Summary
In the retina, it is not well understood how visual processing depends on AMPA- and NMDA-type glutamate receptors. Here, we investigated how these receptors contribute to contrast coding in identified guinea pig ganglion cell types, in vitro. NMDA-mediated responses were negligible in ON α cells but substantial in OFF α and δ cells. OFF δ cell NMDA receptors were composed of GluN2B subunits. Using a novel deconvolution method, we determined the individual contributions of AMPA, NMDA and inhibitory currents to light responses of each cell type. OFF α and δ cells used NMDA receptors for encoding either the full contrast range (α), including near-threshold responses, or only a high range (δ). However, contrast sensitivity depended substantially on NMDA receptors only in OFF α cells. NMDA receptors contribute to visual contrast coding in a cell-type specific manner. Certain cell types generate excitatory responses using primarily AMPA receptors or disinhibition.
Introduction
Excitatory synaptic transmission in the retina, as elsewhere in the CNS, is mediated primarily by glutamate neurotransmission. Cone photoreceptors release glutamate onto bipolar cells, which express either ionotropic (kainate, AMPA) or metabotropic (mGluR6) receptors and thereby initiate parallel OFF and ON pathways (Nakajima et al., 1993; DeVries, 2000; Miller, 2008). Bipolar cells release glutamate onto both amacrine cells (interneurons) and ganglion cells (output neurons), which collectively express multiple receptor types (Wassle, 2004; Dumitrescu et al. 2006; Miller, 2008). Most of the ~15–20 types of ganglion cell express both AMPA and NMDA receptors (Aizenman et al., 1988; Karschin et al., 1988; Massey and Miller, 1990; Mittman et al., 1990; Diamond and Copenhagen, 1993; Lukasiewicz et al., 1997; Cohen, 2000; Cohen and Miller, 1994; Fletcher et al., 2000; Jacoby and Wu, 2001; Grunert et al., 2002; Sagdullaev et al., 2006). However, the precise role for ganglion cell NMDA receptors in visual processing is not well understood.
It has been suggested that ganglion cell AMPA and NMDA receptors play complementary roles in synaptic transmission. Ganglion cell NMDA receptors (NMDARs) show the characteristic voltage-dependence, due to Mg2+ block at hyperpolarized potentials, resulting in a ‘J-shaped’ current-voltage (I-V) relationship (Figure 1) (Dingledine et al., 1999; Erreger et al., 2004). Thus, within the physiological range (−70 to −40 mV), NMDARs experience increased driving force with depolarization, offsetting the decreased driving force of AMPA receptors (AMPARs); the combination could generate excitatory synaptic currents with amplitudes that are voltage-independent (Diamond and Copenhagen, 1993; 1995). Furthermore, in some cells AMPARs encode spontaneous, low frequency presynaptic release, whereas NMDARs encode evoked, high frequency or multivesicular release (Taylor et al., 1995; Matsui et al., 1998; Chen and Diamond, 2002; Sagdullaev et al., 2006). Thus, the two receptors could collectively encode a wide range of release frequencies. NMDARs composed of GluN2B subunits (i.e., new nomenclature for NR2B subunits; Collingridge et al., 2009) localize extrasynaptically, suggesting a specialized role in detecting multivesicular release (Kalbaugh et al., 2009; Zhang and Diamond, 2009). However, it is not clear whether extrasynaptic receptors contribute to visual processing under physiological conditions in intact circuits (Sagdullaev et al., 2006).
Figure 1. Differential NMDA receptor expression across ganglion cell types.
A. Puffed NMDA application generated a response in OFF α cells (top) that persisted in the presence of ifenprodil (10 μM), which blocks NMDARs composed of the GluN2B (NR2B) subunit (bottom). Here and elsewere, Vholds (Vh;in mV) for inset traces are indicated by color. Gray strip shows the time window used to generate the I-V plot.
B. NMDA response in an OFF δ cell (top) was suppressed by ifenprodil (bottom).
C. NMDA response in an ON α cell (top) was suppressed by ifenprodil (bottom). The response represents the largest measured among ON α cells and required a relatively long puff duration (~40 ms, compared to ~5–20 ms in most other cases).
D. NMDA currents at Vhold = −40 mV (± 5 mV; INMDA, −40 mV) for various cell populations in guinea pig or mouse. Each symbol represents a cell. For guinea pig cells, Cd2+ was used in some cases to block synaptic transmission (gray symbols). In all other cases, isradipine and synaptic blockers were used (see Results).
E. In guinea pig, ifenprodil suppressed INMDA, −40 mV for OFF δ cells (circles) and ON α cells (squares), but not OFF α cells (triangles).
F. Example OFF δ cell where block of NMDA puff response by ifenprodil recovered after washing away the drug (Vhold = −40 mV).
The physiological relevance of NMDAR function can be difficult to ascertain, because experiments described above commonly sliced the tissue or blocked inhibitory receptors, and both manipulations can alter bipolar cell glutamate release. Furthermore, many studies using either slice preparations or non-mammalian retina did not target specific ganglion cell types, each of which may use NMDARs differently. Experiments in whole-mount mammalian retina with synaptic inhibition intact also failed to demonstrate a clear role for NMDARs in visual processing. For many well-defined cell types, the I-V relationship of light-evoked responses was relatively linear, suggesting minimal NMDAR contribution (Pang et al., 2003; Taylor and Vaney, 2002; Murphy and Rieke, 2006; van Wyk et al., 2006; 2009; Sivyer et al., 2010). One exception is the cat β cell, which showed a J-shaped I-V relationship in response to a strong stimulus, indicating an apparent NMDAR contribution (Cohen, 2000).
In summary, the proposed roles for NMDARs in visual processing vary widely, and it is not clear that NMDARs play a substantial role for most mammalian ganglion cell types under physiological conditions. To further investigate their role, one straightforward approach would be to block NMDARs with an antagonist; however, this manipulation affects not only the ganglion cell but also many amacrine cells in the circuit leading to non-specific network effects. Instead, we need a method to quantify the NMDAR contribution to light responses under physiological conditions with inhibition intact.
Here, we examined the role of NMDARs in contrast coding of three specific ganglion cell types that could be targeted routinely in the whole-mount guinea pig retina. To test for NMDAR contribution, we developed a deconvolution method, whereby I-V plots of light-evoked responses could be decomposed into the weighted sum of the underlying AMPA, NMDA and inhibitory (GABA/glycine) receptor conductances. We tested between the hypothesis that NMDARs contribute mostly at low contrast, given their long time constant and high affinity for glutamate, and the alternative that they contribute at high contrast, where high release rates might‘spill over’ to reach extrasynaptic receptors (Dingledine et al., 1999; Sagdullaev et al., 2006; Zhang and Diamond, 2009).
Results
We measured responses to NMDA application and visual stimuli in a whole-mount preparation of the intact, in vitro guinea pig retina (see Experimental Procedures). We targeted large cell bodies for patch clamp recording, which led to routine measurements from three cell types: ON α, OFF α and OFF δ. These types could be distinguished by their light-evoked conductances and by their dendritic tree stratification, as described previously (Manookin et al., 2008). Results below are reported as mean ± SEM.
NMDA receptor-mediated conductance is substantial in multiple ganglion cell types but negligible in the ON α cell
We tested for the presence of NMDARs by recording the response to NMDA puffed directly onto ganglion cells. The first goal was to test whether NMDARs were expressed by the three major cell types studied here. The second goal was to characterize the NMDAR I-V relationship in intact cells in order to reveal their contribution to light-evoked responses using a deconvolution method described below.
Following an initial characterization of cell type, based on extracellular and/or whole-cell light-evoked responses, we recorded the NMDA puff response with synaptic transmission strongly attenuated by a combination of Ca2+ channel blockers and ligand-gated receptor antagonists (see Experimental Procedures). In most cells, the NMDA I-V plot showed the characteristic ‘J-shape’ (Mayer et al., 1984; Nowak et al., 1984) (Figure 1A–C). We quantified the response at a holding potential (Vhold) of − 40 ± 5 mV (INMDA, −40mV; averaged over one second following the puff). This current was substantial for both OFF α (−289 ± 33 pA; n = 24) and OFF δ cells (−125 ± 18 pA; n= 15) but was negligible for ON α cells (−8.1 ± 5.4 pA; n = 7) (Figure 1D). In separate recordings, ON α cells responded robustly to puffs of glycine or the GABAA agonist muscimol (data not shown), and thus it was possible to elicit large agonist responses. We conclude that ON α cells express a relatively low level of NMDARs.
To determine further whether weak NMDAR expression in ON α cells was a property specific to this cell type, we measured NMDA responses in cells with small somas (10–20 μm diameter; n = 9). These included ON (n = 2) and ON-OFF types (n = 7) with either monostratified (n = 2) or bistratified dendritic trees (n = 6). Each cell showed an NMDA-mediated response with the characteristic J-shaped I-V relationship (INMDA, −40mV = −206 ± 61 pA) (Figure 1D). While we have not tested exhaustively for the presence of NMDARs in all ~15 ganglion cell types, the collected results suggest that multiple types show robust NMDAR-mediated responses, while ON α cells show weak responses.
We tested whether the weak NMDA response of guinea pig ON α cells generalizes to mouse. Medium to large mouse ganglion cell bodies (~15–30 μm) were targeted for recording (n = 20; including three bistratified cells). The dendritic tree of filled monostratified cells ramified at one of three positions relative to the two cholinergic bands labeled with an antibody against choline acetyltransferase (ChAT bands; see Experimental Procedures) (Margolis et al., 2007; Manookin et al., 2008; van Wyk et al., 2009). Based on the similarity to stratification in guinea pig cells, we grouped the mouse cells as ON α, OFF α and OFF δ (see Experimental Procedures). The INMDA, −40mV responses of each cell group, including the ON α cells, were similar to each other (Figure 1D). Thus, the weak NMDA response in guinea pig ON α cells does not generalize to all species.
OFF δ cells express NMDA receptors composed of the GluN2B subunit
We examined further the NMDAR subunit expression in guinea pig cells. The NMDAR is a heterotetramer comprised of two GluN1 (i.e., NR1) subunits and two other subunits (GluN2 and/or GluN3). The GluN2 subunit comprises four subtypes (A–D) and each confers distinct properties (Erreger et al., 2004; Monyer et al., 1992). In mature retina, GluN2A-containing receptors reportedly locate at the synapse and are expressed primarily by OFF cells (of unknown types); whereas the GluN2B-containing receptors locate outside the synapse (extrasynaptic) and are expressed primarily by ON cells (of unknown types) (Sagdullaev et al., 2006; Zhang and Diamond, 2009).
We tested for the presence of GluN2B subunits by applying ifenprodil (10 mM), an antagonist with a >400-fold higher affinity for NMDARs composed of GluN2B subunits compared to those composed of GluN2A subunits (Williams, 1993). Ifenprodil did not affect the puffed NMDA response in an OFF α cell (INMDA, −40mV = −308 ± 57 pA at baseline, −277 ± 51 pA in drug; n = 9 cells) (Figure 1A, E) but strongly suppressed the response in an OFF δ cell (INMDA, −40mV = −112 ± 13 pA at baseline, −20 ± 5 pA in drug; n = 8 cells; p < 0.01, paired t-test) (Figure 1B, E). In two OFF δ cells, INMDA, −40mV was monitored continuously while applying ifenprodil and then washing it out; the block by ifenprodil was partially reversible (Figure 1F). In two cases, the small NMDA response in ON α cells was suppressed by ifenprodil (INMDA, −40mV = −21 ± 1 pA at baseline, −11 ± 2 pA in drug; n = 2 cells) (Figure 1C, E), suggesting that these relatively weak responses depend partially on receptors composed of GluN2B subunits. In summary, OFF δ cells were the one type with substantial NMDA responses mediated primarily by receptors composed of GluN2B subunits.
Population analysis generates robust ligand-gated receptor basis functions for evaluating light-evoked conductances
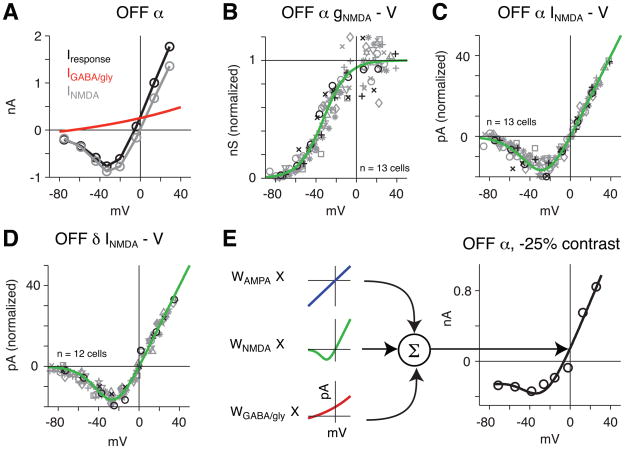
To quantify the contribution of NMDARs to contrast responses, we developed a method for decomposing a light-evoked response into the underlying ligand-gated receptor components. Ganglion cell responses have been studied in this way, but only when the I-V relationship was relatively linear and hence an NMDAR component was not distinguished from an AMPAR component (Murphy and Rieke, 2006; Roska and Werblin, 2001; Taylor and Vaney, 2002; van Wyk et al., 2006).
We considered three major ligand-gated receptor components: AMPA, NMDA and a mixed inhibitory (GABA/glycine) conductance. The ganglion cell AMPAR conductance can be described as a ‘basis function’ that is approximately linear and reverses at ~0 mV (Mittman et al., 1990; Cohen et al., 1994; Zhou et al., 1994; Cohen, 2000; Beaudoin et al., 2008). Below, we established similar basis functions for the other receptor classes. To ensure that voltage-clamp data were of adequate quality for fitting, we focused on cells where light-evoked currents primarily had a voltage error of <10 mV (see Experimental Procedures).
To measure the inhibitory receptor basis function, we initially recorded the response to puffed GABA or glycine receptor agonists. However, these responses resulted in a large apparent shift in ECl, which likely resulted from Cl− loading in the cell (see Experimental Procedures). We therefore took a second approach and recorded the inhibitory ‘ON’ responses of OFF cells in the presence of the NMDAR antagonist D-AP5 (100 μM; Figure 2A, B). The OFF cell’s ON response was measured either at the offset of a negative contrast stimulus or at the onset of a positive contrast stimulus (± 30–50% contrast); the two types of ON response yielded similar results and are combined in the population analysis (Figure 2C). We assumed the ON response was driven by an inhibitory conductance plus the suppression of a basal excitatory conductance (i.e., an active resting AMPAR conductance that was suppressed); the suppressed excitatory conductance corresponds to the outward current recorded at Vhold=ECl (Pang et al., 2003; Manookin and Demb, 2006; Trong and Rieke, 2008; van Wyk et al., 2009). We subtracted the AMPAR conductance (i.e., the reduction in baseline conductance) from the ON response and thereby derived the GABA/glycine receptor conductance (Figure 2B).
Figure 2. Generating the inhibitory receptor basis functions.
A. An OFF α cell was stimulated with a 50% negative contrast flash in the presence of an NMDAR antagonist (D-AP5, 100 μM). Following the excitatory ‘OFF’ response to the spot, there was an inhibitory ‘ON’ response to the termination of the spot. The ‘ON’ response measured in the time window indicated by the gray strip was used to generate the I-V plot in B (Iresponse).
B. The I-V plot for the response in A was separated into two components. A line fitted to the first four points (cyan) was used to estimate the current at ECl (−67 mV). This current was modeled as a decreased excitatory conductance (blue line, IAMPA). Subtracting IAMPA from Iresponse yielded an estimate of the inhibitory current (IGABA/gly, gray symbols).
C. The inhibitory current (IGABA/gly) was converted to conductance (gGABA/gly) by multiplying by the driving force (Vhold ECl), excluding data where Vhold was within 5 mV of ECl. Gray symbols show ‘ON’ response to the termination of a dark spot, whereas green symbols show the ‘ON’ response to the onset of a bright spot. Plotted are 14 measurements from 11 cells (each cell is a different symbol/color combination). Red line shows a fitted conductance (see Experimental Procedures).
D. Measurements and fits from C. were converted to currents by dividing by the driving force at each Vhold. The fit in the I-V plot represents the GABA/glycine receptor basis function for OFF α cells.
E. Same as D. for OFF δ cells (n = eight conditions in seven cells).
F. Same as D. for ON α cells (n = four cells).
We performed a population analysis on the normalized GABA/glycine receptor conductance. The conductance at each Vhold was determined by dividing the current amplitude by the driving force on Cl. The conductance-voltage (g-V) relationship was fit with an exponential function (see Experimental Procedures; Figure 2C). The normalized conductances and population fit were then converted to currents for OFF α cells (Figure 2D). A similar procedure was performed for OFF δ (Figure 2E) and ON α cells (Figure 2F). These fits represent the I-V basis functions for each cell type’s GABA/glycine receptor conductance.
A similar procedure was used to generate the NMDAR basis function. The puff-evoked NMDA responses generated I-V plots that typically reversed negative to 0 mV (OFF α: −7.4 ± 1.4 mV, n = 13 cells; OFF δ: −8.2 ± 1.5 mV, n = 12 cells). We assumed that the negative reversal was due to unblocked ‘feed-forward’ inhibition. We thus subtracted the inhibitory receptor basis function described above to generate the NMDA response in each cell (Figure 3A). These currents were converted to conductance as described above and the population was fit with a sigmoidal function, which represents the voltage-dependence of the conductance (see Experimental Procedures; Figure 3B). The normalized conductances and fit were converted back to currents for both OFF α and δ cells (Figure 3C, D). These fits represent the I-V basis functions for each cell type’s NMDAR conductance.
Figure 3. Generating the NMDAR basis functions.
A. NMDA was puffed onto an OFF α cell at several Vholds (see Figure 1). From the puff-evoked response (Iresponse) a putative inhibitory current (IGABA/gly) was subtracted to generate the NMDA current which reversed at Ecation (INMDA).
B. The INMDA was converted to conductance (gNMDA) by multiplying by the driving force (Vhold Ecation), excluding data where Vhold was within 5 mV of Ecation. Green line shows a fitted conductance (see Experimental Procedures). Other conventions are the same as for Figure 2C.
C. Measurements and fits from B. were converted to currents by dividing by the driving force. The fit in the I-V plot represents the NMDAR basis function for OFF α cells.
D. Same as C. for OFF δ cells.
E. The basic fitting procedure for modeling light-evoked responses. The I-V plot for the response to a −25% contrast spot in an OFF α cell was modeled (black line) as the weighted sum of the underlying AMPA, NMDA and inhibitory (GABA/gly) receptor basis functions.
NMDARs play distinct roles for contrast processing in three cell types
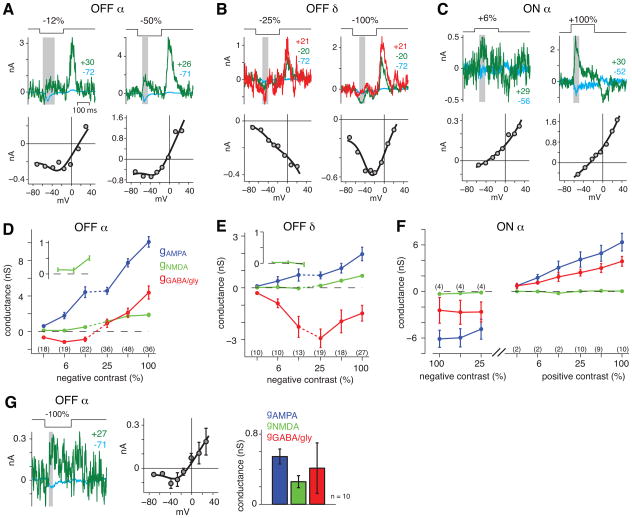
After generating the receptor basis functions, we could describe light-evoked I-V plots as the weighted sum of these functions (Figure 3E). We proceeded to characterize these conductances in each of the three cell types at various contrast levels. For OFF cell types at low contrasts (−3 to −12%), we typically used a large stimulus (0.4-mm dia.) to increase signal-to-noise ratio; whereas for high contrasts (−25 to −100%), we typically used a smaller stimulus (0.2-mm diameter) to limit response amplitude and hence the error in Vhold during the response. OFF α cells showed a J-shaped I-V relationship at both low and high contrast, indicating an NMDAR contribution (Figure 4A). On average there were significant AMPAR and NMDAR conductances (i.e., greater than 0 nS; p < 0.05) at every contrast (−3 to −100%) (Figure 4D). The plotted NMDA value represents the conductance at Vm=−60 mV, but this is only a fraction of the maximal conductance (Figure 3B). Thus, as the cell depolarizes from rest, especially at high contrast, the NMDAR conductance would increase above the plotted value. The inhibitory conductance was negative for the lower contrasts, reflecting removal of a basal inhibitory conductance mediated by the AII amacrine cell (i.e., ‘disinhibition’; Manookin et al., 2008). The inhibitory conductance was positive for high contrasts, reflecting a distinct ‘feed-forward’ inhibitory input that occurs in parallel with the excitation at light offset (Roska and Werblin, 2001). In summary, OFF α cells use NMDARs to encode a wide range of contrasts.
Figure 4. The NMDAR contribution to contrast coding differs between cell types.
A. Responses and I-V plots for 200-ms pulses of low or high contrast in an OFF α cell. Traces at two Vholds are shown (in mV, indicated above the traces). The fitted line was J-shaped in both cases, indicating an NMDAR contribution to the response.
B. Same format as A. for an OFF δ cell. A U-shaped function at −100% contrast reflects an NMDAR contribution.
C. Same format as A. for an ON α cell. Fitted functions are relatively linear at both low and high contrast, indicating a weak NMDAR contribution.
D. Fitted conductances as a function of contrast for OFF α cells. The stimulus size was usually 0.4-mm dia. for low contrasts (3–12%) and 0.2-mm dia. for high contrasts (25–100%). Error bars indicate SEM across cells. The number of cells recorded at each contrast is indicated below the symbols. Inset shows the NMDAR conductance at the lowest three contrasts.
E. Same format as D. for OFF δ cells.
F. Same format as D. for ON α cells. The stimulus was either negative or positive contrast, and spot diameter was always 0.5-mm.
G. OFF α cell response to a 25 x 25 μm square at high contrast (−100%) showed a J-shaped relationship in the I-V plot (error bars indicate SEM across 10 repeats in one cell). The bar graph shows a significant AMPAR and NMDAR conductance across cells (error bars indicated SEM across 10 cells).
OFF δ cells showed different I-V relationships depending on contrast level. At low contrast, the I-V relationship was relatively linear and the slope was negative; whereas at high contrast the I-V relationship was ‘U-shaped’ (Figure 4B). The negative slope at low contrast represents the decrease of a baseline inhibitory conductance that is mediated by the AII amacrine cell (Manookin et al., 2008; Murphy and Rieke, 2008; van Wyk et al., 2009). The U-shape at high contrast is mediated by this disinhibition combined with the AMPAR and NMDAR conductances. On average, the NMDAR conductance was significantly greater than zero (p < 0.05) only at high contrast (−25 to −100%) (Figure 4E); whereas the disinhibition was present at all contrast levels (Murphy and Rieke, 2008; Manookin et al., 2008; van Wyk et al., 2009; Sivyer et al., 2010). Thus, OFF δ cells show an NMDAR conductance selectively at high contrast, and the response at all contrasts was mediated largely by disinhibition.
ON α cells showed only a weak response to NMDA application in some cells (Figure 1), and consistent with this observation, the fitted NMDAR conductance to the contrast response was weak (Figure 4C). At all positive contrasts, the conductance was not significantly greater than zero (Figure 4F). However, AMPAR and inhibitory conductances increased in parallel with contrast. In a subset of cells (n = 4), we probed the response to negative contrasts and found that AMPAR and inhibitory conductances decreased below the baseline level. Thus, the ON α cells received a high tonic level of excitatory (AMPAR-mediated) and inhibitory synaptic input, both of which could modulate either above or below baseline levels depending on contrast sign. These results confirm observations in guinea pig, mouse and primate, that transient, ON α or parasol cells receive high frequency basal glutamate release that can increase or decrease relative to baseline (Manookin et al., 2008; Murphy and Rieke, 2006; Pang et al., 2003; Trong and Rieke, 2008; Zaghloul et al., 2003).
Of the three cell types, only OFF α cells showed a significant NMDAR contribution at low contrast. We followed up this observation with a second test of whether NMDARs contribute to weak stimuli near response threshold by presenting a small spatial stimulus (25 x 25 μm). Responses were small and noisy, and were likely mediated by only one or a few bipolar cells (Berntson and Taylor, 2000; Dacey et al., 2000; Wassle et al., 2009; Zhang and Wu, 2009). However, even for this minimal spatial stimulus, an NMDAR component was significantly greater than zero (p < 0.05; n = 9; Figure 4G). We conclude that OFF α cell NMDARs encode weak stimuli either composed of low contrasts or confined to small spatial regions.
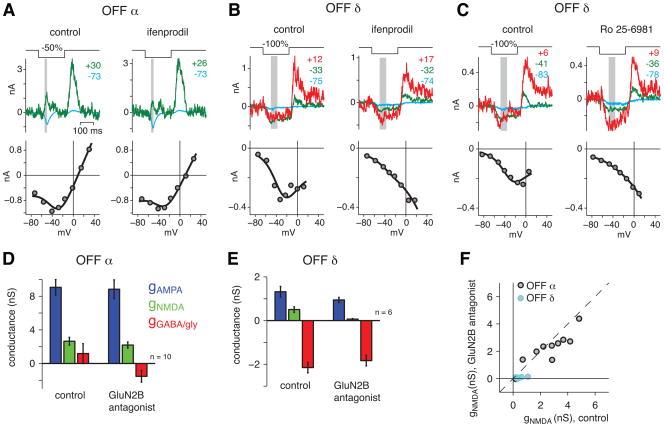
NMDA receptor pharmacology validates the fitting technique
To validate the fitting method, we tested the effect of NMDAR antagonists on the two OFF types, which showed substantial NMDAR conductances in the previous experiments. In one case, we applied the general antagonist D-AP-5 (100 μm). This drug caused the OFF α cell’s J-shaped I-V relationship to become linear (Figure 5A), and the OFF δ cell’s U-shaped I-V relationship to become linear (Figure 5C). On average, the NMDAR conductance was suppressed to levels that were not significantly greater than zero in both cell types (Figure 5B, D). There were additional effects of the drug on the AMPAR and inhibitory receptor conductances, as would be expected given the multiple effects of D-AP-5 throughout retinal circuitry. Nevertheless, these conductances were relatively intact compared to the NMDAR conductance.
Figure 5. The NMDA component of the fitted response is blocked by D-AP-5.
A. Response traces and I-V plots for an OFF α cell under control conditions and in the presence of D-AP-5 (100 μM). The I-V plot becomes more linear in the presence of D-AP-5.
B. Contrast response functions for the three fitted conductances. The NMDA component of the fit was suppressed to near zero values in the presence of D-AP- 5. The number of cells at each contrast is indicated below the points in the D-AP-5 condition.
C. Same format as A. for an OFF δ cell. In the presence of D-AP-5, the U-shaped I-V relationship changed to a negative linear slope, indicating the suppression of a baseline inhibitory conductance (disinhibition).
D. Same format as B. for OFF δ cells.
The NMDA puffing experiments combined with ifenprodil application suggested that OFF δ cells express NMDARs composed of GluN2B subunits. To test whether these subunits were required for contrast responses, we measured the NMDAR contribution to light responses in the presence of GluN2B antagonists ifenprodil (10 μM; n = 11) and Ro-25-6981 (5 μM; n = 5) (Kalbaugh et al., 2009; Zhang and Diamond, 2009). Both drugs affected the responses similarly and were combined in the population analysis. The GluN2B antagonists had little effect on the OFF α cell’s NMDAR conductance but strongly suppressed the OFF δ cell’s NMDAR conductance (Figure 6A-D). On average, the NMDAR component was significantly suppressed to 17 ± 8% of the control value for OFF δ cells (p < 0.01), but to 85 ± 17% of the control value for OFF α cells (Figure 6E). Thus, OFF δ cells, but not OFF α cells, express primarily NMDARs composed of GluN2B subunits, and these receptors mediate contrast responses.
Figure 6. The NMDAR component of the fitted response in OFF δ cells is blocked by GluN2B antagonists.
A. Response traces and I-V plots for an OFF α cell under control conditions and in the presence of the GluN2B antagonist ifenprodil (10 μM). The I-V plot remained J-shaped in both conditions.
B. Same format as A. for an OFF δ cell. In the presence of ifenprodil, the U-shaped I-V relationship changed to a negative linear relationship, indicating the suppression of a baseline inhibitory conductance (disinhibition).
C. Same format as B. for the GluN2B antagonist Ro 25-6981 (5 μM).
D. Bar graphs indicate the fitted conductances for OFF α cells under control conditions and in the presence of GluN2B antagonists (ifenprodil or Ro-25-6981). The stimulus was −50% contrast. Error bars indicate the SEM across cells.
E. Same format as C. for OFF δ cells. The stimulus was −100% contrast. The NMDAR conductance was suppressed by the antagonists.
F. The fitted NMDAR conductance for each cell is plotted for control versus antagonist conditions.
NMDARs support contrast sensitivity of the firing response in OFF α cells
The OFF α and δ cells both showed clear NMDAR components to the contrast-evoked conductance under voltage clamp, but do these receptors contribute to firing? To answer this, we recorded OFF α or δ cells under current-clamp and, in some cases, blocked NMDARs with the open channel blocker MK-801 (1 mM) applied via the recording pipette (Berretta and Jones, 1996; Humeau et al., 2003; Du et al., 2009). Control experiments showed that MK-801 blocked both the NMDA puff response and the NMDA component of the contrast response under voltage clamp (see Experimental Procedures).
For each cell type, we compared two cell groups. We first made loose-patch spike recordings with pipettes filled with extracellular Ames medium. The two OFF α cell groups were well matched in their firing responses to various contrasts (Figure 7A). We then made whole-cell recordings with either control solution (control group; cell 1 in Figure 7) or solution with MK-801 added (MK-801 group; cell 2 in Figure 7). The MK-801 group cells showed reduced contrast sensitivity, compared to control cells, over a range of contrast levels (Figure 7B). The difference curve (extracellular - intracellular firing) showed that MK-801 suppressed spiking at 6–100% negative contrast (Figure 7B, inset). To ensure that MK-801 did not have a nonspecific effect on voltage-gated channels, we injected current through the pipette to elicit firing. The two cell groups showed nearly identical firing rates, which increased linearly with input current (Figure 7C). Thus, MK-801 did not affect general firing properties.
Figure 7. Blocking NMDA receptors suppresses contrast sensitivity of the firing response in OFF α cells but not in OFF δ cells.
A. Loose-patch recordings of cells in the control group and MK-801 group with pipettes filled with the extracellular Ames medium (top). The contrast-response function of the firing response in the two groups was similar (bottom).
B. Same as A. for intracellular recording with control pipette solution or solution with MK-801 added. Inset, The difference curve (extracellular intracellular firing rate) shows that MK-801 suppressed firing over most of the contrast range.
C. Depolarizing current evoked similar firing responses in both cell groups.
D. Average subthreshold Vm showed that the initial depolarization in response to contrast steps was suppressed by MK-801. Spikes were removed by linear interpolation before averaging (Demb et al., 1999).
E.-H. Same as A.-D. for OFF δ cells. Responses were only weakly affected by MK-801.
The MK-801 effect on the contrast response was assessed further by plotting the average subthreshold Vm (Figure 7D). Blocking NMDARs with MK-801 suppressed the initial depolarization compared to control cells (Figure 7D). The MK-801 group cells were also relatively hyperpolarized at rest (−64 mV; SD=2) compared to the control group (−61 mV; SD=2). However, this small difference is unlikely to explain the effect on contrast responses, because the two groups’ firing rates to injected current were similar.
The same experiment was performed on two OFF δ cell groups. The groups were again matched in their extracellular firing rate to contrast stimuli and in their firing rate to current injection (Figure 7E, G). However, in this case, there was only a weak, non-significant effect of MK-801 on the intracellularly recorded contrast response function (Figure 7F, inset). Furthermore, the two groups showed similar subthreshold Vm responses (Figure 7H). Thus, OFF δ cell firing depended little on NMDARs. Instead, the OFF δ cell’s response to negative contrast apparently depended primarily on disinhibition and the AMPAR conductance (Figure 4E).
Discussion
Here, we investigated NMDAR function in three specific retinal ganglion cell types and addressed three questions: (1) does each cell type express NMDARs?; (2) are these receptors used under physiological conditions (i.e., with inhibition intact) to encode excitatory input at various levels of stimulus strength (contrast)?; (3) are these receptors necessary for firing spikes at various contrast levels? Our results show three ways in which ganglion cells encode contrast using ionotropic glutamate receptors (Figure 8). Guinea pig ON α cells showed weak NMDAR expression, as assessed by the response to agonist application (Figure 1), and, correspondingly, they encoded visual contrast using AMPARs (Figure 4). OFF α cells expressed both AMPARs and NMDARs and used both to encode a wide contrast range, including weak responses near threshold (Figures 1, 4). OFF δ cells expressed both AMPARs and NMDARs but used NMDARs only for encoding high contrast (Figures 1, 4).
Figure 8. Three cell types show different contributions of NMDAR conductances to the contrast response.
Three ganglion cell (GC) types encode bipolar cell (BC) glutamate release using distinct patterns of ionotropic glutamate receptor expression and stimulation. Weak expression is indicated by the gray type. Contributions to low and high contrast are indicated by thin or thick arrows.
Our results show that NMDAR usage for contrast coding is cell-type specific, and some types do not require NMDARs for contrast coding. Furthermore, the presence of an NMDAR component to the contrast response measured under voltage clamp was only partly predictive of its impact on firing. The OFF α cell’s high contrast sensitivity depended on NMDARs, whereas the OFF δ cell’s lower sensitivity persisted with NMDARs blocked (Figure 7). Thus, of the three cell types, only OFF α cells depend substantially on NMDARs for contrast coding. The OFF α cell’s dependence on NMDARs at both low and high contrast matches a proposed role for NMDARs in the primary visual cortex (Fox et al., 1990; Daw et al., 1993).
Possible relationship between NMDAR subunit expression and contribution to contrast responses
Based on studies in mouse and rat, we expected that OFF cells would express primarily GluN2A subunits localized to the synapse; whereas ON cells would express primarily GluN2B subunits localized to extrasynaptic locations (Sagdullaev et al., 2006; Zhang and Diamond, 2009). However, we found that OFF δ cells apparently express GluN2B subunits, because their responses to direct NMDA application and the fitted NMDAR component of the contrast responses were both blocked by GluN2B antagonists (Figure 1, 6). Thus, these OFF cells apparently use GluN2B subunits to encode visually-evoked glutamate release. In order to further characterize these receptors, we attempted to record spontaneous excitatory postsynaptic currents (sEPSCs) in the whole-mount preparation but could not isolate individual events with confidence because of the apparently high release rate. Previous sEPSC measurements in mammalian cells used slice preparations, and these conditions may be necessary for lowering release rates and/or the number of functioning synapses so that sEPSCs can be resolved (Chen and Diamond, 2002; Sagdullaev et al., 2006; Zhang and Diamond, 2009). Thus, we speculate that GluN2B subunits in the OFF δ cells indicate an extrasynaptic location, based on previous work in rat, but could not confirm this with direct measurements (Zhang and Diamond, 2009). If the OFF δ cell’s NMDARs were localized extrasynaptically, it could explain why the NMDAR conductance only appears at high contrast, where a relatively high rate of glutamate release might spill-over to extrasynaptic locations on the ganglion cell dendrite.
Variable levels of NMDAR expression and contribution to contrast responses
The guinea pig ON α cell showed weak expression of NMDARs, whereas the mouse ON α cell showed strong expression, suggesting a species difference (Figures 1, 4). In isolated rat ganglion cells of unknown type, a subset of cells showed no response to NMDA application, suggesting NMDAR expression may be absent in some types (Aizenman et al., 1988; Karschin et al., 1988). In cat, one study found little NMDAR expression in ON α cells (Boos et al., 1990), whereas another found stronger expression (Cohen et al., 1994). Thus, NMDAR expression appears to be common to most ganglion cell types but may not be ubiquitous, at least in certain species.
Despite their expression, as indicated by the agonist response, NMDARs may not be used for visual coding in certain cell types. In our recordings from the mouse ON α cell, the I-V relationship for light evoked responses was relatively linear even in cases where puffing NMDA on the same cell evoked a substantial response (data not shown). Furthermore, the rabbit ON-OFF direction selective cell expresses NMDARs, but these receptors do not obviously contribute to motion responses, as indicated by the linear I-V relationship (Taylor and Vaney, 2002; Massey and Miller, 1988). Thus, multiple cell types might express NMDARs but not use these receptors in many stimulus conditions, similar to the guinea pig ON α cell.
The NMDAR may not be useful in retinal circuits where bipolar cells release glutamate at a high frequency. The ON α cell in guinea pig apparently receives high frequency presynaptic release, because the excitatory conductance could be modulated either up or down depending on contrast sign, suggesting that basal release was near the middle of its operating range (Figure 4) (Murphy and Rieke, 2006; Trong and Rieke, 2008; Zaghloul et al., 2003). The exact rate is unknown. However, the number of excitatory synapses on ON and OFF α cell dendrites is similar, and thus the relatively high tonic level of excitatory activity in ON cells must correspond to a relatively high release rate at each synapse (Jakobs et al., 2008; Xu et al., 2008). With high frequency release, the NMDARs, given their long time constant, could effectively be saturated and unable to encode modulations of release. In these cases, it is unclear whether NMDARs could ever play a substantial role, under specific stimulus conditions (Sagdulaev et al., 2006). It is possible that extrasynaptic NMDARs play some role during synaptic development but then become essentially obsolete in mature retina (Blankenship et al., 2009).
Method for modeling ligand-gated receptor contributions to visual responses
Our method for modeling stimulus-evoked I-V relationships should be widely applicable to other cell types and circuits. Here, we have recorded under relatively challenging conditions given the large size of the ganglion cells. Accordingly, we restricted the analysis to cases with low series resistance (Rs), accounted for the voltage drop across the pipette tip when calculating Vhold, analyzed measurements where the voltage error during the response was typically <10 mV, and used small stimuli at high contrast restricted to central dendrites (see Experimental Procedures). To test validity of the method, we blocked NMDARs with antagonists (Figures 5, 6). The fitted NMDAR component was selectively suppressed to levels that were not significantly above zero, and thus the NMDAR component at baseline must reflect an NMDAR-mediated conductance. We likely underestimated the amplitude of this conductance, due to problems inherent with voltage clamping large cells, but our major conclusions regarding the presence of NMDARs and their contribution at various contrast levels should be robust to these errors.
We tested whether fitting OFF α and δ cell responses depended heavily on the shape of the basis functions. We re-fit the contrast responses in Figure 4 after swapping the inhibitory and NMDA basis functions between cell types (i.e., fitting OFF α cell responses with the OFF δ cell’s basis functions, and vice versa). For OFF δ cells, the root mean squared error (RMSE) between the data and fit was unchanged (original fit: 36 ± 4 pA; swapped basis function fit: 35 ± 4 pA; averaged over 25–100% contrasts), whereas for OFF α cells, the RMSE increased slightly (original fit: 62 ± 4 pA; swapped basis function fit: 65 ± 4 pA; averaged over all contrasts) but significantly (difference: 3.1 ± 0.4 pA; p < 0.01). However, the RMSE about doubled if we removed the NMDAR basis function altogether for both OFF δ cells (65 ± 6 pA) and OFF α cells (140 ± 9 pA). Thus, response fitting required an NMDAR basis function, but the main results for OFF α and δ cells did not depend substantially on the precise shape of the basis functions.
Synaptic mechanisms for high contrast sensitivity
There are apparently separate mechanisms for generating high contrast sensitivity in ON and OFF α cells (Dhingra et al., 2003; Demb et al., 2004). The OFF α cell uses NMDARs, which function near Vrest and contribute to minimal stimulation (Figure 4) (Fleidervish et al., 1998; Gottesman and Miller, 2003; Binshtok et al., 2006; Espinosa and Kavalali, 2009). Their long time constant should improve signal-to-noise at low contrast (Demb et al., 2004). This mechanism combines with disinhibition from the AII amacrine cell (Murphy and Rieke, 2008; Manookin et al,. 2008). The ON α cell instead generates sensitive responses to weak stimuli using AMPARs. Given the apparently high release rate onto these cells, it is likely that the AMPARs are relatively non-desensitizing and therefore capable of operating in the presence of high frequency release (Pang et al., 2008). A high release rate should improve the ability to detect low contrasts, because the noisiness of Poisson release is suppressed by increasing the baseline rate (as the square-root of the mean rate).
OFF bipolar cells also use iGluRs to encode modulations around a high basal release rate (i.e., ~20–40 vesicles/sec) (DeVries et al., 2006; Jackman et al., 2009; Singer, 2007; Singer et al., 2004) and apparently do so using several strategies. First, they do not employ NMDARs (Hartveit, 1997), which would apparently be saturated in the presence of high frequency release. Second, they use AMPARs that show little desensitization or recover relatively quickly from desensitization (DeVries, 2000; Pang et al., 2008). Third, some OFF bipolar types use kainate receptors that recover slowly from desensitization, but these types position their dendrites relatively far from synaptic release sites effectively lowering the peak concentration of glutamate, which minimizes desensitization (DeVries et al., 2006). The properties of AMPARs in specific ganglion cell types remain to be elucidated, but we predict that many types, such as the ON α cell, will use a non-desensitizing AMPAR similar to some OFF bipolar cells.
Experimental Procedures
Tissue preparation and electrophysiology
The experimental procedures are identical to those described in detail previously (Manookin et al., 2008; Beaudoin et al., 2008). Following >60 min dark adaptation, a Hartley guinea pig was anesthetized with an IM injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) in dim red light. Under anesthesia, the animal was decapitated and both eyes were removed. All procedures conformed to NIH and University of Michigan guidelines for use and care of animals in research. The retina was prepared and stored as described previously. During recording, the retina was superfused (~6 ml min−1) with oxygenated Ames’ medium (Sigma-Aldrich, St. Louis, MO, USA) heated to 33 35 deg C. The retina and electrode were visualized using a cooled CCD camera (Retiga 1300C, Qcapture software; Qimaging Corporation, Burnaby, British Columbia) mounted on an Olympus BX51WI microscope (Olympus; Center Valley, PA). A glass electrode (tip resistance, 3 5 MΩ) was filled with Ames’ medium for loose-patch recording of spikes or intracellular solution for whole-cell recording of membrane currents. Intracellular solution consisted of the following (in mM): 120 Cs methanesulphonate, 5 TEA-Cl, 10 HEPES, 3 NaCl, 10 BAPTA, 2 QX-314-Cl, 2 ATP-Mg, 0.3 GTP-Na with 0.10% Lucifer Yellow, titrated to pH 7.3. All chemicals were purchased from Sigma-Aldrich (St Louis, MO) except for BAPTA (Invitrogen; Eugene, OR), strychnine (Fisher, Hampton, NH), D-serine, D-AP5, and L-AP4 (Tocris; Bristol, UK).
Membrane current was amplified, sampled at 10 kHz, and stored on a computer (MultiClamp 700A amplifier; Digidata 1322A A–D board; pCLAMP 9 software; Axon Instruments; Union City, CA). Junction potential (−9 mV) was corrected. Light responses were analyzed in Matlab (version 7.4; The Mathworks; Natick, MA). An error in Vhold introduced by Rs was corrected by the formula:
where Vhold,uncorr is the apparent (uncorrected) Vhold (in mV), I leak is the leak current (in nA), RS is the series resistance (14.9 MΩ; S.D. = 4.6; n = 138 cells) and RS,correct is Rs compensation (typically 0.4; higher values sometimes resulted in oscillations that destroyed the seal). We excluded cells with RS >25 MΩ. Uncompensated Rs was 9.4 MΩ (S.D. = 4.0). The above correction accounts for voltage error during the leak current but not additional error during the response. To minimize the impact of errors in Vhold, input resistance (Rin) was typically at least 2x the uncompensated Rs and errors in Vhold during the responses were <10 mV. In a few cases (< 7%), I-V plots had one or two measurements with an error between 10 and 20 mV. In general, the time window analyzed was chosen to avoid periods with large errors. In cases where we compared the response before and after adding a drug, the same time window was used. Vhold started near −75 mV and was stepped up in ~10–15 mV increments; the cell remained at a given Vhold for <=15 sec.
Cell type was confirmed by measuring light responses and in some cases by analyzing dendritic tree stratification, as described (Manookin et al., 2008). OFF α and δ cells were readily distinguished by their responses to square-wave modulated spots (1 Hz, 100% contrast). With Vhold near ECl, the OFF α cell’s leak-subtracted peak inward current was −821 ± 71 pA (0.2-mm dia.) and −1261 ± 102 (0.4-mm dia.; n = 20); whereas the OFF δ cell’s response was −107 ± 36 pA (0.2-mm dia.) and −189 ± 73 pA (0.4-mm dia.; n = 20). For cells that met our criteria for modeling light-evoked conductances (see above), Rin for representative samples was 26±11 MΩ (mean±SD) (ON α; n = 10), 25±4 MΩ (OFF α; n = 48) and 36±9 MΩ (OFF δ; n = 27).
Puff-evoked NMDA response
Responses to puff-evoked NMDA were measured with synaptic transmission strongly attenuated. We bath-applied the L-type Ca2+ channel blocker isradipine (30 μM) plus NMDAR co-agonists D-serine (200 μM) and glycine (6 μM) (Gustafson et al., 2007; Kalbaugh et al., 2009) and antagonists to glycine (strychnine, 2 mM), GABAA (bicuculline, 100 μM) and AMPA/kainate receptors (DNQX, 50 μM). In some cases, we instead applied the Ca2+ channel blocker Cd2+ in an extracellular Ringer that included (in mM): 120 NaCl; 1.15 CaCl2; 1.24 MgSO4; 3.1 KCl; 0.5 K-methylsulfate; 6 glycine; 6 d-glucose; 0.2 D-serine; 22.6 NaHCO3; 1 CdCl2. However, quantitative fitting of the NMDAR basis function was based on measurements in the first condition. NMDA (10 mM) was dissolved in Ames medium with D-serine (200 μM) and applied via a puffer pipette (tip resistance, 3 5 MΩ) positioned either near the cell body or advanced into the inner plexiform layer (Beaudoin et al., 2008).
In early experiments, we attempted to measure an inhibitory receptor basis function by blocking synaptic transmission (6 mM Co2+) and puffing glycine (200 mM) or muscimol (1 mM). These agonists evoked large outward currents as the membrane was stepped positive to ECl (−67 mV). However, upon return to the original Vhold, there was typically both an inward leak current and an inward agonist-evoked current (i.e., the inward current reversed in sign relative to the original outward current). These results suggested a substantial change in intracellular Cl− during the Vholds positive to ECl. We thus took the approach described in Results (Figure 2). With this protocol, responses at the beginning and upon the return to the original Vhold were more similar suggesting that intracellular Cl− was relatively stable.
Visual stimuli
The stimulus was displayed on a miniature monochrome computer monitor (Lucivid MR1-103; Microbrightfield; Colchester, VT, USA) as described (Manookin et al., 2008). The mean luminance evoked an estimated photoisomerization (P*) rate in the rod (R), M-cone (M) and S-cone (S) of ~2×103 PR*, ~103 PM*, and ~102 PS* (Yin et al., 2006). Spots (duration, 200 ms) were centered on the cell body. At low contrasts (3–25%), responses were typically averaged over three repeats.
Basis functions
Based on previous experiments (Beaudoin et al., 2008), the AMPAR basis function was modeled as a linear function of membrane voltage,
where the current (I) is the product of the conductance (g) and the electrical driving force (voltage, V; cation reversal potential, Ecation=0 mV).
The NMDAR basis function was derived from the puff-evoked NMDA response (see above). In each cell, the g-V relationship was fit with the following equation (least-squares fit):
where [Mg2+]o is the extracellular Mg2+ concentration (1.2 mM). The constants representing voltage-dependence and the Mg2+ dependence of the NMDA-receptor (a and b, respectively) (Gerstner and Kistler, 2002; Jahr and Stevens, 1990a, b) were free parameters in the fit (Figure 3).
In each cell, the g-V relationship was normalized to a maximal conductance of 1 nS. The fit was then performed on normalized data for OFF α cells (parameters: α, −0.083 mV−1; β, 18.2 mM; n = 13 cells) and OFF δ cells (α, −0.097 mV−1; β, 26.0 mM; n = 12 cells). The conductances were converted to currents:
The fit of the I-V plot became the NMDAR basis function (Figure 3). The ON α cell showed weak NMDA responses in only a subset of cells. Since these responses were sensitive to ifenprodil, similar to the OFF δ cell, we used the OFF δ cell’s NMDA basis function for the ON α cells.
The GABAA/glycine g-V relationship was modeled as an exponential function with an offset (b):
The (primarily) inhibitory ON responses of OFF cells were generated either by recording the response to the offset of a negative contrast or the onset of a positive contrast. The collected data were fit for OFF α (parameters: a, 5.2 x 10−3; b, 0.1781; n = 11 cells, 14 conditions) and OFF δ cells (a, 1.19 x 10−2; b, 0.3228; n = 7 cells, 8 conditions). A similar analysis was performed for ON α cells in response to a positive contrast (a, 1.79 x 10−2; b, 0.5183; n = 4 cells). Each function was multiplied by a factor to normalize the conductance at ECl to 1 (i.e., multiplying by the inverse of the fit at ECl): 1.13 (OFF α), 1.29 (OFF δ) or 1.22 (ON α). The conductances were then converted to currents:
The fit of the I-V plot became the GABA/glycine receptor basis function (Figure 2).
Analysis
The contrast response was measured by subtracting Ileak (averaged over 0.5 s before stimulus onset) from the response averaged over a time window near peak excitation (~50–100 ms following flash onset). Window size was typically 50 ms but was modified in certain cases (30–100 ms) to increase signal-to-noise or avoid responses with large voltage errors. Responses were modeled as the sum of three ligand-gated currents mediated by AMPA, NMDA, and GABA/glycine receptors (least-squares fit):
Mouse ganglion cell recordings
Ganglion cells were recorded from C57/B6 mice (Jackson Laboratories). Following >60 min dark adaptation, an animal was anesthetized with an IP injection of ketamine (120 mg/kg) and xylazine (10 mg/kg) in dim red light. Under anesthesia, the animal was decapitated and both eyes were removed and prepared under infrared illumination using viewers mounted on a dissection microscope. Other procedures were identical to those described above. Large cell bodies were targeted to bias recordings of large ON and OFF cells (Margolis et al., 2008; Murphy and Rieke, 2008; van Wyk et al., 2009). Following recording, the tissue was fixed and reacted to visualize the ganglion cell and two bands of cholinergic amacrine cell processes (ChAT bands), as described previously (Manookin et al., 2008). We identified large ON cells on the vitreal side of the ON ChAT band as ON α cells (dia., 321 ± 27 μm; n = 5); OFF cells on the vitreal side of the OFF ChAT band as OFF α cells (dia., 298, 333 μm; n = 2); and OFF cells between the OFF ChAT band and the inner nuclear layer as OFF δ cells (dia., 252 ± 34 μm; n = 5). Unfilled cells were grouped with the filled cells based on similarity of the light response (n = 3). Three cells were bistratified with processes that stratified near the ChAT bands (dia., 195, 197, 384 μm).
Recordings with MK-801 added to the pipette solution
Whole-cell recordings were made either with control solution (in mM): 120 K-methanesulfonate; 10 HEPES; 5 NaCl; 0.1 EGTA; 2 ATP-Mg2+; 0.3 GTP-Na+ with 0.10% Lucifer Yellow, titrated to pH 7.3; or the same solution with MK-801 (1 mM). Recordings in Figure 7 were taken from populations of 14 OFF α and 15 OFF δ cells with stable firing rates and Vm and relatively low Rs (< 20 MΩ). For OFF α cells, we chose five cells from the control group (n = 6) and five from the MK-801 group (n = 8) that were well matched in their Vrest (control: −61 ± 2 mV; MK-801: −64 ± 2 mV) (mean ± SD), Rin (control: 40 ± 8 MΩ; MK-801: 40 ± 4 MΩ), firing rate during loose-patch recording (Figure 7A) and firing rate to current injection (Figure 7C). For OFF δ cells, we chose six cells from the control group (n = 8) and six from the MK-801 group (n = 7) that were well matched in their Vrest (control: −63 ± 2 mV; MK-801: −64 ± 3 mV), Rin (control: 58 ± 12 MΩ; MK-801 70± 8 MΩ), firing rate during loose-patch recording (Figure 7E) and firing rate to current injection (Figure 7G). Given the matched properties of the control and MK-801 groups, we interpreted effects on intracellular contrast responses as being mediated by NMDAR blockade.
In control experiments, NMDA puff responses in OFF α or δ cells were completely blocked by intracellular MK-801 (n = 5). Furthermore, we made voltage-clamp recordings of OFF α cell −50% contrast responses and the fitted NMDA component was near zero (0.19 ± 0.13 nS; n = 3). Finally, MK-801 might possibly block some NMDARs as it leaks out of the recording pipette prior to forming the gigaseal. However, we do not think such receptor blockade explains the results in Figure 7. In three OFF α cells shown in Figure 7A-D, spikes were first recorded with an Ames pipette, then recorded with an intracellular pipette with MK-801, and then recorded after establishing the whole-cell configuration. In these cases, the firing response was nearly identical by the loose-patch recording with either Ames or MK-801 solution but suppressed under the whole-cell condition. Thus, suppression of contrast sensitivity in the firing was apparently caused by intracellular blockade of NMDARs by MK-801. After breaking in with the MK-801 pipette, we stimulated repeatedly but analyzed recordings taken after four to seven minutes, to increase the likelihood that MK-801 reached dendritic synapses. In the intact circuit, we could not tightly control glutamate release and determine whether the MK-801 effect was use-dependent, but we assume it acted as an open channel blocker of NMDARs.
Acknowledgments
We thank Dr. Daniel Green for comments on the manuscript and Mania Kupershtok for technical assistance. Supported by a Research to Prevent Blindness Career Development Award, an Alfred P. Sloan Foundation Fellowship, the NIH (EY14454; T32EY13934; Core Grant EY07003) and a Rackham Predoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenman E, Frosch MP, Lipton SA. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol. 1988;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin DL, Manookin MB, Demb JB. Distinct expressions of contrast gain control in parallel synaptic pathways converging on a retinal ganglion cell. J Physiol. 2008;586:5487–5502. doi: 10.1113/jphysiol.2008.156224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Taylor WR. Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J Physiol. 2000;524(Pt 3):879–889. doi: 10.1111/j.1469-7793.2000.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-D-aspartate autoreceptors in the entorhinal cortex. Neuroscience. 1996;75:339–344. doi: 10.1016/0306-4522(96)00301-6. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Fleidervish IA, Sprengel R, Gutnick MJ. NMDA receptors in layer 4 spiny stellate cells of the mouse barrel cortex contain the NR2C subunit. J Neurosci. 2006;26:708–715. doi: 10.1523/JNEUROSCI.4409-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62:230–241. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos R, Muller F, Wassle H. Actions of excitatory amino acids on brisk ganglion cells in the cat retina. J Neurophysiol. 1990;64:1368–1379. doi: 10.1152/jn.1990.64.5.1368. [DOI] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–2173. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED. Light-evoked excitatory synaptic currents of X-type retinal ganglion cells. J Neurophysiol. 2000;83:3217–3229. doi: 10.1152/jn.2000.83.6.3217. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Miller RF. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci. 1994;11:317–332. doi: 10.1017/s0952523800001668. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Zhou ZJ, Fain GL. Ligand-gated currents of alpha and beta ganglion cells in the cat retinal slice. J Neurophysiol. 1994;72:1260–1269. doi: 10.1152/jn.1994.72.3.1260. [DOI] [PubMed] [Google Scholar]
- Dacey D, Packer OS, Diller L, Brainard D, Peterson B, Lee B. Center surround receptive field structure of cone bipolar cells in primate retina. Vision Res. 2000;40:1801–1811. doi: 10.1016/s0042-6989(00)00039-0. [DOI] [PubMed] [Google Scholar]
- Daw NW, Stein PS, Fox K. The role of NMDA receptors in information processing. Annu Rev Neurosci. 1993;16:207–222. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell’s nonlinear receptive field. J Neurosci. 1999;19:9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Sterling P, Freed MA. How retinal ganglion cells prevent synaptic noise from reaching the spike output. J Neurophysiol. 2004;92:2510–2519. doi: 10.1152/jn.00108.2004. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Dhingra NK, Kao YH, Sterling P, Smith RG. Contrast threshold of a brisk-transient ganglion cell in vitro. J Neurophysiol. 2003;89:2360–2369. doi: 10.1152/jn.01042.2002. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Copenhagen DR. The contribution of NMDA and non-NMDA receptors to the light-evoked input-output characteristics of retinal ganglion cells. Neuron. 1993;11:725–738. doi: 10.1016/0896-6273(93)90082-3. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Copenhagen DR. The relationship between light-evoked synaptic excitation and spiking behaviour of salamander ganglion cells. J Physiol. 1995;487:711–725. doi: 10.1113/jphysiol.1995.sp020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Du JL, Wei HP, Wang ZR, Wong ST, Poo MM. Long-range retrograde spread of LTP and LTD from optic tectum to retina. Proc Natl Acad Sci USA. 2009;106:18890–18896. doi: 10.1073/pnas.0910659106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu ON, Protti DA, Majumdar S, Zeilhofer HU, Wassle H. Ionotropic glutamate receptors of amacrine cells of the mouse retina. Vis Neurosci. 2006;23:79–90. doi: 10.1017/S0952523806231079. [DOI] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis SF. Glutamate receptor gating. Crit Rev Neurobiol. 2004;16:187–224. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- Espinosa F, Kavalali ET. NMDA receptor activation by spontaneous glutamatergic neurotransmission. J Neurophysiol. 2009;101:2290–2296. doi: 10.1152/jn.90754.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Binshtok AM, Gutnick MJ. Functionally distinct NMDA receptors mediate horizontal connectivity within layer 4 of mouse barrel cortex. Neuron. 1998;21:1055–1065. doi: 10.1016/s0896-6273(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. J Comp Neurol. 2000;420:98–112. [PubMed] [Google Scholar]
- Fox K, Sato H, Daw N. The effect of varying stimulus intensity on NMDA-receptor activity in cat visual cortex. J Neurophysiol. 1990;64:1413–1428. doi: 10.1152/jn.1990.64.5.1413. [DOI] [PubMed] [Google Scholar]
- Gerstner W, Kistler W. Spiking neuron models. Cambridge, UK: Cambridge University Press; 2002. [Google Scholar]
- Gottesman J, Miller RF. N-methyl-D-aspartate receptors contribute to the baseline noise of retinal ganglion cells. Vis Neurosci. 2003;20:329–333. doi: 10.1017/s0952523803203114. [DOI] [PubMed] [Google Scholar]
- Grunert U, Haverkamp S, Fletcher EL, Wassle H. Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. J Comp Neurol. 2002;447:138–151. doi: 10.1002/cne.10220. [DOI] [PubMed] [Google Scholar]
- Gustafson EC, Stevens ER, Wolosker H, Miller RF. Endogenous D-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina. J Neurophysiol. 2007;98:122–130. doi: 10.1152/jn.00057.2006. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Functional organization of cone bipolar cells in the rat retina. J Neurophysiol. 1997;77:1716–1730. doi: 10.1152/jn.1997.77.4.1716. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- Jackman SL, Choi SY, Thoreson WB, Rabl K, Bartoletti TM, Kramer RH. Role of the synaptic ribbon in transmitting the cone light response. Nat Neurosci. 2009;12:303–310. doi: 10.1038/nn.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Wu SM. AMPA-preferring receptors mediate excitatory non-NMDA responses of primate retinal ganglion cells. Vis Neurosci. 2001;18:703–710. doi: 10.1017/s0952523801185044. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990a;10:1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990b;10:3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs TC, Koizumi A, Masland RH. The spatial distribution of glutamatergic inputs to dendrites of retinal ganglion cells. J Comp Neurol. 2008;510:221–236. doi: 10.1002/cne.21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbaugh TL, Zhang J, Diamond JS. Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells. J Neurosci. 2009;29:1469–1479. doi: 10.1523/JNEUROSCI.4240-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A, Aizenman E, Lipton SA. The interaction of agonists and noncompetitive antagonists at the excitatory amino acid receptors in rat retinal ganglion cells in vitro. J Neurosci. 1988;8:2895–2906. doi: 10.1523/JNEUROSCI.08-08-02895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz PD, Wilson JA, Lawrence JE. AMPA-preferring receptors mediate excitatory synaptic inputs to retinal ganglion cells. J Neurophysiol. 1997;77:57–64. doi: 10.1152/jn.1997.77.1.57. [DOI] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manookin MB, Demb JB. Presynaptic mechanism for slow contrast adaptation in mammalian retinal ganglion cells. Neuron. 2006;50:453–464. doi: 10.1016/j.neuron.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Detwiler PB. Different mechanisms generate maintained activity in ON and OFF retinal ganglion cells. J Neurosci. 2007;27:5994–6005. doi: 10.1523/JNEUROSCI.0130-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SC, Miller RF. Glutamate receptors of ganglion cells in the rabbit retina: evidence for glutamate as a bipolar cell transmitter. J Physiol. 1988;405:635–655. doi: 10.1113/jphysiol.1988.sp017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SC, Miller RF. N-methyl-D-aspartate receptors of ganglion cells in rabbit retina. J Neurophysiol. 1990;63:16–30. doi: 10.1152/jn.1990.63.1.16. [DOI] [PubMed] [Google Scholar]
- Matsui K, Hosoi N, Tachibana M. Excitatory synaptic transmission in the inner retina: paired recordings of bipolar cells and neurons of the ganglion cell layer. J Neurosci. 1998;18:4500–4510. doi: 10.1523/JNEUROSCI.18-12-04500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Miller RF. Cell communication mechanisms in the vertebrate retina the proctor lecture. Invest Ophthalmol Vis Sci. 2008;49:5184–5198. doi: 10.1167/iovs.08-2456. [DOI] [PubMed] [Google Scholar]
- Mittman S, Taylor WR, Copenhagen DR. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J Physiol. 1990;428:175–197. doi: 10.1113/jphysiol.1990.sp018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Munch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron. 2006;52:511–524. doi: 10.1016/j.neuron.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci. 2008 doi: 10.1038/nn2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Barrow A, Jacoby RA, Wu SM. How do tonic glutamatergic synapses evade receptor desensitization? J Physiol. 2008;586:2889–2902. doi: 10.1113/jphysiol.2008.151050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlich P, van Veen T, Szel A. Two different visual pigments in one retinal cone cell. Neuron. 1994;13:1159–1166. doi: 10.1016/0896-6273(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Singer JH. Multivesicular release and saturation of glutamatergic signalling at retinal ribbon synapses. J Physiol. 2007;580:23–29. doi: 10.1113/jphysiol.2006.125302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci. 2004;7:826–833. doi: 10.1038/nn1280. [DOI] [PubMed] [Google Scholar]
- Sivyer B, Taylor WR, Vaney DI. Uniformity detector retinal ganglion cells fire complex spikes and receive only light-evoked inhibition. Proc Natl Acad Sci. 2010;107:5628–5633. doi: 10.1073/pnas.0909621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Chen E, Copenhagen DR. Characterization of spontaneous excitatory synaptic currents in salamander retinal ganglion cells. J Physiol. 1995;486(Pt 1):207–221. doi: 10.1113/jphysiol.1995.sp020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J Neurosci. 2002;22:7712–7720. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trong PK, Rieke F. Origin of correlated activity between parasol retinal ganglion cells. Nat Neurosci. 2008;11:1343–1351. doi: 10.1038/nn.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Taylor WR, Vaney DI. Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. J Neurosci. 2006;26:13250–13263. doi: 10.1523/JNEUROSCI.1991-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Wassle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci. 2009;26:297–308. doi: 10.1017/S0952523809990137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Wassle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Wassle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Xu Y, Vasudeva V, Vardi N, Sterling P, Freed MA. Different types of ganglion cell share a synaptic pattern. J Comp Neurol. 2008;507:1871–1878. doi: 10.1002/cne.21644. [DOI] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Chromatic properties of horizontal and ganglion cell responses follow a dual gradient in cone opsin expression. J Neurosci. 2006;26:12351–12361. doi: 10.1523/JNEUROSCI.1071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci. 2009;29:789–797. doi: 10.1523/JNEUROSCI.4984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Diamond JS. Subunit- and pathway-specific localization of NMDA receptors and scaffolding proteins at ganglion cell synapses in rat retina. J Neurosci. 2009;29:4274–4286. doi: 10.1523/JNEUROSCI.5602-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, Marshak DW, Fain GL. Ligand-gated currents of alpha and beta ganglion cells in the cat retinal slice. J Neurophysiol. 1994;72:1260–1269. doi: 10.1152/jn.1994.72.3.1260. [DOI] [PubMed] [Google Scholar]