Abstract
Tumor hypoxia regulates many cytokines and angiogenic factors (CAFs) and is associated with worse prognosis in head and neck squamous cell cancer (HNSCC). Serum CAF profiling may provide information regarding the biology of the host and tumor, prognosis, and response to therapy. We investigated 38 CAFs in HNSCC patients receiving induction therapy on a Phase II trial of carboplatin, paclitaxel, and cetuximab. CAFs were measured by multiplex bead assay and enzyme-linked immunosorbent assay in 32 patients. Baseline and post-induction CAF levels were correlated with disease progression (PD) and human papilloma virus (HPV) status by Wilcoxon rank sum test. Baseline levels of 8 hypoxia-regulated CAFs (the “high-risk signature” including vascular endothelial growth factor, interleukins-4 and -8, osteopontin, growth-related oncogene-α (Gro-α), eotaxin, granulocyte-colony stimulating factor, and stromal cell derived factor-1α) were associated with subsequent PD. Elevation in ≥6/8 factors was strongly associated with shorter time to progression (p=0.001) and was 73% specific and 100% sensitive for PD. Rising Gro-α from baseline to week six was also associated with PD. Progression free and overall survival were shorter in patients with HPV-negative tumors (p=0.012 and 0.046, respectively), but no individual CAF was associated with HPV-status. However, among 14 HPV-negative patients, the high-risk CAF signature was seen in all 6 patients with PD, but only 2/14 without PD. In conclusion, serum CAF profiling, particularly in HPV-negative patients, may be useful for identifying those at highest risk for recurrence.
Keywords: head and neck squamous cell cancer, serum markers, hypoxia
Introduction
47,500 people present with new HNSCC annually in the United States(1) and 30-40% of patients will relapse within 2-3 years following traditional therapy with radiation or chemoradiation(2). A significant subset of HNSCC tumors have hypoxic regions (3), which are an independent predictor of poor outcome after radiotherapy or combined treatments (4-6). The downstream effects of tumor hypoxia on hypoxia-inducible factor 1-alpha (HIF1α) activation and angiogenesis may contribute to resistance to radiation and to the epidermal growth factor receptor (EGFR) inhibitor cetuximab (7, 8).
In vitro studies suggest that 1-1.5% of all genes are hypoxia-regulated, many of which are part of signaling pathways that promote cancer proliferation, angiogenesis, and progression (4). Recently, Harris, et al. identified a 99 metagene signature of tumor hypoxia that correlates with clinical outcome in HNSCC patients (9, 10). Because many cytokines and angiogenic factors (CAFs) are hypoxia regulated, we hypothesized that a profile of serum CAFs would correlate with clinical outcome. Earlier studies have suggested a potential role for blood-based biomarkers in detecting HNSCC among high risk patients (11-14) and as independent predictors of poor outcome in HNSCC (15, 16). More recently, Allen, et al. observed that rising levels in five nuclear factor kappa-B (NFκB)-modulated cytokines (interleukins (IL)-6 and -8, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), or Gro-α) were associated with shorter cause-specific survival (16). Since hypoxia can modulate secreted proteins through multiple pathways, we investigated a broad panel of 38 CAFs in patients receiving induction therapy for locally advanced HNSCC. In addition, because HPV-positive tumors have better clinical outcomes and appear to have a distinct biology from HPV-negative tumors (17-19), we also investigated whether HPV-status affects a patient’s CAF profile.
Blood-based biomarkers are practical for monitoring during and after treatment. Furthermore, serum factors also reflect the contribution of the microenvironment and host immune response to the behavior of HNSCC, unlike techniques that directly assess only the tumor cells.
In this study, we used multiplex bead assay and ELISA to perform an exploratory analysis of 38 CAFs in serum from patients treated on a Phase II induction chemotherapy trial (20). We identified eight baseline CAFs that were individually associated with outcome. The association was even stronger when they were combined together into a “high-risk” signature. Although individual CAF levels were not associated with HPV-status, elevations in high risk CAFs were observed in HPV-negative patients with subsequent PD.
Methods
Induction chemotherapy study design and treatment outcome
Forty-seven previously untreated patients (33 male, 14 female) with advanced nodal disease (T1-4, N2b/c/3, M0), ECOG performance status 0-1, received 6 weekly cycles of neoadjuvant paclitaxel (135 mg/m2), carboplatin (AUC 2), and cetuximab (400 mg/m2 week 1; 250 mg/m2 weeks 2-6) as part of a Phase II clinical trial at M. D. Anderson Cancer Center (Protocol 2003:0919).(20) A majority of patients enrolled had oropharyngeal primary tumors (n=42). Local therapy following chemotherapy was risk-based, with baseline T1-2 tumors receiving radiation alone (or surgery in one patient with oral primary) and T3-4 tumors receiving concurrent chemoradiation. Neck dissection was performed in nine patients with residual metastasis after locoregional treatment. Written informed consent was obtained from each patient after approval of the study by the University of Texas M. D. Anderson Cancer Center Institutional Review Board.
Following induction therapy, but prior to local therapy, patients were evaluated for clinical and radiographic response in the primary tumor and regional lymph nodes. Nine (19%) out of 47 patients had an overall clinicoradiographic complete response (CR), 36 (77%) had partial responses (PR), and two (4%) had stable disease (SD). CR was more common in never smokers (38.9%) versus former (12.5%) or current smokers (0%).
Patients were followed every three months for routine surveillance. At a minimum follow-up of two years (median 33 months), six patients have had PD (2 local; 1 local and distant; 2 regional and distant; and 1 distant). Four of these died of HNSCC; two are without evidence of disease following salvage surgery. The remaining 41 patients (87%) are alive without evidence of disease (NED).
Serum collection and preparation for CAF analysis
Baseline serum was collected within one week of starting induction therapy. Serum was also collected in 26/32 patients at the end of induction chemotherapy, but prior to definitive treatment. Peripheral blood samples were collected by routine venipuncture technique and allowed to coagulate for 30-60 minutes. Samples were centrifuged for 10 to 15 minutes at 1000g to separate serum which was then frozen and stored at −70 to −80°C.
Multiplex bead assay and ELISA
38 CAFs were measured by multiplex bead assay (36 factors) or ELISA (2 factors). These included hypoxia-induced factors, chemokines, interleukins, angiogenic factors, apoptosis mediators, and hematopoietic growth factors (Table 1). Multiplex bead assays were performed with BioSource Multiplex Assays for Luminex (Invitrogen, Calsbad, CA) in a 96-well format according to the BioSource protocol. Multiplex bead assay is a high-throughput technology that uses antibody-coupled microspheres identified by a unique ratio of two fluorescent dyes to measure up to 100 biomolecules simultaneously. Performance characteristics are comparable to enzyme-linked immunosorbent assay ELISA,(21, 22) but are less expensive and require less blood because of the multiplexed analysis (up to 50 biomarkers can be measured from 200uL serum).
Table 1. Cytokines and angiogenic factors in serum biomarker analysis.
| β-NGF | IL-18 | MIF |
| CTACK | IL-1RA | MIG |
| Eotaxin | IL-2 | MIP-1β |
| G-CSF | IL2RA | Osteopontin |
| GRO-α | IL-3 | PDGF-bb |
| HGF | IL-4 | RANTES |
| ICAM-1 | IL-6 | SCF |
| IFN-a2 | IL-7 | SDF-1α |
| IFN-γ | IL-8 | TNF-b |
| IGF-1 | IP-10 | TRAIL |
| IL-12(p40) | MCP-1 | VCAM-1 |
| IL-13 | MCP-3 | VEGF |
| IL-16 | M-CSF |
Abbreviations: cutaneous T cell-attracting chemokine (CTACK), granulocyte colony stimulating factor (G-CSF), growth-related oncogene (Gro), hepatocyte growth factor (HGF), insulin-like growth factor (IGF-1), inter-cellular adhesion molecule (ICAM), interferon gamma (IFN-γ), interleukin (IL), macrophage inflammatory protein (MIP), macrophage migration inhibitory factor (MIF), monocyte chemotactic protein (MCP), monocyte colony stimulating factor (M-CSF), monokine induced by IFN-Gamma (MIG), nerve growth factor (NGF), osteopontin, platelet derived growth factor (PDGF), regulated upon activation, normal T-cell expressed and secreted (RANTES), stem cell factor (SCF), stromal cell derived factor (SDF), tumor necrosis factor (TNF), tumor-necrosis-factor related apoptosis inducing ligand (TRAIL), vascular cell adhesion molecule (VCAM-1), vascular endothelial growth factor (VEGF)
CAF concentrations were calculated based on a standard curve derived by performing six serial dilutions of a protein standard in assay diluent. Serum samples were tested in duplicate and the mean value used for analysis. If the mean of the duplicate values for all factors in an individual sample varied by >25%, the sample was re-tested. Individual factors were excluded from analysis if ≥50% of the samples were out of range or extrapolated. When out of range values were included in the analysis, the highest value (measured or extrapolated) across all samples was substituted for values above the upper limit of detection. For values below the lower limit of detection, half the lowest value was substituted. ELISA was used to measure insulin-like growth factor-1 (IGF-1) (Diagnostic Systems Laboratories, Inc., Webster, TX) and osteopontin (R&D Systems, Minneapolis, MN) according to the manufacturers’ instructions. Both analytes were measured in duplicate and mean value used for analysis.
HPV
Tumor specimens from 26 of 47 patients were available for HPV DNA detection by in-situ hybridization. Formalin fixed paraffin embedded (FFPE) tissue sections, 4 microns thick were evaluated by in situ hybridization for human papilloma virus (HPV) nucleic acids using the automated BenchMark per manufacturer recommended protocol (Ventana, Tucson, AZ). Ventana inform HPV III Family 16 Probe (Ventana) was used which contains a cocktail of labeled HPV genomic probes targeting the most common HPV genotypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66. Tumor cells were evaluated for positive nuclear expression with concurrent positive and negative controls reviewed. Of 26 tumors evaluated, 12 tested positive for oncogenic HPV DNA. There was no association between HPV status and clinical response to induction chemotherapy. However, patients with HPV-positive tumors had significantly longer progression free survival (PFS) (p=0.012) and overall survival (OS) (p=0.046).(20)
Immunohistochemistry for EGFR
Immunohistochemistry (IHC) was performed on pre-treatment tumor biopsies. FFPE tissue histology sections (5 μm thick) were deparaffinized, hydrated, and heated in a steamer for 30 min with Dako Target Retrieval pH 6.0, for antigen retrieval (Dako North America, Inc., Carpinteria, CA). Peroxide blocking was done with 3% H2O2 in methanol at room temperature for 15 min, followed by 10% fetal bovine serum in TBS-t for 30 min.
Slides were incubated with primary antibody against EGFR (total) clone:31G7 Zymed (Invitrogen, a division of Life Technologies Corporation, Carlsbad, CA) 1:100 at room temperature for 90 min and then probed for 30 min with the secondary antibody Envision Dual Link Plus (DAKO, Carpinteria, CA). Staining was developed with 0.05% DAB + (DAKO) and counterstained with hematoxylin. Tumor staining for EGFR was quantified using a four value intensity score (0, 1+, 2+, or 3+).
Statistical Analysis
PFS and OS were calculated from the first day of induction therapy. Fisher’s exact test was used to assess the relationship between clinicopathologic features and PD. For baseline CAF analyses, CAF levels were log transformed (base 2) prior to analysis and Wilcoxon rank sum test were used to compare CAF expression between patients with and without PD. For these exploratory analyses, a p-value of ≤0.05 was considered significant. The eight CAFs differentially expressed between these two groups were then combined into a “high-risk signature.” The median of each CAF was used as the cutoff value for dichotomizing each marker. Using log-rank analysis, we compared PFS in signature-positive patients (6-8 high-risk markers above the median) versus signature-negative patients (≤5 high-risk markers above the median). For modulation analysis, fold change from baseline to cycle 6 was compared by Wilcoxon rank sum test for each CAF in patients with and without PD. Unsupervised hierarchical clustering was performed on log-transformed, mean-centered baseline CAF levels using Cluster and TreeView software (Eisen Lab, Berkeley, CA) as previously described to identify subsets of patients with similar baseline CAF profiles.
All statistical analyses were performed using SAS v9.2.0 (SAS Institute Inc., Cary, NC) and graphed using the GraphPad Prism software v 5.02 (GraphPad Software, Inc., San Diego, CA).
Results
Clinical and pathological comparison of patients with progression
Of the 47 clinical trial patients, 32 (including all six with PD) had baseline serum for CAF profiling and were included in biomarker analysis. There were no significant differences in demographics (sex, gender, race, smoking status) or baseline clinical characteristics (tumor differentiation, T-stage, N-stage, primary tumor location) between patients with and without biomarker data (p≥0.17 for all clinicopathologic variables by Fisher’s exact t-test).
Among the 32 patients available for CAF analysis, 20 (63%) were male, and 19 (59%) were current or former smokers. All had locally advanced HNSCC, but there was a higher frequency of T1-2 disease than T3-4 (21 patients versus 11). A majority of patients had oropharyngeal primary tumors (n=27). N2B was the most common nodal stage (n=21).
All patients had CR, PR, or stable disease following induction. Since completing definitive local therapy, six of the 32 patients have had disease progression. PD was associated with older age (p=0.049), higher T-stage (p=0.003), and HPV-negative tumors (p=0.008) (Table 2). PD was not associated with response to induction, as 2/6 patients with PD had had CR, 3/6 PR, and 1/6 SD immediately following chemotherapy. Qualitative EGFR levels by IHC were not significantly correlated with stage, clinicoradiographic response, or disease progression.
Table 2. Clinicopathologic characteristics in patients with progression.
| Progressive Disease (PD) | ||||
|---|---|---|---|---|
| No (n=26) | Yes (n=6) | P-value | ||
| Age, median (range) | 52 (21-76) | 59 (52-71) | 0.049+ | |
| Sex | Male | 18(90%) | 2(10%) | 0.17 |
| Female | 8(66.7%) | 4(33.3%) | . | |
| Race | White | 24(82.8%) | 5(17.2%) | 0.48 |
| Black | 0(0%) | 1(100%) | . | |
| Hispanic | 1(100%) | 0(0%) | . | |
| Asian | 1(100%) | 0(0%) | . | |
| Smoking Status | Never Smoker | 12(92.3%) | 1(7.7%) | 0.17 |
| Former smoker | 8(88.9%) | 1(11.1%) | . | |
| Current smoker | 6(60%) | 4(40%) | . | |
| Differentiation | Well | 1(100%) | 0(0%) | 0.70 |
| Mod | 7(87.5%) | 1(12.5%) | . | |
| Poorly | 11(84.6%) | 2(15.4%) | . | |
| Moderately well | 1(50%) | 1(50%) | . | |
| Poorly-Moderately | 2(100%) | 0(0%) | . | |
| T Stage | T1-T2 | 18(86%) | 3(14%) | 0.003 |
| T3-T4 | 8(73%) | 3(27%) | . | |
| N Stage | N2B | 19(90.5%) | 2(9.5%) | 0.090 |
| N2C | 5(62.5%) | 3(37.5%) | . | |
| N3 | 1(50%) | 1(50%) | . | |
| EGFR++ | 0 | 0 (0%) | 0 (0%) | 0.78 |
| 1+ | 4(100%) | 0(0%) | ||
| 2+ | 5(100%) | 0(0%) | ||
| 3+ | 37(82.2%) | 8(17.8%) | ||
| HPV+++ | HPV-positive | 12 (100%) | 0(0%) | 0.008 |
| HPV-negative | 8 (57%) | 6(43%) | ||
p-value from Wilcoxon Rank sum test, all other p-values from the Fisher’s exact test
Available for 24 patients
Available for 26 patients
Baseline serum CAFs associated with subsequent HNSCC progression
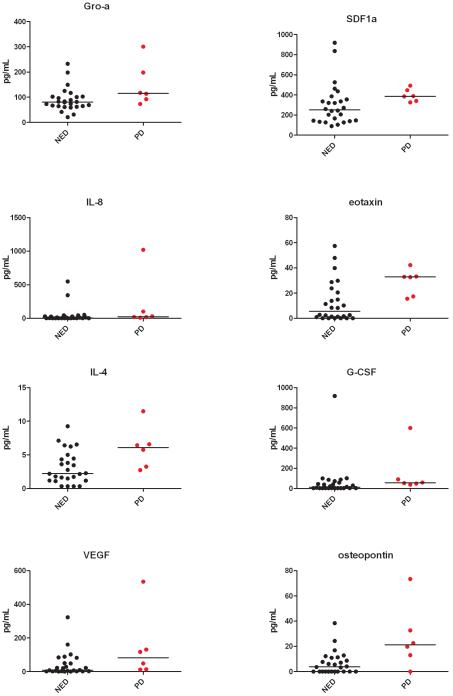
Baseline CAF levels (log2) were compared between patients with (n=6) and without (n=26) PD by Wilcoxon rank sum test. Eight CAFs were significantly higher in patients who later progressed (p≤0.05). These were VEGF, Gro–α, IL-4, IL-8, osteopontin, eotaxin, G-CSF, and SDF-1α (Table 3, Fig. 1). Eotaxin and osteopontin levels were most strongly associated with PD (p=0.016 and 0.021, respectively). Among these eight markers, there was a trend towards higher osteopontin levels in current smokers (median 3.8 pg/mL, range 0-6.2) versus never smokers or former smokers (median 2.3 pg/mL, range 0-5.3), although this was of borderline significance (p=0.073 by Wilcoxon rank sum test).
Table 3. Baseline CAFs associated with progressive disease.
| Covariate | Progression | n | Mean +/− std* | median(min,max)* | p-value+ |
|---|---|---|---|---|---|
| Eotaxin | Yes | 6 | 4.8 +/− 0.6 | 5.1 (4.1, 5.4) | 0.016 |
| No | 26 | 2.6 +/− 2.0 | 2.6 (0, 5.9) | ||
| osteopontin | Yes | 6 | 4.0 +/− 2.1 | 4.5 (0, 6.2) | 0.021 |
| No | 26 | 2.0 +/− 1.8 | 2.3 (0, 5.3) | ||
| G CSF | Yes | 6 | 6.4 +/− 1.5 | 5.9 (5.3, 9.2) | 0.039 |
| No | 26 | 4.2 +/− 2.1 | 3.6 (2.3, 9.8) | ||
| IL 4 | Yes | 6 | 2.7 +/− 0.6 | 2.8 (1.9, 3.6) | 0.040 |
| No | 26 | 1.8 +/− 0.9 | 1.7 (0.4, 3.4) | ||
| SDF 1a | Yes | 6 | 8.6 +/− 0.2 | 8.6 (8.4, 8.9) | 0.044 |
| No | 26 | 8.0 +/− 0.9 | 8.0 (6.5, 9.9) | ||
| VEGF | Yes | 6 | 6.0 +/− 2.1 | 6.3 (3.7, 9.1) | 0.049 |
| No | 26 | 3.6 +/− 2.5 | 3.2 (0.1, 8.3) | ||
| GRO a | Yes | 6 | 7.1 +/− 0.7 | 6.9 (6.2, 8.2) | 0.049 |
| No | 26 | 6.3 +/− 0.7 | 6.3 (4.5, 7.9) | . | |
| IL 8 | Yes | 6 | 5.5 +/− 2.5 | 4.6 (3.2, 10) | 0.050 |
| No | 26 | 2.9 +/− 2.6 | 2.4 (0.5, 9.1) |
values are pg/mL log2 transformed
p-value from the Wilcoxon rank sum test
Figure 1.
Comparison of baseline CAF levels in patients with and without PD for the eight high-risk markers.
High-risk baseline CAF signature predicts PD
We examined whether the combination of the 8 CAFs that were differentially expressed at baseline into a “high-risk” signature would be more sensitive or specific for disease progression. For each patient, the number of CAFs in the high-risk signature that were above the median (across all baseline samples) was counted. PD was not seen in any patient with ≤5 high-risk CAFs above the median. However, among patients with 6-8 elevated high-risk CAFs, 6/13 (47%) of patients had PD (Fig. 2A). Elevation in 6-8 high-risk CAFs was 73% specific and 100% sensitive for PD. As expected, time to progression was also longer in patients with ≤5 elevated high-risk CAFs by Log-rank test (p=0.001) (Fig. 2B).
Figure 2. Elevations in ≥6/8 high-risk CAFs is associated with PD.
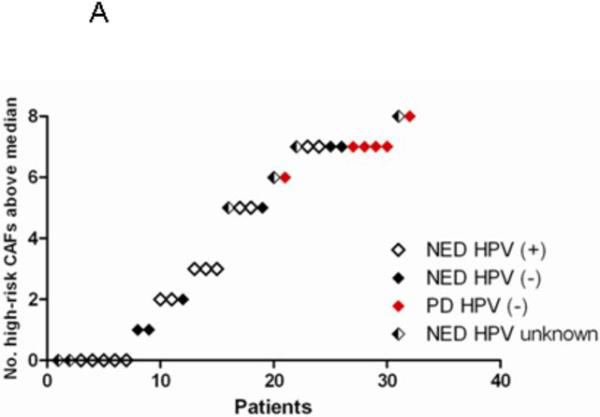
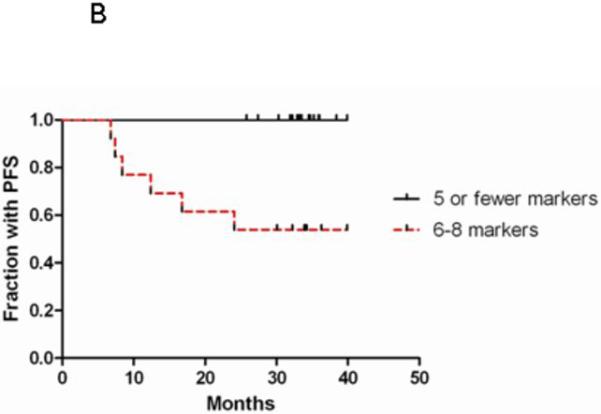
The number of high-risk CAFs (VEGF, IL-4, IL-8, Gro-α, SDF-1α, G-CSF, OPN, and eotaxin) above the median was plotted for each patient in Fig 2A. HPV-status is indicated by circles (HPV-positive) and triangles (HPV-negative). Patients with PD are indicated in red. Kaplan-Meier curve is shown for time to progression in patients with 0-5 versus 6-8 high-risk CAFs above the median (Fig 2B).
Longitudinal rise in CAFs associated with progressive disease
Previously, Allen et al. found that longitudinal changes in five NFKB-mediated cytokines and growth factors (IL-6, IL-8, VEGF, HGF, and Gro-α) predicted worse outcome in patients with advanced or metastatic HNSCC.(16) Here, we measured fold change of these and other factors from baseline to completion of induction therapy (six weeks). CAF levels were available at both time points for 26 patients, including four who ultimately developed PD. As with the Allen study, patients with PD had a significantly greater rise from baseline to cycle 6 in Gro-α (p-value=0.015). There was also a significant rise in βNGF (p=0.039) and borderline significant rise in MCP3 (p=0.053). There was no significant modulation of the other CAFs.
High-risk CAFs elevated in HPV-negative patients with PD
Because all six progression events were in patients with HPV-negative tumors, we investigated whether CAF levels were either surrogates for HPV status or if they might add prognostic information beyond HPV status. HPV status was available for 26/32 patients with CAF profiles, of whom 12 (46%) were HPV-positive and 14 (54%) HPV-negative. No individual CAF was associated with HPV status.
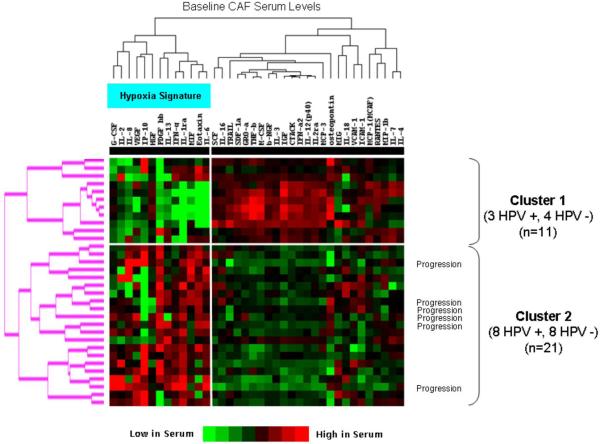
We then performed hierarchical clustering of baseline CAF levels across all 32 patients and evaluated HPV between clusters. Clustering separated the patients into two distinct groups (11 patients in Cluster 1, 21 in Cluster 2) (Fig. 3). Cluster 2 was characterized by higher levels of hypoxia-associated markers and contained all six patients with PD. However, there was a similar number of HPV-positive and negative patients in each cluster (Fig. 3), further suggesting that CAF expression was not primarily HPV driven.
Figure 3. Unsupervised clustering identified two distinct CAF signatures that correlate with clinical outcome, but not with HPV status.
Relative serum levels of 38 baseline CAFs are shown (red indicates high protein levels, green low protein levels). Each row contains CAF levels for an individual patient. Two distinct patient CAF profiles are identified (Cluster 1 and Cluster 2), and all six patients with PD are in Cluster 2 which is characterized by higher levels of hypoxia associated factors, as indicated in the figure. There is no association between HPV status and clusters.
High-risk CAF signature associated with PD among HPV negative patients
Next, we evaluated whether individual CAF levels were different between 6 HPV-negative patients with PD and 8 without. Osteopontin was statistically higher in HPV-negative patients with PD (p=0.04). Gro-α (p=0.06), IL-8 (p=0.08), and VEGF (p=0.08) were also elevated in HPV-negative patients with PD, although these did not reach statistical significance, possibly due to the small sample size.
Among the 14 HPV-negative patients, 8 were signature-positive (6-8 high risk CAFs elevated), 6 of whom had PD. This suggests that the combination of the CAF high-risk signature with HPV status identifies patients at risk for PD beyond that of HPV-status alone. Of note, there was no statistically significant association between smoking status and levels of high-risk CAFs among HPV-negative patients.
Discussion
The availability of multiplexing technologies now permits the simultaneous assessment of large numbers of biologically relevant proteins, such as cytokines, angiogenic factors and receptors, and soluble markers of hypoxia and immune activity using small amounts of serum or plasma. In this investigation, we studied serum biomarkers from a cohort of patients with locally advanced HNSCC on a Phase II trial of induction therapy followed by definitive locoregional treatment. To our knowledge, this is the largest panel of serum markers to be studied in locally advanced HNSCC by multiplex bead analysis, and the only study in the setting of induction chemotherapy.
Our CAF analysis identified 8 individual serum markers (VEGF, IL-4, IL-8, Gro-α, SDF-1α, G-CSF, osteopontin, and eotaxin) higher in patients with subsequent PD. One of these (Gro-α) continued to increase longitudinally from baseline to week 6 to a greater extent in patients with PD. The combination of these 8 CAFs into a high-risk signature correlated better with PD than any individual CAF. Specifically, no patient with ≤5 elevated high-risk CAFs had PD, compared to PD in 6/13 patients with 6-8 high-risk CAFs. The combination of HPV-negativity with high-risk serum CAF status further discriminated patients at greatest risk for PD than either one alone. This may reflect a difference in the biology of HPV-negative tumors or may be the result of the higher incidence of recurrence among this population.
Although this was an exploratory analysis, the results suggest that blood-based biomarkers may have a role as prognostic markers, beyond what is provided by currently available clinical or pathological features. In this study, only age, T-stage, and HPV-negative status were associated with a worse clinical outcome. Consistent with other studies, EGFR did not predict response or outcome, although there was a trend for patients with 3+ staining for EGFR to have shorter DFS and OS despite treatment with cetuximab (23).
Among the individual CAFs associated with a worse prognosis, several have previously been associated with angiogenesis, tumorgenesis, and metastasis in HNSCC and other cancers (24-28). Among these, VEGF and IL-8 are the transcription products of genes included in the Harris metagene hypoxia signature and SDF-1α is the ligand of a receptor (CXCR4) in the signature (10). The elevation of these factors in parallel suggests that we are able to detect the downstream effects of hypoxia in patient serum.
Induction chemotherapy has been shown to decrease metastatic disease in HNSCC patients with locally advanced disease and is being studied in prospective randomized trials (29-31). However, previous studies have shown a correlation between EGFR inhibitor resistance and the upregulation of hypoxia-induced signaling by HIF1α and increased tumor angiogenesis, both of which are downstream effects of tumor hypoxia (7, 8). Therefore, our observation that patients with high levels of hypoxia-associated factors were more likely to relapse following induction therapy that included cetuximab may reflect the role of tumor hypoxia in therapeutic resistance and may be particularly relevant for regimens containing EGFR inhibitors.
Unlike the study by Allen at al., (16) which showed an association between low VEGF and shorter survival, in our study patients with elevated VEGF were more likely to have PD. Both studies observed an association between rising Gro-α and PD, although we did not see a rise in other NFKB factors as they had observed. This discrepancy may be due to the different time course over which we assessed change in CAFs (six weeks in our study versus three month intervals in theirs), differences in therapy, or small sample sizes. In addition, because all of the progression events occurred months to years after the end of induction therapy, it may have been too early to observe a rise in CAFs associated with PD. Because many of the CAF markers in the study can be modulated by inflammation and immune response, it is also possible that CAF levels were additionally influenced by hypoxia independent mechanisms.
We believe that these results support further investigation of CAF profiling to predict clinical outcome in HNSCC patients and as a possible tool for surveillance, particularly among HPV-negative patients. In addition, CAF profiling appears to be a more practical tool for assessing tumor hypoxia than direct intratumoral measurement (6) and more accurate than indirect techniques such as immunohistochemistry of CA9 and HIF1α (32, 33). This is particularly true, since CAF profiling is less invasive than direct tumor biopsy and, therefore, could be assessed longitudinally in the clinical setting with less morbidity and expense. Although this is a small, exploratory analysis, it demonstrates the potential strength of blood biomarker profiling as a clinical tool for risk-stratifying patients. In addition, some of the serum markers in our high-risk signature could be targeted directly (ex., VEGF, IL-4, and IL-8) or indirectly (ex., Gro-α via inhibition of its receptor CXCR2) by new targeted agents and may suggest a rationale for testing these drugs in patients with advanced HNSCC. We plan to validate our findings in an independent group of patients enrolled on a randomized, Phase II trial of this induction regimen. The goal will be to validate the high-risk signature for its prognostic ability as well as to explore its predictive value.
Acknowledgments
Financial support: These studies were supported by the University of Texas M. D. Anderson Cancer Center Head and Neck SPORE (P50 CA097007) and supplemented by Program Project Grant (P01 CA06294) and Cancer Center Support Grant (P30 CA016672). JVH is a Damon Runyon-Lilly Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI 24-04).
MSK received support for the Phase II clinical trial described in this paper from Bristol Myers Squibb Oncology Investigator Initiated trials program and Imclone Systems grant #CS 2004-00011435 WC.
Abbreviation list
- CAF
cytokines and angiogenic factors
- HNSCC
head and neck squamous cell cancer
- ELISA
enzyme-linked immunosorbent assay
- PD
progressive disease
- HPV
human papilloma virus
- Gro-α
growth-related oncogene-alpha
- HIF1α
hypoxia-inducible factor 1-alpha
- EGFR
epithelial growth factor receptor
- IL
interleukin
- NFKB
nuclear factor kappa-B
- VEGF
vascular endothelial growth factor
- HGF
hepatocyte growth factor
- CR
complete response
- PR
partial response
- SD
stable disease
- NED
no evidence of disease
- FFPE
formalin fixed paraffin embedded
- PFS
progression free survival
- IHC
immunohistochemistry
- G-CSF
granulocyte colony stimulating factor
- SDF-1α
stromal cell derived factor
- β-NGF
nerve growth factor
- MCP
monocyte chemotactic protein
Footnotes
Conflicts of Interest: The other authors declared no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Thun MJ, American Cancer Society . Cancer Facts & Figures 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 2.Vikram B, Strong EW, Shah JP, Spiro R. Second malignant neoplasms in patients successfully treated with multimodality treatment for advanced head and neck cancer. Head & neck surgery. 1984;6:734–7. doi: 10.1002/hed.2890060306. [DOI] [PubMed] [Google Scholar]
- 3.Adam MF, Gabalski EC, Bloch DA, et al. Tissue oxygen distribution in head and neck cancer patients. Head & neck. 1999;21:146–53. doi: 10.1002/(sici)1097-0347(199903)21:2<146::aid-hed8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nature reviews. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 5.Kaanders JH, Wijffels KI, Marres HA, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer research. 2002;62:7066–74. [PubMed] [Google Scholar]
- 6.Nordsmark M, Bentzen SM, Rudat V, et al. An international multi-center study Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Viloria-Petit A, Crombet T, Jothy S, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer research. 2001;61:5090–101. [PubMed] [Google Scholar]
- 8.Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem. 2006;281:25903–14. doi: 10.1074/jbc.M603414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denko NC, Fontana LA, Hudson KM, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–14. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- 10.Winter SC, Buffa FM, Silva P, et al. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer research. 2007;67:3441–9. doi: 10.1158/0008-5472.CAN-06-3322. [DOI] [PubMed] [Google Scholar]
- 11.Gourin CG, Xia ZS, Han Y, et al. Serum protein profile analysis in patients with head and neck squamous cell carcinoma. Archives of otolaryngology--head & neck surgery. 2006;132:390–7. doi: 10.1001/archotol.132.4.390. [DOI] [PubMed] [Google Scholar]
- 12.Linkov F, Lisovich A, Yurkovetsky Z, et al. Early detection of head and neck cancer: development of a novel screening tool using multiplexed immunobead-based biomarker profiling. Cancer Epidemiol Biomarkers Prev. 2007;16:102–7. doi: 10.1158/1055-9965.EPI-06-0602. [DOI] [PubMed] [Google Scholar]
- 13.St John MA, Li Y, Zhou X, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Archives of otolaryngology--head & neck surgery. 2004;130:929–35. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- 14.Wadsworth JT, Somers KD, Stack BC, Jr., et al. Identification of patients with head and neck cancer using serum protein profiles. Archives of otolaryngology--head & neck surgery. 2004;130:98–104. doi: 10.1001/archotol.130.1.98. [DOI] [PubMed] [Google Scholar]
- 15.Gourin CG, Moretz WH, 3rd, Weinberger PM, et al. Serum protein profile analysis following definitive treatment in patients with head and neck squamous cell carcinoma. Archives of otolaryngology--head & neck surgery. 2007;133:1125–30. doi: 10.1001/archotol.133.11.1125. [DOI] [PubMed] [Google Scholar]
- 16.Allen C, Duffy S, Teknos T, et al. Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res. 2007;13:3182–90. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- 17.Vidal L, Gillison ML. Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematology/oncology clinics of North America. 2008;22:1125–42. vii. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Braakhuis BJ, Snijders PJ, Keune WJ, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. Journal of the National Cancer Institute. 2004;96:998–1006. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 19.Slebos RJ, Yi Y, Ely K, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:701–9. doi: 10.1158/1078-0432.CCR-05-2017. [DOI] [PubMed] [Google Scholar]
- 20.Kies MSHF, Lee JJ, William WN, Jr., Glisson BS, Lin HY, Lewin JS, Ginsberg LE, Gillaspy KA, Massarelli E, Byers LA, Lippman SM, Hong WK, Garden AS, El-Naggar AK. Papadimitrakopoulou V.Journal of Clinical Oncology, in press. Induction Chemotherapy and Cetuximab for Locally Advanced Squamous Cell Carcinoma of the Head and Neck (SCCHN):Results From a Phase II Prospective Trial. Journal of Clinical Oncology. 2009 doi: 10.1200/JCO.2009.23.0425. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66:175–91. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giavedoni LD. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using luminex technology. J Immunol Methods. 2005;301:89–101. doi: 10.1016/j.jim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Morgillo F, Bareschino MA, Bianco R, Tortora G, Ciardiello F. Primary and acquired resistance to anti-EGFR targeted drugs in cancer therapy. Differentiation. 2007;75:788–99. doi: 10.1111/j.1432-0436.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- 24.Shang ZJ, Li JR, Li ZB. Circulating levels of vascular endothelial growth factor in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2002;31:495–8. doi: 10.1054/ijom.2002.0284. [DOI] [PubMed] [Google Scholar]
- 25.Riedel F, Zaiss I, Herzog D, Gotte K, Naim R, Hormann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer research. 2005;25:2761–5. [PubMed] [Google Scholar]
- 26.Hong SH, Ondrey FG, Avis IM, et al. Cyclooxygenase regulates human oropharyngeal carcinomas via the proinflammatory cytokine IL-6: a general role for inflammation? Faseb J. 2000;14:1499–507. doi: 10.1096/fj.14.11.1499. [DOI] [PubMed] [Google Scholar]
- 27.Teknos TN, Cox C, Yoo S, et al. Elevated serum vascular endothelial growth factor and decreased survival in advanced laryngeal carcinoma. Head & neck. 2002;24:1004–11. doi: 10.1002/hed.10163. [DOI] [PubMed] [Google Scholar]
- 28.Loukinova E, Dong G, Enamorado-Ayalya I, et al. Growth regulated oncogene-alpha expression by murine squamous cell carcinoma promotes tumor growth, metastasis, leukocyte infiltration and angiogenesis by a host CXC receptor-2 dependent mechanism. Oncogene. 2000;19:3477–86. doi: 10.1038/sj.onc.1203687. [DOI] [PubMed] [Google Scholar]
- 29.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–55. [PubMed] [Google Scholar]
- 30.Monnerat C, Faivre S, Temam S, Bourhis J, Raymond E. End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol. 2002;13:995–1006. doi: 10.1093/annonc/mdf172. [DOI] [PubMed] [Google Scholar]
- 31.Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol. 2004;22:1743–52. doi: 10.1200/JCO.2004.06.147. [DOI] [PubMed] [Google Scholar]
- 32.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Hypoxia-regulated carbonic anhydrase-9 (CA9) relates to poor vascularization and resistance of squamous cell head and neck cancer to chemoradiotherapy. Clin Cancer Res. 2001;7:3399–403. [PubMed] [Google Scholar]
- 33.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. International journal of radiation oncology, biology, physics. 2002;53:1192–202. doi: 10.1016/s0360-3016(02)02848-1. [DOI] [PubMed] [Google Scholar]