Abstract
We investigated the expression and role of the dopamine receptor 3 (D3R) in postnatal mouse subventricular zone (SVZ). In situ hybridization detected selective D3R mRNA expression in the SVZ. Fluorescence activated cell sorting (FACS) of adult SVZ subtypes using hGFAP-GFP and Dcx-GFP mice showed that transit amplifying progenitor cells and niche astrocytes expressed D3R whereas stem cell-like astrocytes and neuroblasts did not. To determine D3R’s role in SVZ neurogenesis, we administered U-99194A, a D3R preferential antagonist, and bromodeoxyuridine (BrdU) in postnatal mice. In vivo D3R antagonism decreased the numbers of newborn neurons reaching the core and the periglomerular layer of the olfactory bulb. Moreover, it decreased progenitor cell proliferation but did not change the number of label-retaining (stem) cells, commensurate with its expression on transit amplifying progenitor cells but not SVZ stem cell-like astrocytes. Collectively, this study suggests that dopaminergic stimulation of D3R drives proliferation via rapidly amplifying progenitor cells to promote murine SVZ neurogenesis.
Keywords: Dopamine receptor 3, neurogenesis, proliferation, subventricular zone, U-99194A, stem cells
Introduction
The postnatal subventricular zone (SVZ) is one of the main neurogenic regions and has drawn much attention for its possible therapeutic usage in brain repair. This special area retains many features of embryonic neurogenic niches. For instance, several factors shaping brain development also play important roles in regulating adult SVZ neurogenesis (Alvarez-Buylla and Lim 2004). Recently, neurotransmitters, including dopamine, have become recognized as important modulators of postnatal neurogenesis (Borta and Hoglinger 2007; Hagg 2009). Dopamine as well as dopamine receptor agonists increased postnatal SVZ proliferation whereas dopamine deletion in the nigrostriatal pathway decreased proliferation (Baker et al. 2004; Hoglinger et al. 2004). Moreover, Parkinson’s patients showed decreased proliferation in the SVZ and one of early symptoms of Parkinson’s disorder is olfactory bulb dysfunction (Hoglinger et al. 2004).
While dopamine clearly affects postnatal SVZ neurogenesis, it remains unclear which dopamine receptors mediate this effect. There are five different dopamine receptors: D1R, D2R, D3R, D4R, and D5R. Dopamine 1 like (D1L) receptors (D1R and D5R) are coupled to stimulatory G proteins whereas dopamine 2 like (D2L) receptors (D2R, D3R, and D4R) are linked to inhibitory G proteins (Neves et al. 2002). Among dopamine receptors, D3R is the only one specifically expressed in neurogenic areas, both in the embryonic and postnatal rat SVZ (Diaz et al. 1997; Araki et al. 2007). Therefore in this study we focussed on understanding D3R’s role in postnatal SVZ neurogenesis. The postnatal SVZ is composed of three major neurogenic cell types; slowly proliferating astrocyte-like stem cells, transit amplifying progenitor cells, and migratory neuroblasts (Alvarez-Buylla and Garcia-Verdugo 2002). Since each SVZ subtype has different biological properties, it is essential to understand which specific dopamine receptors are expressed by which SVZ cell types. However, the lack of reliable dopamine receptor antibodies has made it difficult to identify dopamine receptor expression in SVZ cells. To circumvent this issue, we took advantage of FACSorting SVZ subtypes based on marker expression and two different GFP+ reporter mice (Nam et al. 2007; Pastrana et al. 2009).
D3R’s roles in regulating postnatal SVZ neurogenesis remain controversial. D3R receptor stimulation in adult rats increased SVZ proliferation (Coronas et al. 2004; Van Kampen et al. 2004). However, similar treatments with D3R specific agonists failed to show any effect in adult mouse SVZ (Baker et al. 2005). Thus, there are several specific inconsistencies in the literature despite ample evidence that in general dopamine affects postnatal SVZ neurogenesis. Here, we investigated the role and mechanism of D3R in murine postnatal neurogenesis. We provide evidence that D3R is expressed in rapidly amplifying progenitor cells in the postnatal SVZ and drives cell proliferation to regulate olfactory bulb neurogenesis.
Materials and Methods
Animals
Doublecortin-GFP (Dcx-GFP) mice were originally developed by the Rockefeller GENSAT Project (Gong et al. 2003). hGFAP-GFP mice were obtained from the Jackson Laboratory and CD1 mice from Harlan, UK. The animal care and use was conducted in accordance with personal and project license under the UK Animals (Scientific Procedures) 1986 Act and NIH guideline.
In situ hybridization
We performed in situ hybridization for D3R in P4 and 1 month old CD1 mice (N = 4, each age). Total RNA was extracted from P8 mouse brain and the first strand cDNA was synthesized using Superscript III reverse transcriptase together with random hexamers (Invitrogen, Paisley, UK) following the manufacturer’s instructions. DNA fragments corresponding to the region of the mouse D3R cDNA were generated using the following forward (F) and reverse (R) primers: F= 5’-gccctctcctctttggtttc-3’, R= 5’-gtggataacctgccattgct3’. The resulting PCR product, a 565 bp fragment, was ligated into the pST-Blue 1 plasmid (Novagen, Nottingham, UK). The antisense and sense (a negative control) cRNA probes were transcribed using T7 and Sp6 RNA polymerases, respectively, with a digoxigenin (DIG)-labelled RNA mixture (Roche, Penzberg, Germany). Frozen sections were post-fixed with 4% paraformaldehyde in PBS, deproteinised with 0.1N HCL for 5 min, acetylated with acetic anhydride (0.25% in 0.1M triethanolmine hydrochloride) and prehybridized at RT for at least 1hr in a solution containing 50% formamde, 10mM Tris, pH7.6, 200 μg/ml E.Coli tRNA, 1x Denhardt’s solution, 10% dextran sulphate, 600 mM NaCl, 0.25% SDS and 1mM EDTA. The sections were hybridized in the same buffer with the DIG-labeled probes overnight at 68°C. After hybridization, sections were washed to a final stringency of 30 mM NaCl/3 mM sodium citrate at 68°C and detected by anti-DIG-alkaline phosphatase antibody in conjunction with a mixture of nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) (Roche, Penzberg, Germany).
FACSorting and dopamine receptor RT-PCR
To purify neuroblasts, P2, P6 and 8 weeks old Dcx-GFP mice were killed and the brains were sectioned into 1-mm coronal sections approximately 1 mm rostral and caudal to bregma using a brain mold and razor blades. Under a dissection microscope, the SVZ was dissected into dorsal and ventral regions and the tissue was digested using papain (Worthington LK003176) for 20 min at 37°C. The digested tissue was washed with Neurobasal medium (Invitrogen), cell counts obtained with a hemocytometer, and GFP-expressing cells FACS sorted. After samples were sorted, they were centrifuged and Trizol (Invitrogen) added to extract total RNA. First strand cDNA for each sample was synthesized using the SuperScript III first strand synthesis system (Invitrogen). RT-PCR was performed on FACS sorted dorsal and ventral SVZ samples using the following primers for all five dopamine receptors. Forward (F), reverse (R) primers, and expected sizes: F= 5’-gtgactgagattgaccaggaag-3’, R= 5’-accgcaggtgtcgaaacctgat-3’ for D1R (491bp), F= 5’-ccagaatgagtgtatcattgcc-3’, R= 5’-cttcctgcggctcatcgtctta-3’ for D2R (555bp), F= 5’-agtgtatcagcatcagacctgg-3’, R= 5’- ccaagccatgtcgtggctctgt-3’ for D3R (564bp), F= 5’-gtccgctcatgctactgcttta-3’, R= 5’- gagtcttgcggaagacacttcg-3’ for D4R (548bp), F= 5’-cagggagatcgctgctgcctat-3’, R= 5’- agaccataccagcaattgccac-3’ for D5R (590bp). GAPDH was used as a positive control and sizes compared to a 1kb plus DNA ladder (Invitrogen).
The detailed method for simultaneous prospective FACSorting using hGFAP-GFP mice has been published elsewhere (Pastrana et al. 2009). Briefly, the SVZ from 2 months old hGFAP-GFP mice (The Jackson Laboratory) was micro-dissected and dissociated. Cells were incubated with phycoerythrin-conjugated rat anti-mCD24 (1:20, BD Pharmingen) and biotinylated EGF conjugated with Alexa647-streptavidin (2 μl/ml, Molecular Probes) for 30 min. All cell populations were separated in a single sort experiment using a Becton Dickinson FACS ARIA as published previously (Pastrana et al. 2009). Total RNA samples from each FACS purified population were isolated using the RNAqueous-Micro RNA isolation kit from Ambion. cDNA was generated and amplified with WT-Ovation Pico RNA amplification system (NuGEN) as per manufacturer guidelines. We performed D3R RT-PCR in each cell population with the primers used for the in situ hybridization above (PCR performed twice).
U-99194A treatment in vivo
U-99194a (Sigma) was reconstituted in saline. For label retaining cells and olfactory bulb interneuron subtype analysis, 2 mg/kg U-99194a, 20 mg/kg U-99194A, or saline was injected subcutaneously (s.c.) once a day for three days. During the same three days 50-100 mg/kg BrdU was injected twice a day intraperitoneally (i.p.). Mice were killed 4 weeks after the last injection of BrdU (N=3 mice for saline treatment, N=4 mice for 2 mg/kg and 20 mg/kg U-99194A). To detect new born cells reaching the olfactory bulb (OB), the same drug treatment plan was used with 5 days survival (N=4 mice in each group). For proliferation assays for progenitor cells, 2 mg/kg, or 20 mg/kg of U-99194a, or saline was injected from P4 to P6 (3 days) s.c. once a day and a single pulse of 100 mg/kg BrdU injected i.p. with the last dose of U-99194a. Animals were killed 2 hours later (N=5 brains per treatment). Then, mice were perfused with 4% paraformaldehyde in 0.1 M sodium phosphate-buffered saline (PBS) and post fixed overnight, transferred to 30% sucrose overnight, then frozen and stored at −80°C until sectioning. Brains were sectioned with a sliding Microtome (Leica, UK) at 30 μm thickness for immunohistochemistry.
Immunohistochemistry
To detect BrdU immunoreactivity, sections were washed with PBS 3 times for 10 min each, incubated in DNAse buffer (10 mM Tris-HCl, 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, 50% v/v glycerol) with 0.5% Triton X-100 for 20 min, DNAse 2 mg/mL in DNAse buffer with 0.5% Triton X-100 at 37°C for 30 min, washed with PBS 3 X for 10 min each, 50 mM glycine for 15 min once, again PBS 3 times for 10 min each, PBS+ (10% Donkey Serum/0.5% Triton X-100 in PBS) for one hr, and mouse × BrdU (1:200, Dako) or sheep × BrdU (1:200, Abcam) in PBS+ at 4 °C overnight incubation. Then, slices were washed with PBS 3 X for 10 min each, biotinylated Donkey × mouse (1:400, Jackson Immunoresearch) in PBS+ for 1 hr, washed with PBS 3 X for 10 min each, Streptavidin Alexa Fluor 546 (1:500, Jackson Immunoresearch) in PBS+ for 1 hr, PBS 3 X for 10 min each, DAPI (40 mg/mL DAPI stock solution 1:1000 in PBS) for 10 min, PB 3 X for 10 min each, air dry, and coverslipped with Fluorsave (Calbiochem). For double immunohistochemistry, sections were washed with PBS 3 times for 10 min each, 50 mM glycine for 15 min once, again PBS 3 times for 10 min each, PBS+ (10% Donkey Serum/0.1% Triton X-100 in PBS) for one hr, and rabbit × CalB (1:1000, Sigma C9848), rabbit × CalR (1:2000, Chemicon), or rabbit × TH (1:2000, Chemicon) overnight. Sections were washed 3 times with PBS, incubated with secondary antibodies (Alexa488 donkey × rabbit, Invitrogen) for 1hr, and followed by BrdU protocol above. For other immunohistochemistry, we used goat anti-Dcx (1:100, Santa Cruz) or mouse anti-Mash1 (1:200, BDscience) as primaries and Alexa546 donkey anti-goat or anti mouse (Jackson Immunoresearch) as secondaries.
Image acquisition and quantification
Epifluorescent microscopy (Leica) was used with 20x or 40x objectives to obtain images of BrdU+ cells and quantification was done either from captured images or with live counting. Openlab (Improvision) was used to acquire images and Volocity (Improvision) to quantify images. Each section was at least 180 μm apart. For BrdU counting in the periglomerular layer, four different fields were randomly selected per section and 5 different optical sections were taken to count all positive cells in the section (N≥4 section/brain). For the BrdU and CalB, CalR and TH colocalization study, 4 or 5 randomly chosen fields per section were imaged with a confocal microscope (Zeiss LSM510 or LSM710) and 5 optical sections 0.34 μm apart obtained to generate a z-stack. Four corresponding sections ranging from 3.8 mm to 4.4 mm anterior to Bregma were chosen in each animal for imaging. Confocal images were imported into Volocity (Improvision, UK) and target areas were measured. BrdU live counting for progenitor cells in the SVZ was repeated and validated with two different BrdU antibodies (mouse anti-BrdU and sheep anti-BrdU). All image acquisition and quantification was done blindly. Statistical differences were determined by Student’s T-test, and distribution expressed by SEM (Standard error of mean).
Results
D3 receptor mRNA is expressed in the SVZ
We used in situ hybridization (ISH) to detect D3R mRNA in the SVZ. The presence of D3R mRNA has been suggested in the mouse SVZ by laser capture micro-dissection and subsequent RT-PCR (Araki et al. 2007). Although IHC for D3R showed expression in the embryonic and postnatal rat SVZ (Diaz et al. 1997), it has not been performed in the postnatal and adult murine SVZ, to the best of our knowledge. We chose ISH since dopamine receptor antibodies lacked specificity and provided spurious results in our preliminary studies (data not shown). In both P4 and 4 week old mouse brains, D3R was specifically expressed in the SVZ (Fig. 1B-D, H and I). The ventral SVZ showed stronger D3R expression than the dorsal SVZ (Fig. 1C and D). The sense control was negative (Fig, 1E). SVZ D3R mRNA was selectively expressed in the middle SVZ (between A/P bregma +0.5 and +1.42) (Fig. 1A, area 2). D3R mRNA in the very anterior (A/P bregma over +1.42, Fig. 1A, area 1) and posterior SVZ (A/P bregma below +0.5, Fig. 1A, area 3) was weak or below the level of detection (Fig. 1F and G). Similar patterns of D3R expression were observed in the adult brain (Fig. 1H-K). Ventral brain structures such as the olfactory tubercles known to highly express D3R (Diaz et al. 1997), indeed showed clear D3R expression, and served as an internal positive control (Fig. 1J and K).
Fig. 1. Dopamine receptor 3 is expressed in the subventricular zone in P4 and adult brains.
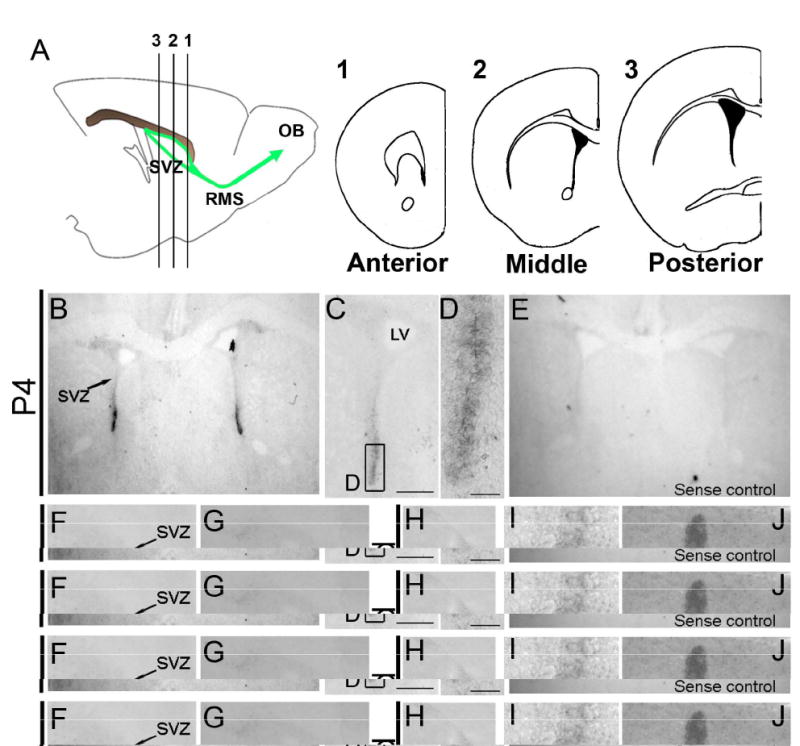
A: SVZ area division, 1: anterior, 2: middle, 3: posterior SVZ, left panel shows sagittal view of the SVZ, right panels coronal schematics of each position.
B: Low magnification picture of P4 brain, note the strong D3R signal in the ventral SVZ.
C, D: Progressively higher magnification views of the SVZ. Scale bars = 250 μm (C), = 50 μm (D).
E: Sense control; no D3R signal was detected.
F: Anterior SVZ in the P4 brain, note lack of signal in the SVZ.
G: Posterior SVZ in the P4 brain showed very weak signal in the SVZ.
H-K: 4 weeks old mouse SVZ.
H: Entire SVZ, Scale bar = 250 μm.
I: ventral SVZ, Scale bar = 50 μm.
J: Olfactory tubercle.
K: Islands of Calleja, Scale bar = 50 μm.
Neuroblasts do not express the D3 receptor
Three major neurogenic cell types play distinct roles in the postnatal SVZ. Astrocyte-like stem cells generate progenitor cells and maintain themselves through asymmetric cell division, progenitor cells proliferate rapidly to expand the progenitor and neuroblast populations, and neuroblasts migrate to the olfactory bulb. The D3R would have different biological effects depending on its expression in these SVZ cell subtypes. Thus, understanding D3R localization in SVZ subtypes is essential to interpret its effect on SVZ neurogenesis. To investigate dopamine receptor expression in neuroblasts, we FACS sorted GFP+ cells in the SVZ from Dcx-GFP mice. GFP expression in the SVZ and the rostral migratory stream (RMS) of Dcx-GFP mice faithfully recapitulates endogenous Dcx expression; almost all GFP+ cells are Dcx+ (a marker for neuroblasts) and Mash1 (a marker for progenitor cells) negative (Fig. 2A-F). We ran RT-PCR in FACSorted GFP+ cells from early postnatal and adult mice for all five dopamine receptors; D1R, D2R, D3R, D4R and D5R (N>3 experiments per age). Whole brain preparations confirmed that all five probes detected specific dopamine receptor subtypes (Fig. 2G). Dcx positive cells in both early postnatal (P2 and P6) and adult brains did not contain detectable D3R, but did express other dopamine receptor subtypes in the dorsal and ventral SVZ (Fig. 2H-J). D4R expression was present at P2 and P6 but was absent in adult SVZ neuroblasts (Fig. 2H-J). These results showed that SVZ neuroblasts express multiple DA receptors but do not express D3R. Thus, we hypothesized that D3R is expressed by SVZ stem cells and/or progenitor cells resulting in the positive ISH described above.
Fig. 2. Neuroblasts do not express the D3R receptor in postnatal and adult brains.
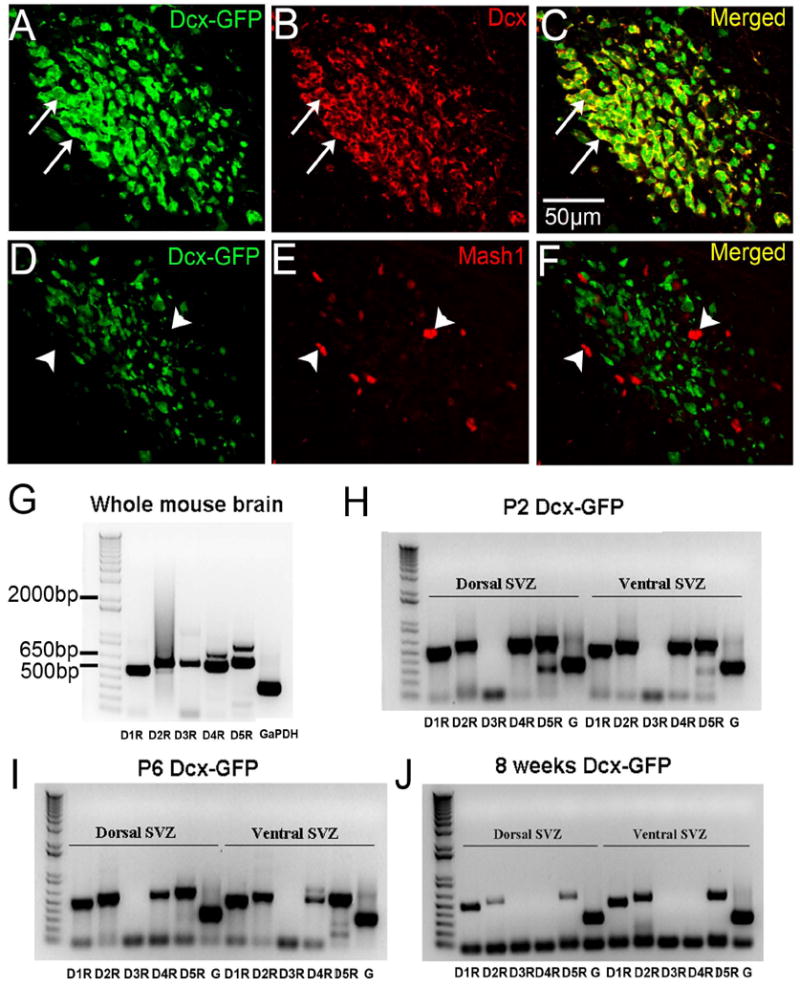
A-C: Dcx immunohistochemistry (red) in Dcx-GFP mouse coronal RMS sections. White arrows indicate examples of colocalized cells, note near perfect colocalization.
D-F: Mash1 immunohistochemistry (red) in Dcx-GFP coronal RMS sections. White arrow heads indicated examples of Mash1+ cells that are Dcx-GFP negative, note lack of colocalization.
G: Dopamine receptor RT-PCR from whole brains of Dcx-GFP mice. All five receptors were detected.
H-J: Dopamine receptor RT-PCR from GFP+ neuroblasts of P2 (H), P6 (I), and adult (J) Dcx-GFP mice. Note the D3R expression was not found in neuroblasts in any of the samples. G = GaPDH.
Transit amplifying progenitor cells in the SVZ express the D3 receptor
To probe D3R expression in other SVZ subtypes, we turned to a recently developed prospective FACSorting method using hGFAP-GFP mice (Pastrana et al. 2009) to purify SVZ subtypes: astrocyte-like stem cells (SC), progenitor cells (PC), neuroblasts (NB) and SVZ niche astrocytes (NA) (Fig. 3A,B). These populations can be distinguished using a combination of hGFAP-GFP, epidermal growth factor receptor (EGFR) and mCD24 expression. EGFR is a marker for stem/progenitor cells (Doetsch et al. 2002) and mCD24 is expressed at low levels in neuroblasts and high levels in ependymal cells, but not in other cell types (Pastrana et al. 2009). Moreover, hGFAP-GFP mice express GFP in SVZ niche astrocytes and stem cells, but not in the progenitor cells or neuroblasts (Tavazoie et al. 2008). Thus, SVZ niche astrocytes are only GFP+ and stem cells GFP+ and EGFR+. Progenitor cells are only EGFR+ and neuroblasts only mCD24+ (low level) (Pastrana et al. 2009) (Fig. 3A). Striatal astrocytes (SA) were also obtained for comparison (Fig. 3B). D3R RT-PCR in whole brain (WB) and negative control (NC) without samples confirmed the specificity of the D3R probe (Fig. 3B). D3R RT-PCR showed that among neurogenic SVZ subtypes, only progenitor cells in the SVZ expressed D3R (Fig. 3B). The lack of D3R expression in neuroblasts matched our previous result from Dcx-GFP FACSorting (Fig. 2). Interestingly, niche astrocytes in the SVZ also showed D3R expression while striatal astrocytes did not (Fig. 3B). Taken together these results show that D3R expression in the SVZ is highly cell specific: amongst the neurogenic cells only the transit amplifying type progenitor cells express it.
Fig. 3. D3R receptor is expressed in SVZ progenitor cells, but not in stem cells or neuroblasts.
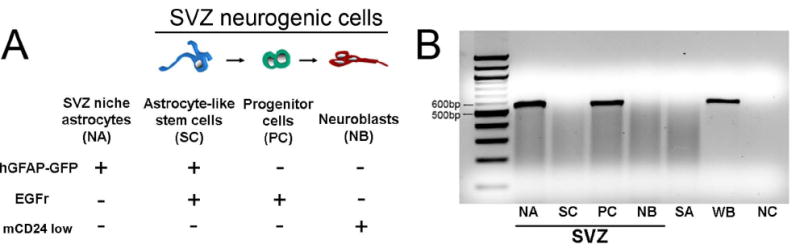
A: SVZ subtype schematic showing differential expression for FACSorting criteria.
B: D3R RT-PCR on SVZ subtypes, NA: SVZ niche astrocytes, SC: astrocyte-like stem cells, PC: progenitor cells, NB: neuroblasts, SA: striatal astrocytes, WB: whole brain, NC: negative control.
D3 receptor antagonism decreases the number of newborn periglomerular neurons
Neuroblasts born in the SVZ migrate to the olfactory bulb (OB) via the RMS and differentiate into interneurons in the OB. To assess the role of D3 receptors on OB neurogenesis, we administered U-99194A (a preferential D3R antagonist) or saline (vehicle for U-99194A) subcutaneously once a day for three days in P4 mice, injected BrdU for three days, and killed mice 4 weeks after the final drug injection (Fig. 4A). This was enough time to detect the majority of SVZ cells reaching the outer layers of the OB. We tested two doses (2 mg/kg and 20 mg/kg) of U-99194A which is known to specifically affect D3R (Laszy et al. 2005). There are three major non-overlapping interneuron subtypes in the periglomerular (PG) layer; Calbindin (CalB), tyrosine hydroxylase (TH), and Calretinin (CalR) positive cells (Parrish-Aungst et al. 2007). To test if specific subpopulations of OB interneurons were preferentially affected by blocking D3 receptors, we carried out CalB, TH, or CalR and BrdU double immunohistochemistry (Fig. 4B-D). In the 2 mg/kg U-99194A treated group, newborn neurons of all three subtypes decreased significantly (Fig. 4E). The 20 mg/kg U-99194A group showed similar, albeit slightly less pronounced, decreases (Fig. 4E). Among PG subtypes, newborn CalR+ cells showed the most significant decreases upon D3R antagonism. Interestingly, this relatively short period of U-99194A treatment did not change the overall number of TH+ and CalB+ cells after 4 weeks of recovery whereas the total number of periglomerular CalR+ cells significantly decreased in the 2 mg/kg U-99194A treatment group (Fig. 4F). In contrast to the periglomerular layer, the number of BrdU+ cells in the granular cell layer was not significantly different between controls and U-99194A treated mice (Sal, 94.0±12.3; 2 mg/kg U-99194A, 86.7±8.2; 20 mg/kg 83.5±4.4).
Fig. 4. D3R antagonism significantly decreased the number of CalB, TH, and CalR cells in the periglomerular layer of the olfactory bulb.
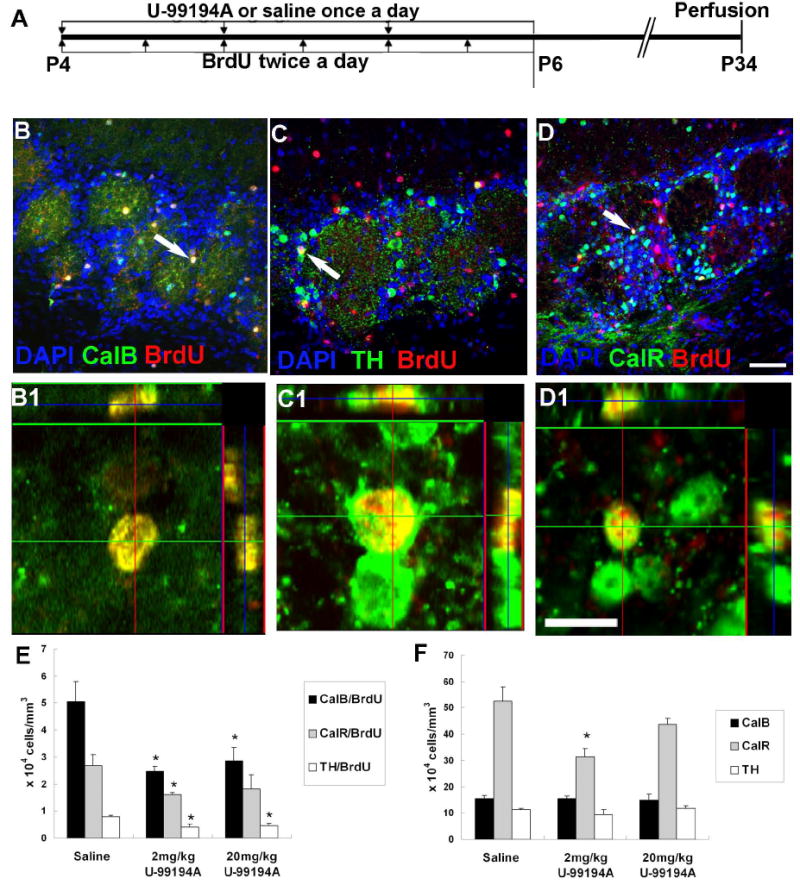
A: Drug treatment plan for long-term survival group.
B-D: Double immunohistochemistry and confocal microscopy showing CalB+ (B), TH+ (C), or CalR+ (D) cells colocalized with BrdU. Scale bar = 50 μm.
B1-D1: High magnification pictures of cells in B-D (white arrows) showing colocalization. Scale bar = 10 μm.
E-F: The number of newborn interneuron subtypes in the PG layer (E). The total number of PG layer cell subtypes shown in (F) * = P<0.05.
D3 receptor blockade decreases the number of newborn cells in the RMS
Once born in the SVZ, neuroblasts migrate in the RMS, the anterior portion of which extends into the core of the olfactory bulb (OB) (Fig. 5B). To measure newborn neuroblast migration into the anterior RMS, BrdU was administered to P4 mice in conjunction with either U-99194A or saline for 3 days and mice were killed 5 days after the last dose (Fig. 5A). We performed BrdU immunohistochemistry and measured the density of BrdU+ cells in the anterior RMS (Fig. 5B-C). As expected, many BrdU+ cells were found in this region after 5 days (Fig. 5D and E). The D3R antagonist treated groups showed a significant decrease in BrdU+ cell density in the anterior RMS (Fig. 5C-E). This data suggests that D3R antagonism reduced the number of cells which arrived in the OB.
Fig. 5. D3R antagonism decreased the number of newborn neurons reaching the anterior RMS.
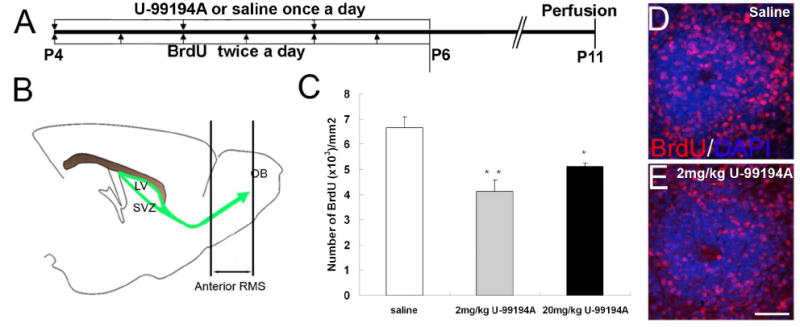
A: Drug treatment plan to label migrating newborn neurons in the anterior RMS/OB core.
B: Schematic of the SVZ system and anterior RMS for the analyzed part.
C: BrdU quantification result in the anterior RMS, * * P<0.01, * means P<0.05
D-E: BrdU staining of the anterior RMS, D: saline treated, E: 2 mg/kg U-99194A treated, scale bar=50 μm.
D3 receptor antagonism decreases proliferation in the SVZ but does not affect label-retaining cells
Based on the specific D3R expression on transit amplifying progenitor cells (Fig. 3), we hypothesized that D3R can mediate dopaminergic effects on SVZ progenitor cell proliferation. To assess the effect of D3R in the transit amplifying progenitors, either U-99194A or saline was given to P4 mice subcutaneously once a day for three days, BrdU administered once with the final dose of U-99194A, and mice killed 2 hours later (Fig. 6A). This short pulse BrdU was designed to primarily detect progenitor cells specifically based on their rapid proliferation, although it would also be predicted to label a small number of mitotic radial glia and neuroblasts (Encinas et al. 2006). D3R antagonism (20 mg/kg U-99194A treatment) significantly decreased the number of BrdU+ cells throughout the SVZ (Fig. 6B-D). These data suggested that proliferation of transit amplifying progenitors was affected by the U-99194A treatment. Next, we examined cells in the SVZ at long term recovery (4 weeks) after BrdU injection (Fig. 6E). These BrdU+ cells (also called label-retaining cells) are likely to be SVZ astrocytes that have derived from radial glia (Tramontin et al. 2003) since slowly dividing and non-motile cells will be the major population retaining BrdU after 4 weeks (Kippin et al. 2005). D3R antagonism did not change the number of label-retaining BrdU+ cells in the entire SVZ (Fig. 6F), suggesting it did not affect rates of SVZ stem cell self-renewal.
Fig. 6. U-99194A treatment decreased the number of progenitor, but not label-retaining cells in the SVZ.
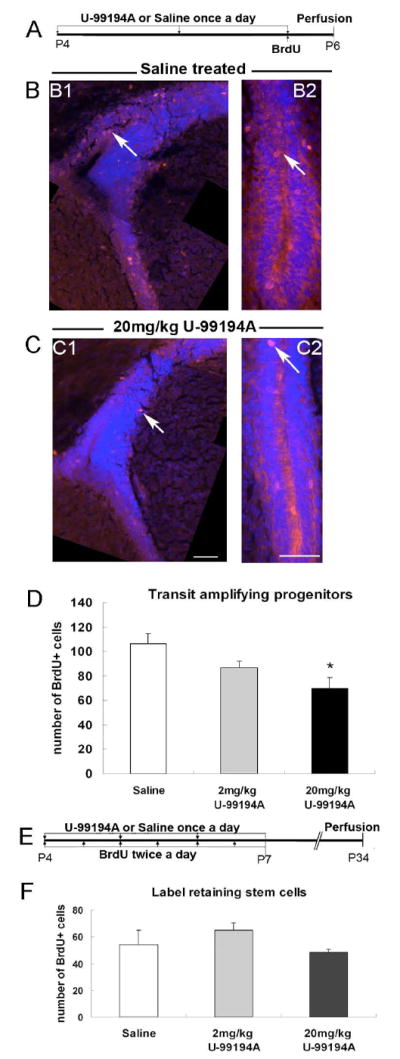
A: Drug treatment plan to detect rapidly proliferating progenitor cells.
B: BrdU immunohistochemistry (red) in saline treated group, B1: dorsal SVZ, B2: ventral SVZ. Arrows point to BrdU+ cells. Blue = Dapi.
C: BrdU immunohistochemistry in 20 mg/kg U-99194A treated group, C1: dorsal SVZ, C2: ventral SVZ, scale bar = 50 μm. Blue = Dapi.
D: Total number of BrdU+ cells in the entire SVZ, * = P<0.05.
E: Drug treatment plan to detect SVZ label-retaining cells.
F: Number of label-retaining cells in the SVZ after D3R antagonism.
Discussion
In this study we show the dopamine D3 receptor is specifically expressed in the murine neurogenic SVZ by transit amplifying progenitor cells and that D3R antagonism decreases SVZ proliferation and OB neurogenesis.
Our D3R expression data in the postnatal mouse SVZ is consistent with previous D3R in situ hybridization studies in rats (Bouthenet et al. 1991; Diaz et al. 1997) as well as with the Allen Brain Atlas (Ng et al. 2009). D3R expression in the SVZ is further supported by a previous report using D3R RT-PCR of laser capture micro-dissected adult SVZ cells (Araki et al. 2007), as well as by D3R-GFP+ SVZ cells in the GENSAT BAC transgenic project (Gong et al. 2003). Elucidating dopamine receptor expression on SVZ subpopulations will help to explain the myriad reported effects of DA on SVZ cells. This has been difficult because of high SVZ cell density, cell surface expression of DA receptors confounding colabeling studies, and lack of reliable DA receptor antibodies. For these reasons, in this study we FACS sorted SVZ cells from genetically labeled reporter mice. FACS sorted Dcx-GFP+ neuroblasts expressed D1R, D2R and D5R receptors at all ages and D4R postnatally. This suggests that perturbations which affect signaling of these receptors may affect neuroblast migration, as has been shown in the embryonic MGE (Crandall et al. 2007). In contrast to the other receptors, D3R was never detected in postnatal and adult SVZ neuroblasts in our studies. The lack of expression by neuroblasts suggested that D3 receptors could be expressed by astrocyte-like stem cells and/or progenitor cells. Indeed, prospective FACSorting using hGFAP-GFP mice to separate SVZ cell types and subsequent RT-PCR indicated that transit amplifying progenitor cells were the sole SVZ neurogenic subtype in which D3R was detected. This expression profile matched previous immuno-electron microscopy (EM) data, showing predominant D2L receptor expression in progenitor cells and D1L expression in neuroblasts (Hoglinger et al. 2004). Thus D3R expression is tightly regulated in the neurogenic lineage, primarily being expressed in the intermediate transit amplifying progenitor cells. Surprisingly, whereas we did not detect D3R in SVZ astrocyte-like stem cells and striatal astrocytes, we did detect it in SVZ niche astrocytes. Unlike striatal astrocytes, niche astrocytes release instructive cues that control neurogenesis (Horner and Palmer 2003). For example, it was recently shown that quinpirole, a D2L agonist, increased SVZ proliferation by increasing ciliary neurotropic factor (CNTF) in SVZ niche astrocytes (Yang et al. 2008). Thus, it is plausible that niche astrocytes also participate to regulate SVZ neurogenesis via D3R signaling.
To study the D3 receptor’s role in the postnatal SVZ, we used a D3R preferential antagonist (U-99194A). U-99194A has 20-fold higher affinity for D3R than D2 receptors and does not bind to any other dopamine receptors (Waters et al. 1993; LaHoste et al. 2000). We provide evidence that D3R antagonism by U-99194A decreased SVZ neurogenesis in vivo by selectively acting on transit amplifying progenitors. Interestingly, U-99194A treatment decreased SVZ proliferation as detected with pulses of BrdU, but our label-retaining data suggests it did not change slow rates of division, which in the adult SVZ are associated with stem cell self-renewal. This well matched our data of D3R expressed by progenitor cells but not stem cells. It also suggests that dopamine normally stimulates SVZ proliferation via D3 receptors. U-99194A decreased proliferation in the dorsal and ventral SVZ despite the fact that D3R was expressed at higher levels ventrally. These results suggest that even low levels of D3R signaling can influence proliferation. We also showed that fewer newborn cells reached the anterior part of the RMS 5 days after BrdU and U-99194A treatment. Although D3 receptors regulate T cell migration (Watanabe et al. 2006), they are unlikely to affect SVZ cell migration because migratory neuroblasts do not express D3R. However, we can not completely rule out the possibility because niche astrocytes might affect neuroblast migration indirectly after D3R stimulation. In fact, 2 mg/kg U-99194A treatment did not change proliferation of progenitor cells, yet significantly fewer cells were found in the RMS 5 days after this U-99194A dose. Thus, this low dose of U-99194A might specifically act on niche astrocytes to regulate SVZ cell migration.
It is possible that, in addition to D3R, U-99194A also blocked D2 receptors. However, we used the same low dose of U-99194A to predominately block D3 receptor effects as in other studies (Rodriguez-Arias et al. 1999; Laszy et al. 2005; Jeanblanc et al. 2006). Moreover, haloperidol treatment in rats and mice increased stem cell self-renewal in vivo via D2R blockade (Kippin et al. 2005) and U-99194A treatment here did not change label-retention of stem cells, suggesting that it did not affect D2R signaling. The effect of D3R stimulation on the rodent SVZ has been controversial. When 7-OH-DPAT, a preferential D3R agonist (Levesque et al. 1992), was given to rats, it stimulated proliferation of stem/progenitor cells in vivo (Van Kampen et al. 2004) and in vitro (Coronas et al. 2004). However, similar treatments by Baker et al., failed to affect cell proliferation in the adult mouse SVZ (Baker et al. 2005). Interesting differences have been noted between rat and mouse neurogenesis (Szele and Chesselet 1996; Goings et al. 2002; Snyder et al. 2009) and it may be that differences in response to the D3R underlie some of these discrepancies. In contrast to Baker et al., our study showed that dopaminergic stimulation of the D3 receptor normally drives mouse postnatal SVZ proliferation and neurogenesis. These discrepancies may be explained by the following differences between our study and Baker’s (Baker et al. 2005). We used early postnatal P4 outbred CD1 mice, and intraperitoneal antagonist (U-99194A) administration for 3 days. We examined its effect on progenitors and stem cells separately by applying either short pulse or label-retaining BrdU protocols and focused our quantification in the anterior and middle part of the SVZ where most D3 receptors are expressed. In contrast, Baker et al., used adult inbred mouse strains and 14 days of continuous D3R agonist (7-OH-DPAT) or U-99194A (Baker et al. 2005) infusion. It is possible that homeostatic feedback loops modulated D3 receptors in Baker’s chronic 14 day experiments and thereby obscured the drugs’ short-term effects. Since we administered U-99194A peripherally, it may have changed dopamine release and thereby affected SVZ neurogenesis. However, similar treatments of U-99194A in vivo did not affect dopamine release in the striatum or the nucleus accumbens (Waters et al. 1993).
Each interneuron subtype in the PG layer is produced in a distinct temporal manner. TH+ cells are born in early development, CalB+ cells in late and early postnatal development, and CalR+ mostly in adults (Batista-Brito et al. 2008). When we blocked the D3R in early postnatal SVZ, CalB+ cell generation was the most significantly decreased among PG layer newborn neuron subtypes. In contrast, CalR+ cell production was not significantly affected in the higher concentration. This data matches the temporal production of PG layer subtypes during our early postnatal treatment. Interestingly, the total number of CalB+ and TH+ cells were at control levels 4 weeks after U-99194A but the total number of CalR+ cells showed significant decreases. This may be explained by late generation of CalR+ cells (Batista-Brito et al. 2008). Decreasing progenitor cells in this critical period could have a profound effect on CalR+ cell genesis.
This study begins to dissect the subregional, cell type, and temporal expression and function of dopamine receptors in the subventricular zone. Recent studies suggested that EGFR+ progenitor cells mediate dopamine’s regulation of SVZ proliferation(O’Keeffe et al. 2009) and over 95% of EGFR+ cells in the SVZ were transit amplifying progenitor cells (Hoglinger et al. 2004). Our current study showed that the D3 receptor is specifically expressed by these neurogenic progenitor cells. Taken together the data suggest that dopaminergic signaling via D3 receptors increases transit amplifying progenitor proliferation and OB neurogenesis. These studies may have clinical relevance: Parkinson’s disease and schizophrenia are characterized by disrupted dopaminergic activity and are treated with drugs that target dopamine receptors. It is possible that reduced olfaction in Parkinson’s patients is due to reduced D3R mediated neurogenesis. Atypical antipsychotics preferentially target the D3R and may also alter human progenitor proliferation. Future studies are needed to clarify the role of the D3R in regulating human neurogenesis.
Acknowledgments
This work was supported by NIH RO1 NS-42253 (FGS). We thank Gregg Stanwood for helpful discussions and advice on the manuscript.
References
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Araki KY, Sims JR, Bhide PG. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res. 2007;1156:31–45. doi: 10.1016/j.brainres.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. D3 dopamine receptors do not regulate neurogenesis in the subventricular zone of adult mice. Neurobiol Dis. 2005;18:523–527. doi: 10.1016/j.nbd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Close J, Machold R, Fishell G. The distinct temporal origins of olfactory bulb interneuron subtypes. J Neurosci. 2008;28:3966–3975. doi: 10.1523/JNEUROSCI.5625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Coronas V, Bantubungi K, Fombonne J, Krantic S, Schiffmann SN, Roger M. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem. 2004;91:1292–1301. doi: 10.1111/j.1471-4159.2004.02823.x. [DOI] [PubMed] [Google Scholar]
- Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Ridray S, Mignon V, Griffon N, Schwartz JC, Sokoloff P. Selective expression of dopamine D3 receptor mRNA in proliferative zones during embryonic development of the rat brain. J Neurosci. 1997;17:4282–4292. doi: 10.1523/JNEUROSCI.17-11-04282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goings G, Wibisono B, Szele F. Cerebral cortex lesions decrease the number of bromodeoxyuridine-positive subventricular zone cells in mice. Neurosci Lett. 2002;329:161–164. doi: 10.1016/s0304-3940(02)00611-0. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Hagg T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist. 2009;15:20–27. doi: 10.1177/1073858408324789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Palmer TD. New roles for astrocytes: the nightlife of an ‘astrocyte’ La vida loca! Trends Neurosci. 2003;26:597–603. doi: 10.1016/j.tins.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, Ron D. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;26:1457–1464. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci. 2005;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaHoste GJ, Henry BL, Marshall JF. Dopamine D1 receptors synergize with D2, but not D3 or D4, receptors in the striatum without the involvement of action potentials. J Neurosci. 2000;20:6666–6671. doi: 10.1523/JNEUROSCI.20-17-06666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszy J, Laszlovszky I, Gyertyan I. Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology (Berl) 2005;179:567–575. doi: 10.1007/s00213-004-2096-z. [DOI] [PubMed] [Google Scholar]
- Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam SC, Kim Y, Dryanovski D, Walker A, Goings G, Woolfrey K, Kang SS, Chu C, Chenn A, Erdelyi F, Szabo G, Hockberger P, Szele FG. Dynamic features of postnatal subventricular zone cell motility: A two-photon time-lapse study. J Comp Neurol. 2007;505:190–208. doi: 10.1002/cne.21473. [DOI] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Ng L, Bernard A, Lau C, Overly CC, Dong HW, Kuan C, Pathak S, Sunkin SM, Dang C, Bohland JW, Bokil H, Mitra PP, Puelles L, Hohmann J, Anderson DJ, Lein ES, Jones AR, Hawrylycz M. An anatomic gene expression atlas of the adult mouse brain. Nat Neurosci. 2009;12:356–362. doi: 10.1038/nn.2281. [DOI] [PubMed] [Google Scholar]
- O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol. 2007;501:825–836. doi: 10.1002/cne.21205. [DOI] [PubMed] [Google Scholar]
- Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Arias M, Felip CM, Broseta I, Minarro J. The dopamine D3 antagonist U-99194A maleate increases social behaviors of isolation-induced aggressive male mice. Psychopharmacology (Berl) 1999;144:90–94. doi: 10.1007/s002130050981. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szele FG, Chesselet MF. Cortical lesions induce an increase in cell number and PSA-N-CAM expression in the subventricular zone of adults rats. J Comp Neurol. 1996;368:439–454. doi: 10.1002/(SICI)1096-9861(19960506)368:3<439::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Hagg T, Robertson HA. Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur J Neurosci. 2004;19:2377–2387. doi: 10.1111/j.0953-816X.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nakayama T, Nagakubo D, Hieshima K, Jin Z, Katou F, Hashimoto K, Yoshie O. Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3. J Immunol. 2006;176:848–856. doi: 10.4049/jimmunol.176.2.848. [DOI] [PubMed] [Google Scholar]
- Waters N, Svensson K, Haadsma-Svensson SR, Smith MW, Carlsson A. The dopamine D3-receptor: a postsynaptic receptor inhibitory on rat locomotor activity. J Neural Transm Gen Sect. 1993;94:11–19. doi: 10.1007/BF01244979. [DOI] [PubMed] [Google Scholar]
- Yang P, Arnold SA, Habas A, Hetman M, Hagg T. Ciliary neurotrophic factor mediates dopamine D2 receptor-induced CNS neurogenesis in adult mice. J Neurosci. 2008;28:2231–2241. doi: 10.1523/JNEUROSCI.3574-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


