Abstract
In order to clarify the probiotic features of immunomodulation, cytokine production by murine spleen and Peyer’s patch (PP) cells was examined in response to probiotic and pathogenic bacteria. In spleen cells, probiotic Lactobacillus casei induced interleukin (IL)-12 production by CD11b+ cells more strongly than pathogenic Gram-positive and Gram-negative bacteria and effectively promoted the development of T helper (Th) type 1 cells followed by high levels of secretion of interferon (IFN)-γ. Although the levels of IL-12 secreted by PP cells in response to L. casei were lower in comparison with spleen cells, Th1 cells developed as a result of this low-level induction of IL-12. However, IFN-γ secretion by the L. casei-induced Th1 cells stimulated with a specific antigen was down-regulated in PP cells. Development of IL-17-producing Th17 cells was efficiently induced in PP cells by antigen stimulation. Lactobacillus casei slightly, but significantly, inhibited the antigen-induced secretion of IL-17 without a decrease in the proportion of Th17 cells. No bacteria tested induced the development of IL-10-producing, transforming growth factor-β-producing or Foxp3-expressing regulatory T cells, thus suggesting that certain probiotics might regulate proinflammatory responses through as yet unidentified mechanisms in PP cells. These data show probiotic L. casei to have considerable potential to induce IL-12 production and promote Th1 cell development, but the secretion of proinflammatory cytokines such as IL-12 and IL-17 may be well controlled in PP cells.
Keywords: Lactobacillus casei, mouse, Peyer’s patch, probiotics, spleen cell, Th1 cells
Introduction
More than 100 trillion commensal bacteria belonging to hundreds of different species living in the human intestine play a pivotal role in the maintenance of intestinal homeostasis in their host.1 The normal formation of intestinal microbiota contributes not only to the prevention of enteritis caused by pathogens but also to immunological development and preservation.2,3 However, intestinal microbiota affected by various factors such as antibiotics and stresses may cause enteric infections or susceptibility to other diseases.4,5
Probiotics are viable cell preparations or foods containing viable bacterial cultures or components of bacterial cells that have beneficial effects on the health of the host, and include bacteria such as lactobacilli and bifidobacteria.6 Orally administered probiotics are expected to have resistance against gastric acid and bile, to be delivered live to the intestinal tract, and to normalize the intestinal bacterial flora and thereby contribute to the reduction of various disease risks.7,8 Moreover, it is becoming clear that probiotics affect the immune system of the host and work effectively against various diseases caused by immune system abnormalities.9,10 The effects of probiotics on the immune system are classified into two major categories. One effect is the activation of cells in the innate immune system, such as phagocytes and natural killer cells, which is expected to have an inhibitory effect against infections and cancers.11,12 The other effect is the inhibition of excessive immune responses, which is expected to have an inhibitory effect against inflammatory bowel diseases (IBDs), allergies, and autoimmune diseases.13–15 Multiple mechanisms have been proposed to account for the expression of the latter anti-inflammatory activities, including: the inhibited production of proinflammatory mediators such as interleukin (IL)-8, the induced production of anti-inflammatory cytokines such as IL-10, and induction of regulatory T cells.
The effects of probiotics on the immune system are, in many cases, exerted through effects on antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs). Among the cytokines that are produced by these cells, particular attention has been paid to the probiotic control of the production of IL-12, which plays a central role in the activation of innate immunity, and IL-10, which, in contrast, acts to inhibit the inflammatory response.16–18 In addition, in vitro studies have revealed that, through their effects on APCs, probiotics may affect differentiation into T helper (Th) cell subsets and the production of cytokines therein. It has been revealed that lactobacilli, such as Lactobacillus casei Shirota or Lactobacillus reuteri ATCC 23272, induce Th1 cells via the induced production of IL-12 generated by macrophages and DCs,19,20 and Bifidobacterium bifidum W23 and Bifidobacterium longum W52 inhibit the production of cytokines generated by Th2 cells via the production of IL-10 generated by monocytes.17 It has also been revealed that L. casei NIZO B255 and B. bifidum W32 induce regulatory T cells, which produce large amounts of IL-10 via the controlling function of DCs.21,22 It has been confirmed that these immunoregulatory effects exhibited by probiotics work effectively against diseases caused by compromised or abnormal immune systems in animal models, and data showing their efficacy in human clinical trials have also been accumulating.23,24
Recently, expectations for the use of probiotics in clinical applications have been growing, and it is essential to determine in detail their effects on immune cells in order to utilize probiotics more effectively and safely. It is believed that orally administered probiotics affect the immune system via the following three pathways: (i) probiotics are introduced through M cells in the Peyer’s patch (PP) follicle-associated epithelium to affect the macrophages and DCs beneath the epithelium; (ii) DCs in the mucosal lamina propria extend their dendrites to sample the intraluminal probiotics; and (iii) intraluminal probiotics stimulate the epithelial cells produce humoral factors which indirectly affect the intestinal immune cells.10,25 Incidentally, the intestinal immune system exhibits a unique responsiveness that is different from that of the systemic immune system. For example, the responsiveness of the macrophages and DCs in the intestinal immune system to the stimulation by bacteria and bacterial cell components that induce the production of proinflammatory cytokines such as IL-12 and tumour necrosis factor (TNF)-α is kept low.26–28 It is believed that homeostasis is usually maintained in the intestinal tract, where various micro-organisms, such as indigenous bacteria, are likely to be found, through various mechanisms that prevent excessive inflammatory responses.29 However, until now, in vitro studies investigating the details of the immunoregulatory effects of probiotics have mainly used peripheral blood mononuclear cells (PBMC), spleen cells, and peritoneal macrophages for their ease of use. Macrophages and DCs prepared from PBMC or spleen cells or differentiated from myeloid cells or PBMC have also been used. Meanwhile, not many studies investigating the immunoregulatory effects of probiotics have been conducted using cells from the intestinal immune system, and there has therefore been no adequate analysis conducted regarding the differences in the effects of probiotics on the systemic immune system and the intestinal immune system. Therefore, in order to clarify these differences, in this study, the cells were prepared from the spleen and PP, which are major organs in the systemic immune system and intestinal immune system, respectively, and the cytokine production responses of APCs to probiotic lactobacilli and bifidobacteria were analysed. We also investigated the effects of probiotics on differentiation into Th cell subsets such as Th1 cells and Th17 cells, as well as the effects on cytokine production and whether these probiotic-induced responses differed from responses induced by pathogenic bacteria. Our results revealed that the probiotic L. casei Shirota is characterized by a potential to strongly induce IL-12 production and thereby strongly induce a Th1 cellular response, and this proinflammatory response is properly controlled in PP cells. These findings are therefore considered to help us to discuss the underlying mechanisms of various beneficial effects of probiotics.
Materials and methods
Animals
Female BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). Female DO11.10 mice30 transgenic for ovalbumin (OVA)323-339-specific and I-Ad restricted T-cell receptor (TCR)-αβ, with a BALB/c genetic background, were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were used at 8–12 weeks of age. The experiments were performed in accordance with the guidelines for the care and use of laboratory animals at the Yakult Central Institute.
Bacteria
Lactobacillus casei strain Shirota (YIT 9029) and B. bifidum strain Yakult (YIT 4007) were originally isolated at Yakult Central Institute, and Lactobacillus plantarum ATCC 14917T and Helicobacter pylori ATCC 43504T were obtained from the American Type Culture Collection (Rockville, MD). Lactobacilli and B. bifidum were cultured at 37° for 20 hr in Lactobacilli-MRS broth (Difco, Detroit, MI) and GAM broth (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 1% glucose, respectively. H. pylori was cultured at 37° for 48 hr in Brucella broth (BD, Franklin Lakes, NJ) supplemented with 10% horse serum. The bacteria were washed with sterile distilled water, heated at 100° for 30 min, and then lyophilized. Escherichia coli ATCC 11303 cells were purchased from Sigma (St Louis, MO), heat-treated at 100° for 30 min, and then lyophilized. Heat-killed Listeria monocytogenes and Staphylococcus aureus were obtained from InvivoGen (San Diego, CA). The lyophilized preparations were suspended in phosphate-buffered saline (PBS) and autoclaved (at 121° for 20 min) before addition to cell cultures.
Culture medium
RPMI-1640 medium (Sigma) supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0·05 mm 2-mercaptoethanol was used for cell cultures.
Cell preparation
Spleens and PPs were removed from BALB/c or DO11.10 mice and treated with collagenase (600 U/ml; Sigma) for 45 and 60 min, respectively, in complete RPMI-1640 medium supplemented with 25 mm HEPES to obtain single-cell suspensions. Erythrocytes were depleted from spleen cells by treatment with 0·144 m NH4Cl in 0·017 m Tris–HCl buffer (pH 7·65).
CD11b+ and CD11c+ cells were enriched from spleen and PP cells by magnetic cell sorting with anti-CD11b and anti-CD11c microbeads (Miltenyi Biotech, Bergish Gladbach, Germany), respectively. The purities of CD11b+ and CD11c+ cells in each fraction were confirmed by flow cytometry to be more than 80%.
Analysis of cell proportions
Spleen and PP cells were double-stained with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD11b (clone M1/70) and phycoerythrin (PE)-conjugated rat anti-mouse CD11c (clone HL3) monoclonal antibodies (BD Pharmingen, San Diego, MO) and analysed on an EPICS Altra flow cytometer using the expo32 software program (Beckman Coulter, Miami, FL).
Cultures for cytokine production
Unfractionated spleen and PP cells (5 × 105 cells) or enriched CD11b+ and CD11c+ cells (1 × 105 cells) from BALB/c mice were cultured with heat-killed bacteria (1–30 μg/ml) in 200 μl of complete RPMI-1640 medium in a 96-well culture plate. Supernatants were collected on day 3 for determination of IL-12p70, TNF-α, IL-6 and IL-10.
Analysis of effector T-cell development and cytokine secretion
Spleen and PP cells (3 × 106 cells) from DO11.10 mice were initially cultured with OVA (300 μg/ml) in the absence or presence of heat-killed bacteria (10 μg/ml) in 2 ml of complete RPMI-1640 medium in a 24-well culture plate for 6 days in order to promote the development of effector T-cell subsets. In some cases, supernatants (100 μl) were collected on days 1, 3 and 5 for analysis of primary cytokine responses. To analyse cytokine-producing effector T-cell development, cultured cells were harvested, washed with RPMI-1640 medium, and stimulated with phorbol 12-myristate 13-acetate and ionomycin in the presence of brefeldin A (Leukocyte Activation Cocktail; BD Pharmingen) for 4 hr. After staining of the cells with FITC-conjugated rat anti-mouse CD4 antibody (clone RM4-5; BD Pharmingen), cytokine-producing cells were stained using intracellular cytokine staining regents from BD Pharmingen (Cytofix/Cytoperm buffer, Perm/Wash buffer, and Stain buffer) and the following antibodies: PE-conjugated rat anti-mouse interferon (IFN)-γ (clone XMG1.2; Immunotech, Marseille, France), IL-4 (clone BVD-24G2; Immunotech), IL-17 (clone TC11-18H10.1; BD Pharmingen), and IL-10 (clone JES5.2A5; Immunotech) monoclonal antibodies and biotinylated rat anti-mouse transforming growth factor (TGF)-β1 antibody (clone A75-3; BD Pharmingen), according to the manufacturer’s instruction manual. The biotinylated anitibody was detected using PE-conjugated streptavidin (Caltag, Burlingame, CA). The cells were analysed on an EPICS Altra flow cytometer.
To examine cytokine secretion by differentiated effector T cells, cultured cells were harvested after the primary stimulation. CD4+ T cells were isolated from the cells by magnetic cell sorting with anti-CD4 microbeads (Miltenyi Biotech). CD4+ T cells (1 × 105 cells) were re-stimulated with OVA (100 μg/ml) plus irradiated (3000 rad) BALB/c spleen cells (4 × 105 cells) as APCs. Supernatants were collected on day 2 for determination of IFN-γ and IL-17.
Analysis of Foxp3-positive T regulatory cells
After the primary stimulation of spleen and PP cells from DO11.10 mice with OVA in the absence or presence of heat-killed bacteria, the cells were stained with FITC-conjugated rat anti-mouse CD4 antibody and intracellularly stained with PE-conjugated rat anti-mouse Foxp3 antibody (clone FJK-16s; eBioscience, San Diego, CA) using intracellular cytokine staining reagents (BD Pharmingen).
Enzyme-linked immunosorbent assay (ELISA) for cytokines
Determination of cytokine levels in culture supernatants was performed by sandwich ELISA. Rat anti-mouse IL-12 (clone 9A5), IL-6 (clone MP5-20F3) and IL-17 (clone TC11-18H10) monoclonal antibodies and rabbit anti-mouse IFN-γ polyclonal antibodies were used as the capture antibody, and biotinylated rat anti-mouse IL-12 (clone C17.8), IL-6 (clone MP5-32C11), IL-17 (clone TC11-8H4), and IFN-γ (clone DB-1) monoclonal antibodies, respectively, were used as the detection antibody. All antibodies and standard recombinant cytokines, except for IFN-γ (Biosource International, Camarillo, CA), were purchased from BD Pharmingen. The mouse IL-10 Opt EIA set (BD Pharmingen) and TNF-α Duoset (Genzyme, Cambridge, MA) were used for determination of IL-10 and TNF-α concentrations, respectively.
Statistical analysis
Differences in cytokine-producing T-cell proportions and cytokine secretion between the groups were analysed using the paired Student’s t-test and Dunnet multiple comparison test, respectively.
Results
Induction of cytokine production by various bacteria
Spleen and PP cells were cultured with probiotic Gram-positive (L. casei Shirota, L. plantarum and B. bifidum Yakult), pathogenic Gram-positive (L. monocytogenes and S. aureus), and pathogenic Gram-negative (E. coli and H. pylori) bacteria for 3 days, and the concentrations of IL-12p70, TNF-α, IL-6 and IL-10 in the supernatants were measured. Lactobacillus casei and L. plantarum strongly induced IL-12 production in spleen cells, but the production of IL-12 decreased in PP cells (Fig. 1). The other bacteria weakly induced IL-12 production in spleen cells, and the low-level production of IL-12 was conserved in PP cells. All Gram-positive bacteria except B. bifidum strongly induced TNF-α production, but the production of TNF-α decreased in PP cells. IL-10 production was strongly induced by E. coli and intermediately by L. plantarum, S. aureus and H. pylori in spleen cells, but the production decreased in PP cells. The production of IL-6, in contrast to other cytokines, was induced by all bacteria tested more strongly in PP cells than in spleen cells. We selected two probiotic strains, L. casei and B. bifidum, and two pathogenic strains, S. aureus and E. coli, for further analysis.
Figure 1.
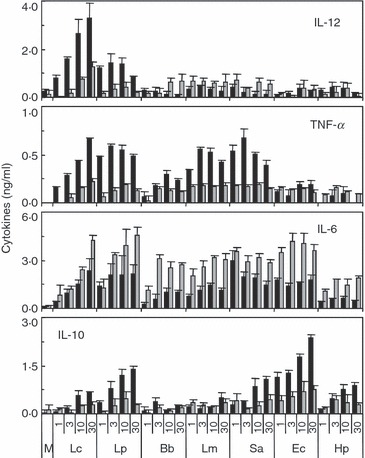
Cytokine secretion by spleen and Peyer’s patch (PP) cells in response to various bacteria. Spleen (black bars) and PP (grey bars) cells prepared from BALB/c mice were cultured with or without (M) various bacteria (1–30 μg/ml) for 3 days. The levels of interleukin (IL)-12, tumour necrosis factor (TNF)-α, IL-6 and IL-10 in culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). The data are the mean ± standard deviation of triplicate cultures. All experiments were repeated three times with similar results. Lc, Lactobacillus casei; Lp, Lactobacillus plantarum; Bb, Bifidobacterium bifidum; Lm, Listeria monocytogenes; Sa, Staphylococcus aureus; Ec, Escherichia coli; Hp, Helicobacter pylori.
Cytokine production by CD11b+ and CD11c+ cells in response to bacterial stimuli
CD11b+ and CD11c+ cells were enriched from spleen and PP cells and cultured with the selected four strains for 3 days, and cytokine production was examined. Lactobacilus casei stimulated spleen CD11b+, but not CD11c+, cells to produce high levels of IL-12, but hardly induced IL-12 production in PP CD11b+ cells (Fig. 2a). In contrast, B. bifidum induced relatively low production of IL-12 in unfractionated PP cells, and the low-level production of IL-12 was observed in PP CD11c+, but not CD11b+, cells. The three Gram-positive strains induced TNF-α production more strongly than E. coli in spleen CD11b+ cells, but the production decreased in PP CD11b+ cells. Escherichia coli induced PP CD11c+ cells to produce a higher level of TNF-α than the Gram-positive strains. PP CD11b+ and CD11c+ cells produced higher levels of IL-6 than those cells in the spleen in response to bacteria, particularly E. coli. All bacteria tested induced IL-10 production in PP CD11b+ and CD11c+ cells more effectively than in those cells in the spleen, although unfractionated spleen cells produced higher levels of IL-10 than unfractionated PP cells, thus indicating the complicated regulation of IL-10 production.
Figure 2.
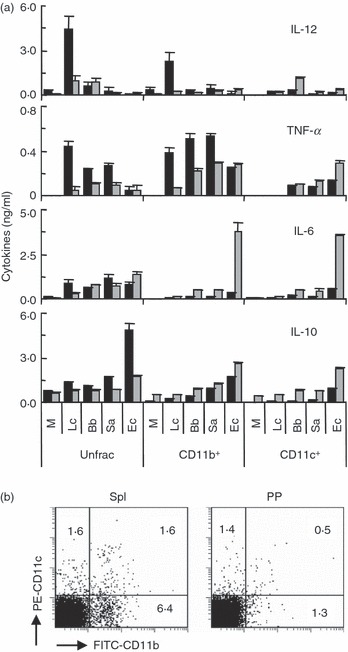
Cytokine secretion by CD11b+ and CD11c+ cells in response to various bacteria. (a) Unfractionated (Unfrac) cells and CD11b+ and CD11c+ cells enriched from BALB/c mouse spleen (black bars) and Peyer’s patch (PP) (grey bars) cells were cultured with or without (M) various bacteria (10 μg/ml) for 3 days. The levels of interleukin (IL)-12, tumour necrosis factor (TNF)-α, IL-6 and IL-10 in the culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). The data are the mean ± standard deviation of triplicate cultures. All experiments were repeated three times with similar results. Lc, Lactobacillus casei; Bb, Bifidobacterium bifidum; Sa, Staphylococcus aureus; Ec, Escherichia coli. (b) Unfractionated spleen and PP cells were stained with fluorescein isothiocyanate (FITC)-labelled anti-CD11b and phycoerythrin (PE)-labelled anti-CD11c antibodies and then analysed by flow cytometry. The values represent the percentages of cells in each quadrant. The experiments were repeated twice with similar results.
Figure 2b shows the proportions of CD11b+ and CD11c+ cells in spleen and PP cells. A higher proportion of CD11b+ cells was observed in spleen cells than PP cells.
Effects of bacteria on the development of T helper cell subsets
Different bacteria induced specific patterns of cytokine production in APCs, so we next examined the effects of these bacteria on the development of Th1, Th2 and Th17 cells. Spleen and PP cells were prepared from unsensitized OVA-specific TCR transgenic (OVA-TCR-Tg) mice and cultured primarily with OVA in the absence or presence of the four bacteria for 6 days in order to induce the development of Th cell subsets. The development of effector T-cell subsets was analysed by staining of intracellular IFN-γ, IL-4 and IL-17 in CD4 + T cells (Fig. 3). OVA stimulation strongly induced the development of IFN-γ-producing Th1 cells in both spleen and PP cells. All bacteria tested significantly enhanced Th1 cell development in PP cells, while only L. casei and E. coli enhanced it in spleen cells. OVA stimulation induced the development of IL-17-producing Th17 cells in PP cells more effectively than in spleen cells. All bacteria tested had no significant effects on the development of Th17 cells. IL-4-producing Th2 cells were hardly induced by any bacteria in these culture conditions.
Figure 3.

An analysis of cytokine-producing T cells developed from spleen and Peyer’s patch (PP) cells in response to various bacteria. Spleen and PP cells prepared from ovalbumin-specific T-cell receptor transgenic (OVA-TCR-Tg) mice were primarily cultured with or without (OVA only) various bacteria (10 μg/ml) in the presence of OVA for 6 days in order to induce the development of effector T helper (Th) cells. The cultured cells and freshly isolated spleen and PP cells were stimulated with phorbol 12-myristate 13-acetate (PMA) plus ionomycin in the presence of brefeldin A for 4 hr, stained with fluorescein isothiocyanate (FITC)-labelled anti-CD4 and phycoerythrin (PE)-labelled anti-interferon (IFN)-γ, interleukin (IL)-4 or IL-17 antibodies, and then analysed by flow cytometry. The values represent the mean ± standard deviation of percentages of cytokine-producing cells in CD4+ T cells in three independent experiments. #P < 0·05, compared with the OVA-only groups. Lc, Lactobacillus casei; Bb, Bifidobacterium bifidum; Sa, Staphylococcus aureus; Ec, Escherichia coli.
The development of Th1 cells is promoted by IL-12, so we examined the relationship between bacterium-induced IL-12 and the development of Th1 cells using probiotic L. casei and B. bifidum. First, we checked IL-12 produced during the primary culture of spleen and PP cells from OVA-TCR-Tg mice. Similar to the results for BALB/c mouse cell cultures without antigen-specific stimulation (Fig. 1), L. casei, but not B. bifidum, induced high levels of secretion of IL-12 during the primary culture of spleen cells (Fig. 4a). In PP cell cultures, B. bifidum induced intermediate levels of IL-12, while only low levels of IL-12 were induced by L. casei. Addition of anti-IL-12 neutralization antibody into the primary cultures abrogated bacterium-induced Th1 cell development in both spleen and PP cells, suggesting that low levels as well as high levels of IL-12 induced by probiotic stimuli played roles in Th1 cell development (Fig. 4b).
Figure 4.
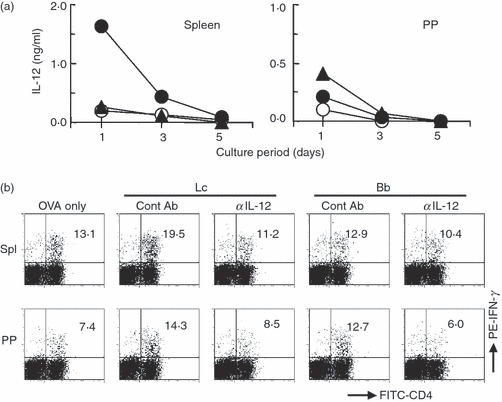
The importance of interleukin (IL)-12 for the development of T helper type 1 (Th1) cells. (a) Spleen and Peyer’s patch (PP) cells prepared from ovalbumin-specific T-cell receptor transgenic (OVA-TCR-Tg) mice were cultured either with or without (open circle) Lactobacillus casei (closed circle) or Bifidobacterium bifidum (closed triangle, 10 μg/ml) in the presence of OVA. The levels of IL-12 in culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA) on days 1, 3 and 5. All experiments were repeated twice with similar results. (b) Spleen and PP cells prepared from OVA-TCR-Tg mice were primarily cultured with or without (OVA only) L. casei (Lc) or B. bifidum (Bb, 10 μg/ml) in the presence of OVA for 6 days in order to induce the development of Th1 cells. Control rat IgG2a (ContAb) or rat anti-mouse IL-12 (αIL-12) antibody was also added to the primary cultures. The cultured cells were harvested, re-stimulated with phorbol 12-myristate 13-acetate (PMA) plus ionomycin in the presence of brefeldin A for 4 hr, stained with fluorescein isothiocyanate (FITC)-labelled anti-CD4 and phycoerythrin (PE)-labelled anti-interferon (IFN)-γ antibodies, and then analysed by flow cytometry. The values represent the percentages of IFN-γ-producing cells in CD4+ T cells. All experiments were repeated twice with similar results.
Effects of bacteria on proinflammatory cytokine secretion by differentiated effector T cells
Cytokine secretion by CD4+ T cells that developed during the primary cultures was examined. After the primary culture of spleen and PP cells in the absence or presence of the four bacteria, CD4+ T cells were isolated from the cultured cells and re-stimulated with OVA in the presence of irradiated BALB/c spleen cells as APCs. Lactobacillus casei-induced spleen CD4+ T cells, but not PP CD4+ T cells, secreted higher levels of IFN-γ (Fig. 5), although L. casei effectively promoted the development of Th1 cells in both spleen and PP cells (Fig. 3). PP CD4+ T cells induced by B. bifidum and S. aureus secreted higher levels of IFN-γ, which was consistent with the results showing that these bacteria induced Th1 cell development in PP, but not spleen, cells. Although E. coli promoted Th1 cell development in spleen and PP cells, increased secretion of IFN-γ was not observed in E. coli-induced CD4+ T cells from spleen and PP cells. Higher levels of IL-17 were secreted by PP CD4+ T cells, which was consistent with the strong induction of Th17 cell development in PP cells. The secretion of IL-17 slightly, but significantly, decreased in the CD4+ T cells induced by L. casei.
Figure 5.
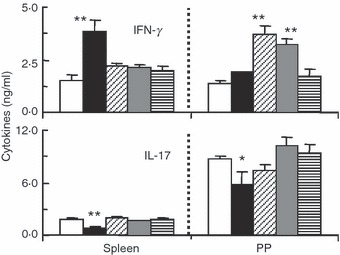
The secretion of cytokines by T cells developed from spleen and Peyer’s patch (PP) cells in response to various bacteria. Spleen and PP cells prepared from ovalbumin-specific T-cell receptor transgenic (OVA-TCR-Tg) mice were primarily cultured with or without various bacteria (10 μg/ml) in the presence of OVA for 6 days in order to induce the development of effector T helper (Th) cells. CD4+ T cells were enriched from the cultured cells and re-stimulated with OVA plus irradiated BALB/c mouse spleen cells as antigen-presenting cells for 2 days. The levels of interferon (IFN)-γ and interleukin (IL)-17 in culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). The data are the mean ± standard deviation of triplicate cultures. All experiments were repeated twice with similar results. *P < 0·05; **P < 0·01, compared with the OVA-only groups. White bars, OVA only; black bars, Lactobacillus casei; bars with oblique lines, Bifidobacterium bifidum; grey bars, Staphylococcus aureus; hatched bars, Escherichia coli.
Effects of bacteria on the development of regulatory T cells
An analysis of cytokine secretion by activated effecter CD4+ T cells suggested that some regulatory mechanisms may have played a role in some cases, such as the stimulation of PP cells with L. casei. Therefore, we examined the development of IL-10-producing, TGF-β-producing, and Foxp3-expressing regulatory T cells after the primary culture. No significant induction of the development of these regulatory T cells was observed for any of the bacteria tested, although L. casei showed a slight tendency to promote IL-10-producing CD4+ regulatory T cells (Fig. 6).
Figure 6.
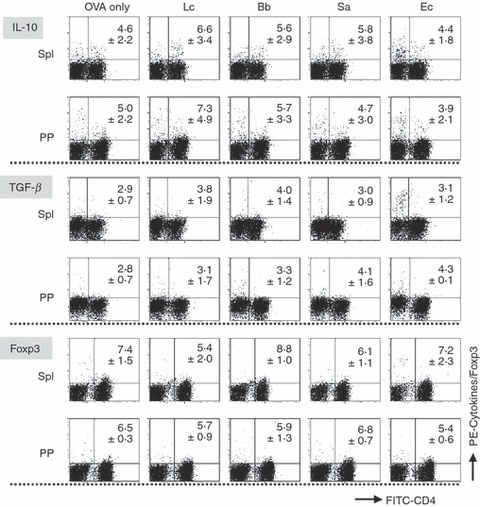
An analysis of the development of regulatory T cells from spleen and Peyer’s patch (PP) cells in response to various bacteria. Spleen and PP cells prepared from ovalbumin-specific T-cell receptor transgenic (OVA-TCR-Tg) mice were primarily cultured with or without (OVA only) various bacteria (10 μg/ml) in the presence of OVA for 6 days in order to induce the development of regulatory T cells. The cultured cells were harvested, re-stimulated with phorbol 12-myristate 13-acetate (PMA) plus ionomycin in the presence of brefeldin A for 4 hr and stained with fluorescein isothiocyanate (FITC)-labelled anti-CD4 and phycoerythrin (PE)-labelled anti-IL-10 or biotinylated anti-transforming growth factor (TGF)-β1 antibodies. Biotinylated antibody was detected using PE-labelled streptavidin. For the analysis of Foxp3-positive cells, the cells after the primary culture were stained with FITC-labelled anti-CD4 and PE-labelled anti-Foxp3 antibodies without re-stimulation. The stained cells were analysed by flow cytometry. The values represent the mean ± standard deviation of the percentages of cytokine-producing or Foxp3-expressing cells in the CD4+ T cells in three independent experiments. Lc, Lactobacillus casei; Bb, Bifidobacterium bifidum; Sa, Staphylococcus aureus; Ec, Escherichia coli.
Discussion
Among the bacteria studied, two strains of lactobacilli strongly induced IL-12 production in BALB/c mice spleen cells, and the effect of L. casei Shirota, which is a major probiotic, on the induction of IL-12 production was particularly significant. Compared with other Gram-positive bacteria, the amount of other cytokines such as TNF-α and IL-6 induced by L. casei Shirota was not particularly significant, and it can be therefore said that one of the main characteristics of L. casei Shirota is its property of inducing high levels of IL-12 production. In addition, in a culture system wherein effector T-cell subsets were induced to differentiate by stimulating spleen cells prepared from antigen-unprimed OVA-TCR-Tg mice with OVA, L. casei Shirota induced the differentiation of the unprimed CD4+ T cells into Th1 cells through the induction of IL-12 production, and the induced Th1 cells produced a large amount of IFN-γ through re-stimulation with antigens. It is believed that the induction of IL-12 production and the resultant Th1 cellular response, as well as the activation of the innate immune system, are important immunoregulatory effects exhibited by probiotics. From this viewpoint, L. casei Shirota can be characterized as a probiotic with great potential for immunostimulation.
However, compared with spleen cells, IL-12 and TNF-α production in the PP cells was low. This result is consistent with reports that the production of proinflammatory cytokines caused by bacterial stimulation is low in intestinal macrophages and DCs.26,28 Regarding differentiation into Th cell subsets, a flow cytometer analysis of intracellular IFN-γ in CD4+ T cells strongly indicated that L. casei Shirota induced differentiation into Th1 cells via low-level induction of IL-12 production; however, high-level IFN-γ production was not observed when CD4+ T cells purified from PP cells after L. casei Shirota was added in the same fashion for culturing were re-stimulated with antigens. Based on these results, it is believed that there is a possibility that L. casei Shirota induced Th1 cell production in the PP cells while simultaneously inducing CD4+ regulatory T-cell production, and as a result IFN-γ production by Th1 cells re-stimulated with antigens was inhibited. However, our analysis of intracellular cytokines indicated that no inhibitory effect was exerted because the secretion of humoral factors by regulatory T cells, which act to inhibit cytokine production, was repressed by the addition of brefeldin A to the culture system in order to inhibit the secretion of proteins such as cytokines. In addition, despite the fact that L. casei Shirota exhibited a tendency to induce the differentiation of Th17 cells, which are important for the induction of inflammatory responses, the amount of IL-17 secreted when the CD4+ T cells were re-stimulated with antigens was low. Again, in this case, it is believed that L. casei Shirota may have inhibited the secretion of IL-17 by Th17 cells re-stimulated with antigen via induced regulatory T cells without inhibiting the differentiation of the Th17 cells.
Regulatory T cells that have been confirmed as being induced by probiotics include: some T cells that produce large amounts of IL-10,21,22 those cells that secrete large amounts of TGF-β,31,32 and other phenotypes that express Foxp3 and/or CD25.33,34 We investigated whether these regulatory T cells could be induced using spleen cells and PP cells prepared from OVA-TCR-Tg mice; no induction of either TGF-β-producing or Foxp3-expressing regulatory T cells was observed for any of the bacteria used under our study conditions. There was a tendency for IL-10-producing regulatory T cells to be induced by the addition of L. casei Shirota in spleen cells and PP cells; however, these increases were not statistically significant, and the probability that they were caused by an immunoregulatory mechanism that inhibits the production of IFN-γ and IL-17, and which was induced in the PP cells by L. casei Shirota, is low. Baba et al.35 reported that human monocyte-derived DCs that were stimulated with L. casei DN-114 001 induced unidentified CD4+ regulatory T cells that were not IL-10-producing, TGF-β-producing, or Foxp3-expressing regulatory T cells, and that IFN-γ production by effector T cells was inhibited as a result. Based on our own experiments, we also believe that there is a possibility that L. casei Shirota controlled the secretion of proinflammatory cytokines by Th1 cells and Th17 cells through the induction of similar CD4+ regulatory T cells in PP cells. As previously described, while L. casei Shirota exhibits the characteristics of a probiotic with a strong potential for activating innate immunity, for PP cells, it may also exert an anti-inflammatory effect, which is another characteristic of the immunoregulatory effects of probiotics.
Among the probiotics studied, B. bifidum exhibited weaker activity than lactobacilli in inducing the production of cytokines such as IL-12 in spleen cells, and there was no observed activity in inducing differentiation into Th1 cells. Based on these observations, B. bifidum can be considered a probiotic with a weak potential for immune stimulation. It has been reported that, compared with lactobacilli, bifidobacteria generally exhibit a weaker effect in inducing IL-12 production in mice spleen cells and human monocyte-derived DCs,36,37 and our results are consistent with these reports. However, although the amount induced was not high compared with L. casei Shirota, B. bifidum induced equal levels of IL-12 production in BALB/c mice PP cells and OVA-TCR-Tg mice PP cells, and it was also able to induce differentiation into Th1 cells. It is also interesting to note that L. casei Shirota strongly induced IL-12 production in spleen CD11b+ cells, while B. bifidum induced IL-12 production in PP CD11c+ cells. We have previously reported that L. casei Shirota strongly induces IL-12 production in mice peritoneal macrophages independently of Toll-like receptor 2 (TLR2) via the three-dimensional structure of the cell wall.16 Meanwhile, Niers et al.22 reported that B. bifidum W32 could effectively stimulate Chinese hamster ovary cells in which TLR2 was forcibly expressed, while the same activity for Lactobacillus salivarius W24 was weak. It has also been reported that IL-12-producing responses to Brucella abortus in DCs are TLR2-dependent; however, it they are TLR2-independent in macrophages.38 Based on these findings, it is possible that lactobacilli induce IL-12 production in macrophages independently of TLR2, while bifidobacteria induce IL-12 production in TLR2-dependent DCs. Therefore, when different types of probiotic strains are used, it is possible for each strain to induce different levels of IL-12 production by stimulating different cell populations via different mechanisms.
When the effects of probiotics and pathogenic Gram-positive bacteria in inducing cytokine production were compared, no significant difference was found in the production of TNF-α, IL-6 or IL-10 in either spleen cells or PP cells. Probiotic lactobacilli strongly induced IL-12 production in spleen cells, but it is highly likely that the property of strongly inducing IL-12 production is one that is unique to each strain or to Lactobacillus bacteria rather than a property that is characteristic of all probiotics. When the effects of Gram-positive bacteria and Gram-negative bacteria in inducing cytokine production were compared, it was found that Gram-positive bacteria strongly induced IL-12 production while Gram-negative bacteria strongly induced IL-10 production in human PBMC.39 In our study using mice spleen cells, Gram-positive lactobacilli strongly induced IL-12 production while Gram-negative E. coli and H. pylori induced IL-10 production strongly and moderately, respectively. However, no such difference was found in PP cells. Karlsson et al have reported that, Gram-negative bacteria strongly induce IL-12 production in the presence of IFN-γ in DCs differentiated from human PBMC.40 On the basis of all these data, it may be better to consider the cytokine-producing patterns induced by each bacterium as variable patterns dependent on differences in the cell populations that the bacteria stimulate rather than as firmly fixed patterns.
In this study, we determined that L. casei Shirota induces immunoregulatory mechanisms to inhibit IL-17 production by Th17 cells. It is becoming clear that Th17 cells play an important role in the induction of inflammatory diseases, including autoimmune diseases and IBD.41 It has been reported that high percentages of Th17 cells are founded throughout the PPs and the mucosal lamina propria in the ileum and colon, and that enteric bacteria are involved in the high density of these Th17 cells.42,43 Therefore, although Th17 cells are attracting attention as a potential new target of immune function control using probiotics, not many investigations on the effects of probiotics on Th17 cells have been conducted to date. Foligne et al.34 transferred bone marrow-derived DCs stimulated with Lactobacillus rhamnosus Lr32 to trinitrobenzene sulphonic acid (TNBS)-induced colitis model mice and discovered that they decreased colon inflammation through the induction of CD25+ regulatory T cells while also significantly inhibiting the expression of IL-17 mRNA in the colon. Tanabe et al.44 have reported that Bifidobacterium infantis JCM 1222 inhibits IL-17 production by mice spleen cells stimulated with TGF-β and IL-6 as well as IL-17 production in a colon tissue culture system stimulated with dextran sodium sulphate (DSS), and they have suggested that these effects may be derived from the induction of IL-10 production. Research in this field has only just begun, and it is expected to expand with future studies on probiotic control of Th17 cells.
In particular, the relationship between the effects of probiotics in inducing IL-12 and IL-10 production and the clinical efficacy thereof is attracting attention. Generally, strains that strongly induce IL-12 production are expected to be effective in preventing infections, lowering the risk of cancer via the activation of the innate immune system, and inhibiting allergies through the control of the Th1/Th2 balance. Strains that strongly induce IL-10 production are expected to be effective in inhibiting excessive immune responses, which would be of benefit in IBD, autoimmune diseases and allergies. In fact, it has been reported that probiotics that induce higher levels of IL-10 and lower levels of IL-12 in human PBMC cultures offer the best protection in mice TNBS-induced colitis.18 Although the involvement of Th17 cells in IBD and autoimmune diseases has been attracting attention in recent years, it has also been revealed that excessive responses of Th1 cells are involved in these diseases.45 For this reason, some researchers have suggested that the increased risk of inflammatory disease may be attributable to probiotics that excessively activate Th1 cellular responses through the induction of IL-12 production.46 In this study, however, it was revealed that IL-12 production generated by APCs and IFN-γ and IL-17 production generated by Th1 cells and Th17 cells can be properly controlled when PP cells are stimulated with L. casei Shirota, which has a strong potential for inducing Th1 cellular responses through the induction of IL-12 production. It is worth noting that, in clinical trials and animal tests, L. casei Shirota not only exerts inhibitory effects against infection,47 cancer,48 and allergies49 but is also effective for IBD50 and autoimmune diseases.51–53 There have been cases in which individual probiotics such as L. rhamnosus GG have been effective against many of the various diseases believed to be caused by a compromised immune system and excessive activation.54 It appears to be difficult to explain the mechanisms of the expression of the multifactorial clinical efficacies shown by these probiotics; however, the phenomena of inducing IL-12 production while inhibiting the production of proinflammatory cytokines in the different types of cells targeted by the probiotics examined in this study may suggest strategies for elucidating the multifactorial clinical efficacies shown by probiotics.
Acknowledgments
We are grateful to Dr Tohru Iino (Yakult Central Institute) for preparation of heat-killed H. pylori. We also gratefully acknowledge the staff of the animal facility of Yakult Central Institute for their expertise in breeding mice.
Glossary
Abbreviations
- APC
antigen-presenting cell
- DC
dendritic cell
- DSS
dextran sodium sulphate
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- OVA
ovalbumin
- OVA-TCR-Tg
OVA-specific T-cell receptor transgenic
- PBMC
peripheral blood mononuclear cells
- PP
Peyer’s patch
- TCR
T-cell receptor
- TGF
transforming growth factor
- Th
T helper
- TLR
Toll-like receptor
- TNBS
trinitrobenzene sulphonic acid
- TNF
tumour necrosis factor
Disclosures
KS, JK-S, MN and KN are employed by Yakult Honsha. The rest of the authors have no conflict of interest.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–40. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Bjorksten B. The intrauterine and postnatal environments. J Allergy Clin Immunol. 1999;104:1119–27. doi: 10.1016/s0091-6749(99)70002-3. [DOI] [PubMed] [Google Scholar]
- 4.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 5.Penders J, Stobberingh EE, van den Brandt PA, Thijs C. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–36. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 6.Joint Food and Agriculture Organization of the United Nations/World Health Organization Expert Consultation. 2001. Health and nutritional properties in food including powder milk with live lactic acid bacteria. Available at http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf.
- 7.Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006;100:1171–85. doi: 10.1111/j.1365-2672.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 8.Morais MB, Jacob CM. The role of probiotics and prebiotics in pediatric practice. J Pediatr (Rio J) 2006;82:S189–97. doi: 10.2223/JPED.1559. [DOI] [PubMed] [Google Scholar]
- 9.Gill H, Prasad J. Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol. 2008;606:423–54. doi: 10.1007/978-0-387-74087-4_17. [DOI] [PubMed] [Google Scholar]
- 10.Shida K, Nanno M. Probiotics and immunology: separating the wheat from the chaff. Trends Immunol. 2008;29:565–73. doi: 10.1016/j.it.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Cross ML. Immunoregulation by probiotic lactobacilli: pro-Th1 signals and their relevance to human health. Clin Applied Immunol Rev. 2002;3:115–25. [Google Scholar]
- 12.Nomoto K. Prevention of infections by probiotics. J Biosci Bioeng. 2005;100:583–92. doi: 10.1263/jbb.100.583. [DOI] [PubMed] [Google Scholar]
- 13.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–33. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Kalliomaki MA, Isolauri E. Probiotics and down-regulation of the allergic response. Immunol Allergy Clin North Am. 2004;24:739–52. doi: 10.1016/j.iac.2004.06.006. viii. [DOI] [PubMed] [Google Scholar]
- 15.Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut. 2005;54:317–20. doi: 10.1136/gut.2004.053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shida K, Kiyoshima-Shibata J, Nagaoka M, Watanabe K, Nanno M. Induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J Dairy Sci. 2006;89:3306–17. doi: 10.3168/jds.S0022-0302(06)72367-0. [DOI] [PubMed] [Google Scholar]
- 17.Niers LE, Timmerman HM, Rijkers GT, et al. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin Exp Allergy. 2005;35:1481–9. doi: 10.1111/j.1365-2222.2005.02375.x. [DOI] [PubMed] [Google Scholar]
- 18.Foligne B, Nutten S, Grangette C, et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236–43. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shida K, Makino K, Morishita A, et al. Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol. 1998;115:278–87. doi: 10.1159/000069458. [DOI] [PubMed] [Google Scholar]
- 20.Mohamadzadeh M, Olson S, Kalina WV, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci U S A. 2005;102:2880–5. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 22.Niers LE, Hoekstra MO, Timmerman HM, et al. Selection of probiotic bacteria for prevention of allergic diseases: immunomodulation of neonatal dendritic cells. Clin Exp Immunol. 2007;149:344–52. doi: 10.1111/j.1365-2249.2007.03421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–72. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116–21. doi: 10.1016/j.jaci.2007.10.043. e11. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 26.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–59. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 27.Kelsall BL, Rescigno M. Mucosal dendritic cells in immunity and inflammation. Nat Immunol. 2004;5:1091–5. doi: 10.1038/ni1104-1091. [DOI] [PubMed] [Google Scholar]
- 28.O’Mahony L, O’Callaghan L, McCarthy J, et al. Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans. Am J Physiol Gastrointest Liver Physiol. 2006;290:G839–45. doi: 10.1152/ajpgi.00112.2005. [DOI] [PubMed] [Google Scholar]
- 29.Honda K, Takeda K. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2009;2:187–96. doi: 10.1038/mi.2009.8. [DOI] [PubMed] [Google Scholar]
- 30.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–3. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 31.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–46. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 32.Zeuthen LH, Fink LN, Frokiaer H. Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology. 2008;123:197–208. doi: 10.1111/j.1365-2567.2007.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feleszko W, Jaworska J, Rha RD, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 34.Foligne B, Zoumpopoulou G, Dewulf J, et al. A key role of dendritic cells in probiotic functionality. PLoS ONE. 2007;2:e313. doi: 10.1371/journal.pone.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J Leukoc Biol. 2008;84:468–76. doi: 10.1189/jlb.0108017. [DOI] [PubMed] [Google Scholar]
- 36.Iwabuchi N, Takahashi N, Xiao JZ, Miyaji K, Iwatsuki K. In vitro Th1 cytokine-independent Th2 suppressive effects of bifidobacteria. Microbiol Immunol. 2007;51:649–60. doi: 10.1111/j.1348-0421.2007.tb03953.x. [DOI] [PubMed] [Google Scholar]
- 37.Zeuthen LH, Christensen HR, Frokiaer H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram-negative bacteria. Clin Vaccine Immunol. 2006;13:365–75. doi: 10.1128/CVI.13.3.365-375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol. 2008;180:1080–7. doi: 10.4049/jimmunol.180.2.1080. [DOI] [PubMed] [Google Scholar]
- 39.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581–6. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karlsson H, Larsson P, Wold AE, Rudin A. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect Immun. 2004;72:2671–8. doi: 10.1128/IAI.72.5.2671-2678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 42.Niess JH, Leithauser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559–68. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 43.Atarashi K, Nishimura J, Shima T, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 44.Tanabe S, Kinuta Y, Saito Y. Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. Int J Mol Med. 2008;22:181–5. [PubMed] [Google Scholar]
- 45.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 46.Baken KA, Ezendam J, Gremmer ER, et al. Evaluation of immunomodulation by Lactobacillus casei Shirota: immune function, autoimmunity and gene expression. Int J Food Microbiol. 2006;112:8–18. doi: 10.1016/j.ijfoodmicro.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Yasui H, Kiyoshima J, Hori T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin Diagn Lab Immunol. 2004;11:675–9. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol. 1995;27:104–9. doi: 10.1159/000475138. [DOI] [PubMed] [Google Scholar]
- 49.Shida K, Takahashi R, Iwadate E, et al. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin Exp Allergy. 2002;32:563–70. doi: 10.1046/j.0954-7894.2002.01354.x. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto S, Hara T, Hori T, et al. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol. 2005;140:417–26. doi: 10.1111/j.1365-2249.2005.02790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato I, Endo-Tanaka K, Yokokura T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998;63:635–44. doi: 10.1016/s0024-3205(98)00315-4. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzaki T, Nagata Y, Kado S, Uchida K, Kato I, Hashimoto S, Yokokura T. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. 1997;105:643–9. doi: 10.1111/j.1699-0463.1997.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 53.Mike A, Nagaoka N, Tagami Y, et al. Prevention of B220+ T cell expansion and prolongation of lifespan induced by Lactobacillus casei in MRL/lpr mice. Clin Exp Immunol. 1999;117:368–75. doi: 10.1046/j.1365-2249.1999.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drisko JA, Giles CK, Bischoff BJ. Probiotics in health maintenance and disease prevention. Altern Med Rev. 2003;8:143–55. [PubMed] [Google Scholar]


