Abstract
Analgesics currently available for the treatment of pain following ophthalmic surgery or injury are limited by transient effectiveness and undesirable or adverse side effects. The cornea is primarily innervated by small-diameter C-fiber sensory neurons expressing TRPV1 (transient receptor potential channel, subfamily V, member 1), a sodium/calcium cation channel expressed abundantly by nociceptive neurons and consequently a target for pain control. Resiniferatoxin (RTX), a potent TRPV1 agonist, produces transient analgesia when injected peripherally by inactivating TRPV1-expressing nerve terminals through excessive calcium influx. The aim of the present study was to evaluate topical RTX as a corneal analgesic. In rat cornea, a single application of RTX dose-dependently eliminated or reduced the capsaicin eye wipe response for 3–5 days, with normal nociceptive responses returning by 5–7 days. RTX alone produced a brief but intense noxious response, similar to capsaicin, necessitating pretreatment of the cornea with a local anesthetic. Topical lidocaine, applied prior to RTX, blocks acute nociceptive responses to RTX without impairing the subsequent analgesic effect. Importantly, RTX analgesia (a) did not impair epithelial wound healing, (b) left the blink reflex intact and (c) occurred without detectable histological damage to the cornea. Immunohistochemistry showed that loss of CGRP immunoreactivity, a surrogate marker for TRPV1-expressing fibers, extended at least to the corneal-scleral boundary and displayed a progressive return, coincident with the return of capsaicin sensitivity. These data suggest RTX may be a safe and effective treatment for postoperative or post-injury ophthalmic pain.
Keywords: resiniferatoxin, vanilloid receptor 1, C-fibers, wound healing, TRPV1, eye wipe test, corneal pain, ophthalmic analgesia, CGRP
Introduction
Safe, long-lasting pain relief following corneal abrasions, corneal ulcers or ophthalmic surgery is difficult to achieve with current analgesics. For example, although effective acutely, continuous topical use of local anesthetics can produce an increased incidence of infection and corneal scarring, as well as impair the blink reflex and other non-nociceptive sensations [16]. Alternative routes of administration such as peribulbar or retrobulbar injections have increased risk and are also limited by transient effectiveness [7]. The use of topical NSAIDs and acetominophen is constrained by their gradual onset and limited efficacy [7], and the adverse side effects of systemic opioids are well known [31]. Postoperative pain remains not only a barrier to the widespread use of clinically effective ophthalmic surgical procedures, such as photorefractive keratectomy [22], but also a burden to patients during the recovery period.
Resiniferatoxin (RTX) is an ultra-potent agonist of the vanilloid receptor 1, now termed the transient receptor potential cation channel, subfamily V, member 1 (TRPV1). TRPV1 is an ion channel permeable to sodium and calcium and highly expressed in nociceptive neurons responsive to noxious heat, various endogenous algesic ligands, and the vanilloid agonist capsaicin (CAP) found in hot peppers [4, 26, 29]. RTX strongly activates TRPV1 producing a large influx of calcium, resulting in calcium-induced cytotoxicity [9, 23]. Intrathecal or intraganglionic administration can delete TRPV1-expressing neurons or lesion the dorsal roots to permanently attenuate thermal, inflammatory and cancer pain [3, 11, 27]. When administered peripherally, a single dose of RTX produces a long-lasting but reversible analgesia by ablating nociceptive nerve terminals [11, 13, 21]. Previous studies have demonstrated that 100 ng RTX injected subcutaneously into the rat hind paw produces thermal analgesia for approximately 20 days [21]. Unlike local anesthetics that target ubiquitous sodium channels in all axons, the specific cellular expression of TRPV1 and the selective action of RTX leaves non-nociceptive neurons and mechanosensitive nociceptive neurons functionally intact [11].
The cornea is densely innervated with sensory nerve fibers whose cell bodies reside in the ipsilateral trigeminal ganglion. Nerve bundles enter the peripheral corneal stroma in its middle third, divide dichotomously as they extend toward the center of the cornea, branch into a sub-basal plexus between the stromal and epithelial layers, and terminate between the epithelial cells of the basal layer and more superficial layers [14, 15, 18]. All the epithelial layers contain nerve terminals except the two most superficial layers. Nerve terminals in the corneal epithelium are unmyelinated and exhibit a high frequency of TRPV1 expression [19, 20], consistent with the cornea’s acute sensitivity to noxious stimuli. A previous study showed that topical CAP reduced corneal sensitivity of Aδ polymodal units to chemical and thermal activation at 5 min [1]. We reasoned that RTX, a far more potent agonist, could produce a long-duration inactivation of TRPV1-expressing nerve endings when applied topically to the corneal surface, providing effective, long-lasting, reversible analgesia without the side effects and limitations presented by other treatments. In this investigation we used gross histology, immunohistochemistry, molecular biological, and behavioral techniques to demonstrate that topical RTX provides prolonged analgesia without damaging the cornea or impairing wound healing.
Methods
Animals
NIH Guidelines for the Care and Use of Laboratory Animals were followed and the National Institute of Dental and Craniofacial Research Animal Care and Use Committee approved the protocol. Male Sprague-Dawley rats (175–300 g) were used for all testing, housed under a 12 hr light-dark cycle, and had access to food and water ad libitum.
Administration of RTX
Rats were anesthetized with 3% isoflurane during RTX administration. RTX was prepared as 0.02, 0.2, 1, 2 or 20 µg (1.6 µM to 1.6 mM) in 20 µL vehicle (7.5% Tween 80 and 0.05% ascorbic acid in phosphate buffered saline, PBS). Ten microliters were pipetted directly onto the corneal surface as the eyelids were gently retracted for 1 min, after which the eye was manually shut. The procedure was repeated with the remaining 10 µl. Each rat received RTX unilaterally while the contralateral eye served as an internal control for behavioral testing (n=5–6 per dose). RTX applied directly to the eye transiently stimulates the nociceptive endings and caused vigorous squinting, even with isoflurane anesthesia.
Lidocaine pretreatment
To reduce the nociceptive, stimulatory component of RTX application to the cornea, we pretreated rats with topical lidocaine 5 to 10 min prior to RTX. A 2% lidocaine solution was applied to the cornea in two consecutive 10 µL doses for 1 min each (similar to above). To evaluate whether lidocaine pretreatment impaired the therapeutic actions of RTX, rats received lidocaine alone, RTX alone, or lidocaine plus RTX (n=5 per group) and were subsequently evaluated with the capsaicin eye wipe test.
Capsaicin eye wipe test
To evaluate the analgesic effect of RTX, we introduced a capsaicin (CAP) solution to the eye and monitored the eye wipe response, as previously described [11]. A 5% CAP (164 mM), 0.2% ascorbic acid (11 mM) stock solution in 75% ethanol was prepared. The CAP stock solution was diluted to 0.02% (655 µM) using saline supplemented with 0.2% ascorbic acid. The awake rat was gently restrained by loosely wrapping it in a surgical towel. The eyelids were retracted by the experimenter and 50 µL 0.02% CAP was rapidly ejected from a pipette onto the cornea. The rat was quickly removed from the towel and placed onto a table top for observation. Eye wipes were counted. The wiping response typically lasted less than 30 sec, consistent with an early burst of Aδ activity recorded from corneal polymodal units [1]. Rats always wiped with the forepaw and sometimes rapidly scratched the eye with the hind paw. Each burst of hind paw scratches was counted as one wipe. Rats were subsequently challenged with 50 µL of the dilute CAP vehicle (0.2% ascorbic acid, 0.3% ethanol in saline) in the control eye. To avoid capsaicin-induced desensitization [1, 11], each eye was only tested once. Thus, a different group of rats was used for each time point. Eye wipe response data was analyzed with 2-way ANOVA (time course) or a paired t-test (dose response), with pairing between the treated and control eye of each rat tested.
Von Frey test
Rats were wrapped in a surgical towel and gently held by hand. Using a set of calibrated von Frey hairs (Stoelting Co., IL, USA), blink response was assessed in untreated controls and rats that received either 2 µg RTX or 20 µL of 2% lidocaine (N=4 per group). RTX and lidocaine were applied 24 hr and 10 min before testing, respectively, as previously described. To assess blink response, von Frey hairs of ascending stiffness (0.005, 0.023, 0.028, 0.068, 0.166, and 0.407 g) were used to mechanically stimulate each cornea 5 times, as previously described [2].
Evaluation of corneal integrity after RTX
Capsaicin spray (pepper spray) has been reported to cause transient, minor corneal epithelial changes [30]. However, the pepper spray formulation includes high concentrations of both CAP (180 mM) and isopropyl alcohol (64%), whereas we used a maximum RTX dose of 1.6 mM formulated in the RTX vehicle (7.5% Tween-80, 0.05% ascorbic acid in PBS). Ophthalmic fluorescein strips were used to assess gross morphological changes in the cornea following RTX administration (n=2). Under ultraviolet light, areas of intense fluorescence indicate regions of epithelial damage (e.g., abrasions). We examined corneas with fluorescein before 2 µg RTX and 24 hr after administration. At 24 hr, the animal was euthanized and the eye imaged under a fluorescent dissecting microscope.
Control and treated eyes (2µg RTX, n=4) were also histologically compared. Eyes were enucleated 24 hr after treatment, post-fixed in 10% formalin, embedded in methyl methacrylate, and sectioned at 2 microns. Tissue was stained with hematoxylin-eosin (H&E) or periodic acid-Schiff (PAS) and evaluated in a masked fashion with respect to treatment status.
Epithelial healing and RTX
Rats received 20 µg RTX in one eye and vehicle contralaterally. While still anesthetized, the corneal epithelial layer was debrided in both eyes as previously described [8, 25] (n=7). Briefly, a 3 mm disk of filter paper was soaked in n-heptanol and placed on the corneal surface for 30 s, after which the eye was flushed repeatedly with excess saline. The wounded area stained intensely with fluorescein and was clearly visible with a dissecting microscope under UV illumination. Wound width was acquired by measuring with digital calipers at 0, 10, 15, 22, 32, 37, and 42 hr and is reported as the average of measurements made in the dorsal-ventral and medial-lateral dimensions. Assessment of significance in average wound width in RTX- and vehicle-treated eyes over time was made using 2-way ANOVA.
RT-PCR
RNA was extracted from excised rat corneas or trigeminal ganglia using a RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA). RNA was quantified fluorometrically using RiboGreen reagent (Invitrogen) and a fluorescent plate reader (Molecular Devices, Sunnyvale, CA, USA). Eight nanograms (ng) of total RNA were used per 25 µl reaction using the Access RT-PCR system (Promega, Madison, WI, USA). The primer sequences used for TRPV1 were 5’-GCACCTAGCTGGTTGCAAAT-3’ (forward) and 5’-TCCTCATAAGGGCAGTCCAG-3’ (reverse). The RT-PCR reaction was carried out using a Robocycler (Stratagene) thermal cycler according to the manufacturer’s instructions. Briefly, one cycle (45 min at 48 °C) was used for reverse transcription. This was followed by one cycle (2 min at 94 °C) of transcriptase inactivation and 36 cycles of denaturation, annealing and extension (94 °C for 30 s, 55 °C for 1 min, and 68 °C for 2 min; respectively). A final extension cycle was done at 68 °C for 7 min. RT-PCR products were resolved on ethidium bromide-stained agarose gels (2%) and the image of the gel was captured with an AlphaImager (Alpha Innotech, San Leandre, CA, USA). GPDH expression was also obtained for normalization using 2 ng of total RNA that was amplified for 23 cycles. The primer sequences used for GPDH are 5’-ACCACAGTCCATGCCATCAC-3’ (forward) and 5’-TCCACCACCCTGTTGCTGTA-3’ (reverse). The RT-PCR methodology has been described in detail [17].
Immunohistochemistry
After euthanasia by CO2 inhalation, rat cornea was excised from the eye using a scalpel. Whole-mount immunofluorescence was performed as previously described [24], with a few modifications. Briefly, the cornea was fixed in a 4:1 solution of methanol and dimethyl sulfoxide for 30 min at room temperature followed by an additional 90 min at −20°C. The cornea was then rinsed twice in pre-chilled methanol for 10 min at −20°C followed by successive rinses in 70%, 50% and 30% methanol at room temperature for 3 min each. After two washes in PBS, the cornea was incubated in blocking solution comprising 10% normal horse serum and 0.1 % Triton X-100 in PBS. For immunostaining, rabbit anti-rat CGRP [28] and mouse anti-β-tubulin III (Abcam, Cambridge, MA, USA) antibodies were combined in blocking solution at a concentration of 1:1000 and 1:200, respectively. Whole cornea was incubated in the primary antibody solution for at least 48 hr at room temperature to allow for adequate penetration through the multiple layers. The corneas were then rinsed three times in PBS and blocked for another 20 min. Donkey anti-mouse and donkey anti-rabbit secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen, Carlsbad, CA, USA), respectively, were used for detection. Secondary antibodies were diluted in blocking solution and incubated on corneas for at least 24 hr. Corneas were then washed twice for 5 min in PBS, incubated overnight in fresh PBS, and whole mounted on slides with Fluoromount-G (Southern Biotech, Birmingham, AL, USA). Immunofluorescence was detected by confocal microscopy using a Leica TCS SP2 microscope (Wetzlar, Germany) and images were captured using Leica software. To label the nuclei, 18.3 mM 4',6-diamidino-2-phenylindole (DAPI) was added to the blocking solution containing secondary antibodies.
To quantify the amount of CGRP staining, cornea whole-mounts were viewed under an epi-fluorescent microscope with a 40× objective. Corneas were labeled 24 hr, 12 d, and 4 mo following treatment with 20 µg RTX or vehicle (n=4–5 per group per time point). Ten fields of view with clear β-tubulin staining in both stromal fibers and the sub-basal plexus were chosen throughout each cornea. Each field was subsequently assessed as positive (any visible fiber staining) or negative for CGRP staining. Data were analyzed using Fisher’s exact test.
Results
RTX dose response
When CAP was applied to control (untreated) eyes, the rats immediately began wiping with their forepaws and occasionally the ipsilateral hindpaw; the wiping response generally persisted for less than 30 sec. The mean number of wipes in the untreated eye was 16.6 ± 0.9, and this response was not affected by treatments to the opposite eye (e.g., RTX). The effect of RTX on the eye wipe response was dose-dependent. The lowest doses of RTX tested (0.02 and 0.2 µg) were ineffective at suppressing the CAP eye wipe response at any time point examined, 1µg of RTX reduced but did not eliminate the wiping response, 2 µg RTX eliminated the response in 3 of 5 rats, and 20 µg RTX eliminated the response in all rats at the one day time point (Figure 1 A).
Figure 1.
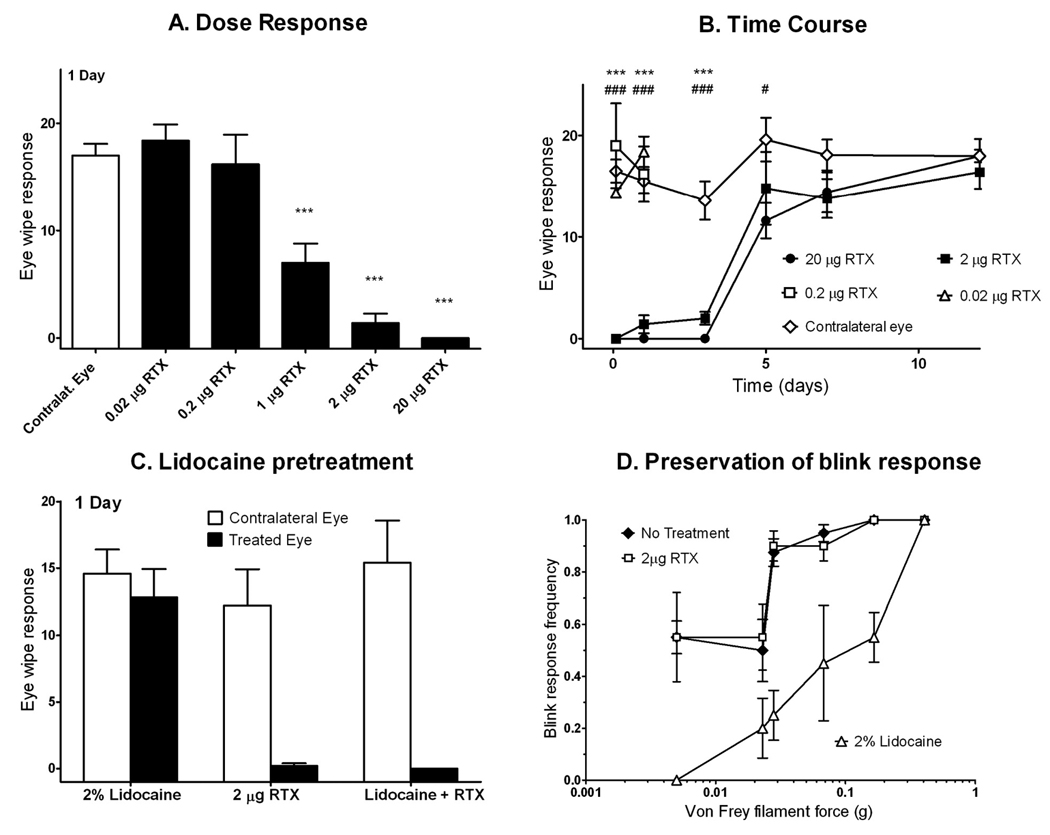
RTX suppression of CAP eye wipe response 1 day after treatment was dose-dependent (A). Doses of 0.02 and 0.2 µg had no significant effect, while doses of 1 µg and above reduced or eliminated eye wipe response. Stars indicate statistical difference from the contralateral eye using a paired t-test; number of stars indicates the p-value (one star, p<0.05; 2 stars, p<0.01; 3 stars, p<0.001). A single dose of RTX applied to the corneal surface ablates the eye wipe response to 0.02% CAP for several days (B). Contralateral eyes received no treatment prior to the CAP eye wipe test. By day 7, response in RTX-treated eyes normalized. Animals treated with 0.02 and 0.2 µg RTX were only assessed at 2 hr and 1 day, as no effect was observed. As determined by 2-way ANOVA, stars indicate statistical difference between 2 µg RTX and vehicle and pound signs indicate statistical difference between 20 µg RTX and vehicle. Lidocaine pretreatment does not interfere with RTX therapeutic action (C). Animals received RTX alone, lidocaine alone, or lidocaine pretreatment 5 to 10 minutes prior to RTX (n=5 per group). The eye wipe test was performed one day later. RTX suppressed eye wipe response, both with and without lidocaine pretreatment. Lidocaine alone had no effect on eye wipe response 24 hr after application. Lidocaine did blunt the nociceptive stimulation that occurred with acute application of RTX. Treatment with 20 µg RTX spares corneal blink response to mechanical stimulation with von Frey hairs (D). 20 µg RTX or 20 µL 2% lidocaine was applied topically onto the cornea 24 hr and 10 min, respectively, before testing, with the contralateral eye receiving no treatment (n=4 per group). Von Frey hairs were touched to the corneal surface to assess blink response. Each hair was touched 5 times to each eye. RTX had no significant effect on the sensitivity of blink response, while lidocaine significantly suppressed response.
RTX Time Course
Rats treated with 2 and 20 µg RTX had a markedly decreased CAP eye wipe response 2 hr after treatment (Figure 1 B). At 1 and 3 days post-treatment with 2 µg RTX, the eye wipe response remained inhibited (p < 0.001 for both times), with mean eye wipes of 1.4 ± 0.9 and 2.0 ± 0.6, respectively. Normal response in eyes treated with 2 µg RTX returned by 5 days. The 20 µg dose completely blocked the eye wipe response at days 1 and 3, and continued to have a significant effect until day 5 (11.6 ± 3.9; p=0.048) before returning to baseline at day 7 (14.4 ± 4.4).
Lidocaine Pretreatment and RTX
Pretreatment with lidocaine 5 to 10 min prior to RTX administration significantly reduced the nocifensive behavior caused by RTX, characterized by vigorous squinting following application. Of 5 rats that received lidocaine and RTX, 3 showed no response to RTX and 2 had a markedly diminished squinting response. Lidocaine did not impair the analgesic effects of RTX (Fig 1 C). The capsaicin eye wiping response one day after receiving lidocaine and RTX did not significantly differ from responses observed after RTX alone. Lidocaine alone produced no significant effects when the CAP eye wipe test was performed at 24 hr after application.
Von Frey test
The blink response, elicited by touching the cornea with von Frey hairs, was not significantly affected by topical application of 2 µg RTX (Fig 1 D). The positive control, 2% lidocaine, markedly reduced the blink response frequency. With a 0.023 g von Frey filament, the mean ± SEM blink response frequency was 50 ± 12% for RTX compared to 55 ± 13% for untreated controls (equivalent to random chance), and was reduced to 20 ± 12% after lidocaine.
Corneal integrity after RTX
Corneal damage was not observed 24 hr following RTX administration. Under gross inspection using a fluorescent dissecting microscope, fluorescein-stained RTX-treated and control eyes were indistinguishable, indicating the absence of corneal surface abrasions or other damage to the epithelium. Neither H&E nor PAS staining revealed an increase in epithelial cell edema, glycoprotein, irregularity, or any other indication of damage (Fig 2 A–D). The stroma, Decement’s membrane, and endothelium were entirely normal. Consistent with the lack of damage to the epithelium, we did not detect TRPV1 expression in corneal epithelial cells (Fig 2 E). The RT-PCR conditions used here (8 ng RNA and 36 cycles) were designed to detect very low levels of TRPV1 [17]. TRPV1 expression was easily detected after 30 cycles (8 ng RNA) in trigeminal ganglia, used as a positive control (Fig 2 E).
Figure 2.
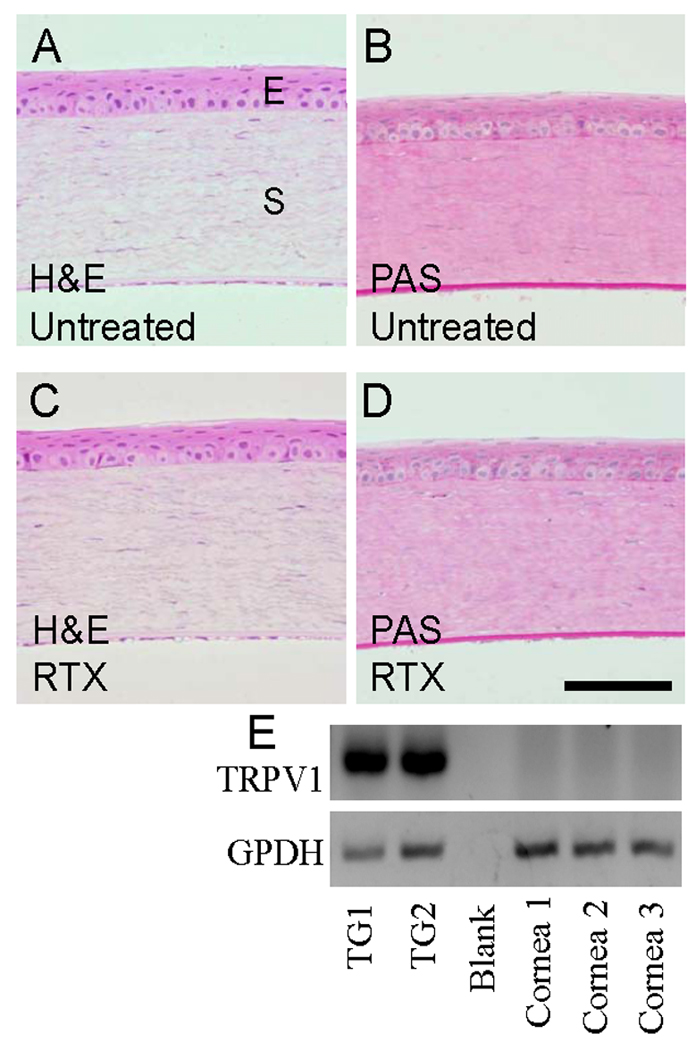
RTX-treated and untreated corneas were histologically indistinguishable using H&E and PAS staining. Corneas were acquired 24 hr after treatment with 2 µg RTX, post-fixed in 10% formalin, and embedded in methyl methacrylate. Sections were stained with H&E or PAS and evaluated by a masked observer. “E” indicates the epithelial layer and “S” indicates the stromal layer. (A) H&E untreated; (B) PAS untreated; (C) H&E 2 µg RTX; (D) PAS 2 µg RTX. Scale bar = 200 µm. (E) RT-PCR shows TRPV1 transcripts are expressed in trigeminal ganglia (TG) but not the cornea, using GPDH as a normalizing control. Thus, RTX likely has no direct effect on resident corneal cells.
Epithelial wound healing
We evaluated the effect of RTX treatment on corneal wound healing following chemical debridement of corneal epithelial cells by n-heptanol. The region of debrided epithelium healed at a similar rate in eyes treated either with 20 µg RTX or vehicle (Fig 3 A). Measurement of average wound width during healing revealed that all wounds healed by 42 hr. At all time points tested (0 to 42 hr), average wound width in RTX- and vehicle-treated eyes was not significantly different (Fig 3 B–D).
Figure 3.
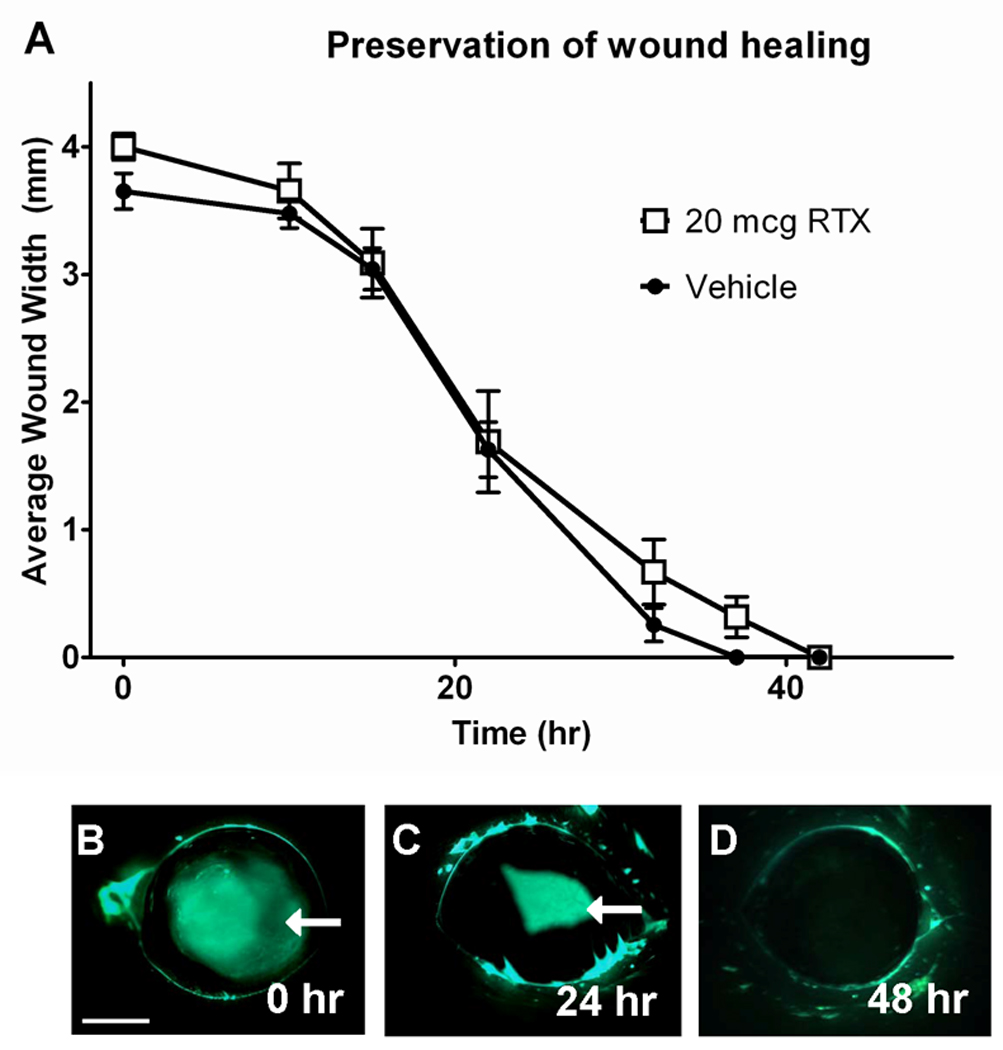
Corneal wound healing rates were not significantly different for eyes treated with 20 µg RTX or vehicle (A). The epithelial layer was removed from an area of the cornea by applying a circle of filter paper soaked in n-heptanol to the cornea for 30 s, followed by copious saline flushing. The wound was visualized at 0, 10, 15, 22, 32, 37, and 42 hr by applying fluorescein solution to the cornea under UV light and the width of the wound was measured with digital calipers in the dorsal-ventral and medial-lateral dimensions. 2-way ANOVA identified no significant difference between average wound widths of RTX- and vehicle-treated eyes at any time point. The area of debridement contracted over time as it was reepithelialized from the edge, and complete reepithelialization occurred by 42 hours in all eyes (B, C, and D). Scale bar: 2 mm.
Immunohistochemistry
To visualize ablation (loss of immunoreactivity) of TRPV1-positive corneal nerve fibers after RTX, we co-stained corneas for the pan-neuronal marker β-tubulin and the neuropeptide CGRP, which has been reported to be expressed primarily in TRPV1-positive corneal nerves [19]. Fig 4 A–C shows β-tubulin-immunoreactivity in fibers penetrating the stromal (arrows) and sub-basal plexus (asterisks) layers as well as in distal nerve terminals projecting towards the surface of the cornea (plus sign). Under our immunohistochemical conditions, in control corneas, CGRP staining was readily visualized in nerve fibers of the stromal layer (Fig 4 D), which are generally bundled and larger [14, 15, 18]. Our procedure was not sensitive enough to resolve CGRP in the very small-caliber distal terminals or in sub-basal plexus fibers. Fig 4 D–F shows that loss of CGRP immunoreactivity was clearly seen at the level of the stromal fibers. Administration of 20 µg RTX significantly reduced CGRP staining at 24 hr and 12 days after treatment (p<0.001 and p=0.005, respectively) while sparing other fibers as indicated by the retention of β-tubulin labeling. Vehicle-treated corneas had CGRP staining in 94 ± 2% of fields examined. RTX-treated corneas had CGRP staining in 18 ± 8% of fields at 24 hr, 68 ± 7% of fields at 12 days, and 100% of fields at four months.
Figure 4.
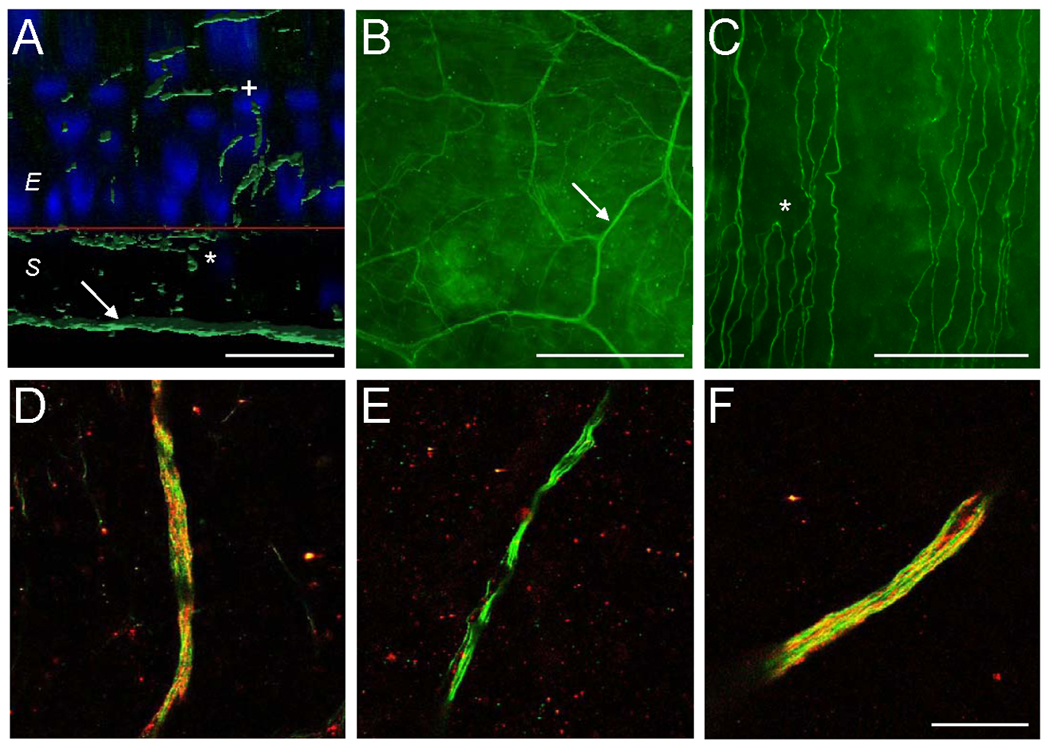
Immunohistochemistry shows selective, long-lasting, reversible removal of CGRP-positive fibers from the rat cornea following topical RTX. Staining of the cornea with the panneuronal marker β-tubulin III (green) reveals large fiber bundles in the stroma (arrows) giving rise to a sub-basal plexus of parallel fibers at the interface of the epithelial and stromal cell layers (stars) and terminating in the superficial epithelium (plus sign) (A,B,C). Red line (A) denotes the boundary between the epithelial and stromal cell layers as seen from a cross-sectional perspective. Scale bars: A – 25 µm; B - 500 µm; C - 100 µm. (D, E, F) CGRP staining (red), a surrogate for TRPV1, is removed following RTX. Vehicle-treated eyes showed strong CGRP staining in stromal fiber bundles (D). 24 hr after treatment with 20 µg RTX, CGRP staining was significantly reduced (E), with most stromal fiber bundles showing no CGRP staining. CGRP staining partially returned by 12 d following RTX treatment (not shown), and had returned completely when assessed at four months (F). CGRP-positive nociceptors returned along bundle tracts. Scale bar is 25 µm.
Discussion
The present data demonstrate that topical application of RTX to the cornea significantly inhibits nociception as assessed by the CAP eye wipe test. The effects are profound but reversible; the maximum effect is present for at least 3 days but less than 5 days. RTX elicits a dose-dependent analgesic effect, with 0.2 µg exhibiting no analgesic actions and 20 µg entirely blocking the CAP eye wipe response. Complete behavioral recovery occurred between 5–7 days. We also observed that RTX resulted in a temporary loss of CGRP-expressing nociceptive fibers; the majority of fibers returned within 12 days and, while intermediate time points were not examined, full recovery was seen when assessed at four months. Importantly, RTX caused no apparent damage to the cornea, did not affect the normal blink response, nor did it impair wound healing.
RTX is a potent vanilloid receptor agonist. Therefore, application of RTX initially evokes a nociceptive response upon binding to and activation of TRPV1. However, since RTX maintains TRPV1 in an open state, causing sustained influx of calcium through the channel, the end result is calcium-induced cytotoxicity and inactivation [13]. RTX has therefore been used to ablate TRPV1-expressing neurons and/or their central projections to obtain permanent analgesia via intraganglionic, intracisternal and intrathecal injection [3, 11]. In contrast, peripheral injections of RTX result in a distal axonopathy (dying back) of TRPV1-expressing nociceptive fibers with significant (or complete) preservation of TRPV1-expressing neurons in the ganglion [21]. This manipulation therefore results in temporary analgesia and subsequent return of normal nociception. It is noteworthy that RTX does not have an impact on sensory neurons and fibers that do not express TRPV1, thus sensory modalities such as proprioception and tactile discrimination are preserved [23] and, in particular for the cornea, the blink reflex.
In the present study, 2 µg RTX was used to achieve prolonged, but temporary, analgesia when applied to the cornea. This dose is 200x greater than that necessary to produce dying back of nociceptive fibers after subcutaneous injection into the paw [21]. Several differences in the delivery method may account for the increased dose. When RTX is directly injected into the paw, the drug has unimpeded access to TRPV1-expressing fibers. In contrast, in the present study, RTX was applied topically to the cornea. The corneal epithelium, which is several cell layers thick (six in humans), appears to present a significant barrier to penetration of the drug. A lower dose might be more effective with increased exposure time of the cornea to RTX. Here, we used two brief (one-minute each) applications of RTX; future studies can be performed to determine whether increasing the exposure time will shift the dose-response. Furthermore, although we applied 20 µl of solution to the cornea, only a thin film makes direct contact with the eye and there is likely a local dilution effect due to tear production.
The stromal layer of the cornea contains bundles of nerve fibers that enter from the peripheral border and extend toward the center of the cornea. In control and vehicle-treated eyes, we observed that all fibers within bundles were positive for β-tubulin III. In contrast, only a subpopulation of the fibers within bundles was positive for CGRP, a surrogate marker used to identify TRPV1-expressing nociceptive fibers [19]. After application of RTX, β-tubulin III fibers were still detected in the bundled fibers. CGRP-staining was largely absent in the bundles 24 h after RTX application, indicating that the CGRP-positive fibers were ablated within bundles or CGRP-containing vesicles were eliminated following RTX treatment. By day 12, the proportion of bundles that contained CGRP-positive fibers significantly increased compared to 24 hr. The majority of fields examined under high power (40× objective) showed staining, suggesting that the fibers had regenerated or had been replenished with CGRP. Importantly, it appeared that CGRP-positive fibers regenerated along the intact bundle tracts, thereby maintaining a normal pattern of stromal innervation. By four months, CGRP staining had returned to baseline, indicating complete restoration of corneal nociceptive innervation. Full return of nociceptive behavior following RTX treatment occurs substantially earlier than complete return of CGRP-positive fibers (5 days vs >12 days, respectively). This is consistent with the idea that a partial return of nociceptive fibers is sufficient to elicit a normal eye wipe response to CAP. The sensory endings in the conjunctiva and eyelid are also exposed to RTX at the same time as the cornea. Thus, it is possible that, while these endings may be similarly inactivated by RTX, they return more quickly than those in the cornea and contribute to reestablishment of a normal eye wipe response.
The present study disclosed no overt adverse side effects associated with corneal application of RTX. When grossly assessed using fluorescein and histology, resident corneal cells appeared unaffected by a single dose of RTX. Local anesthetics, however, are precluded from chronic use (i.e., several days of analgesia) due to direct corneal toxicity [16] and are, therefore, not appropriate agents for post-operative pain control during recovery from ophthalmic surgery. The absence of TRPV1 mRNA in corneal epithelial cells suggests that RTX acts only on TRPV1-expressing nerve fibers innervating the cornea whose cell bodies reside in trigeminal ganglia. In fact, non-TRPV1-expressing cells and processes are resistant to RTX toxicity at doses that are clearly cytotoxic to TRPV1-expressing neurons in sensory ganglia [5, 11]. A previous study which relied primarily on immunohistochemical methods to detect TRPV1 suggested that TRPV1 is expressed in human and mouse corneal epithelial cells [32]. In the present study, we used RT-PCR in the cornea with amplification conditions (36 cycles; 8 ng total RNA each) that were far more sensitive than those required to robustly detect mRNA in our positive control, the trigeminal ganglion (30 cycles; 8 ng total RNA each). Thus, if TRPV1 mRNA is present in corneal epithelial cells, the transcript levels are very low and, likely, the amount of receptor protein produced is too limited to trigger detectable epithelial cell toxicity. It should be mentioned, however, that application of pepper spray, which resulted in varying degrees of corneal desensitization between 30 min and 1 week, did cause epithelial cell toxicity [30]. The pepper spray used contains 64% isopropyl alcohol vehicle and extremely high CAP concentration (180 mM versus 1.6 mM RTX in the present study), and the vehicle, the CAP or the combination may produce toxicity. Mechanical sensitivity was also reduced by pepper spray, which is not likely to be a direct effect of CAP on TRPV1-expressing fibers as it has been reported that response to mechanical stimulation is primarily mediated by endings that are negative for TRPV1 staining [6].
Unlike local anesthetics, we found no effect of RTX on protective mechanosensitive behaviors mediated by non-nociceptive fibers, such as the blink response. RTX-treated rats maintained a blink response to mechanical stimulation identical to untreated controls, whereas the blink reflex was clearly blocked by lidocaine pretreatment. Also, RTX did not affect wound healing, in contrast, local anesthetics have been reported to impair healing [10].
There are several conditions that lead to acute corneal pain including surgery (e.g., photorefractive keratectomy, PRK), corneal abrasions, and corneal ulcers. In the present study, a single application of RTX produced analgesia lasting between 3 and 5 days. Chronic pain conditions may require multiple applications to achieve longer lasting analgesia and the reapplication point would have to be determined empirically. For treating acute corneal pain, it is likely that a single application of RTX is sufficient. In clinical settings, this may be relevant for patients undergoing PRK, a reliable but lesser-used refractive surgery, partially because of the associated acute pain.
RTX may be a valuable tool for managing post-surgical eye pain with many clear advantages over local anesthetics, NSAIDs, and opioids. Examination of translational clinical pain models in, for example, large animals such as the horse, which frequently develop painful eye problems, will allow further optimization of drug delivery and evaluation of the clinical utility of RTX as a corneal analgesic.
Acknowledgments
This research was supported by the Division of Intramural Research, NIDCR. We thank Dr. Rachel Bishop, National Eye Institute for her helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement. We have no financial or other conflicts of interest.
References
- 1.Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat’s cornea. J Physiol. 1991;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boberg-Ans J. Experience in clinical examination of corneal sensitivity; corneal sensitivity and the naso-lacrimal reflex after retrobulbar anaesthesia. Br J Ophthalmol. 1955;39:705–726. doi: 10.1136/bjo.39.12.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, Olah Z, Mannes AJ. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 5.Caudle RM, Karai L, Mena N, Cooper BY, Mannes AJ, Perez FM, Iadarola MJ, Olah Z. Resiniferatoxin induced loss of plasma membrane in vanilloid receptor expressing cells. J. Neurotoxicology. 2003;24:895–908. doi: 10.1016/S0161-813X(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh DJ, Lee H, Lo L, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppens M, Versichelen L, Mortier E. Treatment of postoperative pain after ophthalmic surgery. Bull Soc Belge Ophtalmol. 2002;285:27–32. [PubMed] [Google Scholar]
- 8.Gallar J, Pozo MA, Rebollo I, Belmonte C. Effects of capsaicin on corneal wound healing. Invest Ophthalmol Vis Sci. 1990;31:1968–1974. [PubMed] [Google Scholar]
- 9.Grant ER, Dubin AE, Zhang SP, Zivin RA, Zhong Z. Simultaneous intracellular calcium and sodium flux imaging in human vanilloid receptor 1 (VR1)-transfected human embryonic kidney cells: a method to resolve ionic dependence of VR1-mediated cell death. J Pharmacol Exp Ther. 2002;300:9–17. doi: 10.1124/jpet.300.1.9. [DOI] [PubMed] [Google Scholar]
- 10.Hirata M, Sakaguchi M, Mochida C, Sotozono C, Kageyama K, Kuroda Y, Hirose M. Lidocaine inhibits tyrosine kinase activity of the epidermal growth factor receptor and suppresses proliferation of corneal epithelial cells. Anesthesiology. 2004;100:1206–1210. doi: 10.1097/00000542-200405000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karai LJ, Russell JT, Iadarola MJ, Olah Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem. 2004;279:16377–16387. doi: 10.1074/jbc.M310891200. [DOI] [PubMed] [Google Scholar]
- 13.Kissin I. Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity. Anesth Analg. 2008;107:271–281. doi: 10.1213/ane.0b013e318162cfa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest Ophthalmol Vis Sci. 1989;30:461–472. [PubMed] [Google Scholar]
- 15.Marfurt CF, Murphy CJ, Florczak JL. Morphology and neurochemistry of canine corneal innervation. Invest Ophthalmol Vis Sci. 2001;42:2242–2251. [PubMed] [Google Scholar]
- 16.McGee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;6:637–640. doi: 10.1517/14740338.6.6.637. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell K, Iadarola MJ. RT-PCR analysis of pain genes. In: Szallasi A, editor. Analgesia. Methods in molecular biology series. Totowa, NJ: Springer-Humana Press; 2010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 19.Murata Y, Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006;1085:87–94. doi: 10.1016/j.brainres.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura A, Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M, Mimura O. Morphological and immunohistochemical characterization of the trigeminal ganglion neurons innervating the cornea and upper eyelid of the rat. J Chem Neuroanat. 2007;34:95–101. doi: 10.1016/j.jchemneu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ. Peripherally induced resiniferatoxin analgesia. Pain. 2003;104:219–228. doi: 10.1016/s0304-3959(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 22.Nissman SA, Tractenberg RE, Babbar-Goel A, Pasternak JF. Oral gabapentin for the treatment of postoperative pain after photorefractive keratectomy. Am J Ophthalmol. 2008;145:623–629. doi: 10.1016/j.ajo.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem. 2001;276:11021–11030. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- 24.Sakimoto T, Kim TI, Ellenberg D, Fukai N, Jain S, Azar DT, Chang JH. Collagen XVIII and corneal reinnervation following keratectomy. FEBS Lett. 2008;582:3674–3680. doi: 10.1016/j.febslet.2008.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Invest Ophthalmol Vis Sci. 1981;21:135–142. [PubMed] [Google Scholar]
- 26.Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- 27.Tender GC, Walbridge S, Olah Z, Karai L, Iadarola M, Oldfield EH, Lonser RR. Selective ablation of nociceptive neurons for elimination of hyperalgesia and neurogenic inflammation. J Neurosurg. 2005;102:522–525. doi: 10.3171/jns.2005.102.3.0522. [DOI] [PubMed] [Google Scholar]
- 28.Traub RJ, Iadarola MJ, Ruda MA. Effect of multiple dorsal rhizotomies on calcitonin gene-related peptide-like immunoreactivity in the lumbosacral dorsal spinal cord of the cat: a radioimmunoassay analysis. Peptides. 1989;10:979–983. doi: 10.1016/0196-9781(89)90179-4. [DOI] [PubMed] [Google Scholar]
- 29.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vesaluoma M, Muller L, Gallar J, Lambiase A, Moilanen J, Hack T, Belmonte C, Tervo T. Effects of oleoresin capsicum pepper spray on human corneal morphology and sensitivity. Invest Ophthalmol Vis Sci. 2000;41:2138–2147. [PubMed] [Google Scholar]
- 31.Wishaw K, Billington D, O'Brien D, Davies P. The use of orbital morphine for postoperative analgesia in pterygium surgery. Anaesth Intensive Care. 2000;28:43–45. doi: 10.1177/0310057X0002800107. [DOI] [PubMed] [Google Scholar]
- 32.Zhang F, Yang H, Wang Z, Mergler S, Liu H, Kawakita T, Tachado SD, Pan Z, Capo-Aponte JE, Pleyer U, Koziel H, Kao WW, Reinach PS. Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J Cell Physiol. 2007;213:730–739. doi: 10.1002/jcp.21141. [DOI] [PubMed] [Google Scholar]


