Abstract
Interleukin-7 (IL-7) is a crucial cytokine involved in T-cell survival and development but its signalling in human T cells, particularly in effector/memory T cells, is poorly documented. In this study, we found that IL-7 protects human CD4+ effector/memory T cells from apoptosis induced upon the absence of stimulation and cytokines. We show that IL-7 up-regulates not only Bcl-2 but also Bcl-xL and Mcl-1 as well. Interleukin-7-induced activation of the janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway is sufficient for cell survival and up-regulation of Bcl-2 proteins. In contrast to previous studies with naive T cells, we found that IL-7 is a weak activator of the phosphatidylinositol 3 kinase (PI3K)/AKT (also referred as protein kinase B) pathway and IL-7-mediated cell survival occurs independently from the PI3K/AKT pathway as well as from activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway. Considering the contribution of both IL-7 and CD4+ effector/memory T cells to the pathogenesis of autoimmune diseases such as rheumatoid arthritis and colitis, our study suggests that IL-7 can contribute to these diseases by promoting cell survival. A further understanding of the mechanisms of IL-7 signalling in effector/memory T cells associated with autoimmune inflammatory diseases may lead to potential new therapeutic avenues.
Keywords: Bcl-2, effector/memory T cells, interleukin-7, Janus kinase/signal transducer and activator of transcription, survival
Introduction
Apoptosis is a tightly regulated process that plays an important role in T-cell homeostasis and immune response. During activation, T cells undergo apoptosis triggered via T-cell receptor-mediated activation of the Fas/Fas ligand death pathway.1–3 T cells can also be eliminated through a Fas-independent pathway in response to lack of stimulation or absence of cytokines.2,3 This form of apoptosis, known as passive death or cytokine deprivation-induced apoptosis, occurs both in naive and memory T cells,4,5 and involves the deregulation of members of the Bcl-2 family and activation of the mitochondrial death pathway.2,6,7 Activation of pro-apoptotic Bcl-2 proteins and inactivation of Bcl-2 pro-survival proteins lead to mitochondrial damage, release of apoptogenic factors among which is cytochrome c, activation of caspase-9 and executioner caspases such as caspase-3 and subsequently to cell death. Protection of T cells from this form of apoptosis is believed to be essential for a productive immune response, generation of T-cell memory and persistence of inflammation.4,5
The survival of T cells at different stages of development is believed to be dependent on extrinsic signals delivered by cytokines. Interleukin-7 (IL-7), a member of the IL-2 γ-chain cytokines, has emerged as a crucial T-cell survival factor.8–10 Interleukin-7 is produced by stromal cells, and it is necessary for the development and maturation of lymphocytes in the thymus and their maintenance in the periphery.11,12 It is also necessary for the development of memory T cells,13–15 although some recent reports suggested that the expression levels of IL-7 receptor (IL-7R) may not necessarily identify memory T-cell precursors or that they are insufficient for the formation of memory T cells.16,17.
Several studies conducted with murine models using naive T cells and IL-7-dependent cell lines have associated IL-7 signalling with up-regulation of Bcl-2 pro-survival proteins. The up-regulation of Bcl-2 pro-survival proteins is thought to counteract the activation of the pro-apoptotic Bcl-2 proteins such as Bim and Bax, which are both essential for cytokine deprivation-induced apoptosis.18–21 Cells that express low levels of IL-7R were shown to be susceptible to Bim-induced apoptosis in vivo, whereas those expressing higher levels of IL-7R are protected,22,23 and Bax deficiency partially compensates IL-7R deficiency.9 Furthermore, IL-7 has also been shown to activate cell survival signalling pathways including the Janus kinase/signal transducer and activator of transcription (JAK/STAT) and the phosphatidylinositol 3-kinase (PI3K)/AKT (also referred as protein kinase B) pathways.24
In contrast to mouse T cells, IL-7 signalling is still poorly documented in human T cells, particularly in effector/memory T cells. Therefore, we investigated this issue by examining the mechanisms of IL-7 signalling in the survival of human CD4+ effector/memory T cells. We found that IL-7 protects human CD4+ effector/memory T cells from apoptosis induced upon the absence of stimulation and cytokines and up-regulates not only Bcl-2 but also Bcl-xL and Mcl-1 as well. We further show that IL-7 activates only the JAK/STAT5 pathway, which is sufficient for IL-7-induced cell survival and up-regulation of Bcl-2 proteins. These studies bring additional insights into the mechanisms of IL-7 signalling, which could be important in understanding T-cell homeostasis and persistence during inflammation.
Materials and methods
Antibodies and reagents
Interleukin-7 and IL-2 were purchased from R&D Systems (Minneapolis, MN) and Sigma (St Louis, MO), respectively. The Pan-JAK inhibitor (JAK-Inhibitor I), the mitogen-activated protein kinase kinase 1/2 (MEK 1/2) inhibitor U0126 and the PI3K/AKT inhibitor LY294002 were purchased from Calbiochem (San Diego, CA). Anti-CD3/CD28 Dynabeads were from Invitrogen Dynal AS (Oslo, Norway). Anti-CD3 (OKT3), anti-CD28 (CD28·2), anti-CD127 (hIL-7R-M21), anti-CD45RO (UCLH1), isotype control antibodies and phycoerythrin (PE)-conjugated anti-Bcl-2 and its PE-conjugated isotypic control antibodies were from BD Biosciences (San Diego, CA). Alexa-fluor 488-conjugated anti-mouse antibody was from Invitrogen (Carlsbad, CA). Antibodies against phospho-STAT5 (Tyr-694), Bcl-xL, phospho-AKT (Ser-473), and AKT were from Cell Signaling Technologies (Beverly, MA); and antibodies against Caspase-3, Mcl-1, Bcl-2, phospho-p44/42 mitogen-activated protein kinase (MAPK; E-4), extracellular signal-regulated kinase (ERK2; C-14) and actin (C-2) were from Santa Cruz Biotechnology (Santa Cruz, CA).
Isolation of primary T cells and generation of effector/memory T cells
Peripheral blood mononuclear cells were isolated from healthy adult volunteers on Ficoll gradients. The participating volunteers signed a consent form and the study was approved by the ethical committee of Laval University. Primary human naive CD4+ T cells were then purified by negative selection using magnetic beads from StemCell Technologies (Vancouver BC, Canada) according to the manufacturer’s instructions. Staining with anti-CD3 and anti-CD4 monoclonal antibodies (mAbs) and fluorescence-activated cell sorting (FACS) flow cytometry analysis indicated that more than 97% of the isolated cells were CD3/CD4 double-positive T cells. To generate CD4+ effector/memory T cells, freshly isolated T cells were activated with anti-CD3/CD28 coated beads for 24 hr in complete RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mm/l glutamine and 100 units/ml penicillin and streptomycin. The cells were then washed, transferred to flasks and cultured for a further 7 days in the presence of 50 U/ml recombinant IL-2, which was added every 2 days. Because IL-2 can down-regulate the expression of IL-7R on activated/effector T cells 25 and to avoid any influence of IL-2 on IL-7 signalling, the cells at day 7 were then cultured for 1 day in fresh medium in the absence of IL-2 before being used in subsequent experiments. More than 95% of these cells express CD45RO, the marker of memory T cells, and will be referred to thereafter as effector/memory T cells. This model is suitable for cell signalling studies and has been previously used by different groups and by us.26–28 Upon T-cell receptor restimulation, these cells produce interferon-γ, IL-2 and the osteoclastogenic cytokine receptor activator of nuclear factor-kappa B ligand (RANKL).28
Cell surface molecule expression
The expression of cell surface receptors was determined by immunostaining and flow cytometry analysis. The cells were incubated on ice with 10 μg/ml anti-CD127, anti-CD45RO or with control isotypic mAbs for 45 min in phosphate-buffered saline (PBS) containing 0·1% fetal bovine serum. The cells were washed and incubated with Alexa-fluor-conjugated anti-mouse antibody for another 45 min. Cells were then washed in PBS and analysed by flow cytometry using FACSCalibur instrument (BD Biosciences).
Apoptosis assays
Apoptosis was determined using the Annexin V-PE/7-aminoactinomycin D (7AAD) detection kit from BD Biosciences as previously described.29 After stimulation, the cells were washed in cold PBS and 105 cells were incubated in 1 × buffer containing 2·5 μl Annexin V-PE and 2·5 μl 7-AAD for 15 min at room temperature in the dark. The cells were then washed and analysed by flow cytometry using a FACSCalibur instrument (BD Biosciences) and apoptotic cells were identified as being Annexin V-positive.
Immunoblot analysis
After stimulation, the cells were harvested, washed in cold PBS and cell lysates were prepared in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors as previously described.29 Cell lysates were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and analysed by immunoblot using specific antibodies. The blots were stripped and re-probed with control anti-actin mAb to ensure equal loading. Activation of caspase-3 was determined by immunoblot analysis using specific anti-caspase-3 mAb that recognizes both the inactive pro-caspase form (p32) and the cleaved active form (p17). Activation of STAT5, ERK1/2 and AKT was determined by immunoblot analysis using specific antibodies recognizing the phosphorylated forms of STAT5, ERK1/2 and AKT. The expression levels of Mcl-1, Bcl-xL and Bcl-2 were detected by immunoblot analysis using specific antibodies. In all experiments, immunoblots were visualized using a horseradish peroxidase-conjugated secondary antibody followed by enhanced chemiluminescence detection (Pierce, Rockford, IL).
Intracellular Bcl-2 staining
T cells were permeabilized for 30 min using the CytoFix/CytoPerm kit (BD Biosciences), washed and stained with PE-conjugated anti-Bcl-2 antibody or with its isotypic control antibody for 30 min at room temperature. The cells were washed and analysed by flow cytometry using a FACSCalibur instrument (BD Biosciences).
Statistical analysis
Statistical analysis was performed using paired Student’s t-test. Results with P < 0·05 were considered significant.
Results
Expression of CD45RO and IL-7R on effector/memory T cells
We used human effector/memory T cells that were generated by activating naive T cells with anti-CD3 + anti-CD28 followed by expanding them in IL-2 for 7 days. The cells were then rested for 1 day in complete medium without IL-2 before being used in subsequent experiments. In agreement with previous studies using a similar cell model,26,30 we found that 98% of the generated effector/memory T cells expressed CD45RO (Fig. 1a). We then determined the expression levels of IL-7R on human effector/memory T cells. As shown in Fig. 1(b), effector/memory T cells at day 8 expressed high levels of IL-7R, as determined by immunostaining of the cells for the α-chain (CD127) of the IL-7R. Expression of IL-7R is known to be down-modulated by IL-7 and other γ-chain survival cytokines in peripheral naive T cells.31,32 Therefore, we looked into the regulation of CD127 expression on the cell surface of human effector/memory T cells and found that IL-7 treatment for 24 hr also down-regulated the levels of CD127 on these cells (Fig. 1c).
Figure 1.
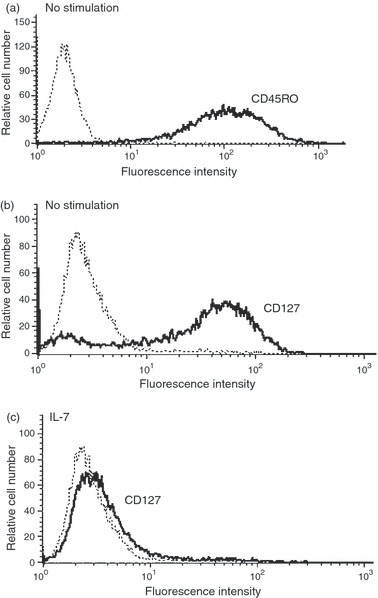
Human effector/memory T cells express CD45RO and the interleukin-7 receptor (IL-7R). The expression of IL-7R and CD45RO on the cell surface was determined by fluorescence-activated cell sorting analysis as described in the Materials and methods. (a) CD45RO expression on effector/memory T cells. (b) Expression of IL-7R on effector/memory T cells without stimulation and (c) upon stimulation with 2 ng/ml of IL-7 for 24 hr. Bold histograms (−) represent IL-7R or CD45RO staining and dashed histograms (…) represent cell staining with matched-control isotypic antibodies. The results are representative of five independent experiments performed with T cells from different blood donors.
IL-7 protects effector/memory T cells from cytokine deprivation-induced apoptosis
Interleukin-7 is known to protect naive T cells from cytokine (IL-2) deprivation-induced apoptosis Therefore, we examined whether IL-7 can rescue effector/memory T cells subjected to lack of stimulation. To this end, the in vitro-generated effector/memory T cells at day 8, which exhibited a low rate of apoptosis (Fig. 2a; 0 hr time-point) were cultured in fresh medium under deprived conditions (without CD3/CD28 stimulation and in the absence of cytokines). Cytokine-deprived cells underwent significant apoptosis as 40% of the total cells became Annexin-V-positive within 24 hr of deprivation. The apoptotic cell population reached 55% and 65% after 48 and 72 hr of culture in deprived conditions (Fig. 2a). The observed apoptosis of cytokine-deprived effector/memory T cells was associated with significant activation of caspase-3, as shown by the reduction of pro-caspase-3 levels and the appearance of the p17 active form of caspase-3 (Fig. 2b). The addition of IL-7 resulted in approximately 50–60% reduction in apoptosis of effector/memory T cells (Fig. 2a), which was accompanied by a remarkable decrease of the p17 fragment indicative of reduced activation of caspase-3 (Fig. 2b). As a control, cells cultured in the presence of IL-2 were also protected from apoptosis and the protective effect of IL-7 was comparable to that of IL-2 (Fig. 2a). The use of the pan-caspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]- fluoromethylketone (zVAD-FMK) strongly protected effector/memory T cells from apoptosis induced in the absence of cytokines (data not shown), indicating the implication of caspases. These results indicate that human effector/memory T cells undergo apoptosis when cultured in the absence of stimulation and are protected by IL-7, which inhibits activation of caspase-3.
Figure 2.
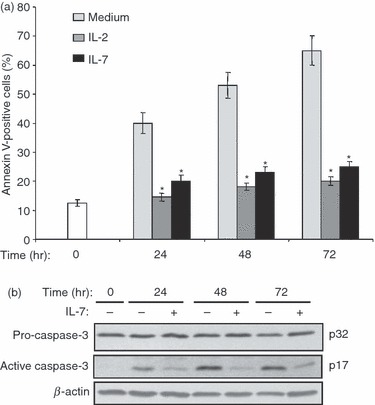
Interleukin-7 (IL-7) protects human effector/memory T cells from apoptosis and inhibits caspase-3 activation. (a) Cells at day 8 (0 hr time-point) were cultured under deprived conditions in the presence or absence of IL-7 (2 ng/ml) or IL-2 (50 U/ml) for different periods of time. Apoptosis was determined using Annexin V/7-aminoactinomycin D staining and flow cytometry analysis. The results are presented as mean percentages (± SE) of Annexin-V-positive cells from three independent experiments performed in triplicate with T cells isolated from different blood donors. *P < 0·05 between IL-7-treated or IL-2-treated samples and non-treated (medium) samples. (b) IL-7 inhibits caspase-3 activation. Effector/memory T cells were cultured under deprived conditions in the presence or absence of IL-7 for different periods of time. Cells at day 8 (0 hr time-point) were used as controls. Whole cell lysates were prepared and activation of caspase-3 was determined by immunoblot analysis using an anti-caspase-3 monoclonal antibody recognizing both pro-caspase-3 (p32) and the active form of caspase-3 (p17). The blot was stripped and re-probed with anti-β-actin monoclonal antibody to ensure equal loading. Results are representative of five independent experiments performed with T cells from different blood donors.
IL-7 up-regulates Bcl-2 proteins Mcl-1, Bcl-xL and Bcl-2
Interleukin-7 signalling is associated with up-regulation of Bcl-2 anti-apoptotic proteins, which regulate cytokine deprivation-induced apoptosis.2,6,7 We therefore examined the expression levels of Bcl-2, Bcl-xL and Mcl-1 proteins in effector/memory T cells cultured under deprived conditions in the presence or absence of IL-7. As shown in Fig. 3(a), effector/memory T cells before starvation (0 hr time-point) expressed all three Bcl-2 proteins including Bcl-2, Mcl-1 and BcL-xL. However, culture of effector/memory T cells in deprived conditions led to a drop in the levels of all three Bcl-2 proteins, which was more pronounced for Mcl-1 and Bcl-xL. Addition of IL-7 to deprived cells up-regulated the levels of Mcl-1, Bcl-2 and Bcl-xL. The high levels of all three proteins induced by IL-7 were sustained up to 72 hr. The levels of β-actin were similar between non-treated cells and IL-7-treated cells. Densitometric analysis showed that IL-7 treatment for 24 hr led to a significant twofold to threefold increase in the levels of Mcl-1, Bcl-2 and Bcl-xL (Fig. 3b). Similar results were also obtained in cells treated with IL-7 for 48 and 72 hr (data not shown). The capacity of IL-7 to up-regulate pro-survival Bcl-2 proteins after 24 hr of stimulation was comparable to that of IL-2, with the exception of Bcl-xL because IL-2 is slightly more potent that IL-7 (Fig. 3c). Similar results were also obtained when the cells were stimulated for 48 and 72 hr (data not shown).
Figure 3.
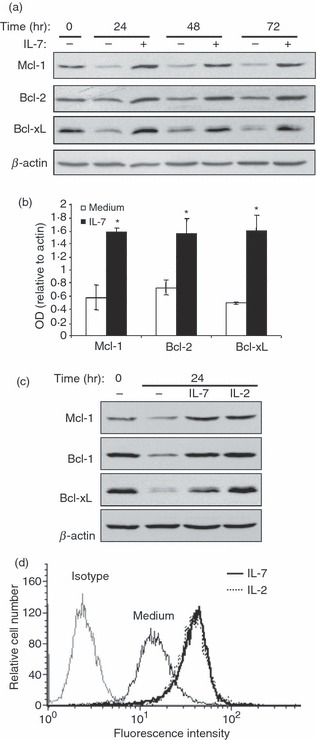
Interleukin-7 (IL-7) up-regulates Bcl-2 proteins in human effector/memory T cells. (a) Cells at day 8 (0 hr time-point) were cultured under deprived conditions in the presence or absence of IL-7 (2 ng/ml) for the indicated periods of time. Whole cell lysates were then prepared and subjected to immunoblot analysis with specific antibodies against Mcl-1, Bcl-2, or Bcl-xL. The blot was stripped and re-probed with anti-β-actin monoclonal antibody (mAb) to ensure equal loading. The results are representative of five independent experiments performed with T cells from different blood donors. (b) Densitometric quantification of relative increase in Bcl-2 proteins in cells cultured for 24 hr under deprived conditions in the absence (medium) or the presence of IL-7. The results represent mean values (± SE) from three independent experiments and are expressed as the ratio between Mcl-1, Bcl-2 or Bcl-xL values and β-actin values. *P < 0·05 between IL-7-treated samples and non-treated samples (medium). (c) The cells were cultured under deprived conditions in the presence or absence of IL-7 (2 ng/ml) or IL-2 (50 U/ml) for 24 hr. The expression of Mcl-1, Bcl-2 and Bcl-xL was determined by immunoblot analysis. The results are representative of three independent experiments performed with T cells from different blood donors. (d) The cells were left unstimulated (medium) or stimulated with IL-7, IL-2 for 24 hr. The cells were then washed, stained with phycoerythrin-conjugated anti-Bcl-2 or phycoerythrin-conjugated isotypic control antibodies and analysed by fluorescence-activated cell sorting analysis. The control isotypic staining is shown for unstimulated cells, which is similar to control isotypic staining of IL-2- and IL-7-stimulated cells (data not shown). The results are representative of three different experiments performed with T cells from different blood donors.
To determine if the observed increase in the levels of Bcl-2 proteins also occurred at the cellular level, we measured the levels of intracellular Bcl-2 in cells stimulated or not with IL-7 or with IL-2. As shown in Fig. 3(d), IL-7 also up-regulated the levels of intracellular Bcl-2 protein and this effect was comparable with that of IL-2. Together, these results indicate that IL-7 up-regulates all three major Bcl-2 anti-apoptotic proteins in human effector/memory T cells.
IL-7 promotes cell survival and up-regulates Bcl-2 proteins via JAK/STAT signalling pathway
The JAK/STAT, PI3K/AKT and MAPK/ERK signalling molecules are major cell survival pathways, which can all be activated by IL-7. To provide insights into the signalling pathways contributing to IL-7-induced effector/memory T-cell survival, we have first assessed the effects of the JAK/STAT inhibitor Pan-Jak, the PI3K/AKT inhibitor LY294002 and the MEK1-2/ERK inhibitor U0126, on the ability of IL-7 to protect effector/memory T cells from apoptosis. Cells were cultured under deprived conditions with the different inhibitors or with IL-7 plus inhibitors, and their apoptosis was determined. The protective effect of IL-7 was only abrogated in the presence of the JAK/STAT inhibitor, but not in the presence of the MAPK/ERK or PI3K/AKT inhibitors (Fig. 4a). Interestingly, cells cultured under deprived conditions with LY294002 inhibitor led to a significant increase in cell apoptosis compared with cells cultured only in deprived conditions or with Pan-Jak or U0126 inhibitors (Fig. 4a), suggesting that the PI3K/AKT pathway can modulate the basal viability of effector/memory T cells. In addition, the presence of LY294002 also slightly reverses the protective effect of IL-7 (Fig. 4a) but statistical analysis indicated that the differences between IL-7 and IL-7 + LY294002 samples did not reach significance (P = 0·078). Similarly and as shown by immunoblot and densitometric analysis, only the JAK/STAT inhibitor abrogated the capacity of IL-7 to up-regulate the pro-survival Bcl-2 proteins (Fig. 4b,c) suggesting that the IL-7 pro-survival function is mediated through the JAK/STAT pathway.
Figure 4.
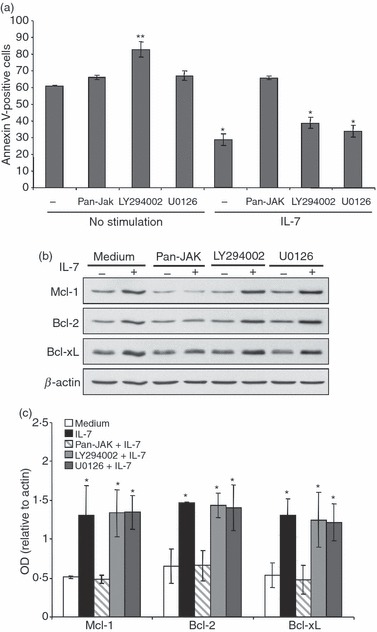
Interleukin-7 (IL-7) -mediated cell survival is abrogated by the Janus kinase/signal transducer and activator of transcription (JAK/STAT) inhibitor. (a) IL-7-mediated cell survival is reversed by the Pan-JAK inhibitor. Human effector/memory T cells were treated or not in the absence of cytokines with the Pan-JAK inhibitor (1 μm), the MEK-1 inhibitor U0126 (20 μm), or with the phosphatidylinositol 3-kinase (PI3K)/AKT inhibitor LY294002 (25 μm) for 1 hr. The cells were then stimulated or not with IL-7 (2 ng/ml) for 72 hr. Cells were collected and apoptosis was determined using Annexin V/7-aminoactinomycin D staining and flow cytometry analysis. The results are presented as mean percentages (± SE) of Annexin-V-positive cells from three independent experiments performed in triplicate with T cells isolated from different blood donors. *P < 0·05 between IL-7- or IL-7 + LY294002- or IL-7 + U0126-treated samples and non-treated samples (no stimulation) or IL-7 + Pan-JAK-treated samples. **P < 0·05 between LY294002-treated samples and non-treated or Pan-Jak- or U0126-treated samples. (b) IL-7-up-regulation of Bcl-2 proteins is abrogated by the Pan-JAK inhibitor. Cells were treated as described in (a) and the expression levels of Bcl-2 proteins were detected by immunoblot analysis. The results are representative of five independent experiments performed with T cells from different blood donors. (c) Densitometric quantification of Bcl-2 proteins in non-stimulated cells, versus cells stimulated with IL-7 and cells stimulated with IL-7 plus the different inhibitors as indicated. The results represent mean values (± SE) from three independent experiments and are expressed as the ratio between Mcl-1, Bcl-2 or Bcl-xL values and β-actin values. *P < 0·05 between IL-7- or IL-7 + LY294002- or IL-7 + U0126-treated samples and -non-treated samples (medium) or IL-7 + Pan-JAK-treated samples.
We then examined the ability of IL-7 to activate the JAK/STAT, PI3K/AKT and MAPK/ERK signalling pathways in effector/memory T cells. Interleukin-7 within 15 min of stimulation induced significant phosphorylation of STAT5, the major IL-7-activated STAT isoform, which remained detectable after 1 hr of stimulation with IL-7 (Fig. 5a). The presence of the Pan-Jak inhibitor completely abrogated the observed phosphorylation of STAT5 (Fig. 5a). The capacity of IL-7 to induce STAT5 phosphorylation is comparable to that of IL-2 (Fig. 5b). Conversely, we found that IL-7 is a weak activator of AKT and ERK phosphorylation in human effector/memory T cells (Fig. 5c). Extending the time of activation up to 6 hr or increasing the concentration of IL-7 for up to 10 ng/ml did not result in any further activation of AKT and ERK (data not shown). As a control, and in contrast to IL-7, cell stimulation with IL-2 induced significant phosphorylation of AKT and ERK1/2 kinases (Fig. 5c). Together these data indicate that the protective effect of IL-7 is dependent on the JAK/STAT5 pathway and is independent from the PI3K/AKT and MAPK/ERK signalling pathways in human effector/memory T cells.
Figure 5.
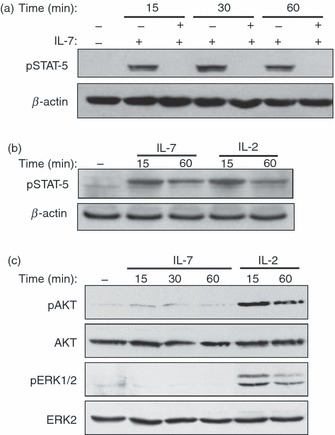
Activation of Janus kinase/signal transducer and activator of transcription (JAK/STAT), AKT and extracellular signal-regulated kinase (ERK) by interleukin-7 (IL-7) in human effector/memory T cells. (a) IL-7 activates the JAK/STAT signalling pathway. Human effector/memory T cells cultured under deprived conditions were stimulated or not with IL-7 (2 ng/ml) for different periods of time in the presence or absence of the Pan-JAK inhibitor. Non-stimulated cells (−) were maintained in medium alone for 1 hr. Activation of STAT5 was determined by immunoblot analysis using specific antibody that recognizes the phosphorylated form of STAT5. The blot was stripped and re-probed with anti-β-actin monoclonal antibody to ensure equal loading. (b) The cells were activated as in (a) with IL-7 or with IL-2 (50 U/ml) and STAT5 phosphorylation was determined by immunoblot analysis. (c) IL-7 is a weak activator of AKT and ERK in human effector/memory T cells. The cells were activated under deprived conditions with IL-7 for different periods of time. Non-stimulated cells (−) were maintained in medium alone for 1 hr. In some cases, the cells were also activated with IL-2 (50 U/ml) as indicated. AKT and ERK activation was determined by immunoblot analysis using antibodies that recognize the phosphorylated forms of AKT and ERK1/2. The blot was stripped and re-probed with anti-ERK2 and anti-AKT antibodies to ensure equal loading. The results in (a) and (c) and in (b) are respectively representative of five and three independent experiments performed with T cells from different blood donors.
Discussion
Elimination of activated T cells through apoptosis is a critical mechanism of immune homeostasis. Interleukin-7 is a crucial T-cell survival factor but its signalling in human T cells has been poorly addressed. In this study, we show that IL-7 protects human CD4+ effector/memory T cells from apoptosis by activating the JAK/STAT signalling pathway and by up-regulating the levels of Bcl-xL, Bcl-2 and Mcl-1.
Apoptosis induced by lack of stimulation is regulated by Bcl-2 proteins and the mitochondrial death pathway.2,6,7 Our results show that IL-7-induced effector/memory T-cell survival is associated with increased expression of anti-apoptotic proteins Bcl-2, Mcl-1 and Bcl-xL. Interleukin-7 has been reported to increase the expression of either Bcl-2 or Bcl-xL in human and murine naive T cells as well as in murine IL-7-dependent T-cell lines.15–35 However, the up-regulation of Mcl-1 was observed only in a few studies.36,37 Our study further extends the function of IL-7 to the regulation of all three major Bcl-2 proteins in human effector/memory T cells.
Our inhibition studies demonstrated that IL-7 protects effector/memory T cells from apoptosis by activating the JAK/STAT signalling pathway. Previous studies on IL-7 signalling in mouse naive T cells and IL-7-dependent T-cell clones indicated that IL-7 is connected to the JAK1/3-STAT5 and PI3K/AKT cell signalling pathways.38–41 The IL-7-induced PI3K/AKT activation was implicated in cell survival mainly through the phosphorylation and inactivation of the Bcl-2 proapoptotic protein Bad 42,43 and through glucose uptake, which is necessary to maintain T-cell viability.44 However, a recent study suggested that IL-7-induced survival of mouse naive T cells can rely solely on the up-regulation of Bcl-2 and can be independent from the PI3K/AKT pathway despite the fact that Bad is inactivated by AKT.33 Our results showed that IL-7 is a weak activator of AKT in human effector/memory T cells and the use of specific inhibitors of the PI3K/AKT pathway did not abrogate the effect of IL-7 on cell survival and up-regulation of Bcl-2 pro-survival proteins. Hence, although activation of the PI3K/AKT pathway may contribute to IL-7 signalling and survival in certain mouse T-cell models, our study clearly showed that IL-7-induced survival of human effector/memory T cells is independent from the PI3K/AKT pathway. Interestingly, human effector/memory T cells express a basal level of phosphorylated AKT and treating them with the PI3K/AKT inhibitor slightly enhanced their apoptosis, suggesting that PI3K/AKT can be rather important in maintaining basal cell viability.
Interleukin-7 can also activate the MAPK/ERK signalling pathway as seen in immature thymocytes and acute T-cell leukaemia blasts.41,45 However, our study indicates that IL-7 does not activate the MAPK/ERK in human effector/memory T cells and the use of the MEK-1/2 inhibitor did not abrogate the pro-survival effect of IL-7. Together these results indicate that IL-7 protects human effector/memory T cells from cytokine deprivation-induced apoptosis by activating the JAK/STAT signalling pathway independently from the PI3K/AKT and MAPK/ERK signalling pathways.
We have not addressed whether IL-7 regulates the function of pro-apoptotic proteins such as Bad and Bim, which are known to be inactivated through phosphorylation and subsequent degradation. Phosphorylation of Bim and Bad is a complex process involving multiple sites and different kinases. Recent evidence indicates that Bad can be phosphorylated by AKT, c-Raf/ERK kinases and other kinases as well,46 whereas Bim seems to be inactivated mainly by MAPK/ERK.47,48 In our cell model IL-7 does not activate the PI3K/AKT and MAPK/ERK pathways and IL-7-mediated cell survival is independent from these pathways; it is therefore unlikely that IL-7 regulates the phosphorylation of Bad or Bim through PI3K/AKT and MAPK/ERK. Notably, we found that Bad phosphorylation at serine-112, which has been reported to be regulated by AKT,43 c-Raf 49 or pim kinase 50 is not affected by IL-7 (data not shown). Whether IL-7 regulates Bim or Bad phosphorylation and function through other kinases and/or other mechanisms is unknown and warrants further investigation.
In addition to promoting the development and maintenance of memory T cells during protective immunity, IL-7 is also associated with inflammation and autoimmunity. High levels of IL-7 have been associated with T-cell activation and immunopathogenesis of rheumatoid arthritis and inflammatory bowel disease.51–53 Given that CD4+ effector/memory T cells are the cells that are associated with tissue damage in autoimmune diseases, it is conceivable that by enhancing cell survival, IL-7 contributes to the persistence of effector/memory T cells in the inflammatory sites, which will further exacerbate tissue damage and inflammation. The cells used in this study produce two major inflammatory cytokines including interferon-γ and the osteoclastogenic cytokine RANKL 28 and both cytokines are known to induce tissue inflammation and bone erosion in diseases like rheumatoid arthritis and inflammatory bowel disease. Therefore, our study suggests that targeting the JAK/STAT pathway and anti-apoptotic Bcl-2 proteins can be beneficial for these inflammatory diseases. A further understanding of IL-7 signalling in effector/memory T cells associated with autoimmunity may lead to the development of novel therapeutic strategies.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) to F.A., who was a recipient of a CIHR New Investigator Award. M.B. is a recipient of a scholarship from the Canadian Arthritis Network Centre of Excellence and a scholarship from the Fonds de Recherche en santé du Québec. The authors are grateful to Dr Reem Al-Daccak (INSERM-UMRS 940, Paris) for critical discussions.
Glossary
Abbreviations
- 7-AAD
7-aminoactinomycin D
- ERK
extracellular signal-regulated kinase
- FACS
fluorescence-activated cell sorter
- JAK
Janus kinase
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- PI3K
phosphatidylinositol-3 kinase
- RANKL
receptor activator of nuclear factor kappa B ligand
- STAT
signal transducer and activator of transcription
- Th
T helper
Disclosures
Authors have no conflicts of interest.
References
- 1.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 2.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–42. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 3.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–5. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–87. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 5.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Strasser A, Puthalakath H, O’Reilly LA, Bouillet P. What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86:57–66. doi: 10.1038/sj.icb.7100141. [DOI] [PubMed] [Google Scholar]
- 7.Hildeman D, Jorgensen T, Kappler J, Marrack P. Apoptosis and the homeostatic control of immune responses. Curr Opin Immunol. 2007;19:516–21. doi: 10.1016/j.coi.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol Invest. 2004;33:109–42. doi: 10.1081/imm-120030732. [DOI] [PubMed] [Google Scholar]
- 9.Khaled AR, Durum SK. The role of cytokines in lymphocyte homeostasis. BioTechniques. 2002;33(Suppl):40–5. [PubMed] [Google Scholar]
- 10.Dai Z, Arakelov A, Wagener M, Konieczny BT, Lakkis FG. The role of the common cytokine receptor γ-chain in regulating IL-2-dependent, activation-induced CD8+ T cell death. J Immunol. 1999;163:3131–7. [PubMed] [Google Scholar]
- 11.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 12.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrio R, Rolle CE, Malek TR. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol. 2007;37:3078–88. doi: 10.1002/eji.200737585. [DOI] [PubMed] [Google Scholar]
- 15.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–7. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 17.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor α is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–5. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutcheson J, Perlman H. Loss of Bim results in abnormal accumulation of mature CD4– CD8– CD44– CD25– thymocytes. Immunobiology. 2007;212:629–36. doi: 10.1016/j.imbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaled AR, Durum SK. Death and Baxes: mechanisms of lymphotrophic cytokines. Immunol Rev. 2003;193:48–57. doi: 10.1034/j.1600-065x.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 20.Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci U S A. 1999;96:14476–81. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seward RJ, Von Haller PD, Aebersold R, Huber BT. Phosphorylation of the pro-apoptotic protein Bim in lymphocytes is associated with protection from apoptosis. Mol Immunol. 2003;39:983–93. doi: 10.1016/s0161-5890(03)00047-6. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–95. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127lo effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–33. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Xue HH, Kovanen PE, Pise-Masison CA, Berg M, Radovich MF, Brady JN, Leonard WJ. IL-2 negatively regulates IL-7 receptor α chain expression in activated T lymphocytes. Proc Natl Acad Sci U S A. 2002;99:13759–64. doi: 10.1073/pnas.212214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das L, Levine AD. TGF-β inhibits IL-2 production and promotes cell cycle arrest in TCR-activated effector/memory T cells in the presence of sustained TCR signal transduction. J Immunol. 2008;180:1490–8. doi: 10.4049/jimmunol.180.3.1490. [DOI] [PubMed] [Google Scholar]
- 27.Strauss G, Knape I, Melzner I, Debatin KM. Constitutive caspase activation and impaired death-inducing signaling complex formation in CD95-resistant, long-term activated, antigen-specific T cells. J Immunol. 2003;171:1172–82. doi: 10.4049/jimmunol.171.3.1172. [DOI] [PubMed] [Google Scholar]
- 28.Gendron S, Boisvert M, Chetoui N, Aoudjit F. α1β1 integrin and interleukin-7 receptor up-regulate the expression of RANKL in human T cells and enhance their osteoclastogenic function. Immunology. 2008;125:359–69. doi: 10.1111/j.1365-2567.2008.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gendron S, Couture J, Aoudjit F. Integrin α2β1 inhibits Fas-mediated apoptosis in T lymphocytes by protein phosphatase 2A-dependent activation of the MAPK/ERK pathway. J Biol Chem. 2003;278:48633–43. doi: 10.1074/jbc.M305169200. [DOI] [PubMed] [Google Scholar]
- 30.Franko JL, Levine AD. Antigen-independent adhesion and cell spreading by inducible costimulator engagement inhibits T cell migration in a PI-3K-dependent manner. J Leukoc Biol. 2009;85:526–38. doi: 10.1189/jlb.0808505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Rα transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Swainson L, Verhoeyen E, Cosset FL, Taylor N. IL-7R α gene expression is inversely correlated with cell cycle progression in IL-7-stimulated T lymphocytes. J Immunol. 2006;176:6702–8. doi: 10.4049/jimmunol.176.11.6702. [DOI] [PubMed] [Google Scholar]
- 33.Ostiguy V, Allard EL, Marquis M, Leignadier J, Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J Leukoc Biol. 2007;82:645–56. doi: 10.1189/jlb.0806494. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Q, Li WQ, Hofmeister RR, Young HA, Hodge DR, Keller JR, Khaled AR, Durum SK. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol Cell Biol. 2004;24:6501–13. doi: 10.1128/MCB.24.14.6501-6513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–54. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 36.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–6. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 37.Dzhagalov I, Dunkle A, He YW. The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol. 2008;181:521–8. doi: 10.4049/jimmunol.181.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foxwell BM, Beadling C, Guschin D, Kerr I, Cantrell D. Interleukin-7 can induce the activation of Jak 1, Jak 3 and STAT 5 proteins in murine T cells. Eur J Immunol. 1995;25:3041–6. doi: 10.1002/eji.1830251109. [DOI] [PubMed] [Google Scholar]
- 39.Kittipatarin C, Khaled AR. Interlinking interleukin-7. Cytokine. 2007;39:75–83. doi: 10.1016/j.cyto.2007.07.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pernis A, Gupta S, Yopp J, Garfein E, Kashleva H, Schindler C, Rothman P. γ chain-associated cytokine receptors signal through distinct transducing factors. J Biol Chem. 1995;270:14517–22. doi: 10.1074/jbc.270.24.14517. [DOI] [PubMed] [Google Scholar]
- 41.Johnson SE, Shah N, Bajer AA, LeBien TW. IL-7 activates the phosphatidylinositol 3-kinase/AKT pathway in normal human thymocytes but not normal human B cell precursors. J Immunol. 2008;180:8109–17. doi: 10.4049/jimmunol.180.12.8109. [DOI] [PubMed] [Google Scholar]
- 42.Sade H, Sarin A. IL-7 inhibits dexamethasone-induced apoptosis via Akt/PKB in mature, peripheral T cells. Eur J Immunol. 2003;33:913–9. doi: 10.1002/eji.200323782. [DOI] [PubMed] [Google Scholar]
- 43.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–6. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 44.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–11. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200:659–69. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl. 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 47.O’Reilly LA, Kruse EA, Puthalakath H, Kelly PN, Kaufmann T, Huang DC, Strasser A. MEK/ERK-mediated phosphorylation of Bim is required to ensure survival of T and B lymphocytes during mitogenic stimulation. J Immunol. 2009;183:261–9. doi: 10.4049/jimmunol.0803853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30:415–25. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polzien L, Baljuls A, Rennefahrt UE, et al. Identification of novel in vivo phosphorylation sites of the human proapoptotic protein BAD: pore-forming activity of BAD is regulated by phosphorylation. J Biol Chem. 2009;284:28004–20. doi: 10.1074/jbc.M109.010702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–54. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanai T, Nemoto Y, Kamada N, Totsuka T, Hisamatsu T, Watanabe M, Hibi T. Homeostatic (IL-7) and effector (IL-17) cytokines as distinct but complementary target for an optimal therapeutic strategy in inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:306–13. doi: 10.1097/MOG.0b013e32832bc627. [DOI] [PubMed] [Google Scholar]
- 52.Totsuka T, Kanai T, Nemoto Y, Makita S, Okamoto R, Tsuchiya K, Watanabe M. IL-7 is essential for the development and the persistence of chronic colitis. J Immunol. 2007;178:4737–48. doi: 10.4049/jimmunol.178.8.4737. [DOI] [PubMed] [Google Scholar]
- 53.Hartgring SA, Bijlsma JW, Lafeber FP, van Roon JA. Interleukin-7 induced immunopathology in arthritis. Ann Rheum Dis. 2006;65(Suppl. 3):iii69–74. doi: 10.1136/ard.2006.058479. [DOI] [PMC free article] [PubMed] [Google Scholar]


