Abstract
Neurotransmitter transporters play essential roles in the process of neurotransmission. Vesicular neurotransmitter transporters mediate storage inside secretory vesicles in a process that involves the exchange of lumenal H+ for cytoplasmic transmitter. Retrieval of the neurotransmitter from the synaptic cleft catalyzed by sodium-coupled transporters is critical for the termination of the synaptic actions of the released neurotransmitter. Our current understanding of the mechanism of these transporters is based on functional and biochemical characterization but is lacking high-resolution structural information. Very few structures of membrane transport systems from mammalian origin have been solved to atomic resolution, mainly because of the difficulty in obtaining large amounts of purified protein. Development of high yield heterologous expression systems suitable for mammalian neurotransmitter transporters is essential to enable the production of purified protein for structural studies. Such a system makes possible also the production of mutants that can be used in biochemical and biophysical studies.
We describe here a screen for the expression of the vesicular monoamine transporter 2 (VMAT2) in cell-free and baculovirus expression systems and discuss the expression of VMAT2 in other systems as well (bacterial, yeast and mammalian cell lines). After screening and optimization, we achieved high yield (2–2.5 mg/liter) expression of functional VMAT2 in insect cells. The system was also used for the expression of three additional plasma membrane neurotransmitter transporters. All were functional and expressed to high levels. Our results demonstrate the advantages of the baculovirus expression system for the expression of mammalian neurotransmitter transporters in a functional state.
Introduction
Synaptic transmission involves the concerted function of two classes of neurotransmitter transporters: the plasma membrane sodium-coupled neurotransmitter transporters and the proton-gradient dependent vesicular transporters (SLC18 family). Crystal structures of prokaryotic homologues of the neurotransporters had a major impact on the field. Notably in the case of the plasma membrane transporters they have served as structural paradigms for interpretation of the wealth of functional data available on the eukaryotic counterparts from biochemical and electrophysiological experiments [1–3]. However, the work with the prokaryotic homologues, while informative at the mechanistic level can provide only an incomplete view of the mammalian proteins [4]. Despite their importance and clinical relevance, no high-resolution structures of mammalian neurotransmitter transporter are yet available. A basic reason for the lack of high resolution atomic data for mammalian integral membrane proteins in general is the difficulty of establishing high yield heterologous expression systems and obtaining large amounts of functional purified protein [5]. Some of the structures of mammalian membrane proteins have been solved to high resolution with proteins purified from native source [6, 7] while some others from heterologous expression systems [8–12], but these are still hardly a handful of examples.
The vesicular storage of monoamines, namely serotonin, dopamine, norepinephrine, epinephrine, and histamine, is mediated by the vesicular monoamine transporter family (VMATs) that operate by exploiting the proton gradient formed by the V-type ATPase [13]. Two mammalian genes encode for two vesicular monoamine transporters that share 62% identity. Both proteins exchange two protons per substrate molecule, but they display different pharmacological profile and tissue distribution [14–16]. Immunohistochemistry analysis showed that VMAT2 is the more common isoform in most tissues, and it is the only one expressed in neuronal cells, while VMAT1 is found only in some types of endocrine cells. Both genes are expressed in chromaffin cells of the human adrenal medulla [14].
VMAT2 has been shown to be an essential protein and homozygous VMAT2 knockout mice die shortly after birth [17, 18]. VMAT2 displays higher affinities towards all the native substrates and also to the inhibitors reserpine and tetrabenazine. In addition to the native substrates and the above-mentioned inhibitors, the VMATs interact with many clinically relevant drugs, including the psychostimulants 3,4-methylenedioxymethamphetamine (MDMA) and amphetamines and the parkinsonian toxin 1-methyl-4-phenylpyridinium (MPP+). Expression of VMAT confers to mammalian and yeast cells resistance to MPP+, a process accomplished by compartmentalization of the drug in intracellular acidic compartments, thus removing it from its presumed target [1, 19, 20].
In the present study, the vesicular monoamine transporter VMAT2 served as a case study for the heterologous over-expression of neurotransmitter transporters in quality and amounts high enough for structural analysis. We present a systematic study of various expression platforms including two cell free systems and three baculovirus vectors used for the expression of VMAT2 in insect cells. After optimization for VMAT2 of the baculovirus system, the same conditions were shown appropriate for expression of three plasma-membrane sodium-coupled neurotransmitter transporters.
Materials and Methods
Materials
RTS 100 E. coli HY and RTS 100 WG HY kits, Proteoexpert software license, pIVEX vectors, mouse anti His6 monoclonal antibody were purchased from Roche Diagnostics GmbH (Manheim, Germany). flashBAC™ baculovirus vectors were purchased from Oxford Expression Technologies Ltd (Oxford, UK). ESF 921 protein-Free Insect Cell Culture Medium from Expression Systems LLC (Woodland, CA), Sf9 insect cells from Invitrogen (Carlsbad, CA), pVL1393 vector from BD Biosciences (San Jose, CA), Escort™ transfection reagent, protease inhibitors, DNaseI, Concanavalin A Sepharose 4B and Methyl-α-D-Manno-Pyranoside were from Sigma (St. Louis, MO). Texas Red-conjugated anti-mouse secondary IgG from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Detergents used, n-dodecyl β-maltoside (DDM) and n-octyl β-D-glucopyranoside (OG) were purchased from Glycon Biochemicals GmbH (Luckenwalde, Germany). Antibiotic solution of Penicillin-Streptomycin-Amphotericin B was purchased from Biological Industries (Beit Ha'emek, Israel).
Methods
Expression of Neurotransmitter Transporters using cell-free expression systems
The cDNAs coding for rVMAT2 (a rat VMAT2 gene with hemagglutinin (HA) tag in the first loop between positions 96 and 105, a generous gift from RH Edwards, UCSF), GAT1 and EAAC1 were cloned into the appropriate pIVEX vectors using the NdeI and XhoI restriction sites. The vectors used were pIVEX 1.3, 1.4, 2.3d and 2.4d. Protein was synthesized using the Rapid Translation System RTS 100 E. coli HY and Rapid Translation System RTS 100 WG HY kits, according to the manufacturer instruction manual. Radiolabeling of VMAT2 was achieved by addition of 1–3 μCi of [35S]methionine (>1000 Ci/mmol, Amersham Life Sciences, Arlington Heights, IL).
Expression of VMAT2 in insect cells
cDNAs coding for rVMAT2 with or without a His tag at the C-terminus were cloned into a pVL1393 transfer vector (BD Pharmigen) using the EcoRI and NotI restriction sites. Top10 E. coli cells (Invitrogen) were used throughout cloning and mutagenesis processes of VMAT2. The same procedure was applied also for the VMAT2 mutants (termed GlyD and GlyQ) and for the cDNAs coding for rat GAT1, rat GLT1 and rabbit EAAC1. GlyD is a VMAT2 mutant in which 5 putative glycosylation sites have been cancelled by mutating Asn residues at positions: 56, 80, 81, 89 and 111 to Asp. In the mutant GlyQ Asn residues in the same positions were changed to Gln. Mutations in VMAT2 were introduced using the QuikChange® II site-directed mutagenesis kit (Stratagene, La Jolla, CA). Sequences of all constructs were verified by DNA sequencing.
Recombinant virus was produced by co-transfection of the transfer vector DNA together with the flashBAC™ DNA into Sf9 insect cells, as instructed by manufacturer. Recombinant baculovirus stocks were subjected to three rounds of amplification in Sf9 cells to produce high-titer viral stock. For assessing virus amounts suitable for expression, we performed calibration infections in suspension cultures using 40–50 ml of ~2.5×106 Sf9 cells/ml per assay.
VMAT2 expression in Sf9 cells and purification
Sf9 cells grown in suspension were infected with rVMAT2 recombinant baculovirus. Sf9 cells were grown at 27°C in a serum-free protein-free medium supplemented with antibiotics in sterile shaker flasks. Cells expressing VMAT2 were harvested 72–76 hrs post infection, re-suspended in lysis buffer (0.3 M sucrose, 15 mM HEPES pH 7.4, 5 mM MgCl2, 15 μg/mL DNase I (Sigma) and protease inhibitor cocktail (Sigma)) and disrupted using a Sonics Vibra Cell probe sonicator. The membrane fraction was collected by ultracentrifugation at 213500×g for 1 h at 4 °C and re-suspended in buffer containing 150 mM NaCl and 15 mM Tris pH 7.5. Membranes were solubilized with n-dodecyl β-maltoside (DDM) at a final concentration of 2 %. After 1.5 hrs incubation at 4°C, unsolubilized material was removed by centrifugation, imidazole was added to 40 mM, and the sample was loaded onto HiTrap™ chelating HP column (GE Healthcare, Fairfield, CT) mounted on Äkta™ Explorer (Amersham Biosciences) and washed with 0.08% DDM, 150 mM NaCl, 15 mM Tris-HCl, and 40 mM imidazole, pH 7.5, till A280 of the flow-through decreased below 0.05. VMAT2 was eluted with a gradient of up to 400 mM imidazole. Major peak fractions were pooled, MnCl2 and CaCl2 were added to 1 mM final concentration and the protein solution was bound to Concanavalin A Sepharose 4B (Sigma) for 1 hr at 4°C. The column was washed with a buffer containing 0.08 % DDM, 150 mM NaCl, 15 mM Tris-HCl, 1 mM MnCl2 and 1 mM CaCl2 and protein was eluted in the same buffer supplemented with 0.5 M Methyl-α-D-Manno-Pyranoside (Sigma).
Identity of the purified protein was verified by mass spectrometry performed at the Smoler Proteomics Center, Technion, Israel. The sample was cleaved with trypsin and chymotrypsin in parallel and analyzed by LC-MS/MS on the Orbitrap mass spectrometer (Thermo). The fragments were identified by Sequest 3.31 software against the IPI mouse database and against a decoy database. The results were filtered according to the Xcore value.
Western Blot analysis
Samples were separated by SDS-PAGE on 12.5 % Laemmli gels [21] and Western blot analysis was performed essentially as described in [22].
Immunocytochemistry
Cells grown on coverslips at 50 % confluency were infected with recombinant baculovirus. Two days post-infection, cells were fixed with methanol at −20°C. Cover slips were incubated with mouse anti His6 monoclonal antibody at a 1:300 dilution followed by Texas Red-conjugated anti-mouse secondary IgG at a 1:500 dilution. Cells were visualized with an FV-1000 confocal microscope (Olympus, Tokyo, Japan).
[3H] Tetrabenazine binding in cell lysates
Cells were treated as described above and cell lysates were added to 200 μl of reaction buffer containing 150 mM NaCl, 15 mM Tris·HCl pH 7.5, and increasing concentrations of [3H] dihydrotetrabenazine ([3H] TBZOH, 20 Ci/mmol) (American Radiolabeled Chemicals, St. Louis, MO) at room temperature. The reaction was stopped after 20 min by dilution in ice-cold buffer without radiolabeled ligand and with 125 μM tetrabenazine and was filtered through 0.45 μm HAWP filters (Millipore) presoaked in the same solution. Nonspecific binding measured in the presence of 125 μM tetrabenazine was subtracted from the total binding levels.
[3H] Tetrabenazine binding of detergent solubilized VMAT2
Cell lysates were solubilized in 2 % DDM for 20 min in cold room and VMAT2 was immobilized onto Ni-NTA beads (Qiagen, Hilden, Germany). After two washes with 200 μl of buffer containing 0.08 % DDM, 150 mM NaCl, 15 mM Tris-HCl, 200 μl of [3H]TBZOH at the indicated concentration in the same buffer were added for 20 min at 4°C. Beads were then spun down, the buffer was discarded and, after 10 min in 450 μl elution buffer with 200 mM imidazole at room temperature and an additional spin, 200 μl were sampled to measure the bound fraction. Radioactivity was measured using liquid scintillation.
Reconstitution of VMAT2 into Proteoliposomes
Cell lysate, from 5ml Sf9 cells expressing VMAT2 was supplemented with n-octyl-β-D-glucoside (OG; Glycon; final concentration of 2 %) and polar brain lipids (Avanti Polar Lipids, Inc, Alabaster, Alabama) to a final concentration of 0.5 mg/ml and incubated for 30 min at 4°C in rotation. The suspension was centrifuged for 10 min, 20000 × g at 4°C and the solubilized protein was mixed with 500μl of reconstitution mix containing 150 mM NaCl, 15 mM Tris-HCl pH=7.5 (Na-buffer), 1.2 % OG, 10 mg/ml polar brain lipids and 1 mg/ml asolectin, and sonicated (G112SP1 Special Ultrasonic Cleaner, Laboratory Supplies Co. Inc) to clarity. The mixture was then dialyzed at 4°C against 300 volumes of ammonium buffer containing 140 mM (NH4)2-tartrate and 15 mM Tris-HCl pH 7.5. After overnight dialysis the external buffer was replaced with fresh one for an additional 2 hours of dialysis. The liposomes mixture was then ultracentrifuged at 213,500 × g, 70 min, 4 °C. The supernatant was discarded and the liposome pellet was re-suspended in 150 μl of ammonium buffer, divided into aliquots, and kept at −70°C until use.
Purified VMAT2 was also reconstituted into proteoliposomes and the transport measured displayed properties very similar to the ones shown with the crude preparation. In this case, DDM solubilized protein was immobilized on NiNTA, washed with 0.08 % DDM /Na-buffer followed by 0.5 % OG /Na-buffer washes and elution in the same buffer supplemented with 200 mM imidazole. The eluted fractions were mixed with the reconstitution mix as described above.
Uptake of [3H] Serotonin into Proteoliposomes
Liposomes were thawed and sonicated to clarity. The uptake assay was performed in reaction buffer containing 140 mM K2-tartrate, 10 mM Tricine, 10 mM Tris, and 5 mM MgCl2, adjusted to pH 8.5. Liposomes (2 μl) were diluted into 200 μl of reaction buffer containing 50 nM valinomycin and the indicated concentration of the radiolabeled serotonin, usually 100 nM [3H]-serotonin (28.1 Ci/mmol) (Perkin Elmer). Nonspecific accumulation of [3H]-serotonin was measured in the presence of 125 μM tetrabenazine and was subtracted from the total transport. Reactions were stopped at the indicated times by dilution of the mixture in 2 ml ice-cold reaction buffer and filtering on 0.22 μm GSWP filters. Radioactivity on the filters was measured using liquid scintillation.
Reconstitution and Uptake Assays with Plasma Membrane Transporters
Reconstitution of the transporters into liposomes using spin columns and the subsequent with 120 mM KPi, pH 7.4 as internal [23, 24]transport assay was done as described medium. For each transport reaction 10 μl of reconstituted proteoliposomes was added to 360 μl of transport solution containing 150 mM NaCl supplemented with 2.8 μM valinomycin and 1 μCi of either [3H] GABA (94 Ci/mmol) or D-[3H] Aspartate (23.9.[23, 24]Ci/mmol). Reactions were terminated after 10 min as described
Results
Expression of VMAT2 in Cell-free systems
Cell free-coupled transcription and translation systems have been used successfully for the functional expression of various integral membrane proteins. A few examples are the prokaryotic proteins: EmrE, an E. coli multidrug resistance protein shown to be fully functional when synthesized in vitro [25, 26], the seven-transmembrane proton pump proteorhodopsin [27] and the flagellar motor proteins PomA/PomB [28]. Several mammalian membrane proteins have been functionally expressed in cell free systems as well, for example: human GPCRs [29] and the rat SLC22 transporters OCT1, OCT2 and OAT1 [30].
Two different in vitro transcription/translation systems were tested for the functional expression of VMAT2: one based on an E. coli based extract and another based on eukaryotic wheat germ extract. We chose to express VMAT in vitro because the cell-free system has the advantage of being independent of cell physiology. Moreover, it provides an open system that allows addition of supplements such as detergents or chaperones that may facilitate the synthesis of a properly folded protein.
Using E. coli based extracts, expression was low and detected only by 35S-Met labeling, while in wheat germ extracts expression was somewhat higher but still in the range of ~2 μg/ml. Fig 1A lane 1 shows the expression of rat VMAT2 in the E. coli based extract and Fig 1B (lanes 1 and 2) shows expression of the same protein in the wheat germ based extract. To achieve higher expression levels with the E. coli based system, we altered the 5' end of the VMAT2 gene using the ProteoExpert algorithm (Roche Applied Science). Several mutants bearing silent mutations were constructed, cloned into pIVEX 2.3d expression plasmid and compared for expression levels. Some mutants exhibited higher expression levels compared to the original construct and some exhibited a lower level of expression (examples in Fig 1A lane 2 versus lane 3). To further improve expression levels in the E. coli extract, a synthetic VMAT2 gene was prepared (Kosan Biosciences Inc., Hayward, CA), with E. coli codon usage. This construct expressed successfully in vitro (Fig 1A, lane 4).
Fig 1. Cell-free expression of VMAT2.
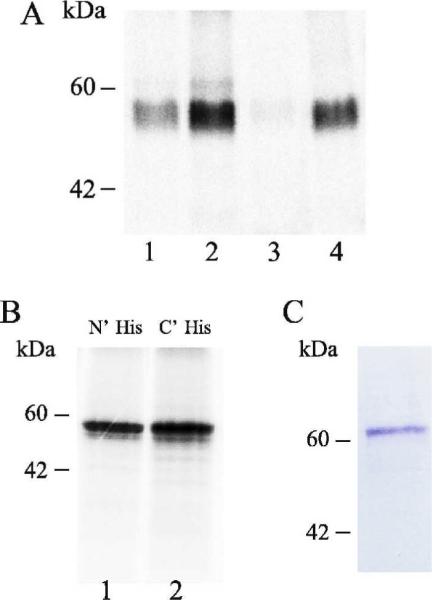
A. Native VMAT2 (lane 1), VMAT2 harboring silent mutations (lanes 2 and 3) and synthetic VMAT2 (lane 4) were synthesized by using the rapid-translation system 100 E. coli HY kit. Reaction was supplemented with 35S-Methionine. Samples were separated on SDS-PAGE and radioactive bands were visualized with a FLA-3000 Phosphor-Imager (Fujifilm, Tokyo). B. His tagged VMAT2 (lane 1 N' tag, lane 2 C' tag) was synthesized using the rapid-translation system 100 Wheat Germ HY kit. Reaction was supplemented with 35S-Methionine. Samples were separated on SDS-PAGE and radioactive bands were visualized with a FLA-3000 Phosphor-Imager. C. EAAC1-His synthesized in the rapid-translation system 100 E. coli HY kit was incubated with Ni-NTA beads. After purification and elution from the beads, sample (equivalent to half of the original reaction) was separated by SDS-PAGE and detected by Coomassie blue staining.
In both E. coli and Wheat germ cell free systems, the protein was synthesized with and without the addition of detergent during reaction. The detergents that were used as additives during synthesis are listed in Table 1. In the presence of most detergents, the protein aggregated, or showed extremely low solubility. In the presence of dimethyldodecylamineoxide (LDAO), Fos-Choline 14 or Anzergent 3–14 the protein was soluble to some extent (40–70%). Solubility was assessed after incubation of the radiolabeled protein with detergent (at a concentration at least 10 times the CMC value) followed by an ultracentrifugation step and then calculating the ratio between radioactivity in supernatant versus pellet fractions. When native protein from chromaffin granules was solubilized in either one of the three detergents in which in vitro VMAT2 showed some solubility, (Fos Choline 14, Anzergent 3–14 or LDAO) its activity was reduced by 80–99 % (data not shown). The only detergent in which the native protein retained 100 % activity was DDM.
Table 1.
Cell-free synthesis reactions were carried out in the presence of various detergents.
| Detergent present during synthesis | concentartion | Expression |
|---|---|---|
| CHAPS | 8mM | + |
| DDM | 0.02% | + |
| Triton ×100 | 0.021% | + |
| Cholate | 0.6% | − |
| Anzergent 3–14 | 0.2mM | + |
| LDAO | 2mM | + |
| C12E8 | 0.11mM | + |
| Fos-Choline 14 | 0.12mM | + |
| Brij 35 | 0.1% | + |
| Brij 58 | 0.15% | + |
| Brij 78 | 0.1% | + |
| Brij 98 | 0.1% | + |
We therefore screened for conditions in which the protein will retain its activity. Screening conditions included additives such as substrates, inhibitors, detergents, lipids and glycerol. Activity was tested by the ability of the synthesized protein to bind a radioactive labeled tetrabenazine, a known non-competitive inhibitor of VMAT2, and by the ability of the protein to transport radioactively labeled serotonin upon reconstitution into proteoliposomes. All of the expressed constructs, in all of the conditions, failed to show any activity.
In addition to VMAT2, two plasma membrane mammalian transporters, the neuronal glutamate transporter EAAC1 and the neuronal GABA transporter GAT1, were subcloned into a pIVEX 2.3d vector and tested for cell free expression. EAAC1 expressed in an estimated amount of a few micrograms per ml (Fig 1C), while GAT1 did not express at all. Cell-free expressed EAAC1 reconstituted into proteoliposomes failed to show any transport activity (data not shown).
Expression of VMAT2 in insect cells
Baculoviruses are lethal pathogens of insects, predominantly of the order Lepidoptera. Common baculovirus derived expression vectors are engineered derivatives of Autographa californica multiple capsid nucleopolyhedrovirus (AcMNPV). Insect cells can be infected either in monolayer or in suspension, which enables the system to be scaled- up to hundreds of liters of culture. This expression system has been used for the successful high yield production of mammalian membrane proteins such as bovine rhodopsin [31]. Recently, several high resolution structures of membrane proteins expressed in insect cells have been published: the turkey β1 adrenergic receptor [9], the human β2 adrenergic receptor [8], the human A2A adenosine receptor [10], the chicken acid-sensing ion channel (ASIC1) at 1.9Å resolution [11] and the rat GluA2 Glutamate receptor (AMPA-subtype) at 3.60 Å [12].
In the past, the baculovirus expression system was used for the expression of VMAT2. It was reported that the produced protein bound the inhibitor tetrabenazine, but that difficulties in purifying resulted in extremely low yields [32]. In a more recent report from the same group, the same system was used and the substrate binding region was studied using a photoprobe [33].
In the present study we used the flashBAC™ viruses to optimize the expression of VMAT2 in insect cells. In this system, the baculovirus genome is produced as a circular genome within bacterial cells, and it lacks the essential ORF1629, that prevents viral replication within insect cells. Following co-transfection of the virus with a transfer vector containing the target gene, the essential ORF1629 is restored, and the recombinant virus can replicate. In this work we have compared the expression of VMAT2 in three different genomes of the bacmid. The flashBAC-VMAT2 construct (FB-VMAT2), which contains a deletion of the chitinase gene (ChiA), the flashBACGOLD-VMAT2 construct (FBG-VMAT2) which contains a double deletion of both the chitinase gene and protease v-cathepsin (v-cath), and the flashBACULTRA-VMAT2(FBU-VMAT2) bearing the deletions of three more genes (p10, p26 and p74) in addition to the chitinase and v-cathepsin deletions. Deletions of the chitinase and v-cathepsin genes are modifications designed to improve the movement of recombinant proteins through the cellular secretory pathway, and to reduce their degradation [34] and the further deletions of three more genes (p10, p26 and p74) are designed to remove un-necessary genetic burden and thus improve the yields of the target protein.
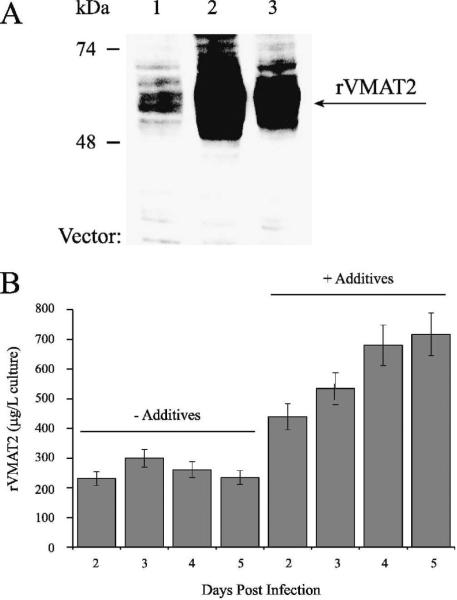
VMAT2 was expressed in insect cells using the above three baculovirus vectors. Western analysis using an antibody directed to the HA epitope located on the first loop revealed that all three baculovirus constructs yielded VMAT2 expression in Sf9 cells, but to different degrees (Fig 2A), as can be judged from Western analysis results and supported by tetrabenazine binding levels (not shown). VMAT2 expressed in all three vectors was functional, as judged from tetrabenazine binding and serotonin transport upon reconstitution into proteoliposomes (data not shown).
Fig 2. Expression screen in insect cells using three baculovirus vectors.
A. Three baculovirus vectors were used for the infection of Sf9 cells: FB-VMAT2 (lane 1), FBG-VMAT2 (lane 2) and FBU-VMAT2 (lane 3). Sf9 cells were harvested 72 hrs post infection, washed in lysis buffer and sonicated. Samples of the lysates (equal to original volume of 10 μl infected cells in suspension) were separated by SDS-PAGE and Western blot analysis with anti-HA antibody was performed. B. Sf9 cells infected using the FB-VMAT2 vector were grown without or with the addition of glucose, Asn, Gln and FBS after the first 24 hours of infection. Cells were harvested 2,3,4 or 5 days (as indicated) post infection and cell lysates were prepared and assayed for [3H]TBZOH binding. The amount of VMAT2 was calculated from the TBZ binding results.
Out of the three vectors FB-VMAT2 had the lowest yield of about ~200 μg functional protein per liter of cells grown in suspension (Fig 2A). Optimization of the expression levels by adding glucose, glutamine, asparagine and fetal calf serum (FCS) to the growth medium (according to [35]) raised the yields of functional protein up to ~ 800 μg per 1 liter of culture (Fig 2B). FBG-VMAT2 had the highest yield of ~2.5 mg functional protein per liter and FBU-VMAT2 had intermediate yields; hence we chose to use FBG-VMAT2 for further purification trials and analysis of the protein.
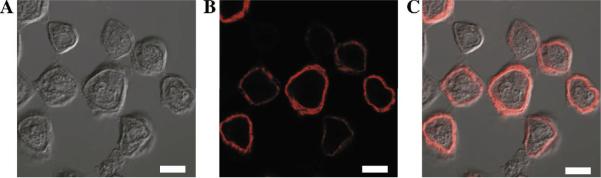
Immunocytochemistry of Sf9 cells expressing VMAT2 showed that the protein is arranged in what seems like a perfect ring around the cell. This ring seems thicker than what would be expected from the membrane width alone, and might represent proteins that are localized partly in the plasma membrane and partly in ER membranes adjacent to the plasma membrane (Fig 3A–C).
Fig 3. Expression and localization of VMAT2 expressed in insect cells.
A. Immunocytochemistry: cells grown on cover slips at 50 % confluency were infected with FBG-VMAT2. Two days post-infection, cells were fixed with methanol at −20°C. Cover slips were incubated with mouse anti His6 monoclonal antibody, followed by Texas-Red conjugated secondary IgG. Cells were observed with a confocal microscope. A. Differential interference contrast microscopy (DIC) image B. Fluorescence C. Merged image. Cells expressing the wild-type protein without His tag showed no background fluorescence. White bars represent 10 μm.
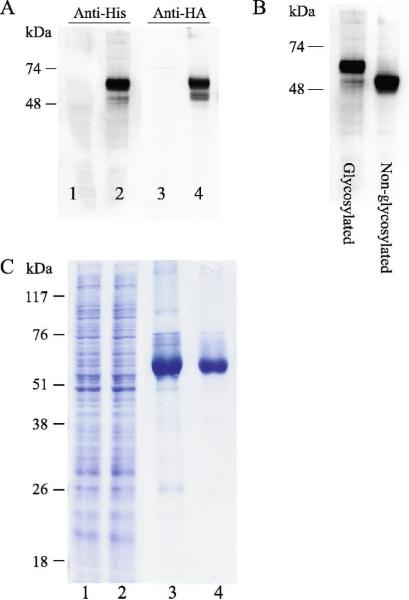
The glycosylated form of the protein migrates at ~ 60 kDa. Western analysis using both an antibody against the HA epitope located in the first loop of the protein and an antibody against the C' His tag yield bands of the same molecular weight implying the expression of the full length glycoprotein. No proteolytic fragments of the protein were detected (Fig 4A, lanes 2 and 4).
Fig 4. Expression and purification of VMAT2 expressed in insect cells.
A. Sf9 cells were infected using the baculovirus FBG-VMAT2 vector (lanes 2 and 4). Samples were analyzed by SDS-PAGE and transferred onto PVDF membrane. Western blot was performed using anti-His (lanes 1–2) or anti HA (lanes 3–4) antibodies. Lanes 1 and 3 are un-infected control cells. B. Western analysis using anti-His antibody of Sf9 expressed native VMAT2 (lane 1) and GlyQ in which putative glycosylation sites have been mutated, as described under Material and Methods. C. Purification of Sf9 expressed native VMAT2. Sf9 membranes from 800 ml cells infected using FBG-VMAT2 vector were solubilized (lane 1, 0.5 μl from a total of 60 ml solution) and loaded onto NiNTA column (lane 2 unbound material, same amount as lane 1) and eluted with imidazole (lane 3, 5 μl from a total of 6 ml solution). rVMAT2 was further purified on a ConA column as described (lane 4, 2 μl from a total of 1.5 ml solution). Samples were analyzed on SDS-PAGE and stained with coomassie.
Expression of VMAT2 was tested in Sf9 and High-Five cells that are assumed to increase expression of secreted proteins, but expression of VMAT2 was higher in Sf9 cells (data not shown).
VMAT2 expressed in insect cells is functional
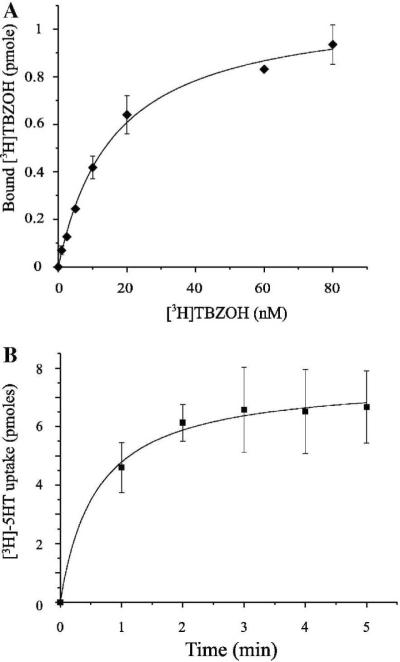
Functionality of the protein was assessed both by its ability to bind the non-competitive inhibitor [3H]TBZOH (Fig 5A), and its ability to transport [3H]5-HT upon reconstitution into brain lipid proteoliposomes (Fig 5B). Sf9 expressed VMAT2 displayed high affinity tetrabenazine binding with a Kd of 16.27±1.46 nM. The Km for the transport of serotonin was 228±56 nM and it was inhibited by tetrabenazine and reserpine, a known competitive inhibitor of VMAT2 (data not shown). The kinetic parameters of the Sf9 expressed protein are similar to those of VMAT2 expressed in mammalian cell lines [22].
Fig 5. VMAT2 expressed in insect cells is functional.
A. Binding of the noncompetitive inhibitor of VMAT2, [3H] TBZOH to lysates of Sf9 cells infected by FBG-VMAT2. Constant amount of cell lysate was bound to increasing [3H] TBZOH concentrations for 20 min, followed by fast dilution and filtration on 0.45 μm filters. The calculated Kd is 16.27±1.46 nM, and the Bmax is 1.1±0.03 pmole per 30 μl of original cell volume (R-square = 0.995; the experiment was performed in duplicates and repeated three times). B. Uptake of [3H]-serotonin in VMAT2 reconstituted in proteoliposomes. VMAT2 expressed in Sf9 cells was reconstituted into proteoliposomes as described under “materials and methods”. Proteoliposomes (2 μl samples) were diluted into 200 μl reaction buffer containing 50 nM valinomycin and 100nM [3H]-serotonin. Reaction was stopped at the indicated time points by fast dilution and filtration on 0.22 μm filters. The calculated Km for the transport of serotonin was 228±56 nM.
Non- glycosylated VMAT2 expressed in insect cells is functional
It is usually assumed that glycosylation maybe detrimental to some structural studies. Among other effects, glycosylations are known to be involved in protein stabilization, therefore, we decided to test the effect of glycosylation on Sf9 expressed VMAT2. Though it has been shown before that for VMAT1 glycosylation is not essential for the protein activity [36], in order to verify that the same applies to VMAT2, two mutants were constructed in which all five putative glycosylation sites were mutated. FBG-GlyQ (coding for VMAT2 in which five putative Asn glycosylation sites were mutated into Gln) and FBG-GlyD (coding for VMAT2 in which five putative Asn glycosylation sites were mutated into Asp) were constructed and tested for expression in Sf9 cells and for function. The non-glycosylated form of the protein migrates at lower molecular weight than the glycosylated form (~ 50 kDa) (Fig 4B). Both proteins expressed in similar levels to that of the wild-type VMAT2 and both were functional as assessed by [3H]TBZOH binding activity and [3H]5-HT transport activity upon reconstitution into brain lipid proteoliposomes (data not shown).
Purification
Glycosylated FBG-VMAT2 bearing a C' His tag was purified in a two-step chromatography. Solubilized Sf9 membranes, from Sf9 expressing cells at day 3 post infection, were loaded on a Ni2+-column followed by a lectin (ConA) column (Fig 4C). The yield of purified VMAT2 was ~1.5 mg/L as determined by absorption at 280 nm. Purified VMAT2 was analyzed and its identity confirmed by mass spectrometry. Purified VMAT2 was stable at 4°C for over two weeks with no detectable degradation or aggregation (data not shown).
Baculovirus expression system supports the functional expression of various mammalian neurotransmitter transporters
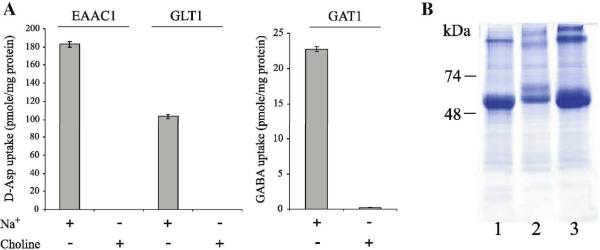
We used the baculovirus expression system for the expression of three mammalian plasma membrane neurotransmitters transporters: the glutamate transporters EAAC1 and GLT1/EAAT2 and the GABA transporter GAT1. All three transporters were expressed to high yields (ranging from estimated 2.5 mg/L for GAT1 and up to 6 mg/L for GLT1) in Sf9 cells (Fig 6B). The proteins were reconstituted into proteoliposomes and tested for transport activity. All three proteins demonstrated transport activity of their specific substrates in a sodium-dependent manner (Fig 6A).
Fig 6. EAAC1, GLT1 and GAT1 expressed in insect cells are functional.
A. Protein from Sf9 cells expressing EAAC1, GAT1 or GLT1 (5 ml each) was used for reconstitution of the transporters into brain lipids. Transport activity was assayed in the presence and absence of sodium as indicated. B. Partial purification of 5 ml cells expressing EAAC1 (lane 1), GAT1 (lane 2) and GLT1 (lane 3). Cell lysates were prepared, and protein was solubilized by addition of 2 % DDM. Solubilizate was bound to 30 μl NiNTA beads for 1 h, then eluted with 450 mM Imidazole, analyzed by SDS-PAGE and visualized with Coomassie stain.
Discussion
Mammalian integral membrane proteins pose a challenge to those who wish to elucidate their mechanisms of action and their structures. Obtaining high yield expression of these proteins is usually the first major bottleneck on the way to successful biochemical and structural analysis.
Purification of the native vesicular monoamine transporter from bovine adrenal medullae was previously described [37]. Working with proteins isolated from their native source allows for functional characterization of the protein, and in some cases where the protein is naturally highly abundant, it allows purification for structural purposes. A few membrane proteins were purified from their native source and crystallized, for example bovine rhodopsin isolated from rod outer segments [6] and human aquaporin-1 purified from red blood cells [38]. However, purification of proteins from native source is practical only when they are relatively abundant and the biological material for purification is readily available. Moreover, working with the native protein is confined to characterization of the wild type protein, while establishing an expression system for the recombinant protein allows for modifications such as mutations, deletions and additions of tags or fusion proteins. The goal of this study was to establish an expression system for the functional expression of the recombinant vesicular monoamine transporter 2 (VMAT2) for both functional and structural characterizations. We present the successful high-yield functional expression of the vesicular monoamine transporter VMAT2 as a case study for the expression of mammalian integral membrane proteins. Using the conditions optimal for VMAT2 we also obtained expression of three plasma membrane neurotransmitter transporters.
E. coli is the most commonly used expression system due to its advantages as an easy-to-set-up, highly commercialized and low-cost system and so naturally, our first trials focused on expression of VMAT2 in E. coli cells. When considering the over-expression of membrane proteins for structural analysis, E. coli cells are the most popular heterologous expression system used. Most bacterial and archaeal proteins that have been crystallized were expressed in this system [39], but the limitations of the system appear when challenging this bacterial system with the expression of mammalian membrane proteins. Very few mammalian membrane proteins expressed functionally in E. coli and crystallized to yields high enough for structural studies [40, 41]. Though we screened different bacterial strains, none managed to express VMAT. In an attempt to overcome a possible codon usage barrier, a synthetic cDNA coding for VMAT2 in which DNA codons were optimized for bacterial expression. Nevertheless, synthetic VMAT2 could not be expressed in any of strains the E. coli tested (data not shown).
Interestingly, however, when we assayed both native and synthetic VMAT2 constructs for bacterial cell free expression in cell-free E. coli extracts we detected expression of a full-length protein. This expression system that is free from physiological constrains managed to synthesize the polypeptide that could not be detected in living cells. VMAT2 expressed also in the eukaryotic wheat-germ extracts based cell free system. Here too, as in the bacterial based system, a full-length polypeptide was synthesized. Unfortunately, though highly promising at first glance, both cell free systems failed to express functional proteins and no activity was observed. We screened for detergents that will support both solubility and function of the protein. In some detergents (Fos-Choline 14, Anzergent 3–14 and LDAO) the protein was soluble to a certain degree. Activity was measured using two assays: by the ability of the protein to bind the inhibitor tetrabenazine and by the reconstituted protein to perform transport of serotonin. Though other membrane proteins have been shown to be functional when expressed in cell free systems (for a very thorough review: [42]) neither VMAT2 nor the other two plasma membrane neurotransmitter transporters EAAC1 and GAT1 displayed any activity. Both the bacterial and eukaryotic cell free coupled transcription and translation systems do not support post translational modifications such as glycosylation. This however is not the direct cause for loss of function of the cell free VMAT2 proteins since we have shown that VMAT is active after cancellation of all its putative glycosylation sites. We assume that the lack of activity is due to improper folding and aggregation of the protein.
In the past, VMAT2 was functionally expressed in various mammalian cells lines [20, 22, 36, 43–46]. Though these systems provide valuable platform for the expression of functional protein in a close-to-native environment in terms of translation machinery, they are time-consuming, expensive, and the yield is still low, forcing us to search for a more appropriate system for the high-yield over-expression of functional VMAT2. VMAT2 was also expressed in the yeast Saccharomyces cerevisiae with either partial [47] or full activity [1], but expression levels of the protein in yeast are very low.
In the baculovirus expression system we managed to express high amounts of functional VMAT2 (up to 2.5 mg per 1 liter). VMAT2 produced in insect cells was functional, as judged from its ability to bind known inhibitors such as tetrabenazine and reserpine. The recombinant protein was also purified taking advantage of a His affinity tag, reconstituted into polar lipid brain liposomes and serotonin transport activity was measured. VMAT2 produced in insect cells demonstrated similar pharmacological and kinetic properties to the native protein.
Though in the past there were reports that suggested this system might not be suitable for the expression of mammalian integral membrane proteins due to low percentage of active protein [48] this is not the case for VMAT2. Recently several high-resolution structures of mammalian membrane proteins expressed in insect cells have appeared [8–11].
We have expressed four different neurotransmitter transporters in insect cells: VMAT2, GLT1, EAAC1 and GAT1. All four are functional and express at high levels in Sf9 cells and VMAT2 was further purified to high homogeneity.
Acknowledgements
We wish to thank Etay Benovich for assistance and guidance in the very early stages of work with the baculovirus expression system, Hila Frenkel for excellent technical support regarding cell-free expression systems, Dr. Mario Lebendiker from the protein purification facility for his excellent guidance and advice in the purification of VMAT2, Shmulik Ittah and Dr. Naomi Melamed-Book for assistance in preparing and analyzing samples using confocal microscopy, Sonia Steiner-Mordoch for assistance in screening VMAT2 expression in bacterial strains and Annie Bendahan for sub-cloning GAT1, EAAC1 and GLT1 into pVL1393 and for the reconstitution of these proteins. Y.E. is supported by the Adams Fellowship Program of the Israel Academy of Sciences and Humanities. This work was supported by grant NS16708 from the National Institute of Health and by The Center for Innovation in Membrane Protein Production (P50 GM73210). SS is Mathilda Marks-Kennedy Professor of Biochemistry at the Hebrew University of Jerusalem.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gros Y, Schuldiner S. Directed evolution reveals hidden properties of VMAT, a neurotransmitter transporter. J Biol Chem. 2010;285:5076–5084. doi: 10.1074/jbc.M109.081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vardy E, Arkin IT, Gottschalk KE, Kaback HR, Schuldiner S. Structural conservation in the major facilitator superfamily as revealed by comparative modeling. Protein Sci. 2004;13:1832–1840. doi: 10.1110/ps.04657704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gouaux E. The molecular logic of sodium-coupled neurotransmitter transporters. Philos Trans R Soc Lond B Biol Sci. 2009;364:149–154. doi: 10.1098/rstb.2008.0181. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Singh SK. LeuT: A prokaryotic stepping stone on the way to a eukaryotic neurotransmitter transporter structure. Channels (Austin) 2008;2 doi: 10.4161/chan.2.5.6904. [DOI] [PubMed] [Google Scholar]
- [5].Wiener MC. A pedestrian guide to membrane protein crystallization. Methods. 2004;34:364–372. doi: 10.1016/j.ymeth.2004.03.025. [DOI] [PubMed] [Google Scholar]
- [6].Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- [7].Toyoshima C, Nomura H, Tsuda T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature. 2004;432:361–368. doi: 10.1038/nature02981. [DOI] [PubMed] [Google Scholar]
- [8].Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- [9].Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- [12].Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- [14].Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eiden LE. The vesicular neurotransmitter transporters: current perspectives and future prospects. FASEB J. 2000;14:2396–2400. doi: 10.1096/fj.00-0817rev. [DOI] [PubMed] [Google Scholar]
- [16].Peter D, Jimenez J, Liu YJ, Kim J, Edwards RH. The Chromaffin Granule and Synaptic Vesicle Amine Transporters Differ in Substrate Recognition and Sensitivity to Inhibitors. Journal of Biological Chemistry. 1994;269:7231–7237. [PubMed] [Google Scholar]
- [17].Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM, Caron MG. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- [19].Liu Y, Roghani A, Edwards R. Gene transfer of a reserpine-sensitive mechanism of resistance to N-methyl-4-phenylpyridinium. Proc Natl Acad Sci U S A. 1992;89:9074–9078. doi: 10.1073/pnas.89.19.9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu Y, Peter D, Roghani A, Schuldiner S, Prive GG, Eisenberg D, Brecha N, Edwards RH. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992;70:539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- [21].Laemmli U. Cleavage of structural proteins during assembly of head ofbacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [22].Adam Y, Edwards RH, Schuldiner S. Expression and function of the rat vesicular monoamine transporter 2. Am J Physiol Cell Physiol. 2008;294:1004–1011. doi: 10.1152/ajpcell.00348.2007. [DOI] [PubMed] [Google Scholar]
- [23].Bennett ER, Su H, Kanner BI. Mutation of arginine 44 of GAT-1, a (Na(+) + Cl(−))-coupled gamma-aminobutyric acid transporter from rat brain, impairs net flux but not exchange. J Biol Chem. 2000;275:34106–34113. doi: 10.1074/jbc.M004229200. [DOI] [PubMed] [Google Scholar]
- [24].Kavanaugh MP, Bendahan A, Zerangue N, Zhang Y, Kanner BI. Mutation of an amino acid residue influencing potassium coupling in the glutamate transporter GLT-1 induces obligate exchange. J-Biol-Chem. 1997;272:1703–1708. doi: 10.1074/jbc.272.3.1703. [DOI] [PubMed] [Google Scholar]
- [25].Klammt C, Lohr F, Schafer B, Haase W, Dotsch V, Ruterjans H, Glaubitz C, Bernhard F. High level cell-free expression and specific labeling of integral membrane proteins. Eur J Biochem. 2004;271:568–580. doi: 10.1111/j.1432-1033.2003.03959.x. [DOI] [PubMed] [Google Scholar]
- [26].Elbaz Y, Steiner-Mordoch S, Danieli T, Schuldiner S. In vitro synthesis of fully functional EmrE, a multidrug transporter, and study of its oligomeric state. Proc Natl Acad Sci U S A. 2004;101:1519–1524. doi: 10.1073/pnas.0306533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gourdon P, Alfredsson A, Pedersen A, Malmerberg E, Nyblom M, Widell M, Berntsson R, Pinhassi J, Braiman M, Hansson O, Bonander N, Karlsson G, Neutze R. Optimized in vitro and in vivo expression of proteorhodopsin: a seven-transmembrane proton pump. Protein Expr Purif. 2008;58:103–113. doi: 10.1016/j.pep.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [28].Terashima H, Abe-Yoshizumi R, Kojima S, Homma M. Cell-free synthesis of the torque-generating membrane proteins, PomA and PomB, of the Na+-driven flagellar motor in Vibrio alginolyticus. J Biochem. 2008;144:635–642. doi: 10.1093/jb/mvn110. [DOI] [PubMed] [Google Scholar]
- [29].Ishihara G, Goto M, Saeki M, Ito K, Hori T, Kigawa T, Shirouzu M, Yokoyama S. Expression of G protein coupled receptors in a cell-free translational system using detergents and thioredoxin-fusion vectors. Protein Expr Purif. 2005;41:27–37. doi: 10.1016/j.pep.2005.01.013. [DOI] [PubMed] [Google Scholar]
- [30].Keller T, Schwarz D, Bernhard F, Dotsch V, Hunte C, Gorboulev V, Koepsell H. Cell free expression and functional reconstitution of eukaryotic drug transporters. Biochemistry. 2008;47:4552–4564. doi: 10.1021/bi800060w. [DOI] [PubMed] [Google Scholar]
- [31].Klaassen CH, Bovee-Geurts PH, Decaluwe GL, DeGrip WJ. Large-scale production and purification of functional recombinant bovine rhodopsin with the use of the baculovirus expression system. Biochem J. 1999;342(Pt 2):293–300. [PMC free article] [PubMed] [Google Scholar]
- [32].Sievert MK, Thiriot DS, Edwards RH, Ruoho AE. High-efficiency expression and characterization of the synaptic-vesicle monoamine transporter from baculovirus-infected insect cells. Biochem J. 1998;330(Pt 2):959–966. doi: 10.1042/bj3300959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gopalakrishnan A, Sievert M, Ruoho AE. Identification of the Substrate Binding Region of Vesicular Monoamine Transporter-2 (VMAT-2) Using Iodoaminoflisopolol as a Novel Photoprobe. Mol Pharmacol. 2007;72:1567–1575. doi: 10.1124/mol.107.034439. [DOI] [PubMed] [Google Scholar]
- [34].Kaba SA, Salcedo AM, Wafula PO, Vlak JM, van Oers MM. Development of a chitinase and v-cathepsin negative bacmid for improved integrity of secreted recombinant proteins. J Virol Methods. 2004;122:113–118. doi: 10.1016/j.jviromet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- [35].Vallazza M, Petri T. Optimization of the production of triabin, a novel thrombin inhibitor, in High Five (TM) insect cells infected with a recombinant baculovirus. Cytotechnology. 1999;29:85–92. doi: 10.1023/A:1008008023779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yelin R, Steiner-Mordoch S, Aroeti B, Schuldiner S. Glycosylation of a Vesicular Monoamine Transporter: A Mutation in a Conserved Proline Residue Affects the Activity, Glycosylation, and Localization of the Transporter. J. Neurochem. 1998;71:2518–2527. doi: 10.1046/j.1471-4159.1998.71062518.x. [DOI] [PubMed] [Google Scholar]
- [37].Stern-Bach Y, Greenberg-Ofrath N, Flechner I, Schuldiner S. Identification and Purification of a Functional Amine Transporter from Bovine Chromaffin Granules. Journal of Biological Chemistry. 1990;265:3961–3966. [PubMed] [Google Scholar]
- [38].Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- [39].Junge F, Schneider B, Reckel S, Schwarz D, Dotsch V, Bernhard F. Large-scale production of functional membrane proteins. Cell Mol Life Sci. 2008;65:1729–1755. doi: 10.1007/s00018-008-8067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ferguson AD, McKeever BM, Xu S, Wisniewski D, Miller DK, Yamin TT, Spencer RH, Chu L, Ujjainwalla F, Cunningham BR, Evans JF, Becker JW. Crystal structure of inhibitor-bound human 5-lipoxygenase-activating protein. Science. 2007;317:510–512. doi: 10.1126/science.1144346. [DOI] [PubMed] [Google Scholar]
- [41].Yang J, Ma YQ, Page RC, Misra S, Plow EF, Qin J. Structure of an integrin alphaIIb beta3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proc Natl Acad Sci U S A. 2009;106:17729–17734. doi: 10.1073/pnas.0909589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schwarz D, Dotsch V, Bernhard F. Production of membrane proteins using cell-free expression systems. Proteomics. 2008;8:3933–3946. doi: 10.1002/pmic.200800171. [DOI] [PubMed] [Google Scholar]
- [43].Shirvan A, Laskar O, Steiner-Mordoch S, Schuldiner S. Histidine-419 plays a role in energy coupling in the vesicular monoamine transporter from rat. FEBS LETTERS. 1994;356:145–150. doi: 10.1016/0014-5793(94)01252-0. [DOI] [PubMed] [Google Scholar]
- [44].Schuldiner S, Liu Y, Edwards RH. Reserpine binding to a vesicular amine transporter expressed in Chinese hamster ovary fibroblasts. J Biol Chem. 1993;268:29–34. [PubMed] [Google Scholar]
- [45].Gasnier B, Krejci E, Botton D, Massoulie J, Henry JP. Expression of a Bovine Vesicular Monoamine Transporter in COS Cells. FEBS LETTERS. 1994;342:225–229. doi: 10.1016/0014-5793(94)80506-7. [DOI] [PubMed] [Google Scholar]
- [46].Erickson J, Eiden L, Hoffman B. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1992;89:10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yelin R, Schuldiner S. Vesicular monoamine transporters heterologously expressed in the yeast Saccharomyces cerevisiae display high-affinity tetrabenazine binding. Biochim Biophys Acta. 2001;1510:426–441. doi: 10.1016/s0005-2736(00)00374-6. [DOI] [PubMed] [Google Scholar]
- [48].Tate CG, Haase J, Baker C, Boorsma M, Magnani F, Vallis Y, Williams DC. Comparison of seven different heterologous protein expression systems for the production of the serotonin transporter. Biochim Biophys Acta. 2003;1610:141–153. doi: 10.1016/s0005-2736(02)00719-8. [DOI] [PubMed] [Google Scholar]