Abstract
B7-H3 is a B7-family co-stimulatory molecule and is broadly expressed on various tissues and immune cells. Transduction of B7-H3 into some tumours enhances anti-tumour responses. We have recently found that a triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a receptor for B7-H3. Here, we examined the roles of tumour-associated B7-H3 and the involvement of TLT-2 in anti-tumour immunity. Ovalbumin (OVA)257–264-specific OT-I CD8+ T cells exhibited higher cytotoxicity against B7-H3-transduced OVA-expressing tumour cells (B7-H3/E.G7) in vitro and selectively eliminated B7-H3/E.G7 cells in vivo. The presence of B7-H3 on target cells efficiently augmented CD8+ T-cell-mediated cytotoxicity against alloantigen or OVA, whereas the presence of B7-H3 in the priming phase did not affect the induced cytotoxicity. B7-H3 transduction into five tumour cell lines efficiently reduced their tumorigenicity and regressed growth. Treatment with either anti-B7-H3 or anti-TLT-2 monoclonal antibody accelerated growth of a tumour that expressed endogenous B7-H3, suggesting a co-stimulatory role of the B7-H3–TLT-2 pathway. The TLT-2 was preferentially expressed on CD8+ T cells in regional lymph nodes, but was down-regulated in tumour-infiltrating CD8+ T cells. Transduction of TLT-2 into OT-I CD8+ T cells enhanced antigen-specific cytotoxicity against both parental and B7-H3-transduced tumour cells. Our results suggest that tumour-associated B7-H3 directly augments CD8+ T-cell effector function, possibly by ligation of TLT-2 on tumour-infiltrating CD8+ T cells at the local tumour site.
Keywords: anti-tumour immunity, B7-H3, cytotoxic T lymphocyte, effector function, tumour-infiltrating lymphocytes, triggering receptor expressed on myeloid cell-like transcript 2
Introduction
Optimal activation of T cells requires co-signals in addition to T-cell receptor (TCR) engagement. Co-signal molecules regulate T-cell responses, positively or negatively. B7-H3 (CD276) is a member of the B7 family and is expressed on lymphoid cells, such as dendritic cells, monocytes/macrophages and activated T cells, as well as non-lymphoid tissue cells, such as epithelial cells, anterior pituitary progenitor cells, muscle cells and fibroblast-like synoviocytes.1–8 Mouse B7-H3 consists of immunoglobulin variable (IgV)-constant (IgC) domains. The human B7-H3 homologue has another isoform (B7-H3b), consisting of two pairs of IgV-IgC domains, and B7-H3b is the major form in humans.9–12 B7-H3 was initially identified as a co-stimulator, which enhanced proliferation and interferon-γ (IFN-γ) production in human T cells.1 However, subsequent human and mouse studies suggest that B7-H3 plays inhibitory roles in T-cell activation. Human and mouse B7-H3 fusion proteins inhibit T-cell activation and effector cytokine production in vitro, and B7-H3 deficiency or blockade of B7-H3 by anti-B7-H3 monoclonal antibody (mAb) exacerbates murine experimental autoimmune encephalomyelitis and experimental allergic conjunctivitis.9–15 Hence, the immunological function of B7-H3 is controversial.
Tumour-associated B7-H3 is expressed in non-small cell lung cancer, prostate cancer, neuroblastoma and renal cell carcinoma.2–21 Tumour-associated B7-H3 seems to correlate with clinicopathological features or poor prognosis.19,21,22 In contrast, there is one report demonstrating better survival in patients with gastric carcinoma B7-H3+ tumours.23 Most reports in humans suggest negative roles for tumour-associated B7-H3 in anti-tumour immunity. In contrast, murine tumour experiments have demonstrated the immune-enhancing function of tumour-associated B7-H3. Intra-tumoral injection of an expression plasmid encoding B7-H3 led to regression of EL-4 lymphomas, which was dependent on CD8+ T cells and natural killer cells, and transduction of B7-H3 into P815 mastocytoma or C26 colon carcinoma caused regression of tumour growth and reduced metastasis.24–27 P815 cells expressing B7-H3 induce tumour-specific CD8+ cytotoxic T lymphocyte (CTL) expansion and enhance cytotoxicity.25
We have recently found that a counter-receptor for B7-H3 is a triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2, TREML2), which is a member of the TREM family of proteins that belongs to the immunoglobulin superfamily.28 Like other TREM family proteins, TLT-2 is expressed on B cells, granulocytes and macrophages.28,29 TLT-2 expression on splenic and bone marrow-derived dendritic cells is limited. Interestingly, TLT-2 is also expressed constitutively on CD8+ T cells and is induced on CD4+ T cells after activation.28 The transduction of either B7-H3 into tumour cells lines as stimulator/target cells or TLT-2 into T cells enhances CD8+ T-cell effector function and blockade of the B7-H3–TLT-2 pathway at the time of elicitation and efficiently inhibits contact hypersensitivity responses, demonstrating a co-stimulatory function of the B7-H3–TLT-2 pathway.28 These findings prompted us to investigate the effects of B7-H3-transduced tumour cells on anti-tumour immunity, because CD8+ T cells are the major effector cells in most cases of tumour eradication. In this study, we examined mechanisms of enhanced anti-tumour immunity induced by tumour-associated B7H3 and the involvement of its TLT-2 receptor.
Materials and methods
Mice and cell lines
Female C3H/HeN, DBA/2, BALB/c, C57BL/6 (B6) and BALB/c nude mice were purchased from Japan SLC (Hamamatsu, Japan), Charles River Japan (Tokyo, Japan) and CLEA Japan (Tokyo, Japan). Chicken ovalbumin (OVA)257–264-specific TCR transgenic OT-I mice were generously provided by Dr William R. Heath (The Walter and Eliza Hall Institute of Medical Research, Victoria, Australia).30 Mice were 6–10 weeks of age at the start of the experiments. All experiments were approved by the Animal Care and Use Committee of Tokyo Medical and Dental University.
The T lymphoma EL4, OVA-expressing EL4 (E.G7), plasmacytoma J558L, mastocytoma P815 and melanoma B16 cell lines were cultured in RPMI-1640, supplemented with 10% fetal bovine serum and 10 μg/ml gentamicin. A squamous cell carcinoma SCCVII cell line was maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and 10 μg/ml gentamicin.
Monoclonal antibodies and flow cytometry
Anti-B7-H3 [MIH32 and MIH35, both rat immunoglobulin G2a (IgG2a), κ] and anti-TLT-2 mAb (MIH47, rat IgG2a, κ and MIH49, rat IgM, κ) were generated as described previously.28 These mAbs were biotinylated or conjugated with fluorescein isothiocyanate (FITC), according to a standard protocol. Peridinin-chlorophyll-protein complex-carbocyanin 5.5 (PerCP-Cy5.5) -conjugated-anti-CD4 (GK1.5), anti-CD8 (53-6.72), and anti-CD3 (145-2C11); FITC-conjugated anti-CD45 (3F11.1); anti-major histocompatibility complex (MHC) class I (SF1-1.1, 36-7-5 and AF6-88.5 for Kd, Kk and Kb, respectively); phycoerythrin-conjugated anti-CD8 (53-6.72), anti-CD25 (PC61), anti-CD69 (H1.2F3), anti-CD54 (YN1/1.7.4), anti-CD80 (1G10) and anti-CD86 (GL1) mAbs; and appropriate fluorochrome-conjugated isotype control immunoglobulins were used. All fluorochrome-conjugated antibodies except FITC were obtained from eBioscience (San Diego, CA) or BD-Pharmingen (San Diego, CA). Culture supernatant from the 2.4G2 hybridoma (anti-CD16/CD32 mAb) was used to block Fc-mediated binding. Phycoerythrin-streptavidin or allophycocyanin-streptavidin was used for the biotinylated mAbs. Cells were stained and analysed using a fluorescence-acitvated cell sorter (FACSCalibur; BD Biosciences, Sparks, MD) and the CellQuest (BD Biosciences) or flowJo (TreeStar, Ashland, OR) software.
Gene transduction
Mouse B7-H3 complementary DNA28 was inserted into the pMKITneo, pMXC and pMXs-neo (kindly provided by T. Kitamura) expression vectors. P815 cells were transfected with B7-H3/pMKITneo by electroporation on a GenePulser Xcell Complete system (Bio-Rad, Hercules, CA). B7-H3/pMXC and B7-H3/pMXs-neo were used for SCCVII, EL4, E.G7, B16 cells and J558L cells, respectively. Tumour cells were retrovirally transduced with B7-H3.28 For infecting EL4, SCCVII and B16 cells, pVSV-G was co-transfected to generate pan-tropic retrovirus. After drug selection, transfectants expressing high levels of B7-H3 were sorted by flow cytometry as described previously.31
The TLT-2 complementary DNA was inserted into pMXs-IG, and control IRES-GFP (pMXs-IG) or TLT-2/pMXs-IG was retrovirally transduced into OT-I CD8+ T cells stimulated with OVA peptide (SIINFEKL).28 GFP+ cells were sorted by flow cytometry and used as mock- or TLT-2-transduced OT-I CD8+ T cells.
T-cell co-stimulation assay using P815 cells in vitro
CD4+ and CD8+ T cells from BALB/c mice were isolated by negative selection, as described previously.28 The purity of the CD4+ and CD8+ T cells was over 95% and 90%, respectively, as confirmed by flow cytometry. For the anti-CD3 mAb-induced co-stimulation assay, isolated T cells (2 × 105/well) were co-cultured with mitomycin C-treated parental P815 or B7-H3-transduced P815 (B7-H3/P815) cells at the indicated responder : stimulator ratios, in the presence of anti-CD3 mAb (145-2C11, 0·2 μg/ml in CD4+ T cells and 1·0 μg/ml in CD8+ T cells). The proliferative responses for the final 18 hr of the 3-day culture and IFN-γ production in the culture supernatants at 72 hr were then measured.32 Anti-CD3 mAb-induced redirected cytotoxicity against P815 and B7-H3/P815 cells was measured by the 6-hr JAM test.33,34
Measurement of cytotoxicity against E.G7 cells
Splenocytes from OT-1 mice were cocultured with mitomycin C-treated E.G7 cells for 3 days for in vitro sensitization. The cells were harvested, separated into CD8+ T cells, and used as in vitro-sensitized OT-I CD8+ T cells. Cytotoxicity against E.G7 and B7-H3/E.G7 was measured by a 6-hr JAM test. For the in vivo cytotoxicity assay, E.G7 and B7-H3/E.G7 cells were labelled with CellTracker Orange [5-(and-6)-(((4-chloromethyl)benzoyl)amino)] tetramethylrhodamine (CMTMR; 10 μm, Invitrogen, Carlsbad, CA) and/or carboxyfluorescein diacetate succinimidyl ester (CFSE; 10 μm, Invitrogen). The CMTMR-labelled cells (2 × 106) were mixed with a twofold number of CFSE-labelled parental E.G7 (A-mix) or B7-H3/E.G7 (B-mix) cells (4 × 106) and then the mixed cells were injected intraperitoneally (i.p.) into OT-I mice. Peritoneal exudate cells (PEC) were analysed by flow cytometry after 24 hr.
Induction of CTL against the alloantigen and OVA by in vivosensitization
B6 mice were sensitized in vivo by peritoneal injection with DBA/2-originated allogeneic P815 or B7-H3/P815 cells (2 × 107 cells) to evaluate CTL against the alloantigen. After 8 days, PEC were collected and cytotoxicity against P815 and B7-H3/P815 was measured as described above. The OT-I mice received a peritoneal injection of mitomycin C-treated OVA-expressing EL4 (E.G7 or B7-H3/E.G7) cells (2 × 107) to induce OVA-specific CTL. Three days later, PEC were harvested and cytotoxicity against E.G7 and B7-H3/E.G7 was assessed as described above.
Tumour inoculation and evaluation of tumour growth
The P815 (DBA/2-originated, 5 × 104), EL-4 (B6-originated, 2 × 104), J558L (BALB/c-originated, 5 × 106), SCCVII (C3H-originated, 2 × 105), B16 (B6-originated, 1 × 105) parental cells and each transfectant were injected subcutaneously into the shaved right flank of syngeneic mice, and tumour volumes were evaluated.35,36 At the final examination day (day 42), tumours with an estimated size < 30 mm3 were defined as ‘rejected’ and the final rejected ratios were calculated. In some selected experiments, P815 and B7-H3/P815 (5 × 104) cells were injected into BALB/c nude mice and tumour volumes were evaluated. To deplete CD4+, CD8+, or both T cells, 0·5 mg of anti-CD4 (GK1.5), anti-CD8 (53-6.72), or both mAbs were administrated i.p. on days − 5, − 1 and 3. In experiments examining the effects of anti-B7-H3 or anti-TLT-2 mAb, 200 μg each of anti-B7-H3 (MIH35), anti-TLT-2 (MIH49) mAb, or control immunoglobulin was injected i.p. every other day after tumour inoculation.
Isolation of tumour-infiltrating lymphocytes
For isolating tumour-infiltrating lymphocytes (TIL), the skin with a small tumour mass at the parental SCCVII or B7-H3/SCCVII tumour-inoculated sites was resected after 7 days and single-cell suspensions were obtained by digestion with collagenase I (400 U/ml; Sigma, St Louis, MO), DNase (10 U/ml; Wako, Tokyo, Japan) and hyaluronidase (2·5 U/ml; Sigma), followed by a density gradient.37 The cells were then subjected to flow cytometry.
CD8+ T-cell stimulation
OT-1 CD8+ T cells (1 × 106 cells) were co-cultured with equal numbers of B7-H3/E.G7 for 24 hr and then expression of TLT-2, CD8, CD3 and CD69 or CD25 was analysed by flow cytometry. For experiments to see the effects of cytokines on TLT-2 expression, CD8+ T cells (8 × 105 cells/well) from naive B6 mice were stimulated with immobilized anti-CD3 mAb (145-2C11, 5 μg/ml) in the presence of either interleukin-2 (IL-2; 10 ng/ml), IL-10 (20 ng/ml), tumour necrosis factor-α (TNF-α; 40 ng/ml), IFN-γ (10 ng/ml) or transforming growth factor-β (TGF-β; 10 ng/ml) in 24-well plates for 3 days. The cells were collected and subjected to flow cytometric analyses for TLT-2 expression. All cytokines were obtained from eBioscience or BD Pharmingen.
Results
Tumour-associated B7-H3 enhances T-cell effector functions
A co-stimulation assay using Fcγ receptor-bearing P815 cells and sub-optimal doses of anti-CD3 mAb has been used to evaluate co-signal function of various B7 and TNF family molecules.28,33,38–41 We examined the effect of B7-H3 transduction in P815 cells on anti-CD3 mAb-induced CD4+ or CD8+ T-cell responses including proliferative responses, cytokine production and cytotoxicity. P815 cells expressed endogenously low levels of B7-H3 but the transduction of B7-H3 induced dramatically higher levels (∼ 50-fold; Fig. S1 and ref. 28). Splenic CD4+ or CD8+ T cells were co-cultured with either parental P815 or B7-H3/P815 cells in the presence of a suboptimal dose of anti-CD3 mAb. Consistent with our previous report,28 proliferative responses and IFN-γ production by CD4+ T cells were not affected by B7-H3 transduction, whereas proliferation and IFN-γ production by CD8+ T cells were efficiently augmented by B7-H3/P815. Anti-CD3 mAb-induced redirected cytotoxicity against B7-H3/P815 cells was higher than that against P815 in both CD4+ and CD8+ T cells (Fig. 1c).
Figure 1.
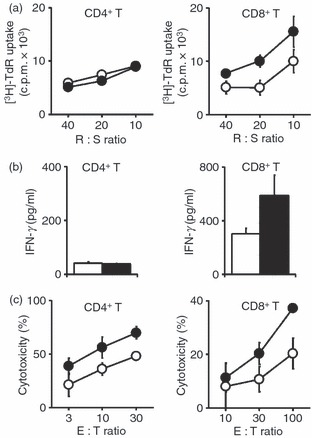
Enhancement of CD8+ T-cell effector function by B7-H3. CD4+ or CD8+ T cells were co-cultured with either P815 (white circles or columns) or B7-H3/P815 (black circles or columns) in the presence of a sub-optimal dose of anti-CD3 monoclonal antibody at the indicated responder : stimulator (R : S) or effector : target (E : T) ratios. Proliferative responses for 3 days (a), interferon-γ (IFN-γ) production for 4 days culture (b) and cytotoxicity for 6 hr (c) were measured as described in the Materials and methods. Values shown are the means ± SD from triplicate cultures. The data are representative of three independent experiments.
Tumour-associated B7-H3 enhances antigen-specific cytotoxicity by CD8+ T cells
We examined OVA-specific cytotoxicity against E.G7 cells that express peptide antigens derived from OVA protein, using OT-I-derived CD8+ T cells to investigate whether B7-H3 on target cells up-regulated antigen-specific cytotoxicity of CD8+ T cells. B7-H3 expression on parental E.G7 and B7-H3/E.G7 cells is shown in Fig. S1. Cytotoxicity against B7-H3/E.G7 cells by freshly isolated OT-I CD8+ T cells was consistently higher than that against parental E.G7 cells (Fig. 2a). When the in vitro-sensitized OT-I CD8+ T cells were used as effectors, cytotoxicity against B7-H3/E.G7 was seen even at lower effector : target (E : T) ratios (E : T = 1 and E : T = 5) and consistently showed higher cytotoxicity than that against parental E.G7 cells (Fig. 2b). These results indicate that tumour-associated B7-H3 enhanced antigen-specific cytotoxicity of CD8+ T cells.
Figure 2.
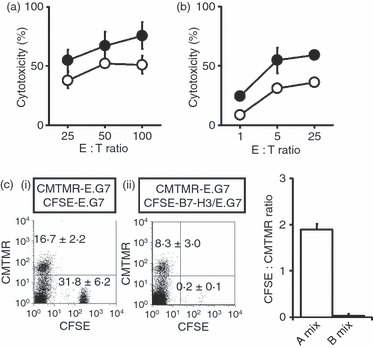
Selective lysis in B7-H3-transduced tumours. Freshly isolated OT-I CD8+ T cells (a), in vitro-sensitized OT-I CD8+ T cells (b) were used as effector cells. Cytotoxicity against parental E.G7 (white circles) or B7-H3/E.G7 (black circles) was measured. Values shown are means ± SD. (c) The mixture of [5-(and-6)-(((4-chloromethyl)benzoyl)amino)] tetramethylrhodamine (CMTMR) -labelled E.G7 and carboxyfluorescein diacetate succinimidyl ester (CFSE) -labelled E.G7 (1 : 2, A-mix) or CMTMR-labelled E.G7 and CFSE-labelled B7-H3/E.G7 (1 : 2, B-mix) was injected into the peritoneal cavity of OT-I mice. After 24 hr, peritoneal exudate cells (PEC) were analysed by flow cytometry. The same number (5 × 103 cells) of CMTMR+ cells was acquired, and data are displayed as dotted plots with CFSE (x-axis) and CMTMR (y-axis) intensity. Representative profiles from two independent experiments are shown. Values in the profiles are the mean positive percentages ± SD from each group of three mice. In the right panel, the values shown are the CFSE : CMTMR ratios of the isolated PEC after the injection of A and B mix (the mean ± SD).
To investigate whether CD8+ T cells selectively lyse tumour cells that express B7-H3, different fluorochrome-labelled parental E.G7 and/or B7-H3/E.G7 cell combinations were injected into the peritoneal cavity of OT-I mice, and PEC were analysed after 24 hr by flow cytometry. In the mix of CMTMR-labelled E.G7 and CFSE-labelled E.G7 (1 : 2) (A-mix; i) cells, the ratio of recovered CFSE-labelled cells : CMTMR-labelled cells was approximately 2 (Fig. 2c). This was similar to the injected cell ratio, suggesting that the respective fluorochrome-labelled E.G7 cells were lysed equally. In contrast, for the mix of CMTMR-labelled E.G7 and CFSE-labelled B7-H3/E.G7 (1 : 2) (B-mix; ii), the ratio of CFSE-labelled B7-H3/E.G7 to CMTMR-labelled WT E.G7 was dramatically reduced (Fig. 2c; centre and right panels), suggesting a selective deletion of B7-H3/E.G7 cells. Similar experiments with different fluorescent protein-expressing J588L and B7-H3/J558L cells injected into syngeneic mice also showed the selective elimination of B7-H3/J558L at 14 days (data not shown). The selective elimination of the B7-H3-expressing target cells suggests preferential activation of CD8+ T cells in the interactions with CD8+ T cells and B7-H3-expressing tumour cells.
Tumour-associated B7-H3 augments antigen-specific CTL function in the effector phase
We next examined whether B7-H3 on tumour cells enhances CD8+ T-cell activation at either the induction or effector phases using two different models. B6 and OT-I mice were sensitized in vivo with P815 or B7-H3/P815 cells as alloantigen-expressing cells and E.G7 or B7-H3/E.G7 cells as OVA-peptide-expressing cells, respectively, and then cytotoxicity against parental tumour cells was analysed. The in vivo sensitization with either alloantigen or OVA antigen by B7-H3-expressing tumour cells did not affect the induced cytotoxicity (Fig. 3a). These results suggest that B7-H3 expressed on tumour cells did not enhance antigen-specific priming of CD8+ T cells in the induction phase. Next, similar mice were sensitized with parental tumour cells, and cytotoxicity against parental versus B7-H3-transfected tumour cells was compared. In both systems, considerably higher cytotoxicity was elicited against respective B7-H3-transfected tumour cells (Fig. 3b), suggesting that B7-H3 on tumour cells augments the cytolytic effector function of antigen-specific CD8+ T cells in vivo during the effector phase.
Figure 3.

Enhanced cytotoxic T lymphocytes (CTL) against alloantigen and ovalbumin (OVA) by B7-H3-expressing target cells. (a) B6 (left panel) and OT-I (right panel) mice were sensitized in vivo with allogeneic tumour cells (P815 or B7-H3/P815) and OVA-expressing tumour cells (E.G7 or B7-H3/E.G7), respectively. After the sensitization, cytotoxicity against respective parental tumour cells was measured using peritoneal exudate cells as effector cells. (b) B6 (left panel) and OT-I (right panel) mice were sensitized in vivo with P815 and E.G7 cells, respectively. After the sensitization, cytotoxicity against respective parental and B7-H3-transfected tumour cells was measured. Values shown are the means ± SD. The data are representative of three independent experiments.
B7-H3 transduction into tumour cells reduces tumorigenicity
We obtained five types of in vivo transplantable tumour cells including mastocytoma (P815), T lymphoma (EL4), plasmacytoma (J558L), squamous cell carcinoma (SCCVII) and melanoma (B16) to investigate the effects of B7-H3 transduction on anti-tumour immunity. All tumour cells expressed endogenous cell surface B7-H3, although the levels were low (Fig. S1). Four tumours, but not the B16 melanoma, expressed substantial levels of MHC class I, but none of the tumours expressed endogenous CD80 or CD86. P815 and J558L cells expressed CD54. We established respective B7-H3 transfectants that stably expressed B7-H3 at high levels. B7-H3 transduction did not affect other cell-surface expression including MHC class I, CD54, CD80 and CD86 (Fig. S1). All B7-H3-transduced tumour cell lines showed comparable growth in culture and the addition of anti-B7-H3 mAb did not clearly affect their growth (data not shown). Five B7-H3-transduced tumours and their respective parental tumours were injected subcutaneously into syngeneic mice, and tumour growth was monitored to examine tumorigenicity. All of the parental tumours grew progressively, whereas the growth of B7-H3-transduced tumours was efficiently inhibited (Fig. 4). The inoculation of parental or B7-H3-transduced P815 cells into immunodeficient BALB/c nude mice showed a comparable growth curve (Fig. 4f), suggesting T-cell-dependent action in the rejection of B7-H3/P815 tumours. These results indicate that B7-H3 transduction into tumours markedly reduced tumorigenicity.
Figure 4.

Reduced tumorigenicity by B7-H3 transduction. Parental (white circles) and B7-H3-transduced (black circles) tumour cells (a, P815; b, EL4; c, J558L; d, SCCVII; e, B16) were subcutaneously injected into syngeneic mice and tumour volume was monitored. In (f ), parental and B7-H3-transduced P815 tumour cells were injected into BALB/c nude mice as described above. The mean tumour volume ± SD (mm3) was determined in each group of five or six mice, and the data are representative of two independent experiments. The final tumour rejected ratios are shown in brackets.
Requirements for B7-H3+ tumour-induced anti-tumour immunity
To examine the requirements of CD8+ and CD4+ T cells for tumour-associated B7-H3-induced anti-tumour immunity, we pre-treated with anti-CD4, anti-CD8 mAb, or a mixture of both mAbs to deplete CD4+, CD8+, or both T cells, and then B7-H3/SCCVII cells were inoculated. Depletion of either CD4+ or CD8+ T cells slightly enhanced mean tumour volume and four out of five mice failed to reject the tumours from CD4-depleted mice, whereas all of the mice failed to reject the tumours from CD8-depleted mice (Fig. 5a). The depletion of both CD4+ and CD8+ T cells dramatically promoted tumour growth, resulting in a reversal of the B7-H3 transduction effects. These results suggest that both CD4+ and CD8+ T cells are required, and that CD8+ T cells alone are insufficient for eradicating B7-H3/SCCVII tumours.
Figure 5.
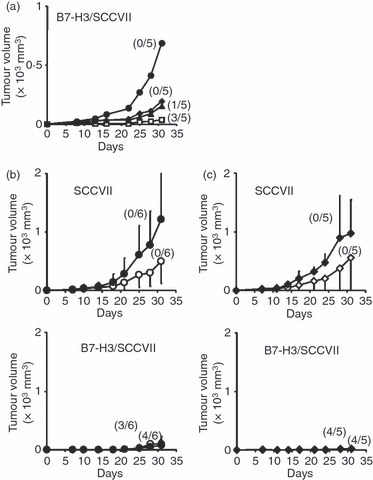
Requirements for B7-H3-induced anti-tumour immunity (a) Mice were pre-treated with control rat immunoglobulin G (IgG; white squares), anti-CD4 (black triangles), anti-CD8 (black diamonds), or both anti-CD4 and anti-CD8 (black circles) monoclonal antibodies (mAbs), and then B7-H3/SCCVII tumour cells were inoculated and tumour volume was monitored. The mean tumour volume ± SD (mm3) was determined. The final tumour rejected ratios are shown in brackets. The data shown are representative of two independent experiments. (b) and (c) SCCVII or B7-H3/SCCVII tumour-inoculated mice received intraperitoneal injections of control rat IgG (white circles) or anti-B7-H3 mAb (black circles) (in b), and control rat IgM (white diamonds) or anti-TLT-2 mAb (black diamonds) (in c). The tumour volume was monitored. The mean tumour volume ± SD (mm3) was determined in each group of 11 mice from two independent experiments.
We have recently reported that TLT-2 is a counter-receptor for B7-H3.28 Despite the binding inhibition in the B7-H3 and TLT-2 interaction assessed by flow cytometry, the addition of anti-B7-H3 (MIH35) and anti-TLT-2 (MIH49) mAbs could not reverse the enhanced T-cell responses induced by B7-H3 transduction and TLT-2 transduction.28 However, treatment with anti-TLT-2 (MIH49) mAb as well as anti-B7-H3 mAb at both sensitization and challenge of hapten-induced contact hypersensitivity efficiently inhibits ear swelling.28 We therefore examined the effects of anti-B7-H3 (MIH35) or anti-TLT-2 (MIH49) mAb treatment on the growth of parental and B7-H3-transduced SCCVII tumours. Treatment with anti-B7-H3 mAb significantly enhanced (P = 0·0005) tumour growth of parental SCCVII, but similar treatment with anti-B7-H3 mAb did not alter the reduced tumour growth induced by B7-H3 transduction (Fig. 5b). Similar to treatment with anti-B7-H3 mAb, anti-TLT-2 mAb treatment also enhanced SCCVII tumour growth (Fig. 5c), suggesting the involvement of the B7-H3–TLT-2 pathway in parental SCCVII tumour-mediated immunity. Treatment with anti-TLT-2 mAb in B7-H3/SCCVII-inoculated mice did not reverse the eradication of tumour induced by B7-H3 transduction. It is unlikely that the administration of anti-TLT-2 mAb depleted the TLT-2-expressing target cells, because no differences were observed in the ratios of CD8+ and CD4+ T cells, CD45RB+ B cells, CD11b+ macrophages and CD11c+ dendritic cells (data not shown).
Similar results were obtained by the treatment with either anti-B7-H3 or anti-TLT-2 mAb in B7-H3/EL-4 or B7-H3/P815 tumour cell inoculation (data not shown).
TLT-2 expression in tumour-infiltrating CD8+ T cells and its function
If the B7-H3–TLT-2 pathway is involved in anti-tumour immunity, T cells in tumour-bearing mice should express the TLT-2 counterpart receptor. We examined TLT-2 expression on T cells in regional lymph nodes (RLNs) and TIL 7 days after either parental SCCVII or B7-H3/SCCVII tumour inoculation. In intact LNs, TLT-2 was preferentially expressed on CD8+ T cells, but not on CD4+ T cells, and the expression levels on CD8+ T cells were not changed in the RLNs of both types of tumour-inoculated mice (Fig. 6a, left panels). Histological analyses of the tumour-inoculated tissues showed more abundant lymphocyte infiltration in the periphery of and inside the B7-H3/SCCVII tumour mass compared with the parental SCCVII-inoculated tissues (Fig. 7). In flow cytometric analyses, TIL from parental SCCVII-inoculated sites consistently expressed TLT-2. Surprisingly, in the TIL from B7-H3/SCCVII tumour-inoculated sites, only a sub-population of CD8+ TIL expressed TLT-2, and the residual population did not express TLT-2. TLT-2− CD8+ TIL revealed a larger cell size, as assessed by forward scatter on flow cytometry, than TLT-2+ CD8+ TIL (data not shown). To investigate whether the down-regulation of TLT-2 was induced after activation, the levels of TLT-2 expression in CD8+ T cells stimulated with B7-H3+ tumour cells were compared between CD69+, CD69–, CD25+ and CD25– fractions. TLT-2 expression in the CD69+ CD8+ TIL fraction was lower than that in the CD69– fraction (Fig. 6b), In addition, OT-I CD8+ T cells cultured with B7-H3/E.G7 tumour cells showed that TLT-2 expression was lower in both CD69+ and CD25+ fractions compared with CD69− and CD25− fractions (Fig. 6b). These results indicated that TLT-2 expression was down-regulated after activation. We further investigated cytokines that affect TLT-2 expression. Although IL-2, IFN-γ, TNF-α and IL-10 did not clearly affect TLT-2 expression on CD8+ T cells stimulated with anti-CD3 mAb, the addition of TGF-β markedly decreased the TLT-2 expression (Fig. 6c).
Figure 6.

Modulation of TLT-2 expression on CD8+ T cells and enhanced cytotoxicity by TLT-2. (a) Lymph node cells from naive mice and regional lymph node cells and tumour-infiltrating lymphocytes (TILs) were obtained from SCCVII or B7-H3/SCCVII tumour-inoculated mice. Cells were stained with fluorescein isothiocyanate-conjugated (FITC-) anti-CD45, peridinin chlorophyll protein Cychrome 5.5-conjugated (PerCP-Cy5.5-) anti-CD3, and either phycoerythrin-conjugated (PE-) anti-CD4 or anti-CD8 mAbs, and biotinylated anti-TLT-2 mAb, followed by allophycocyanin (APC) -streptavidin or with the appropriate fluorochrome-conjugated control immunoglobulins and were then analysed by flow cytometry. An electronic gate was placed on CD45+ CD3+ CD8+ lymphocytes and TLT-2 expression is displayed as histograms with the control histograms nearest the ordinate (shaded). The data are representative of two independent experiments. (b) TILs from B7-H3/SCCVII-inoculated mice were stained with FITC-anti-CD45, PerCP-Cy5.5-anti-CD8, PE-anti-CD69 mAbs and biotinylated anti-TLT-2 mAb, followed by APC-streptavidin. CD8+ T cells from naive OT-I mice were labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and co-cultured with E.G7 or B7-H3/E.G7 cells for 24 hr. The harvested cells were stained with either PE-anti-CD69 or anti-CD25, and PerCP-Cy5.5-anti-CD8 mAbs and biotinylated anti-TLT-2 mAb, followed by APC-streptavidin. The cells were analysed by flow cytometry. An electronic gate was first placed on CFSE+ CD8+ lymphocytes and then the secondary gates were set on CD69- or CD25-positive and negative cells. TLT-2 expression is displayed as histograms with the control histograms nearest the ordinate (shaded). The values of upper right are the mean fluorescence intensity (MFI) of TLT-2 stained cells. The data are representative of two independent experiments. (c) CD8+ T cells from naive B6 mice were stimulated with immobilized anti-CD3 mAb in the presence of the indicated cytokines for 3 days. The cells were harvested and stained with FITC-anti-CD8, PerCP-Cy5.5-anti-CD3 mAbs and biotinylated anti-TLT-2 mAb, followed by APC-streptavidin and analysed by flow cytometry. The data are representative of two independent experiments. The values of upper right are the MFI of TLT-2 stained cells. (d) Mock- or TLT-2-transduced OT-I CD8+ T cells were stained with biotinylated anti-TLT-2 mAb, followed by PE-streptavidin, and analysed by flow cytometry. The data are displayed as histograms with the control histograms nearest the ordinate (shaded). Mock-transduced (white circles) or TLT-2-transduced (black circles) OT-I CD8+ T cells were used as effector cells. Cytotoxicity against E.G7 or B7-H3/E.G7 cells was measured. Values shown are the means ± SD. The data are representative of two independent experiments.
Figure 7.
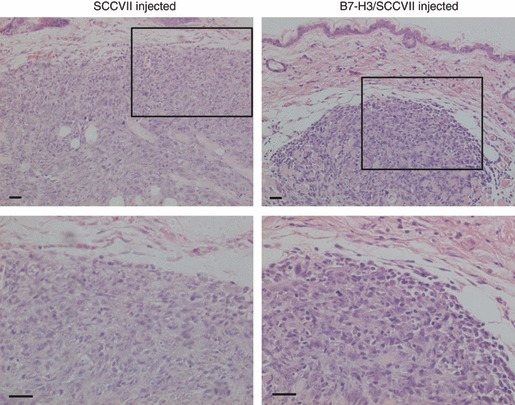
Abundant tumour-infiltrating lymphocytes (TIL) in B7-H3/SCCVII-inoculated sites. The abdominal skin of SCCVII or B7-H3/SCCVII tumour (1 × 105 cells) inoculated sites was surgically resected after 7 days. The formalin-fixed, paraffin-embedded tissue sections were stained with haematoxylin & eosin. Representative images of the tumour mass near the epithelium from three individuals are shown. Lower images show the selected field of the upper images at higher magnification. Scale bars = 100 μm.
Finally, we examined whether TLT-2 over-expressed on CD8+ T cells directly enhanced antigen-specific cytotoxicity against B7-H3-transduced tumour cells. TLT-2 was retrovirally transduced into OT-I CD8+ T cells and cytotoxicity against parental E.G7 or B7-H3/E.G7 was measured. The mean fluorescence intensity of TLT-2/GFP-transduced OT-I CD8+ T cells was sixfold higher than that of mock/GFP-transfected cells (Fig. 6d). The transduction of TLT-2 did not alter the activation status assessed by cell size and proliferation and IFN-γ production stimulated with anti-CD3 or phorbol 12-myristate 13-acetate plus ionomycin (data not shown). TLT-2-transduced OT-I CD8+ T cells showed higher cytotoxicity against both E.G7 and B7-H3/E.G7 than the mock-transduced OT-I CD8+ T cells. B7-H3 over-expression on tumours did not dramatically enhance cytotoxicity when there was sufficient TLT-2 expression on OT-I CD8+ T cells. These results suggest that TLT-2, which is expressed on CD8+ T cells, enhanced antigen-specific cytotoxicity by direct interaction with B7-H3 on tumour cells.
Discussion
We demonstrated that CD8+ T cells showed higher antigen-specific cytotoxicity against B7-H3-transduced tumour cells in vitro, and that B7-H3-transduced tumour cells were preferentially eliminated in vivo. The presence of B7-H3 on tumours during antigen sensitization did not enhance the induced cytotoxicity against alloantigen and OVA, whereas the presence of B7-H3 on target tumour cells did efficiently enhance the cytotoxicity. Transduction of B7-H3 into five different types of tumours markedly reduced their tumorigenicity, and the inoculated tumours were largely eradicated. Administration of either anti-B7-H3 or anti-TLT-2 mAb accelerated parental tumour growth, but not growth of B7-H3-transduced tumours. The RLN CD8+ T cells from tumour-bearing mice expressed substantial levels of TLT-2, but a considerable proportion of CD8+ T cells within TIL lost TLT-2 expression. Finally, TLT-2-transduced OT-I CD8+ T cells displayed greater cytotoxicity against both parental and B7-H3-transduced tumour cells.
Because B7-H3 expression is ubiquitous,1,42 all tumour cell lines examined expressed endogenous B7-H3 at low-to-moderate levels. We transduced B7-H3 into such tumour cells and obtained the B7-H3 transfectants that expressed at least a 20-fold higher level of B7-H3 than parental cells, as assessed by fluorescence intensity. Our results indicate that B7-H3 over-expression in tumours reduces tumorigenicity by enhancing immunogenicity, and the presence of B7-H3 on tumour cells up-regulated the cytolytic ability of already antigen-primed CD8+ T cells. Tumour-associated B7-H3 was unlikely to be involved in an initial antigen-priming phase of CD8+ T-cell responses. A similar observation has been reported using B7-H3-transfected P815 cells and adoptive transfer in a P1A-specific CTL model system.25 B7-H3 expression on P815 tumour cells enhanced CD8-mediated tumour immunity by amplifying local expansion of tumour-specific CTL in the absence of professional antigen-presenting cells. Unfortunately, the P815 cells used in our study lacked a P1A tumour antigen so we used OVA-specific TCR-transgenic CD8+ (OT-I CD8+) T cells and an OVA-expressing tumour (E.G7) cell system to assess antigen-specific CTL responses. Another report also demonstrated enhanced tumour immunity by B7-H3 introduction into Colon 26 colon carcinoma cells.26 IFN-γ production from splenic CD8+ T cells of tumour-bearing mice was enhanced by co-culture with B7-H3+ tumour cells. In both reports, B7-H3-introduced tumours were not completely rejected in all individuals and some mice developed large tumours and died. Our results also showed a failure of complete tumour rejection. Although we have not observed this in parallel studies, it seems that the effects of introducing B7-H3 is not as strong as those of CD80, CD86, 4-1BBL or GITRL both in vitro and in vivo.35,36,40,43–45 We also examined tumour vaccine effects of B7-H3-transduced tumours following several injections of B7-H3/SCCVII after pre-inoculation of live parental tumours; however, there was no effect on tumour growth (data not shown). These observations are consistent with a previous report on B7-H3/P815 tumour vaccine effects.25 It is likely that the reason for the limited effect of B7-H3-transduced tumour cells was the few or no enhancing effects of B7-H3 during the priming phase. The de novo induction of regulatory co-stimulatory ligands like B7-H1 and B7-H4 in tumour cells and others may override the effects of B7-H3-mediated anti-tumour immunity.22
The major reason for dominant involvement of CD8+ T cells in B7-H3-enhanced immunity could be the result of counter-receptor expression. In the steady state, TLT-2 is clearly expressed on splenic CD8+ T cells, whereas TLT-2 on CD4+ T cells is either weak or null (Fig. S2 and ref. 28). Nevertheless, we observed preferentially higher anti-CD3 mAb-induced re-directed cytotoxicity of CD4+ T cells against both parental P815 and B7-H3/P815 cells (Fig. 1). We have previously shown that the anti-CD3 mAb-induced re-directed cytotoxicity was greatly dependent on the Fas–Fas ligand pathway.33 In fact, the re-directed cytotoxicity of CD4+ T cells against P815 and B7-H3/P815 cells was efficiently inhibited by blocking anti-Fas ligand mAb (data not shown). CD4+ T cells rapidly increased TLT-2 expression by anti-CD3 mAb stimulation alone (Fig. S2) and the interaction of TLT-2 with B7-H3 on P815 cells preferentially up-regulated Fas ligand expression on the CD4+ T cells, resulting in higher re-directed cytotoxicity. We consistently observed constitutive expression of TLT-2 in LN CD8+ T cells from naive mice, and its expression was comparable in RLN CD8+ T cells from tumour-bearing mice. Here, we examined TLT-2 expression in TIL for the first time. We found a marked lymphocyte infiltration within the B7-H3/SCCVII tumour mass, indicating active anti-tumour immune responses in the B7-H3+ tumour sites. Surprisingly, the majority of CD8+ TIL in the B7-H3/SCCVII-inoculated mice lost TLT-2 expression, and the cells expressing activation marker down-regulated TLT-2 expression. These findings suggest that activation signals to CD8+ T cells induce down-regulation of TLT-2. Although we tried to detect TLT-2 expression by immunofluorescence histostaining, TLT-2 expression was undetectable so we could not examine the distribution of TLT-2+ versus TLT-2− CD8 TIL in the tissues. We also found that TGF-β, which is often secreted from solid tumour cells like squamous cell carcinomas or tumour-associated cells, down-regulated TLT-2 expression. It is therefore possible that some tumour-related environmental factor(s) may have caused TLT-2 down-regulation. TLT-2 down-regulation occurred at the local tumour sites and this may have contributed to the limited efficacy of B7-H3-transduced tumours. Our results from the TLT-2-transduced CD8+ T-cell study suggest that the TLT-2 expression level is more critical than that of B7-H3 to deciding whether there is a contribution of the B7-H3–TLT-2 pathway. Over-expression of B7-H3 is no longer required when sufficient TLT-2 expression is provided on the surface of CD8+ T cells (Fig. 6d). In contrast to broad and abundant B7-H3 expression, TLT-2 expression levels in T cells are tightly regulated. Additional approaches for preventing TLT-2 down-regulation or enhancing TLT-2 expression at tumour sites may be needed.
We performed experiments to block the B7-H3–TLT-2 pathway, using anti-B7-H3 and anti-TLT-2 mAbs, to confirm the functional contribution of B7-H3 and TLT-2 in B7-H3-introduced tumour-mediated immunity. Unfortunately, there was no effect on the tumour regression induced by B7-H3-introduced tumours that expressed high levels of B7-H3. Interestingly, growth of the parental tumour, which expressed endogenously low levels of B7-H3, was accelerated by treatment with either anti-B7-H3 or anti-TLT-2 mAb. This suggests the immunoenhancing effects of the B7-H3–TLT-2 pathway in tumour immunity against parental tumours. We have previously attempted and failed to reverse the enhanced responses induced by B7-H3- or TLT-2-transduced cells using the same anti-B7-H3 and TLT-2 mAbs in vitro, although these mAbs could inhibit B7-H3 immunoglobulin binding, assessed by flow cytometry, and the functional endogenous TLT-2 and B7-H3 interaction in contact hypersensitivity in vivo.28 The low affinity of our blocking mAbs may explain the failure. The mAbs may not be able to completely neutralize the high levels of B7-H3 and TLT-2 that are introduced on transfectants. Another explanation is the presence of soluble forms of B7-H3 and TLT-2. Indeed, secretion of a soluble form of human B7-H3 has been reported in patients with cancer16 and we have also observed a soluble form of TLT-2 in culture supernatants of TLT-2-transduced cells (M.H., unpublished observation). Excess molecule expression in the transduced cells may produce a soluble form and neutralize the mAb action. Additionally, the presence of an opposite function from an unknown B7-H3 receptor may have neutralized the co-stimulatory action of the B7-H3–TLT-2 pathway. Unfortunately, we could not induce agonistic signals by ligation of TLT-2 using immobilized anti-TLT-2 mAbs. This causes further difficulty for the direct analyses of TLT-2 function in T cells. Further studies are needed to clarify the direct interaction of TLT-2 with B7-H3 in T-cell responses.
Most reports describing the role of B7-H3 in humans suggest regulatory roles for tumour-associated B7-H3,18,19,21,22 and all murine tumour B7-H3-transduction experiments, including our study, demonstrate positive co-stimulatory functions for tumour-associated B7-H3.24–27 However, a number of mouse studies using various assay systems in vitro and disease models in vivo still support the regulatory role of B7-H3.13–15,46,47 The discrepancy in B7-H3 function is not simply explained by the different forms of B7-H3 found in humans and mice. Further studies to clarify the real function(s) of B7-H3 will be required before developing cancer immunotherapy targeting B7-H3.
Acknowledgments
We thank T. Kitamura (University of Tokyo, Tokyo, Japan) for kindly providing the retrovirus vector and the packaging cell line Plat-E, Dr W. R. Heath for OT-I mice, and A. Yoshino and S. Miyakoshi for cell sorting. This study was supported by a Grant-In-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to M.A.) and grants from the Japan Society for the Promotion of Science (to M.H. and M.A.).
Disclosures
The authors declare no conflict of interests.
Supporting Information
Additional Supporting information may be found in the online version of this article:
Figure S1. Expression of cell surface antigenson parental and B7-H3-transduced tumor cell lines. B7-H3-transducedtumor cells were generated as described in the Materials andMethods. Parental and B7-H3-transduced P815, EL4, J558L, SCCVII,B16 and E.G7 cells were stained with FITC-anti-B7-H3, FITC-anti-MHCclass I, PE-anti-CD54, PE-anti-CD80, and PE-anti-CD86 mAbs or withthe appropriate fluorochrome-conjugated control immunoglobulin.Data are displayed as histograms (4-decade logarithm scales) withthe control histograms nearest the ordinate (shaded).
Figure S2. Expression of TLT-2 onCD4+ and CD8+ T cells. Splenocytes fromBALB/c mice were stimulated with anti-CD3 mAb (10 μg/ml) for 6and 24 h. Freshly isolated and activated splenocytes were stainedwith PerCP-Cy5.5-anti-CD4, PE-anti-CD8, and biotinylated anti-TLT-2mAbs or with the appropriate isotype control Ig, followed byAPC-streptavidin. Data are displayed as histograms (4-decadelogarithm scales) with the control histograms nearest the ordinate(shaded). The values of lower left and upper right are theMFI of control and TLT-2-stainings, respectively.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 2.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang GB, Dong QM, Xu Y, Yu GH, Zhang XG. B7-H3: another molecule marker for Mo-DCs? Cell Mol Immunol. 2005;2:307–11. [PubMed] [Google Scholar]
- 4.Kim J, Myers AC, Chen L, et al. Constitutive and inducible expression of b7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;33:280–9. doi: 10.1165/rcmb.2004-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saatian B, Yu XY, Lane AP, Doyle T, Casolaro V, Spannhake EW. Expression of genes for B7-H3 and other T cell ligands by nasal epithelial cells during differentiation and activation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L217–25. doi: 10.1152/ajplung.00132.2003. [DOI] [PubMed] [Google Scholar]
- 6.Nagai Y, Aso H, Ogasawara H, et al. Anterior pituitary progenitor cells express costimulatory molecule 4Ig-B7-H3. J Immunol. 2008;181:6073–81. doi: 10.4049/jimmunol.181.9.6073. [DOI] [PubMed] [Google Scholar]
- 7.Waschbisch A, Wintterle S, Lochmuller H, Walter MC, Wischhusen J, Kieseier BC, Wiendl H. Human muscle cells express the costimulatory molecule B7-H3, which modulates muscle–immune interactions. Arthritis Rheum. 2008;58:3600–8. doi: 10.1002/art.23997. [DOI] [PubMed] [Google Scholar]
- 8.Chen YW, Tekle C, Fodstad O. The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets. 2008;8:404–13. doi: 10.2174/156800908785133141. [DOI] [PubMed] [Google Scholar]
- 9.Ling V, Wu PW, Spaulding V, Kieleczawa J, Luxenberg D, Carreno BM, Collins M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82:365–77. doi: 10.1016/s0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 10.Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294–7. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 11.Zhou YH, Chen YJ, Ma ZY, et al. 4IgB7-H3 is the major isoform expressed on immunocytes as well as malignant cells. Tissue Antigens. 2007;70:96–104. doi: 10.1111/j.1399-0039.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 12.Tran CN, Thacker SG, Louie DM, et al. Interactions of T cells with fibroblast-like synoviocytes: role of the B7 family costimulatory ligand B7-H3. J Immunol. 2008;180:2989–98. doi: 10.4049/jimmunol.180.5.2989. [DOI] [PubMed] [Google Scholar]
- 13.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173:2500–6. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 14.Suh WK, Gajewska BU, Okada H, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima A, Sumi T, Fukuda K, et al. B7-H3 regulates the development of experimental allergic conjunctivitis in mice. Immunol Lett. 2007;113:52–7. doi: 10.1016/j.imlet.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B, Zhang X. Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.01.017. DOI: 10.1016/j.lungcan.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Chavin G, Sheinin Y, Crispen PL, et al. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res. 2009;15:2174–80. doi: 10.1158/1078-0432.CCR-08-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth TJ, Sheinin Y, Lohse CM, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 19.Zang X, Thompson RH, Al-Ahmadie HA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104:19458–63. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castriconi R, Dondero A, Augugliaro R, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA. 2004;101:12640–5. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crispen PL, Sheinin Y, Roth TJ, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–7. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–51. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Wu CP, Jiang JT, Tan M, et al. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12:457–9. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW. Mouse B7-H3 induces antitumor immunity. Gene Ther. 2003;10:1728–34. doi: 10.1038/sj.gt.3302070. [DOI] [PubMed] [Google Scholar]
- 25.Luo L, Chapoval AI, Flies DB, et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173:5445–50. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 26.Lupu CM, Eisenbach C, Kuefner MA, Schmidt J, Lupu AD, Stremmel W, Encke J. An orthotopic colon cancer model for studying the B7-H3 antitumor effect in vivo. J Gastrointest Surg. 2006;10:635–45. doi: 10.1007/BF03239969. [DOI] [PubMed] [Google Scholar]
- 27.Lupu CM, Eisenbach C, Lupu AD, Kuefner MA, Hoyler B, Stremmel W, Encke J. Adenoviral B7-H3 therapy induces tumor specific immune responses and reduces secondary metastasis in a murine model of colon cancer. Oncol Rep. 2007;18:745–8. [PubMed] [Google Scholar]
- 28.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci USA. 2008;105:10495–500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King RG, Herrin BR, Justement LB. Trem-like transcript 2 is expressed on cells of the myeloid/granuloid and B lymphoid lineage and is up-regulated in response to inflammation. J Immunol. 2006;176:6012–21. doi: 10.4049/jimmunol.176.10.6012. [DOI] [PubMed] [Google Scholar]
- 30.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 31.Otsuki N, Kamimura Y, Hashiguchi M, Azuma M. Expression and function of the B and T lymphocyte attenuator (BTLA/CD272) on human T cells. Biochem Biophys Res Commun. 2006;344:1121–7. doi: 10.1016/j.bbrc.2006.03.242. [DOI] [PubMed] [Google Scholar]
- 32.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–14. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 33.Ebata T, Mogi S, Hata Y, Fujimoto JI, Yagita H, Okumura K, Azuma M. Rapid induction of CD95 ligand and CD4+ T cell-mediated apoptosis by CD137 (4-1BB) costimulation. Eur J Immunol. 2001;31:1410–6. doi: 10.1002/1521-4141(200105)31:5<1410::AID-IMMU1410>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Oikawa T, Kamimura Y, Akiba H, et al. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol. 2006;177:4281–7. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 35.Mogi S, Sakurai J, Kohsaka T, Enomoto S, Yagita H, Okumura K, Azuma M. Tumour rejection by gene transfer of 4-1BB ligand into a CD80+ murine squamous cell carcinoma and the requirements of co-stimulatory molecules on tumour and host cells. Immunology. 2000;101:541–7. doi: 10.1046/j.1365-2567.2000.t01-1-00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piao J, Kamimura Y, Iwai H, et al. Enhancement of T cell-mediated anti-tumor immunity via the ectopically expressed glucocorticoid-induced tumor necrosis factor receptor-related receptor ligand (GITRL) on tumors. Immunology. 2009;127:489–99. doi: 10.1111/j.1365-2567.2008.03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao TY, Chu TM. Effect of indomethacin on tumor-infiltrating lymphocytes of a spontaneously developed murine mammary adenocarcinoma. Cancer Immunol Immunother. 1989;30:158–64. doi: 10.1007/BF01669424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azuma M, Cayabyab M, Phillips JH, Lanier LL. Requirements for CD28-dependent T cell-mediated cytotoxicity. J Immunol. 1993;150:2091–101. [PubMed] [Google Scholar]
- 39.Lanier LL, O’Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 40.Nakajima A, Kodama T, Morimoto S, et al. Antitumor effect of CD40 ligand: elicitation of local and systemic antitumor responses by IL-12 and B7. J Immunol. 1998;161:1901–7. [PubMed] [Google Scholar]
- 41.Igarashi H, Cao Y, Iwai H, Piao J, Kamimura Y, Hashiguchi M, Amagasa T, Azuma M. GITR ligand-costimulation activates effector and regulatory functions of CD4+ T cells. Biochem Biophys Res Commun. 2008;369:1134–8. doi: 10.1016/j.bbrc.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Suh WK, Wang SX, Jheon AH, et al. The immune regulatory protein B7-H3 promotes osteoblast differentiation and bone mineralization. Proc Natl Acad Sci USA. 2004;101:12969–73. doi: 10.1073/pnas.0405259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 44.Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellstrom I, Hellstrom KE. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523–32. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gajewski TF. B7-1 but not B7-2 efficiently costimulates CD8+ T lymphocytes in the P815 tumor system in vitro. J Immunol. 1996;156:465–72. [PubMed] [Google Scholar]
- 46.Wang L, Fraser CC, Kikly K, Wells AD, Han R, Coyle AJ, Chen L, Hancock WW. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol. 2005;35:428–38. doi: 10.1002/eji.200425518. [DOI] [PubMed] [Google Scholar]
- 47.Nagashima O, Harada N, Usui Y, Yamazaki T, Yagita H, Okumura K, Takahashi K, Akiba H. B7-H3 contributes to the development of pathogenic Th2 cells in a murine model of asthma. J Immunol. 2008;181:4062–71. doi: 10.4049/jimmunol.181.6.4062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


