n=11; Figure 2B, E, G). In contrast, decreasing activity, by overexpressing Kir channels, led to a 27% increase in the number of Lmx1b-IR plus TPH-IR neurons (control, 127±7; Kir, 161±8; n=12; Figure 2C, F, G). In larvae overexpressing Kir or Nav, as in controls, all TPH-IR neurons were Lmx1b-IR as well. Nkx2.2-IR neurons were also Sox2-IR (data not shown), consistent with their previously identified status as progenitor cells (Graham et al., 2003). The stability of the number of Nkx2.2-IR neurons indicates that expression of Nkx2.2 does not depend on the level of Ca spike activity (Figure 2A-C, G). To ensure that newly generated neurons were producing functional TPH we also stained for 5-HT directly. The number of 5-HT-IR neurons in the raphe followed the same pattern as TPH-IR neurons in larvae from embryos overexpressing Kir and Nav (data not shown). These results show that the specification of 5-HT is modulated by signaling initiated by Ca spikes and indicate that the expression of X. laevis Lmx1b (xLmx1b) is activity-dependent whereas the expression of X. laevis Nkx2.2 (xNkx2.2) is not.
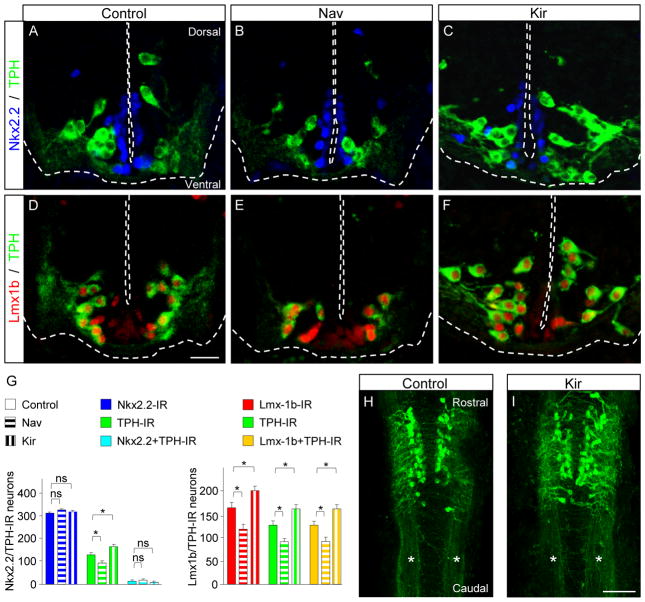
Figure 2. Calcium spikes regulate Lmx1b and TPH but not Nkx2.2 expression.
(A-F) Transverse sections of the rhombencephalon of stage 41 larvae stained for Nkx2.2 and TPH (A-C) or Lmx1b and TPH (D-E). Nkx2.2-IR neurons are located ventrally, symmetrically along the ventricle. The profile of expression of Nkx2.2 is the same in control larvae (A) and in larvae overexpressing Nav (B) and Kir (C) mRNA. Lmx1b-IR (D-F) and TPH-IR (A-F) neurons extend more laterally. A subset of Lmx1b-IR cells, closest to the midline, are not TPH-IR. In Nav mRNA-overexpressing larvae, the extent of Lmx1b and TPH staining is reduced (B, E). In Kir mRNA-overexpressing larvae, in contrast, the domain of expression of Lmx1b-IR and TPH extends laterally and dorsally along the midline (C, F). Lmx1b staining in the center of TPH-IR neurons (D) persists in Nav and Kir mRNA-overexpressing larvae (E, F). Scale bar, 40 μm.
(G) Quantitative analysis of Nkx2.2- and TPH-IR cells (left) and Lmx1b- and TPH-IR cells (right), demonstrating stability in the number of cells expressing Nkx2.2 and the decrease and increase in number of Lmx1b-IR and TPH–IR neurons in larvae overexpressing Nav and Kir mRNA, respectively.
(H,I) Wholemount preparations of the rhombencephalon of a control embryo (H) and a Kir mRNA-injected embryo (I) immunostained for TPH and Lmx1b. Stage 41. The general organization of the raphe nucleus and the compact lateral bundles of axons (*) are similar in both conditions. Scale bar, 160 μm. n≥9 (A-G), n≥6 (H,I); ns, no significant difference, * p<0.05. Kruskal-Wallis test with Conover post-hoc analysis.
Newly TPH-IR neurons observed in response to suppression of Ca spike activity significantly increased the number of serotonergic neurons. The axonal projections of all TPH-IR neurons observed in wholemount preparations appeared to be similar, projecting rostrally and caudally, suggesting that newly induced TPH-IR neurons and primary TPH-IR neurons send their axons to similar target regions (Figure 2H, I).
Alterations of activity regulate differentiation and not proliferation or patterning
Because activity can regulate proliferation of progenitor pools (Spitzer, 2006) and control neuronal patterning (Buonanno & Fields, 1999), we tested the involvement of these mechanisms in the change in incidence of TPH-IR by alterations of Ca spike activity. The stability of the number of Nkx2.2-IR neurons suggested that the effects of perturbing activity on the incidence of Lmx1b-IR and TPH-IR neurons do not engage these mechanisms. In addition, nuclear staining revealed that the number of cells is not altered by manipulation of Ca spike activity (Figure S4), consistent with the lack of effect on cell proliferation and cell death in the spinal cord and hypothalamus (Borodinsky et al., 2004; Dulcis and Spitzer, 2008). Specific labelling of proliferating cells by exposure of embryos to BrdU between stages 13 and 33/34 was unaffected by overexpression of Nav or Kir (Figure 3A-D). Because stages 13–41 constitute the period during which the neurons are generated and the number of TPH-IR neurons increases throughout this period (Figure S2), a proportion of TPH-IR neurons are labeled with BrdU in each condition. Overexpression of Nav or Kir had no discernable effect on patterning, evaluated by Otx2-IR identifying the isthmic organizer at the midbrain-hindbrain boundary (Figure 3E-G). Although these experiments do not exclude the possibility of changes in proliferation and patterning, they suggest that any such changes are small.
Figure 3. Calcium spikes regulate TPH expression through respecification of neurotransmitter phenotype rather than modulation of proliferation or patterning.
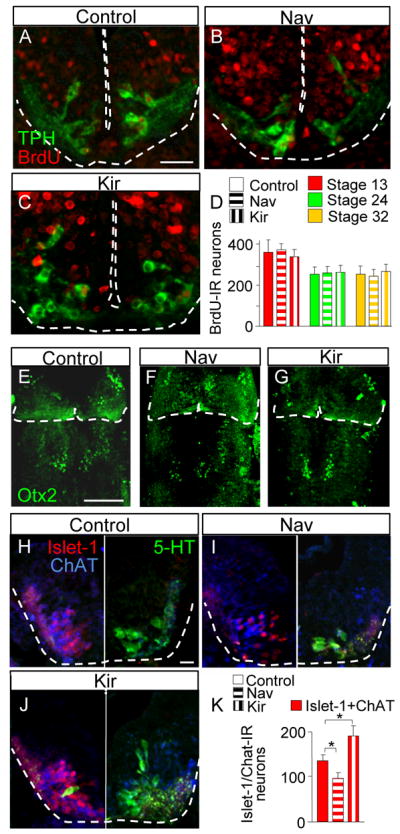
(A-C) Transverse sections of the rhombencephalon of stage 41 larvae from control embryos and embryos overexpressing Kir or Nav and exposed to BrdU for 6 hr during three periods of development. Scale bar, 60 μm.
(D) Quantitative analysis of BrdU-labeling, demonstrating stability in the number of cells expressing BrdU.
(E-G) Whole mounts of the hindbrain of stage 41 larvae from control embryos and embryos overexpressing Kir or Nav and stained for Otx2. Alteration of activity does not alter the midbrain-hindbrain boundary defined by expression of Otx2. Scale bar, 100 μm.
(H-J) Transverse sections of the rhombencephalon of stage 41 larvae from control embryos and embryos overexpressing Kir or Nav. Each image is a composite of two sections from the same embryo: the left half shows ChAT and islet-1 staining and the right half shows TPH staining 50 μm rostrally. Scale bar, 20 μm.
(K) The numbers of ChAT- and TPH-labeled cells vary in parallel rather than inversely in response to changes in activity. Thus the two populations are unlikely to be derived by redirecting the fates of cells in a common pool of Foxa2 progenitors and appear to reflect transfating of differentiating neurons. D, n≤3; K, n≤6. * p<0.05. Kruskal-Wallis test with Conover post-hoc analysis.
In contrast, activity appears to regulate transmitter transfating. To determine whether activity respecifies transmitter expression in progenitors, we took advantage of the origin of Lmx1b and Foxa2 cells from a common Nkx2.2 progenitor (Jacob et al., 2007). If activity respecifies Nkx2.2 progeny to Foxa2 cells that express ChAT, we expected to observe an inverse relationship between ChAT-IR and TPH-IR following manipulations of Ca spike activity. However perturbing activity led the number of ChAT- and TPH-IR cells to vary in parallel (ChAT-IR neurons in control, 137±12; Nav, 97±13; Kir, 190±23; n=6; Figure 3H-K). This result is expected from the homeostatic model proposed by Borodinsky et al. (2004), since both ACh and 5-HT are excitatory transmitters in this region of the nervous system, and suggests that activity is changing the transmitter fate of differentiating neurons (Figure 3H-K).
Previous work demonstrating the link between Lmx1b and the serotonergic phenotype in other species, in combination with the correlation between the changes in Lmx1b-IR and TPH-IR populations, raises the possibility of a causal connection between the two.
Altering xLmx1b expression changes the number of TPH-IR neurons
To ascertain whether xLmx1b expression can affect 5-HT specification, we tested the effect of knockdown and overexpression of this transcription factor on the number of TPH-IR neurons. The xLmx1b gene has been sequenced (Haldin et al., 2003; Haldin et al., 2008), enabling the design of a translation-blocking morpholino, xLmx1b-MO (Gene Tools, OR); a MO that does not recognize any known sequence in X. laevis was used as a control (CMO). We also generated xLmx1b RNA encoding the xLmx1b protein.
Bilateral injection of the xLmx1b-MO at the 2-cell stage led to a 27% decrease in the number of TPH-IR neurons in stage 41 larvae whereas the injection of the CMO had no effect (control, 127±7; xLmx1b-MO, 93±8; CMO, 138±12; n=9; Figure 4A, B, E, F). This result is commensurate with the reduction in the number of Lmx1b-IR neurons (control, 163±11; xLmx1b-MO, 128±6; CMO, 171±18; n=6). It is not clear why the reduction is not greater; the transcription factor cascade for 5-HT expression may be different in Xenopus and the mouse. Overexpression of Lmx1b using blastomere injection of xLmx1b mRNA led to a 31% increase in the number of TPH-IR neurons (control, 127±7; xLmx1b mRNA, 167±10; n=6; Figure 4A, C, F), again localized in the region of the raphe, and commensurate with the increase in the number of Lmx1b-IR neurons; no TPH-IR neurons were observed in the rostral spinal cord, up to 200 μm posterior to the most caudal TPH-IR neurons in the raphe. This result demonstrates the ability of the Lmx1b-RNA to induce TPH specification in the raphe and suggests that local environment and other factors are also required as shown in chick embryos (Cheng et al., 2003). To test the specificity of the effect of the xLmx1b-MO, we co-injected it with xLmx1b mRNA containing a 3 base pair mutation in the sequence targeted by the MO to suppress blockade. In larvae from co-injected embryos the presence of the xLmx1b-MO no longer induced a decrease in the number of TPH-IR neurons (xLmx1b-MO, 93±8; xLmx1b-MO+xLmx1b mRNA, 169±4; n=5; Figure 4A, D, F). These results confirm the role of Lmx1b expression in specification of the serotonergic phenotype in the raphe of X. laevis.
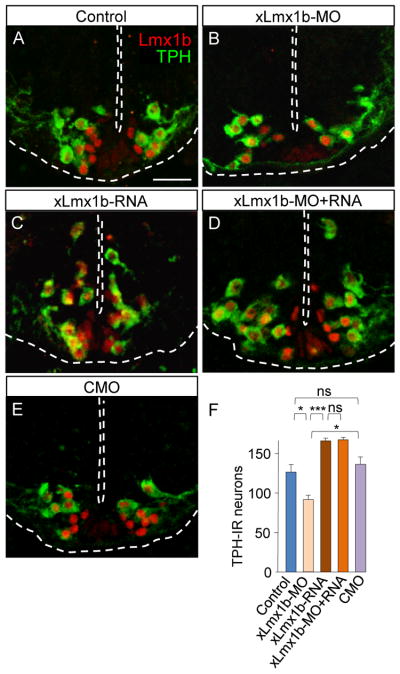
Figure 4. Changes in xLmx1b transcript expression alter the number of TPH-IR neurons.
(A-E) Transverse sections of the rhombencephalon of stage 41 larvae from control and xLmx1b-MO, xLmx1b-RNA, xLmx1b-MO+RNA, and CMO-injected embryos. Injection of the xLmx1b-MO reduces TPH staining (B). Overexpressing xLmx1b RNA increases the number of TPH-IR neurons (C). Overexpression of xLmx1b RNA rescues the xLmx1b-MO phenotype (D). Injection of a CMO does not change the number of TPH-IR neurons (E). Scale bar, 40 μm.
(F) Quantitative analysis of these data.
n≥7; ns, no significant difference,* p<0.05, ***p<0.001. Kruskal-Wallis test with Conover post-hoc analysis.
Manipulations of xLmx1b occlude the effects of alterations of activity
To investigate further the relationship between activity and xLmx1b expression in generation of the serotonergic phenotype, we combined alteration of activity with the manipulation of xLmx1b expression that produced the opposite effect on the number of TPH-IR neurons in stage 41 larvae. Coexpression of Nav and xLmx1b yielded a number of TPH-IR neurons similar to that obtained by xLmx1b overexpression alone (Nav +xLmx1b mRNA, 164±10; xLmx1b mRNA, 167±10; n=6; Figure 5A-D, I). There was no significant change in the number of Nkx2.2-IR neurons (data not shown). Overexpression of Kir combined with knockdown of xLmx1b led to a number of TPH-IR neurons similar to that achieved by xLmx1b knockdown alone (Kir+xLmx1b-MO, 83±4; xLmx1b-MO, 93±8; n=6; Figure 5E-H, I). Again the number of Nkx2.2-IR neurons was not significantly different from control (data not shown). We also examined the effect of Nav overexpression + xLmx1b knockdown and the effect of Kir overexpression + xLmx1b overexpression, and found that the results were not different from those of overexpression of the xLmx1b-MO and xLmx1b overexpression alone (Nav+xLmx1b-MO, 84±9; Kir+xLmx1b-RNA, 168±10; n=6; Figure 5E-H, I). Because the combined manipulation of activity and xLmx1b expression yielded a phenotype that was the same in all cases as the manipulation of xLmx1b alone, activity appears to exert its effect via Lmx1b. Furthermore, the stability of xNkx2.2 expression indicates that it is not regulated by activity, placing it upstream of the effect of activity on the modulation of 5-HT specification (Figure 5J). These results indicate that the interplay between events initiated by Ca signaling and xLmx1b expression is a critical modulator of serotonergic differentiation. It remains to be determined whether intracellular levels of Ca affect xLmx1b expression directly.
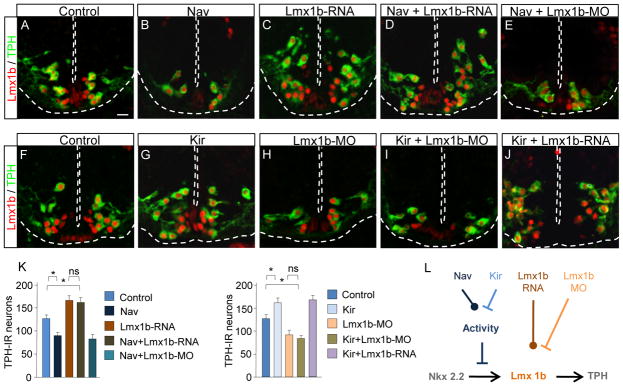
Figure 5. Changes in xLmx1b transcript expression occlude the effects of altered activity.
(A-H) Transverse sections of the rhombencephalon of stage 41 larvae. Reduction in the number of TPH-IR neurons by overexpression of Nav is reversed by overexpression of xLmx1b (A-D, I left). Increase in the number of TPH-IR neurons by overexpression of Kir is reversed by expression of the xLmx1b-MO (E-H, I right). Scale bar 40 μm.
(I) Quantitative analysis of TPH-IR neurons in the conditions described above.
(J) Schematic representation of the interactions between Ca spike activity and Lmx1b that regulate TPH expression. Activity acting downstream of Nkx2.2 reduces Lmx1b expression that in turn decreases expression of TPH and 5-HT. n≤7; ns, no significant difference, * p<0.05. Kruskal-Wallis test with Conover post-hoc analysis.
Activity-dependent changes in the number of TPH-IR neurons influence fictive swimming
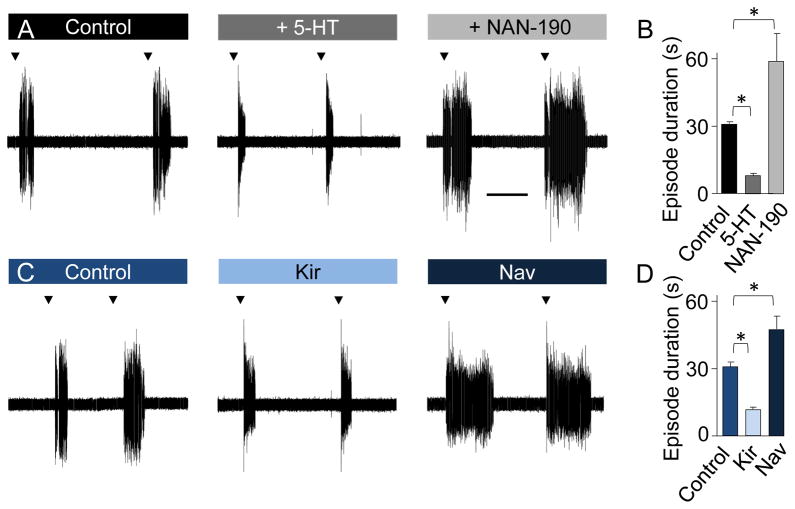
Since newly serotonergic neurons induced by suppression of Ca spike activity project their axons in alignment with those from the core population (Figure 2H, I), we hypothesized that activity-dependent modifications of the number of serotonergic neurons could have functional consequences. We studied fictive swimming because of the central role of locomotion in vertebrate behavior and the well-described role of 5-HT in its modulation (Sillar et al., 1998). Fictive swimming patterns consist of periods of successive action potential bursting called swimming episodes followed by periods of inactivity (Figure S5A). At stage 41, swimming is rarely initiated spontaneously but begins promptly in response to sensory stimulation. The termination of swimming episodes in Xenopus is largely due to the progressive activation of Ca-sensitive potassium channels, KCa, which hyperpolarize neurons (Dale and Kuenzi, 1997). Since 5-HT increases action potential burst duration and amplitude (Sillar et al., 1998), it is expected to induce an increased build-up of intracellular Ca leading to faster activation of KCa channels and more rapid hyperpolarization of interneurons that causes quicker arrest of swimming episodes. The basic parameters of the episodes we recorded in control larvae, such as their threshold for initiation and duration, were comparable to published values. The developmental evolution of burst parameters observed between stage 37/38 and stage 42/43 was also similar (Figure S5B) (Sillar et al., 1998). 5-HT reliably induces a decrease in episode duration mediated by activation of 5-HT1A receptors (Wedderburn and Sillar, 1994).
At stage 41, bath application of 5-HT (5 μM) reversibly decreased the swimming episode duration while bath application of NAN-190 (100 μM), a 5-HT1A receptor antagonist, reversibly increased the episode duration (control, 32±1 s; 5-HT, 8±1 s; NAN-190, 61±13s; n=11; Figure 6A, B). To test whether changes in the number of 5-HT neurons in the hindbrain led to similar modulation of fictive swimming, we performed ventral root recordings in control larvae and in larvae from embryos in which Kir or Nav mRNA had been overexpressed. The effect of increasing the number of TPH-IR neurons obtained by Kir overexpression paralleled the effect of bath application of 5 μM 5-HT in decreasing episode duration. In contrast, overexpression of Nav mRNA, decreasing the number of TPH-IR neurons, induced a significant increase in the episode duration, similar to the effect of application of NAN-190 (control, 32±1 s; Kir, 8±2s; Nav, 48±8s; n=5; Figure 6C, D).
Figure 6. Outcomes of calcium spike alteration mimic serotonin-dependent regulation of fictive swimming behavior.
(A) Traces showing 2 successive evoked swimming episodes (arrowheads) recorded from ventral roots of stage 41 control larvae (left), after application of 5-HT (5 μM, middle) and the 5-HT1A receptor antagonist, NAN-190 (100 μM, right). Application of exogenous 5-HT significantly reduces the duration of swimming episodes. The duration of episodes in the presence of NAN-190 is longer than in controls, indicating an ongoing basal level of activation of the network by endogenous 5-HT. Scale bar for (A) and (C), 20 sec.
(B) Average swimming episode durations in (A).
(C) Traces showing 2 successive swimming episodes recorded from ventral roots of control stage 41 larvae (left), and from larvae from embryos injected with Kir mRNA (middle) and embryos injected with Nav mRNA (right). The episode duration in Kir mRNA-overexpressing larvae is decreased, consistent with an increased basal level of serotonin. In contrast, the duration of episodes in larvae overexpressing Nav is longer than that in controls, suggesting a decrease in basal release of endogenous serotonin.
(D) Average swimming episode durations in (C).
n≥7; * p<0.05. Kruskal-Wallis test with Conover post-hoc analysis.
Because the neurons of the swimming central pattern generator could be directly hyperpolarized or depolarized by the presence of overexpressed potassium or sodium channels during our manipulations, we used hKir2.1 covalently linked to GFP to track the period of expression of the channels. Using an antibody to GFP, fluorescence was detected in injected embryos until stage 35/36 (2.5 days of development), one full day after the onset of serotonergic specification, but not when examined at stage 41 (between 3 and 4 days after injection) (Figure S3B-D). Thus, early global perturbations of Ca activity may exert effects on fictive swimming by changing the numbers of neurons expressing different transmitters (Borodinsky et al., 2004).
Decrease of TPH by targeted suppression of xLmx1b expression alters fictive swimming
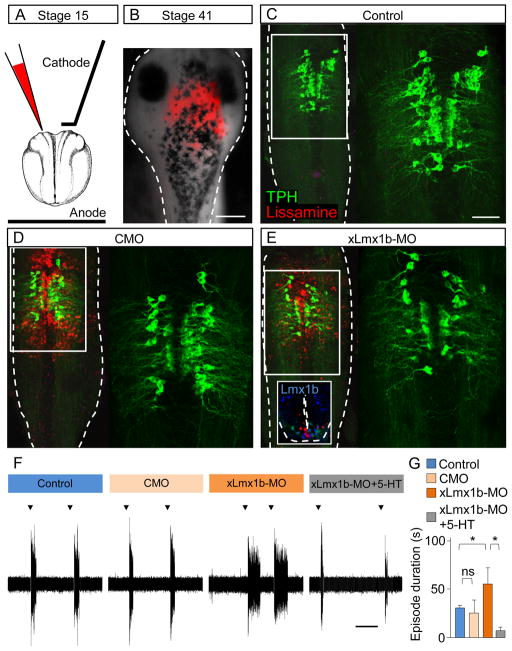
We then tested the hypothesis that manipulations of activity impact the duration of fictive swimming episodes principally through changes in the number of serotonergic neurons in the raphe achieved by changes in expression of xLmx1b. If this is the case, changing only the number of TPH-IR neurons in the raphe is expected to induce changes in the duration of swimming episodes similar to those observed following manipulations of activity. However global knockdown experiments are complicated by the fact that expression of xLmx1b is not limited to the raphe and a subpopulation of spinal cord neurons also express this gene. The behavioral phenotype of larvae injected with the xLmx1b-MO at the 2-cell stage could potentially arise from the knockdown of this transcription factor in the spinal cord. To overcome this problem we expressed the xLmx1b-MO in a restricted area of the hindbrain, including the raphe, by local electroporation.
MO oligonucleotides have a negative charge that makes them suitable for electroporation and a lissamine tag allows efficient screening of electroporation efficacy. We targeted the region of interest identified by the map of presumptive brain regions of the neural tube of stage 15, neurula stage, embryos (Eagleson and Harris, 1990) using either the xLmx1b-MO or the CMO (Figure 7A). Electroporated embryos were grown at 18°C to slow development, promote healing and allow a longer period of action. Larvae were analyzed at stage 41, approximately 5 days after electroporation (Figure 7B).
Figure 7. Focal suppression of endogenous xLmx1b transcript expression reduces 5-HT-dependent regulation of swimming behavior.
(A) Stage 15 embryos were electroporated with lissamine-tagged xLmx1b-MO or CMO. The presumptive raphe was targeted using a combination of local injection and a small platinum cathode.
(B) Effective targeting of the raphe was assessed by examining lissamine distribution (red) in living stage 41 larvae. A brightfield image is superimposed on the red fluorescence image. Scale bar, 200 μm.
(C-E) Wholemount preparations of stage 41 brains from control larvae (C), larvae from embryos electroporated with CMO (D), and larvae from embryos electroporated with xLmx1b-MO (E). TPH staining in the boxed region on the left of each image is shown on the right at higher magnification. Note the decreased number of TPH-IR neurons in xLmx1b-MO electroporated larvae (E) compared to controls and CMO-electroporated larvae (C,D). Scale bar 80 μm. The inset in panel (E) shows the ventral restriction of the electroporated Lmx1b-MO in a transverse section of the rhombencephalon of a stage 41 larva.
(F) Traces of evoked swimming episodes (black arrowheads) recorded from ventral roots illustrate the increase in episode duration in xLmx1b-MO-electroporated larvae (orange) compared to controls and CMO-electroporated larvae (blue and salmon respectively). Sensitivity to 5-HT is not completely abolished, as shown by the shortening of episode durations by application of 5μM 5-HT (grey). Scale bar, 60 sec.
(G) Mean swimming episode durations in these conditions.
n≥9; ns, no significant difference, * p<0.05. Kruskal-Wallis test with Conover post-hoc analysis.
The extent of the region of expression and the number of TPH-IR neurons were assessed using wholemount immunostaining (Figure 7C-E) and transverse sections (inset in Figure 7E). Larvae with lissamine fluorescence extending over a region of 300 to 600 μm encompassing the raphe were retained for examination. Only ventral cells were electroporated, so dorsal Lmx1b-expressing cells were likely unaffected; other cell types in the target zone do not express Lmx1b. Only larvae with no detectable expression of lissamine in the spinal cord were analyzed. Brains of larvae from xLmx1b-MO electroporated embryos had a decreased number of Lmx1b-IR and TPH-IR neurons in the raphe (control, 163±11 Lmx1b-IR, 127±7 TPH-IR; x-Lmx1b-MO, 127±6 Lmx1b-IR, 91±8 TPH-IR; n=4; Figure 7E). Local electroporation of the MO had no effect on patterning assessed by Otx2 expression (data not shown).
We next examined the effect on fictive swimming of changing the number of TPH-IR neurons in these larvae. When larvae were locally electroporated with the xLmx1b-MO, fictive swimming involved longer episodes than in control larvae or larvae from embryos electroporated with the CMO. Significantly, 5-HT decreased the duration of swimming episodes of xLmx1b-MO larvae, consistent with reduction in the level of 5-HT by the MO (control, 32±1 s; CMO, 26±13 s; xLmx1b-MO, 56±17 s; xLmx1b-MO+5-HT, 7±4 s; n≥9; Figure 7F, G). These results demonstrate the ability of local neurotransmitter respecification to influence neuronal activity at a network level.
Decreased incidence of TPH-IR neurons achieved by targeted knock-down of xLmx1b expression changes the behavior of freely swimming larvae
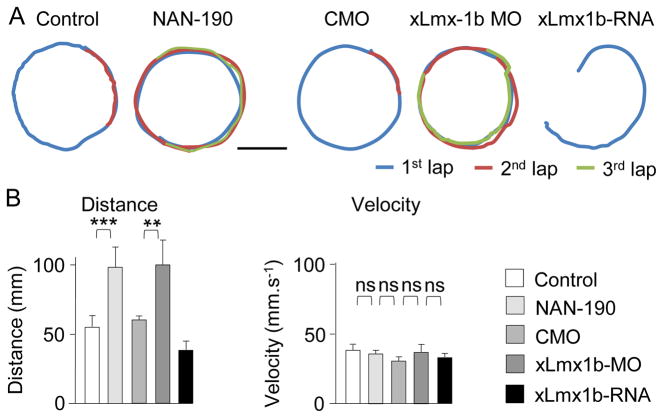
To address the relevance of local transmitter respecification for awake behaving animals, we took advantage of the effect of 5-HT on episode duration and investigated the impact of local Lmx1b-dependent recruitment of serotonergic neurons on freely swimming larvae. We hypothesized that free-swimming episode durations would be related to the number of TPH-IR neurons and tested the effect of local xLmx1b-MO electroporation using video analysis. A single larva was placed in a circular dish and swimming episodes were elicited by a tail poke. During an episode, the larva would typically swim a roughly circular trajectory along the edge of the dish for several seconds.
The fictive swimming data predicted that the lower the levels of 5-HT, the longer the swimming episodes. We first tested whether pharmacological blockade of 5-HT receptors affected swimming in these recording conditions. Bath application of NAN-190 to control larvae induced a rapid increase in the distance covered after each stimulus (control, 55±8 mm; NAN-190, 99±15 mm; n≥12; Figure 8). We then tested the effects of manipulations of Lmx1b expression. Overexpression of xLmx1b mRNA led to a shortened swimming distance (control, 55±8 mm; xLmx1b mRNA, 39±6 mm; n≥5) consistent with an increase in the incidence of serotonergic neurons. Larvae with raphe electroporation of the xLmx1b-MO swam greater distances than controls or larvae from embryos electroporated with the CMO (CMO, 61±3 mm; xLmx1b-MO, 100±18 mm; n≥12; Figure 8; Movie S2). The average trajectory around the edge of the dish and the average velocity were equivalent in all conditions tested, indicating that the duration of the episode is the principal parameter affected by the level of 5-HT (control, 42±4 mm.s−1; NAN-190, 46±5 mm.s−1; CMO, 53±2 mm.s−1; xLmx1b-MO, 54±3 mm.s−1; n≥12; Figure 8B). Free swimming episodes are shorter than fictive swimming episodes, likely because of more active sensory inputs. However these data are consistent with the longer episodes observed in fictive swimming experiments for xLmx1b-MO-electroporated larvae and suggest that the plasticity of neurotransmitter phenotype has functional consequences at the behavioral level.
Figure 8. Suppression of endogenous xLmx1b transcript expression alters free-swimming larval behavior.
(A) Average trajectories of stage 41 larvae during evoked free-swimming episodes in different conditions. In the control condition and for larvae from embryos electroporated with the CMO, the average distance corresponds to one lap of the dish. Acute application of NAN-190 to unelectroporated larvae or embryonic raphe electroporation with xLmx1b-MO increases the average distance covered during single evoked swimming episodes and larvae frequently achieve two laps. Scale bar, 10 mm.
(B) Average distance (left) and velocity (right) for each condition, demonstrating the parallel effects of NAN-190 application and local xLmx1b-MO electroporation on distance covered. The average velocity of larvae during an evoked swimming cycle remains approximately constant across conditions.
n≥7; ns, no significant difference, ** p<0.01, ***p<0.001. Kruskal-Wallis test with Conover post-hoc analysis.
DISCUSSION
Our results demonstrate Ca spike activity-dependent regulation of the specification of 5-HT in the raphe of X. laevis during development. Serotonergic specification of a core population of neurons is independent of activity, but decreasing or increasing activity generates an increase or decrease in the number of serotonergic neurons that is functionally significant. The expression of xLmx1b, a transcription factor involved in the specification of 5-HT, is also regulated by Ca activity: the lower the activity, the larger the number of neurons expressing xLmx1b. The expression of this transcription factor is required for the activity-dependent increase in incidence of serotonergic neurons. Proliferation of progenitor pools and neuronal patterning appear to be unaffected. We demonstrate the impact of the change in numbers of serotonergic neurons on swimming of Xenopus larvae. These data link the activity-dependent expression of a transcription factor with its effect on transmitter specification and functional consequences for freely behaving animals.
Ca spikes and plasticity of the serotonergic phenotype
We report spontaneous Ca spikes generated in the rhombencephalon of developing Xenopus embryos. They are produced during the period of serotonergic specification and their incidence, frequency, and duration are in the same range as those in the spinal cord, making them good candidates for activity-dependent modulation of developmental processes (Spitzer, 2006). They are present at later stages than those reported for the spinal cord, consistent with a caudal-to-rostral gradient of development. The presence of spontaneous Ca spikes in neurons already expressing 5-HT suggests that Ca activity may fulfill another function such as specification of additional neurotransmitters or modulation of other aspects of differentiation. The higher frequency of spontaneous spiking in these cells implies that a low frequency is not necessary for the maintenance of the serotonergic phenotype. Plasticity of the phenotype may remain at later stages, as described for dopaminergic neurons (Dulcis and Spitzer, 2008), and different mechanisms may be involved in more mature networks due to different sources of Ca entry such as those of synaptic origin (Bading et al., 1993; Cohen and Greenberg, 2008; Hardingham et al., 2001).
Given the predominantly excitatory effects of 5-HT in Xenopus (Roubos et al., 2005; Sillar et al., 1998), the changes in numbers of serotonergic neurons induced by perturbations of activity are consistent with the homeostatic model of neurotransmitter respecification whereby suppression of activity leads to increases in the numbers of neurons expressing excitatory transmitters (Borodinsky et al., 2004). Low spiking frequency may be necessary to promote the switch to a serotonergic phenotype, and higher frequencies of Ca spikes in Nav mRNA-overexpressing embryos may block this switch. Higher spike frequencies are predicted to lead to a decrease in the number of neurons displaying an excitatory transmitter phenotype, as a compensatory mechanism in response to increased spontaneous activity (Borodinsky et al., 2004).
Activity-dependent and -independent factors delimit domains of expression for xLmx1b and the serotonergic phenotype
Transmitter phenotype is initially specified through a regulatory network of transcription factors (Goulding and Pfaff, 2005). Our findings provide evidence for activity-dependent regulation of this network. The expression of xLmx1b is affected by the endogenous level of Ca spiking, whereas the expression of xNkx2.2 is not. This result indicates that activity does not exert a global effect on all transcription factors involved in the specification of 5-HT and supports the hypothesis that activity influences choices of differentiation at critical crossroads for fate selection (Marek et al., in press). Future experiments will determine whether this is specific to the synthesis of 5-HT or a general rule for activity-dependent specification of transmitters. Our results demonstrate that Lmx1b is necessary for activity-dependent specification of 5-HT in the X. laevis raphe, extending the conserved role of this transcription factor in the specification of serotonergic neurons (Cheng et al., 2003; Ding et al., 2003) to amphibia. Indeed, a decrease in spontaneous Ca spike activity no longer induces an increase in 5-HT specification if xLmx1b expression has been knocked down.
The spatial restriction of activity-dependent 5-HT overexpression in the ventral half of the larval hindbrain, to the region of the raphe, suggests that limiting factors are involved. In contrast, no spatial restrictions were detected for the changes in expression of the four transmitters studied in the larval spinal cord (Borodinsky et al., 2004). Nkx2.2, Lmx1b and Pet1 have been shown to be necessary to induce expression of the serotonergic phenotype in mice (Cheng et al., 2003). The ability of neurons to express Lmx1b in an activity-dependent manner may be one of the limiting factors. On this view, expression of activity-dependent transcription factors is expected to define domains within which the number of neurons synthesizing a given transmitter is fine-tuned and adjusted to the environment.
The inverse correlation between activity level and xLmx1b expression could result from activity-dependent repression of xLmx1b, through regulatory elements such as REST or MeCP2 in the xLmx1b promoter. Alternatively, the effect of activity may be indirect, and activity-dependent activation of a set of transcription factors through CRE, USF or CaRF sites could in turn induce the repression of xLmx1b. 5-HT itself may be involved in a regulatory feedback loop influencing its own specification, since the 5-HT2A receptor modulates early spontaneous activity in mouse hindbrain (Hunt et al., 2006). Examination of the effect of local suppression of these receptors on Ca spike frequency and transmitter phenotype could be revealing.
Functional consequences of transmitter phenotype plasticity
We have demonstrated the influence of activity-driven, Lmx1b-dependent specification of 5-HT on swimming behavior. Manipulations of activity led to changes in numbers of serotonergic neurons, but this took place in the context of changes in the numbers of neurons expressing other transmitters (Borodinsky et al., 2004). Moreover, persistence of low levels of exogenously expressed currents could have confounded measurements of fictive swimming parameters. To address these concerns we used electroporation to provide greater spatial and temporal control. Local knockdown of xLmx1b expression induced a specific decrease in the number of TPH neurons in the raphe.
Genetic deletion of Lmx1b in mouse serotonergic neurons leads to their death and behavioral compensation, precluding tests of serotonin-dependent behavior (Zhao et al., 2006). However, targeted alterations of activity (Deisseroth et al., 2006) provide powerful tools with which to change the transmitter phenotype in identified neuronal populations. Expression of key activity-dependent transcription factors in a cell type-specific manner (Goulding, 2009; Zhang et al., 2008) could be used to restrict the extent of these manipulations.
Advantages and implications of activity-dependent specification of transmitters
Genetic programs are critical to the appropriate unfolding of developmental processes. However a standardized and stereotyped application of genetically encoded development would be unlikely to satisfactorily accommodate the diversity of different environments in which development takes place. Other mechanisms provide the nervous system with ways in which to calibrate expression of different molecules and make adjustments to achieve optimal balance (Burrone et al., 2002; Lin et al., 2008). The plasticity of neurotransmitter phenotype constitutes an adaptive mechanism through which epigenetic factors can be taken into account.
The regulation of 5-HT specification by Ca spikes provides the nervous system with a powerful way to adjust the properties of locomotor networks at the cellular level. This form of regulation can have broad impact on these networks since several neuronal populations express 5-HT receptors. For instance, 5-HT has been shown to modulate maturation of the GABAergic system in the rat spinal cord (Branchereau et al., 2002) as well as synaptogenesis (Gaspar et al., 2003; Niitsu et al., 1995). The serotonergic system is also involved in recovery of locomotor function after spinal cord lesions in the rat (Antri et al., 2003), for which plasticity in the number of serotonergic neurons could be beneficial.
Further studies are needed to determine whether physiological stimuli relevant to the embryos’ behavior, such as mechanical stimuli, stress, and temperature, can trigger activity-dependent 5-HT specification. Xenopus is a poikilothermic species, which renders it dependent on external temperature. Animals acclimated at a given temperature perform better at their acclimated temperature than non-acclimated peers (Wilson et al., 2000). More recently, temperature has been shown to have an acute affect on the fictive swimming of stage 42 larvae (Sillar and Robertson, 2009). It will be interesting to investigate the role of temperature on spontaneous Ca spike activity and neurotransmitter specification.
Pathological activity also affects specification of transmitters. Epileptic seizures respecify transmitter expression in the mouse dentate gyrus, promoting appearance of GABA in glutamatergic granule cells (Gutierrez et al., 2003). Serotonin 5-HT1A receptor immunoreactivity is decreased in CA1 interneurons of seizure-prone gerbils (Kim et al., 2007). A recent study in which pentylenetetrazole and other classic epileptogenic drugs induced reproducible seizure behavior show that Xenopus is a potent model for epilepsy studies (Hewapathirane et al., 2008). The consequences of such ictal activities on transmitter specification have yet to be determined. The clinical importance of epilepsy, its high prevalence during development and the links between epilepsy and the serotonergic system (Bagdy et al., 2007) make this issue a compelling one to investigate.
EXPERIMENTAL PROCEDURES
Embryos and larvae
Adult Xenopus laevis females were primed and injected with human chorionic gonadotropin (Sigma, St. Louis, MO) to induce ovulation. Eggs were fertilized in vitro and embryos and larvae were grown at 21–23 °C, unless specified otherwise, in 0.1 Marc’s modified Ringer’s solution (MMR; 1 MMR contains 100 mM NaCl, 2 mM KCl, 1 mM MgSO4, 5 mM HEPES, 0.1 mM EDTA, 2 mM CaCl2, pH adjusted to 7.8) and staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). All animal procedures were performed in accordance with institutional guidelines and approved by the UCSD Institutional Animal Care and Use Committee.
Immunocytochemistry
Embryos and larvae were fixed in 4% paraformaldehyde phosphate-buffered saline (pH 7.4) for 2 hr at 4°C, incubated in 30% sucrose for 2.5 hr or overnight and embedded in OCT (Fisher Scientific). Transverse 10 μm frozen sections were made through the whole brain and regions of interest were identified anatomically afterwards. For wholemount analysis, specimens were incubated in PBS-Triton 0.5% for 3 days after the fixation protocol. The CNS was then dissected and processed through the immunostaining protocol below. Slides and wholemounts were incubated in a blocking solution of 1% fish gelatin for 0.5 hr at 20 C, followed by overnight incubation with primary antibodies at 4 °C and 2 hr incubation with fluorescently tagged secondary antibodies at 20 °C. After incubation with secondary antibodies, wholemount preparations were incubated in benzyl-benzoate until cleared and then mounted. The following primary antibodies were used: TPH (sheep, 1:250), 5-HT (rat, 1:50), GABA (guinea pig, 1:100) and ChAT (goat, 1:100) from Chemicon (Temecula, CA); Lmx-1b (mouse, 1:20 - supernatant), Nkx2.2 (mouse, 1:50 - concentrate), Lim1/2, and Islet-1 from the Developmental Studies Hybridoma Bank (Chicago, IL); GFP (chicken, 1:500) and Otx2 (rabbit, 1:100) from Abcam (Cambridge, MA); BrdU (rat, 1:20) from AbD Serotec (Raleigh, NC); and Sox2 (goat, 1:100) from R&D Systems (Minneapolis, MN). Immunoreactivity was examined on a Leica SP5 laser confocal system (Leica Microsystems, Wetzlar, Germany) with a 20× 0.7 N.A. water immersion objective, or on a Zeiss Axioskop (Carl Zeiss imaging, Thornwood, NY) with a 40× 0.65 N.A. air objective or a 20× 0.5 N.A. air objective using a Xenon arc lamp, attenuated by neutral density filters and the appropriate excitation and emission filters for Alexa 488, Alexa 350, Alexa 594 and Alexa 647 fluorophores. Images were acquired and analyzed with NIH Image, Image J (W. Rasband, NIH), Axiovision (Carl Zeiss imaging) and Leica Application Suite (Leica Microsystems). Cell counts were performed based on DAPI (Vector, Burlingame, CA) or DRAQ5 (Biostatus, Leicester, UK) nuclear staining using the Image-based Tools for Counting Nuclei plugin for Image J (Center for Bio-image Informatics, Santa Barbara, CA).
BrdU staining
The birthdates of cells in the Xenopus raphe have been established by thymidine labeling (Van Mier P., 1986). The peak of proliferation occurs around stage 13 but significant proliferation is detected at all later stages studied. Dividing cells in control, Kir-expressing and Nav-expressing embryos were labeled with BrdU (Sigma, 4 mg/mL) during three 6 hr periods (stages 13–19, 25–28, and 32–33/34) and stained for TPH at stage 41.
Imaging
Fluorescence of the Ca indicator Fluo4-AM (Invitrogen, Carlsbad, CA) was used to study elevations of [Ca]i in neurons. Hindbrain serotonergic neurons were exposed by ventral dissection of embryos at stages 28 and 35/36 and incubated for 30 min to 1 hr in saline containing 2–5 μM of indicator and 0.01% Pluronic F-127. Images were acquired at 0.2 Hz for 30 min or 1 hr periods with a BioRad MRC 1024 with a 20× 0.4 N.A. water immersion objective or Leica SP5 laser confocal system with a 20× 0.7 N.A. water immersion objective and transferred to a computer. Image stacks were imported into Image J and analyzed using Multi Measure (W. Rasband, B. Dougherty), Measure Stack (Optinav, Redmond, WA) and custom Image J plugins. Traces from selected regions of interest are presented as F-F0/F0. Ca spike incidence was scored as the number of cells generating transients divided by the number of cells in the imaged field and expressed as percent. Frequency was calculated as the total number of transients in a cell divided by the total acquisition time and expressed as spikes per hr.
To match Ca spike profile with cell identity the imaged brains were processed as described above for wholemounts except that preparations were kept immobile in the same position through the procedure to facilitate matching images of Fluo-4 and immunoreactivity. 20 μm (one cell diameter) z-stacks of immunostained hindbrains were used to align the images.
Plasmids and morpholinos
hKir2.1, hKir2.1-GFP, rNav1.2 and xLmx1b DNA constructs were generous gifts of Eduardo Marban, William Catterall and Elizabeth Jones. These genes were subcloned into a pBluescript or pcDNA3 vector and mRNA was transcribed using the mMessage mMachine kit (Ambion, Austin, TX). Capped RNA (5–10 nl of a 0.01–0.1 mg/ml RNA solution in 10% MMR, 6% Ficoll) was co-injected with a lineage tracer, either Cascade Blue or Rhodamine Red 30 kDa dextran (30 mg/ml) or Alexa 568 10 kDa dextran (15 mg/ml), into both blastomeres at the 2-cell stage using a picospritzer (Picospritzer III, Parker Instruments, Cleveland, OH). Control injections consisted of fluorescent dextran alone. A 5′-lissamine-tagged xLmx1b morpholino was designed and supplied by GeneTools (5′ ATCCATGCCACTCTCCAAAACTCAC-3′). A control MO (CMO) was used as a control for the specificity of the effect of the xLmx1b-MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′). 8–10 ng of MO were injected in each blastomere at the two-cell stage.
Electrophysiology
Glass suction electrodes placed on axon bundles in intermyotomal clefts of Xenopus laevis larvae provide extracellular recordings of the activity of motoneuron axons in anesthetized animals. Because this is the motor control that would have induced swimming in the awake animal it is referred to as “fictive swimming.” Incisions in the dorsal and ventral fins of 3-day old larvae were made under MS222 anesthesia and larvae were exposed to 10 μM α-bungarotoxin (Tocris, Ellisville, MO) until immobilization (~15 minutes). Larvae were transferred to a bath with 21–23 C saline (in mM: 120 NaCl, 2.5 KCl, 4 CaCl2, 1 MgCl2, 15 NaHCO3; pH 7.6) perfused at a rate of 2 ml.s−1. They were pinned through the notochord and the trunk skin was removed on one side to expose upper-trunk myotomes. The impulse activity of motor axons was recorded extracellularly from ventral roots by placing glass suction electrodes over the 5th to 13th intermyotomal clefts, numbered rostro-caudally from the level of the otic capsule. Electrodes were made from glass capillaries without inner filament (1 mm outer diameter; Clarke Electromedical Instruments, Reading, UK), pulled with a micropipette puller (model P-87, Sutter Instruments, Novato, CA) to produce an approximately 50 μm tip opening when fire polished over an open flame. Ventral root activity was recorded with a differential amplifier (model 1700; A-M Systems, Sequim, Wash., USA) and fictive swimming episodes were elicited by brief electrical pulses through another suction electrode applied to the tail skin (Kahn and Roberts, 1982; Sillar et al., 1991; Sillar and Roberts, 1988). Data were digitized with a Digidata interface card (Axon Instruments - MDS, Toronto, Canada) to a computer and analyzed with pClamp 8.1 (Axon Instruments). 5-HT (5 μM) (Sigma Aldrich, St Louis, MO) and NAN-190 (100 μM) (Tocris) were applied through the bath perfusion system.
Electroporation
Local electroporation was performed on stage 15/16 embryos, ~18 h post fertilization, using a protocol based on the method developed by Sasagawa et al. (Sasagawa et al., 2002). Embryos were placed on an agarose bed over a circular platinum anode (20 mm in diameter, NEPA GENE Co., Ltd., Chiba, Japan), in a 1X MMR solution to decrease the resistance between the 2 electrodes. 5 nl of 0.25 μg/μl morpholino solutions were injected into the presumptive raphe region of the closing neural tube (Eagleson and Harris, 1990). A platinum cathode (platinum wire, 0.5 mm diameter; Sigma) was then placed over the region of interest immediately after injection. A Grass stimulator was used to deliver 6 square pulses of 5 msec, 40 volts, at 500 msec intervals. Electroporated embryos were transferred to 10% MMR and kept at 18° C overnight to facilitate healing. Non-electroporated embryos grown in the same conditions served as controls.
Swimming analysis
Single stage 41 larvae were placed in a 22.1 mm diameter circular dish in 3 ml of 10% MMR. Since swimming performance is affected by temperature (Sillar and Robertson, 2009), the temperature of the solution was monitored during the course of the experiments and remained between 21–23 C. The order of conditions tested was varied randomly to avoid pattern effects. Swimming episodes were elicited by a touch of the tail with a fine glass pipette. Images (640×480 pixel resolution) were acquired at 15 Hz using a Wild dissection microscope with a C-Mounted Hitachi KP-M1 monochrome CCD camera (Image Labs International, Bozeman, MT), digitized using a Data Translation frame grabber card (DT3155) controlled with the Matlab (Mathworks Natick, MA) Image Acquisition Toolbox on a computer. Each embryo was tested at least 6 times with a minimum of 1 minute interval between trials. The distance covered, the trajectory, and the duration of swimming were measured and the velocity was calculated using the Manual Tracking plugin (Fabrice Cordelieres, France) for Image J; values were averaged using custom Excel tables.
Statistical analysis
Data are presented as mean±SEM. Statistical differences were determined with the online software Brightstat (brightstat.com) using either the Mann Whitney U test or the Kruskal-Wallis test with Conover post-hoc analysis for k independent samples (* p<0.05; ** p,0.01; *** p<0.001).
Supplementary Material
Acknowledgments
We thank Xavier Nicol, Krista Todd and the Kristan lab for technical support, members of our laboratory for thoughtful discussion, and Davide Dulcis, Kurt Marek and Xavier Nicol for their comments on the manuscript. This work was supported by NIH NS15918 to N.C.S.
Footnotes
The supplemental data include 5 figures and 3 videos and can be found online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alenina N, Bashammakh S, Bader M. Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2006;2:5–10. doi: 10.1007/s12015-006-0002-2. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Popa D, Fabre V, Bouali S, Venault P, Lesch KP, Hamon M, Adrien J. Early life blockade of 5-hydroxytryptamine 1A receptors normalizes sleep and depression-like behavior in adult knock-out mice lacking the serotonin transporter. J Neurosci. 2006;26:5554–5564. doi: 10.1523/JNEUROSCI.5156-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur J Neurosci. 2003;18:1963–1972. doi: 10.1046/j.1460-9568.2003.02916.x. [DOI] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem. 2007;100:857–873. doi: 10.1111/j.1471-4159.2006.04277.x. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Chapron J, Meyrand P. Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. J Neurosci. 2002;22:2598–2606. doi: 10.1523/JNEUROSCI.22-07-02598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Cheng L, Chen CL, Luo P, Tan M, Qiu M, Johnson R, Ma Q. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes SP. Molecular genetics of the early development of hindbrain serotonergic neurons. Clin Genet. 2005;68:487–494. doi: 10.1111/j.1399-0004.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- Dale N, Kuenzi FM. Ion channels and the control of swimming in the Xenopus embryo. Prog Neurobiol. 1997;53:729–756. doi: 10.1016/s0301-0082(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Spitzer NC. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature. 2008;456:195–201. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson GW, Harris WA. Mapping of the presumptive brain regions in the neural plate of Xenopus laevis. J Neurobiol. 1990;21:427–440. doi: 10.1002/neu.480210305. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr Opin Neurobiol. 2005;15:14–20. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the “glutamatergic” granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldin CE, Masse KL, Bhamra S, Simrick S, Kyuno J, Jones EA. The lmx1b gene is pivotal in glomus development in Xenopus laevis. Dev Biol. 2008;322:74–85. doi: 10.1016/j.ydbio.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Haldin CE, Nijjar S, Masse K, Barnett MW, Jones EA. Isolation and growth factor inducibility of the Xenopus laevis Lmx1b gene. Int J Dev Biol. 2003;47:253–262. [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998;83:1185–1201. doi: 10.1016/s0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hewapathirane DS, Dunfield D, Yen W, Chen S, Haas K. In vivo imaging of seizure activity in a novel developmental seizure model. Exp Neurol. 2008;211:480–488. doi: 10.1016/j.expneurol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Hunt PN, Gust J, McCabe AK, Bosma MM. Primary role of the serotonergic midline system in synchronized spontaneous activity during development of the embryonic mouse hindbrain. J Neurobiol. 2006;66:1239–1252. doi: 10.1002/neu.20259. [DOI] [PubMed] [Google Scholar]
- Kahn JA, Roberts A. Experiments on the central pattern generator for swimming in amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982;296:229–243. doi: 10.1098/rstb.1982.0004. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kim JE, Kwak SE, Kim DW, Choi SY, Kwon OS, Kang TC. Seizure activity selectively reduces 5-HT1A receptor immunoreactivity in CA1 interneurons in the hippocampus of seizure-prone gerbils. Brain Res. 2007;1154:181–193. doi: 10.1016/j.brainres.2007.03.084. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El MS, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Tannhauser B, Stigloher C, Kremmer E, Bally-Cuif L. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev Dyn. 2007;236:1072–1084. doi: 10.1002/dvdy.21095. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus Laevis. Daudin; North Holland, Amsterdam: 1967. [Google Scholar]
- Niitsu Y, Hamada S, Hamaguchi K, Mikuni M, Okado N. Regulation of synapse density by 5-HT2A receptor agonist and antagonist in the spinal cord of chicken embryo. Neurosci Lett. 1995;195:159–162. doi: 10.1016/0304-3940(95)11805-7. [DOI] [PubMed] [Google Scholar]
- Root CM, Velazquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubos EW, Scheenen WJ, Jenks BG. Neuronal, neurohormonal, and autocrine control of Xenopus melanotrope cell activity. Ann N Y Acad Sci. 2005;1040:172–183. doi: 10.1196/annals.1327.022. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Eaton MJ, Mather P, Lindsay RM, Whittemore SR. CNTF induces raphe neuronal precursors to switch from a serotonergic to a cholinergic phenotype in vitro. Mol Cell Neurosci. 1996;7:204–221. doi: 10.1006/mcne.1996.0016. [DOI] [PubMed] [Google Scholar]
- Rumajogee P, Madeira A, Verge D, Hamon M, Miquel MC. Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms. J Neurochem. 2002;83:1525–1528. doi: 10.1046/j.1471-4159.2002.01264.x. [DOI] [PubMed] [Google Scholar]
- Sasagawa S, Takabatake T, Takabatake Y, Muramatsu T, Takeshima K. Improved mRNA electroporation method for Xenopus neurula embryos. Genesis. 2002;33:81–85. doi: 10.1002/gene.10094. [DOI] [PubMed] [Google Scholar]
- Scott MM, Deneris ES. Making and breaking serotonin neurons and autism. Int J Dev Neurosci. 2005;23:277–285. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Reith CA, McDearmid JR. Development and aminergic neuromodulation of a spinal locomotor network controlling swimming in Xenopus larvae. Ann N Y Acad Sci. 1998;860:318–332. doi: 10.1111/j.1749-6632.1998.tb09059.x. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Roberts A. A neuronal mechanism for sensory gating during locomotion in a vertebrate. Nature. 1988;331:262–265. doi: 10.1038/331262a0. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Robertson RM. Thermal activation of escape swimming in post-hatching Xenopus laevis frog larvae. J Exp Biol. 2009;212:2356–2364. doi: 10.1242/jeb.029892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillar KT, Wedderburn JF, Simmers AJ. The development of swimming rhythmicity in post-embryonic Xenopus laevis. Proc Biol Sci. 1991;246:147–153. doi: 10.1098/rspb.1991.0137. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- van Mier P, Joosten HW, van RR, ten Donkelaar HJ. The development of serotonergic raphespinal projections in Xenopus laevis. Int J Dev Neurosci. 1986;4:465–475. doi: 10.1016/0736-5748(86)90028-6. [DOI] [PubMed] [Google Scholar]
- Wedderburn JF, Sillar KT. Modulation of rhythmic swimming activity in post-embryonic Xenopus laevis tadpoles by 5-hydroxytryptamine acting at 5HT1a receptors. Proc Biol Sci. 1994;257:59–66. doi: 10.1098/rspb.1994.0094. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56:479–485. doi: 10.1016/s0361-9230(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Wilson RS, James RS, Johnston IA. Thermal acclimation of locomotor performance in tadpoles and adults of the aquatic frog Xenopus laevis. J Comp Physiol B. 2000;170:117–124. doi: 10.1007/s003600050266. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Narayan S, Geiman E, Lanuza GM, Velasquez T, Shanks B, Akay T, Dyck J, Pearson K, Gosgnach S, Fan CM, Goulding M. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60:84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RW, Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Brain Res Dev Brain Res. 2000;119:33–45. doi: 10.1016/s0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.