Abstract
Rationale: Airway hyperreactivity and remodeling are characteristic features of asthma. Interactions between the airway epithelium and environmental allergens are believed to be important in driving development of pathology, particularly because altered epithelial gene expression is common in individuals with asthma.
Objectives: To investigate the interactions between a modified airway epithelium and a common aeroallergen in vivo.
Methods: We used an adenoviral vector to generate mice overexpressing the transforming growth factor-β signaling molecule, Smad2, in the airway epithelium and exposed them to house dust mite (HDM) extract intranasally.
Measurements and Main Results: Smad2 overexpression resulted in enhanced airway hyperreactivity after allergen challenge concomitant with changes in airway remodeling. Subepithelial collagen deposition was increased and smooth muscle hyperplasia was evident resulting in thickening of the airway smooth muscle layer. However, there was no increase in airway inflammation in mice given the Smad2 vector compared with the control vector. Enhanced airway hyperreactivity and remodeling did not correlate with elevated levels of Th2 cytokines, such as IL-13 or IL-4. However, mice overexpressing Smad2 in the airway epithelium showed significantly enhanced levels of IL-25 and activin A after HDM exposure. Blocking activin A with a neutralizing antibody prevented the increase in lung IL-25 and inhibited subsequent collagen deposition and also the enhanced airway hyperreactivity observed in the Smad2 overexpressing HDM-exposed mice.
Conclusions: Epithelial overexpression of Smad2 can specifically alter airway hyperreactivity and remodeling in response to an aeroallergen. Moreover, we have identified novel roles for IL-25 and activin A in driving airway hyperreactivity and remodeling.
Keywords: asthma, lung, epithelium, smooth muscle, collagen
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Airway epithelial cells are crucial to the origins and progression of asthma. However, the link between altered epithelial gene expression and the development of airway remodeling and hyperreactivity has not yet been explored.
What This Study Adds to the Field
A novel role for activin A and IL-25 in mediating airway remodeling and hyperreactivity in response to the aeroallergen house dust mite in mice with altered epithelial Smad2 gene expression is identified.
Asthma is a complex disease typically characterized by a Th2-type pulmonary inflammatory response and airway hyperreactivity (AHR). Structural changes to the airway wall, termed airway remodeling, are believed to contribute to the decline in lung function observed during chronic disease. Airway remodeling encompasses subepithelial fibrosis, deposition of extracellular matrix (ECM) proteins, increased smooth muscle mass, and mucus gland hyperplasia. It is a generally held belief that continued cycles of Th2 inflammation driven by allergen exposure ultimately lead to the development of remodeling. Recently however, it has been proposed that inflammation and remodeling may occur in parallel rather than sequentially, and that the interaction of the immune system with the lung structural cells is critical in both initiation and development of chronic disease (1).
Airway epithelium represents the site of initial exposure to environmental antigens, which induce allergic airway inflammation in a proportion of atopic individuals. The airway epithelium is crucial to both the origins and progression of asthma (2), and as a result of activation by environmental triggers susceptible asthmatic epithelium creates a microenvironment that supports chronic cycles of injury, inflammation, and airway remodeling (3). Epithelial cells isolated from patients with asthma demonstrate delayed epithelial repair, a change to a mucus-secreting phenotype, and generation of growth factors that drive mesenchymal cell proliferation and differentiation toward increased matrix deposition and smooth muscle production (1, 4, 5).
Growth factors involved in fetal lung development (such as members of the transforming growth factor [TGF]-β family) are also involved in airway wall remodeling in asthma (6). The similarities between wound healing and organ morphogenesis have led to the development of a new concept of chronic tissue inflammation in which there is reactivation of the processes that drive branching morphogenesis in the fetus, where the epithelium and the mesenchyme function as a trophic unit. It is proposed that epithelial damage and Th2 cytokines cooperate to promote functional disturbance of this epithelial mesenchymal trophic unit (EMTU), leading to myofibroblast activation and induction of airway inflammation and remodeling, characteristic of chronic asthma (1, 6, 7).
To investigate the contribution of altered epithelial gene expression to airway inflammation and remodeling we have used an adenovirus encoding Smad2 (a downstream signaling molecule in the TGF-β/activin cascade) to overexpress this protein in airway epithelium before exposure to the environmentally relevant allergen house dust mite (HDM).
Using this model we have determined that epithelial overexpression of Smad2 increased expression of IL-25 and activin A in the lung and potentiated airway remodeling and AHR without affecting inflammatory responses. These data reinforce the vital contribution of the epithelium to allergic pathology and outline a novel in vivo role for activin A and IL-25 in mediating AHR and remodeling after allergen exposure.
Some of the results of these studies have been previously reported in the form of an abstract (8).
METHODS
Animals
Female BALB/c mice 6 to 8 weeks old (Charles River, Morgate, UK) received 15 μg HDM extract (Dermatophagoides pteronyssinus in phosphate-buffered saline [PBS]) (Greer Laboratories, Lenoir, NC) or 15 μl PBS intranasally 3 days a week for up to 3 weeks. Some groups received a first-generation replication-deficient adenovirus serotype 5 containing murine Smad2 cDNA (AdSmad2) (2 × 109 viral particles in 25 μl PBS) or an empty vector lacking a transgene (AdC) 2 days before commencing installation of either HDM or PBS. In blocking experiments mice received either 20 μg of neutralizing antibody to murine activin A or control IgG (R&D Systems, Abingdon, UK) intraperitoneally 2 hours before intranasal challenge with either PBS or HDM.
Measurement of AHR
AHR was determined by direct measurements of resistance and compliance in anesthetized and tracheostomized mice in response to inhaled methacholine (MCh; Sigma, Cambridge, UK) at concentrations of 3 to 100 mg/ml for 1 minute in an EMMS system (EMMS, Hampshire, UK) in a modified version of previously described methods (9, 10).
Sample Preparation
Collection of bronchoalveolar lavage (BAL) and lung cells were performed as previously described (11). Differential cell counts were performed on Wright-Giemsa–stained cytospins.
Western Blotting
Expression of total and phosphorylated Smad2 were assessed after protein fractionation, transfer to polyvinylidene difluoride membranes, and sequential reaction with a rabbit anti-mouse Smad2 (Zymed Laboratories, San Francisco, CA) or pSer465/467 Smad2 Ab (Calbiochem, Nottingham, UK). (More detailed methods are provided in the online supplement.) Immunoblots were incubated with peroxidase-conjugated secondary antibodies and developed using ECL Western blotting detection system (Amersham, Buckinghamshire, UK). Data were normalized to mouse α-tubulin. Densitometry analysis was performed using ImageJ 1.41 software.
Pathology
Paraffin-embedded sections were stained with hematoxylin/eosin to evaluate general morphology. Goblet cells were visualized on periodic acid-Schiff (PAS)-stained lung sections and scored as previously described (12). Collagen deposition was assessed on Sirius red–stained sections. Image analysis was performed using Scion Image (Scion Corporation, Frederick, MD) (13). Epithelial cell height and thickness of the airway smooth muscle layer around medium-sized conducting airways measuring between 150 and 250 μm in diameter were measured. (Additional details are provided in the online supplement.).
Immunohistochemistry
Paraffin sections were stained with rabbit anti-mouse proliferating cell nuclear antigen (PCNA) (Abcam, Cambridge, UK), goat anti-mouse activin A (R&D Systems), and α- smooth muscle actin (α-SMA) (Abcam) using an avidin/biotin staining.
Quantification of Total Lung Collagen
Recently synthesized acid-soluble collagens were measured in lung tissue by biochemical assay according to the manufacturer's instructions (Sircol collagen assay; Biocolor, Belfast, UK) and normalized for tissue weight.
Cytokine Analysis
Lung tissue was homogenized and the supernatant collected for analysis by ELISA. Paired antibodies for murine IL-4, IL-5, TGF-β1, and IFN-γ (PharMingen, Oxford, UK), IL-25, and activin A (R&D Systems) were used in standardized sandwich ELISAs. Kits to measure IL-13 were purchased from R&D Systems. All presented data have been normalized for tissue weight.
Statistical Analysis
Data were analyzed using Prism 4 for Windows from GraphPad Software Inc. Multiple comparisons were performed using Kruskal-Wallis test for nonparametric data and where statistical differences were observed the data sets were further analyzed using a paired Mann-Whitney test. Data are presented as averages ± SEM.
RESULTS
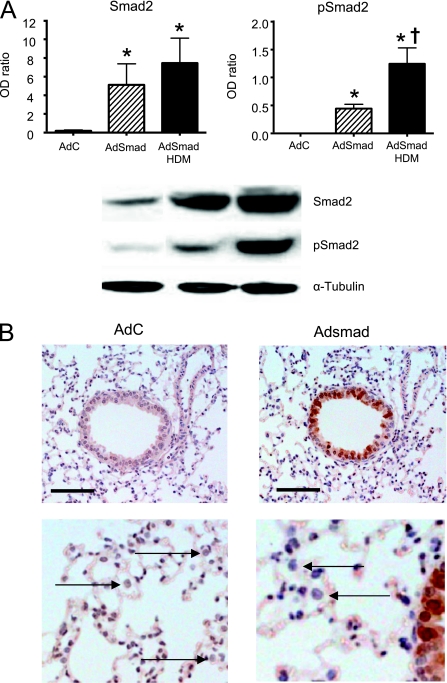
Intranasal instillation of HDM three times a week resulted in lung eosinophilia, a Th2-type immune response, AHR, and airway remodeling similar to that previously reported (14), despite lower and less-frequent dosing. This regime was chosen for all future experiments to enable us to observe any abrogation or enhancement of airway inflammation and/or remodeling parameters as a result of overexpression of Smad2 in the airway epithelium. In preliminary experiments we investigated the effect of administration of AdC on the development of inflammation and airway remodeling compared with mice treated with either PBS or HDM alone. Mice exposed to PBS alone or with control virus showed no significant change in BAL or lung cellular profile compared with naive, untreated mice. Likewise, the inflammatory profile, AHR, and remodeling parameters in mice exposed to HDM alone or with control virus were not different from each other (see online supplement Figure E1). In conclusion, examination of both differential cell counts and lung histology confirmed that the dose of viral vector used did not cause viral-mediated inflammation. Thus, in all subsequent experiments the effects of treatment with AdSmad2 were compared with mice treated with an equal number of AdC viral particles as an appropriate control. An optimal dose of 2 × 109 viral particles delivered in 30 μl of PBS was arrived at based on observations using an adenovirus encoding the marker protein β-galactosidase that resulted in epithelial transgene expression in 30 to 70% of the medium airways 48 hours post intranasal administration in the absence of cellular infiltrate in the lung (data not shown). Using this dosing regimen expression of the transgene was limited to the conducting airways with no significant expression observed in alveolar epithelial cells. Western blotting for total Smad2 in the lung 48 hours post intranasal AdC or Adsmad2 clearly showed an increase in Smad2 protein expression (the inactive form encoded by the adenoviral vector) in the Adsmad2-treated mice indicating expression of the transgene after vector administration (Figure 1). Immunohistochemistry confirmed increased expression of Smad2 in the airway epithelium in these mice with negligible transduction of alveolar epithelial cells or macrophages (Figure 1). At 48 hours post adenoviral administration exogenous gene expression was present in less than 3% of alveolar macrophages and thus does not contribute significantly to viral-mediated gene expression during the allergen challenge phase of the experiment. TGF-β family ligands bind type II receptors, which then recruit type I receptors, which phosphorylate the serine residues of Smad2 and 3 enabling them to form a complex with the co-smad, Smad4. The phosphorylated Smad complex then enters the nucleus where it binds transcription promoters/cofactors of target genes resulting in DNA transcription. Levels of phosphorylated Smad2 (pSmad2) were also increased in the AdSmad2-treated mice and were further increased in the presence of HDM challenge in both AdC and Adsmad2 groups, indicative of active cellular signaling via the Smad2 pathway. Importantly, the amount of pSmad2 detected was greater in the AdSmad2 HDM mice compared with the AdC HDM group (Figure 1).
Figure 1.
Increased protein expression of Smad2 in airway epithelium 2 days after intranasal installation of AdSmad2. (A) Protein levels of total Smad2 and phosphorylated Smad2 (pSmad2) and the housekeeping protein α-tubulin were determined in the lung by Western blotting and quantitated by densitometry. (B) Immunohistochemistry for total smad2 expression demonstrates positively stained epithelial cells of the conducting airways. Staining was absent in alveolar epithelial cells and macrophages (arrows). Photographs are representative examples from each group. Original magnification, ×40. Scale bar = 50 μm. AdC = control adenoviral vector; Adsmad = Smad-expressing adenoviral vector; HDM = house dust mite;
Overexpression of Smad2 in the Airways Does Not Influence HDM-induced Airway Inflammation
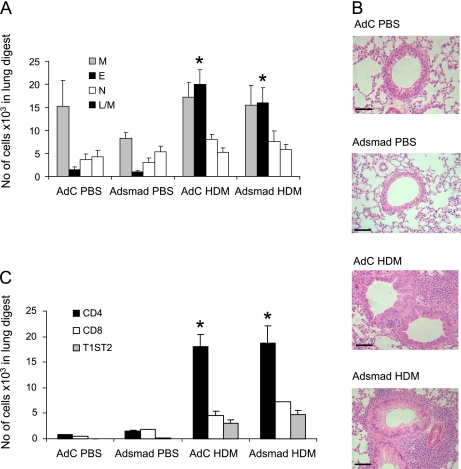
Recruitment of leukocytes to the lung is a characteristic feature of allergen challenge. We found that total cell recruitment to the lung and airway lumen was enhanced in the HDM-exposed groups compared with PBS and was largely due to robust eosinophilic inflammation (Figure 2A). Inflammatory infiltrates were absent from lungs of mice in the AdC and AdSmad2 groups treated with PBS, and there were no observable differences in lung morphology between these two groups (Figure 2B). In contrast, mice administered HDM showed significant perivascular and bronchial infiltrates in the lung. There were no significant differences in the quantity of infiltrating cells or their distribution between the AdC-treated and AdSmad HDM-exposed mice. CD4+ and CD8+ subsets and the proportion of CD4 cells staining for the surrogate Th2 marker T1/ST2 were determined in cells isolated from lung digests by flow cytometry. A significant increase in the population of CD4+ cells (12-fold increase) and T1ST2+/CD4+ cells were detected after 3 weeks of HDM exposure compared with PBS in the lung (Figure 2C). Similar increases in CD4+ and T1/ST2+ T cells were observed in the BAL fluid (data not shown). However, no significant differences in the inflammatory profile were detectable between animals overexpressing epithelial smad2 and those treated with the control vector.
Figure 2.
Inflammatory response in the lung after exposure to house dust mite (HDM) in the presence or absence of epithelial overexpression of Smad2. (A) Differential counts. E = eosinophil; L/M = lymphocyte monocyte; M = macrophage; N = neutrophil. (B) Lung sections stained with hematoxylin and eosin showing peribronchial and perivascular cellular infiltrate. Scale bar = 50 μm. (C) Flow cytometric analysis of T-cell subsets. Data shown represent means ± SEM (n = 6–12). Photographs are representative examples from each group. Original magnification ×40. *P < 0.05 compared with phosphate-buffered saline (PBS) controls.
Epithelial Smad2 Expression Enhances AHR
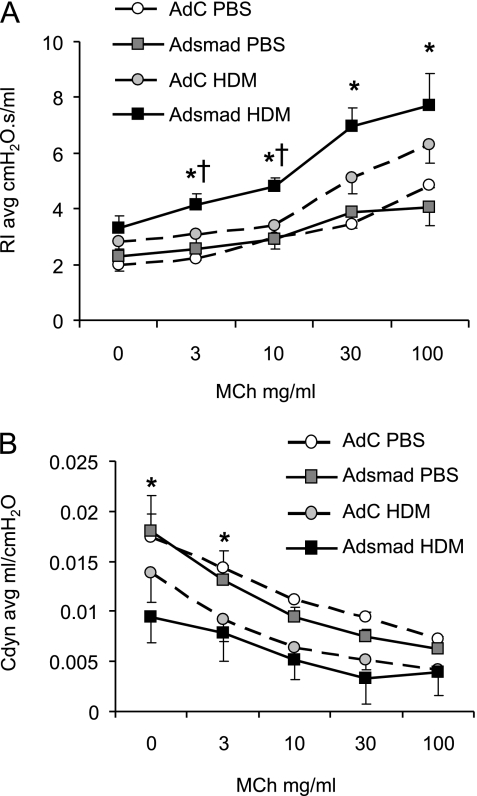
AHR to MCh was determined in anesthetized ventilated mice. Overexpression of Smad2 had no effect on lung function in mice 24 hours after the final challenge because no significant differences were measured between AdC and AdSmad2 groups of mice receiving PBS. However, AdSmad2 mice exposed to HDM showed significantly increased airway resistance in response to increasing doses of MCh when compared with the AdC HDM mice (Figure 3A). Baseline airway compliance was significantly decreased in the AdSmad2 HDM mice compared with PBS-treated controls and lung compliance was further decreased in response to escalating MCh doses (Figure 3B).
Figure 3.
Analysis of airway hyperreactivity to methacholine (MCh) as determined by resistance/compliance measurements in tracheotomized restrained animals at 3 weeks. (A) Increased airway resistance (RI) and (B) decreased airway compliance (Cdyn) in response to increasing doses of MCh. Data shown represent means ± SEM (n = 6). *P < 0.05 compared with phosphate-buffered saline (PBS) control. †P < 0.05 comparing control adenoviral vector (AdC) house dust mite (HDM) with Smad-expressing adenoviral vector (AdSmad) HDM groups.
Overexpression of Epithelial Smad2 Augments Airway Remodeling
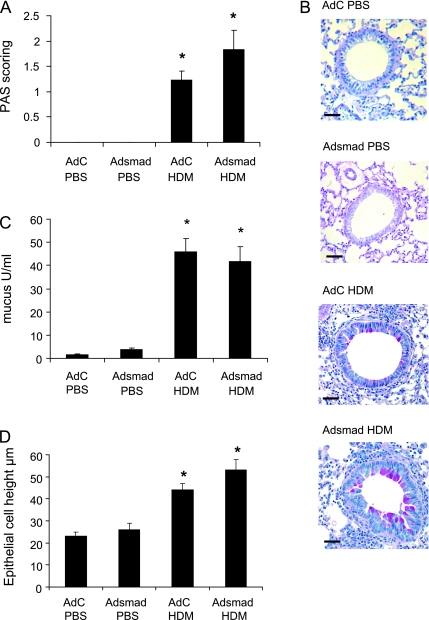
The effect of epithelial Smad2 overexpression on lung tissue pathology is shown in Figures 4–6. PAS staining for mucus was absent from lungs of mice in the AdC and AdSmad2 groups treated with PBS, and there were no observable differences in lung morphology or Sirius red staining for collagen between these two groups.
Figure 4.
Epithelial remodeling in response to house dust mite (HDM) and ectopic epithelial smad2 expression. (A) Scoring of periodic acid-Shiff (PAS)-stained sections. (B) PAS staining demonstrates pink/purple-colored mucin containing cells in the epithelium. Scale bar = 50 μm. (C) Quantification of mucus in the bronchoalveolar lavage fluid by ELISA. (D) Quantitative measurements of epithelial cell height. Data shown represent means ± SEM (n = 6). Photographs are representative examples from each group. Original magnification ×40. *P < 0.05 compared with phosphate-buffered saline (PBS) controls. AdC = control adenoviral vector; Adsmad = Smad-expressing adenoviral vector.
Figure 5.
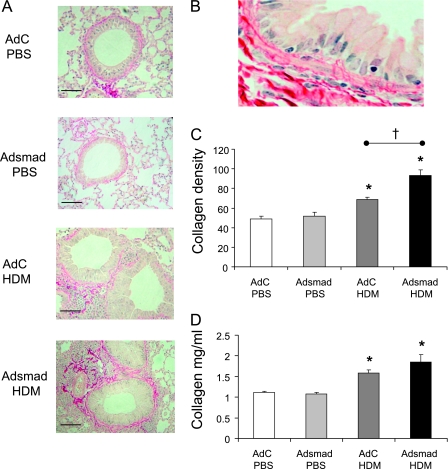
Quantitation of extracellular matrix deposition in mice exposed to house dust mite (HDM) in the context of altered epithelial smad2 expression. (A) Sirius red staining of lung sections depicts perivascular and peribronchiolar collagen (red). (B) High-power magnification of collagen deposition around the airway smooth muscle. (C) Quantitative analysis of subepithelial peribronchiolar collagen density determined by measuring Sirius red–stained collagen in lung sections under polarized light. (D) Total lung collagen was quantified by a biochemical Sircol assay. Data shown represent means ± SEM (n = 6). Photographs are representative examples from each group. Original magnification ×40. Scale bar = 50 μm. *P < 0.05 compared with phosphate-buffered saline (PBS) controls. †P < 0.05 comparing control adenoviral vector (AdC) HDM with Smad-expressing adenoviral vector (AdSmad) HDM groups.
Figure 6.
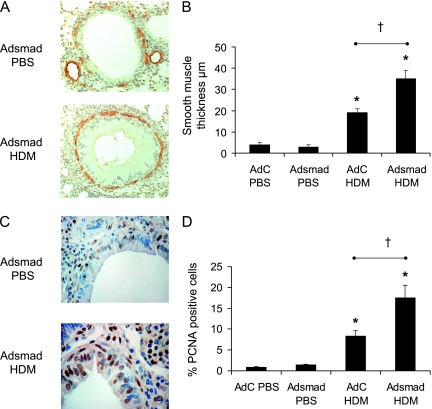
Hypertrophy and hyperplasia of smooth muscle cells. (A) Smooth muscle cells and myofibroblasts were identified by immunohistochemical staining of α–smooth muscle actin (α-SMA). (B) The thickness of the peribronchial smooth muscle layer was measured from α-SMA stained sections. (C) Proliferating cells were identified as those stained positively with an antibody against proliferating cell nuclear antigen (PCNA). (D) Percentage PCNA-positive cells. Data shown represent means ± SEM (n = 6). Photographs are representative examples from each group. Original magnification ×40. Scale bar = 50 μm. *P < 0.05 compared with phosphate-buffered saline (PBS) controls. †P < 0.05 comparing control adenoviral vector (AdC) house dust mite (HDM) with Smad-expressing adenoviral vector (AdSmad) HDM groups.
Epithelial changes.
Marked morphological changes were observed in the HDM-treated groups. PAS staining demonstrated the presence of mucus containing goblet cells in all HDM-treated animals (Figures 4A and 4B). This same trend was observed when mucus in the BALF was measured by ELISA (Figure 4C) and the change in the epithelium to a mucus-secreting phenotype in the HDM-treated animals was associated with an increase in the height of the epithelium (Figure 4D). In PBS-treated animals the average epithelial cell height was 27 μm compared with the height of the hyperplastic goblet cells present in the HDM-exposed animals, which averaged 49 μm. However, despite the significant effect of HDM exposure on epithelial cells, there was no enhanced change after overexpression of Smad2.
Extracellular matrix deposition.
Significant alterations to the subepithelium, most notably an increase in the deposition of collagen, were observed in the mice exposed to HDM (Figure 5). PBS control mice showed little collagen staining around the airways and most of the lung collagen was distributed perivascularly. Peribronchial collagen deposition was clearly increased in the AdC HDM-treated mice and further increased in the HDM mice overexpressing epithelial Smad2 (Figure 5A). It was clear from high-power examination of the histological sections that the collagen was laid down subepithelially between and around the smooth muscle cells (Figure 5B). The density of the peribronchial collagen was determined quantitatively by image analysis on Sirius red–stained lung sections (Figure 5C). Peribronchial collagen deposition was significantly increased by 41% in the AdC HDM-exposed mice compared with control mice. In the AdSmad2 HDM mice the subepithelial collagen accumulation was further increased by 32%. The recently synthesized acid-soluble collagens in the lung were also quantified by a biochemical assay and were increased compared with the PBS-treated animals. However, unlike the peribronchial collagen, which was significantly greater in the AdSmad2 HDM mice compared with AdC HDM-treated animals, total lung collagen, which includes perivascular collagen, was not significantly different between these two groups (Figure 5D).
Smooth muscle cell changes.
From the hematoxylin and eosin–stained lungs in Figure 2B, changes to the smooth muscle layer were visible when AdSmad2 HDM mice were compared with those of either the PBS groups or AdC HDM. To further investigate these changes, sections were immunostained with α-SMA to positively identify myofibroblasts and smooth muscle cells. Single positive-stained cells were observed in the PBS-treated mice and these did not form a continuous layer around the airways (Figure 6A). In contrast, a continuous layer of smooth muscle cells and myofibroblasts were observed around the bronchioles of the AdC HDM mice and this layer was frequently up to two or three cells deep in the AdSmad2 HDM mice. To quantify the increase in smooth muscle mass in the HDM mice the thickness of the airway smooth muscle layer was measured (Figure 6B). There was an increase in the depth of the smooth muscle layer surrounding the bronchioles in the AdC HDM group compared with controls; however, the increased smooth muscle mass was significantly greater (10-fold compared with control mice) in the AdSmad2 HDM-treated group. To determine the extent of airway smooth muscle cell proliferation, sections were immunostained with PCNA (Figure 6C). PCNA is a cell surface antigen expressed by proliferating cells in S-phase of the cell cycle. The number of smooth muscle cells expressing PCNA was calculated as a percentage of the total number of airway smooth muscle cells around a given airway (as described in Reference 11). Smooth muscle hyperplasia, as determined by the percentage of PCNA-positive cells, was significantly increased from less than 1% in PBS treated mice to 8% in the AdC HDM-exposed mice. This index of hyperplasia was further doubled in the Adsmad2 HDM group (Figure 6D).
Enhanced Airway Remodeling Is Associated with Early Increases in Activin A and Interleukin-25
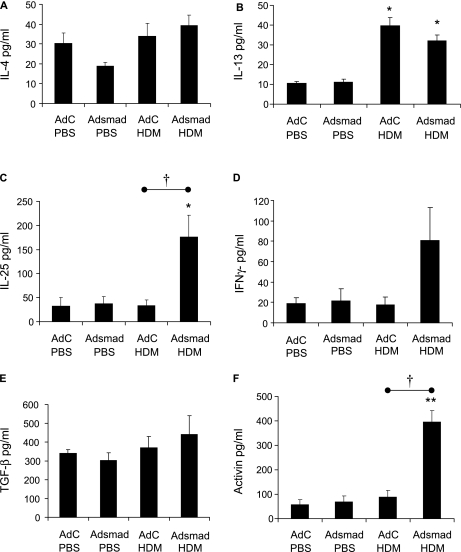
We next sought to identify a mediator that could be responsible for the increased airway remodeling changes observed in the Adsmad2 HDM mice. Significant increases in IL-4, IL-5, IL-13, TGF-β, and activin A were measured in the lung homogenate in the HDM-treated mice compared with the PBS groups (Figure E2). In contrast, the levels of IL-25 and IFN-γ were unchanged. However, despite the increased AHR and airway remodeling in the AdSmad2 HDM-treated mice compared with the AdC HDM group no differences in the levels of any of the mediators tested were observed. We therefore examined groups of mice treated with either the AdC or AdSmad2 after three intranasal challenges with PBS or HDM (1-week protocol) to determine cytokine levels before the induction of airway remodeling. At this early time point there were no significant changes in the levels of IL-4, IL-5, IFN-γ, or TGF-β in the lung between any of the treatment groups (Figure 7). In contrast, IL-13 was increased three- to fourfold in the lungs after HDM treatment, although there was no significant difference between AdC and AdSmad2 HDM-treated mice. However, both IL-25 and activin A were significantly increased in lungs from AdSmad2 HDM mice compared with AdC HDM-treated mice. These data suggest that activin A and IL-25 play a fundamental role in the enhanced AHR and airway remodeling observed in the lung after exogenous expression of Smad2 in the airway epithelium in the HDM-exposed mice. Increased immunohistochemical staining for activin A was observed in the airway epithelium of AdSmad2 HDM-treated mice compared with the AdC HDM group (Figure E3).
Figure 7.
Cytokine and growth factor levels in the lung after 1-week exposure to house dust mite (HDM) in the presence or absence of epithelial overexpression of Smad2. Mediator levels were determined by ELISA in lung homogenate. (A) IL-4, (B) IL-13, (C) IL-25, (D) IFN-γ, (E) transforming growth factor (TGF)-β, (F) activin A. Data shown represent means ± SEM (n = 6). *P < 0.05 compared with phosphate-buffered saline (PBS) controls. †P < 0.05 comparing control adenoviral vector (AdC) house dust mite (HDM) with Smad-expressing adenoviral vector 2 (AdSmad) HDM groups.
Neutralizing Activin A Abrogates Enhanced Airway Remodeling and AHR
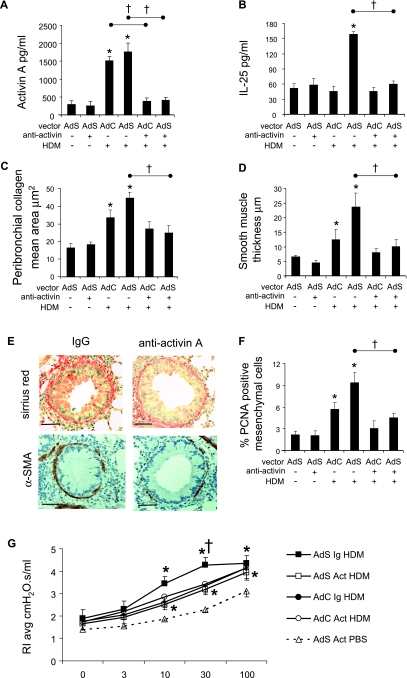
To confirm a role for activin A in promoting airway remodeling we administered a neutralizing antibody to this cytokine 2 hours before each HDM challenge. Blocking activin A had no effect on recruitment of inflammatory cells to the lung or airway lumen and the level of airway eosinophilia was similar in AdC and AdSmad2 HDM-treated mice whether pretreated with the neutralizing antibody or isotype control (Figure E4). Likewise, there was no effect on epithelial mucus secretion. Levels of activin A in the lungs of HDM-exposed anti-activin A–treated mice were comparable with PBS-treated control groups (Figure 8A). Blockade of activin A did not impact the levels of the Th2 cytokines IL-4, IL-5, or IL-13, or the pleiotropic mediator TGF-β measured after 3 weeks of allergen challenge (data not shown). However, the early increase in IL-25 in the lungs of Adsmad2 HDM mice was completely inhibited (Figure 8B). Blocking activin A in the lung resulted in complete abrogation of peribronchial collagen deposition in response to HDM (Figure 8C). Similarly, changes in the smooth muscle layer surrounding the bronchioles were absent in HDM-treated mice receiving the blocking antibody compared with those administered the control IgG. There was an increase in smooth muscle mass surrounding the airways in the AdSmad2 HDM IgG-treated mice as previously described, but there was no enhanced remodeling in the AdSmad2 HDM mice receiving the anti-activin (Figures 8D and 8E). In agreement with these observations there was also no evidence of smooth muscle hyperplasia as assessed by the percentage of peribronchiolar PCNA-positive smooth muscle cells (Figure 8F).
Figure 8.
Blocking activin A in vivo prevents Smad2-mediated airway remodeling and airway hyperresponsiveness (AHR) in response to house dust mite (HDM). Mediator levels were determined by ELISA in lung homogenate prepared from mice treated with either the isotype control (Ig) or neutralizing activin antibody (Act). (A) Activin A after 3-week HDM challenge. (B) IL-25 after 1-week HDM challenge. (C) Quantitative analysis of subepithelial peribronchiolar collagen density determined by measuring Sirius red–stained collagen in lung sections under polarized light. (D) The thickness of the peribronchial smooth muscle layer was measured from α-SMA stained sections. (E) Collagen was stained by Sirius red. Smooth muscle cells and myofibroblasts were identified by immunohistochemical staining of α-smooth muscle actin (α-SMA). Photographs are representative examples from each group. Original magnification ×20. Scale bar = 50 μm. (F) The percentage of peribronchial proliferating cell nuclear antigen (PCNA)-positive mesenchymal cells. (G) Analysis of airway hyperreactivity to methacholine (MCh) as determined by resistance measurements in tracheotomized restrained animals at 3 weeks. Increased airway resistance in response to increasing doses of MCh (for clarity only a single phosphate-buffered saline [PBS] dose-response curve is shown, but all PBS treatment groups were not different from one another). Data shown represent means ± SEM (n = 6). *P < 0.05 compared with PBS controls. †P < 0.05 comparing anti-activin HDM-treated mice with the appropriate Ig control HDM group. AdC = control adenoviral vector; AdS = Smad-expressing adenoviral vector.
Blocking Activin A Prevents the Smad2 Mediated Enhancement of AHR
Airway resistance in response to increasing doses of MCh was significantly increased in AdSmad2 HDM IgG mice compared with AdC HDM IgG as previously described, and this increase in airway hyperreactivity was completely abolished in the AdSmad2 HDM-exposed mice receiving the activin A neutralizing antibody (Figure 8G). Collectively, these studies directly demonstrate that activin A signaling is critically involved in the enhanced AHR observed in AdSmad2-treated mice exposed to the clinically relevant aeroallergen, HDM.
DISCUSSION
We set out to investigate the role of altered epithelial gene expression on the development of airway remodeling in an HDM model of allergic airways disease and chose to use an adenoviral vector encoding Smad2 to perturb the airway epithelium. We have determined that epithelial overexpression of Smad2 specifically promoted enhanced airway hyperreactivity and smooth muscle and matrix remodeling without increasing airway inflammation. Interestingly, this effect was associated with the specific induction of activin-A and IL-25, rather than IL-13, in the lungs of allergen-exposed mice. Blocking activin A with a neutralizing antibody prevented the increase in IL-25 and abolished the observed collagen deposition, smooth muscle hyperplasia, and enhanced AHR. These data provide evidence that development of AHR and airway remodeling are not necessarily dependent on inflammation but may be driven directly by cytokines such as activin A and IL-25. These data further contribute to our understanding of disease evolution in asthma and are important given that there is a lack of effective treatments that can directly impact airway remodeling in this disease.
Smad2 is an essential arm of both the smad-dependent TGF-β and activin signaling pathways, and there is extensive evidence documenting the importance of TGF-β in airway remodeling (15). Smad2 is of interest since phosphorylated Smad2 (pSmad2) expression has been found to be increased in the epithelium of individuals with asthma as determined by immunostaining in clinical biopsies (16) and in the lungs of ovalbumin (OVA)-challenged mice (17). Ectopic Smad2 expression has also been shown to enhance TGF-β–induced epithelial–mesenchymal transition in vitro (18). Epithelial overexpression of Smad2 in vivo did not modulate collagen deposition or other markers of lung fibrosis in the absence of allergen challenge (AdSmad2 PBS group). This is not unexpected because Smad2 needs to be phosphorylated to translocate to the nucleus and bind smad-responsive elements in the promoter region of target genes.
Th2-type inflammation is characteristic of asthma and is believed to be critically important for disease pathogenesis (19, 20). In our study, exposure of mice to inhaled low-dose HDM was characterized by the hallmarks of allergic asthma, namely lung eosinophilia, Th2-type cytokines, AHR, and airway remodeling, as previously described (14). Epithelial overexpression of Smad2 did not seem to modulate T-cell function, because there was no change in the numbers of T-cell subsets or in levels of Th2-associated cytokines (IL-4, 5, 13) in the lung at 3 weeks. There was also no effect on humoral immunity because serum levels of IgE and IgG1 were unchanged between the AdC and AdSmad2 HDM groups of mice. Previous studies have shown that IL-13 plays a pivotal role in the development of AHR, eosinophil recruitment (via the secretion of chemokines such as eotaxin), as well as the structural changes to the lung that encompass airway remodeling (13, 21, 22). Although we detected IL-13 in the lung and BAL after only 1 week of HDM challenge, there was no significant increase with Smad2 overexpression in the epithelium. Additionally, epithelial mucous production and goblet cell hyperplasia, which are induced by IL-13, were not greater in the Adsmad2 mice compared with the AdC group. Therefore, it is unlikely that the enhanced AHR and remodeling initiated by Smad2 overexpression was due to IL-13. In contrast, we observed greatly enhanced production of activin A and IL-25 specifically in the lungs of mice overexpressing Smad2 in the airway epithelium.
Both TGF-β and activin A were up-regulated in the lung tissue in response to 3 weeks of inhaled HDM in the present study. However, at an early time point, corresponding to only three challenges with allergen, the levels of activin A in the lung are differentially increased in the AdSmad2 HDM mice, whereas there are no significant changes in the TGF-β activity in the lung at this time point in response to either allergen challenge or altered epithelial gene expression. The most potent activator of TGF-β1 expression is TGF-β itself (23). Epithelial overexpression of Smad2 did not affect auto-induction of TGF-β expression, although this is not unexpected as the autocrine signaling loop is believed to be mediated via smad 3/4 complexes as opposed to Smad2/4 (24, 25). Activin A is a dimeric glycoprotein belonging to the TGF-β superfamily (26). In common with TGF-β, it shares Smad2 as an intracellular signaling target and both cytokines share functions in inflammatory reactions, including tissue repair. This overlap in function and signal transduction is also observed for the target genes induced by both (27). Activin A is expressed in biopsy specimens from patients with asthma and in mouse models of asthma and has been suggested to provide a link between acute allergen-specific T-cell responses and chronic TGF-β1–mediated airway remodeling in asthma (26). Activin A has previously been shown to have an immunomodulatory role in a murine model of acute allergic airway inflammation using OVA as a surrogate allergen (28). However, in the HDM model, in which sensitization occurs at the mucosal surfaces of the lung, blocking activin A had no effect on the pulmonary inflammatory profile. Instead, the effects of activin A are fibrogenic, with this mediator having a profound effect on collagen deposition. The differential requirements to develop airway remodeling between OVA- and HDM-based models has been extensively discussed by Fattouh and Jordana (29). It has previously been shown using neutralizing antibodies to TGF-β that this cytokine mediates OVA-induced airway remodeling (11); however, remodeling can develop independently of TGF-β after respiratory exposure to HDM extract (30). The data presented in the current study suggest that activin A signaling via Smad2 is responsible for driving airway remodeling after HDM challenge. The relative importance of different mediators in different murine models of allergic airway disease is indicative of the heterogeneity of the disease and may also reflect the existence of distinct asthma phenotypes (31). Activin A has been demonstrated to stimulate the proliferation of various cell types, including lung fibroblasts and smooth muscle cells (32–34) and like TGF-β can induce the expression of collagen and other components of the ECM (27, 35). Importantly, in this context, TGF-β–induced collagen production has been shown to depend on an autocrine activin A signaling loop (36). Additionally, α-SMA expression in myofibroblasts also depends on activin A (36). In keeping with the hypothesis that reactivation of the EMTU is fundamental to airway remodeling in asthma, activin A is induced in the wound-healing process in skin within 15 to 24 hours after injury (37, 38). Activin could therefore act as an inducer of ECM molecules in the mesenchymal compartment as it has been shown to stimulate fibronectin expression and type I collagen mRNA (35, 38). This is the first study to link activin A with IL-25 and airway remodeling. However, in vitro treatment of primary normal human bronchial epithelial cells with activin A did not induce IL-25 and likewise activin A was not induced by treatment of the cells with IL-25, suggesting that regulation of IL-25 by activin A is complex and requires interaction with additional cell types and mediators likely present in the EMTU in vivo (data not shown). Blocking activin A prevented the allergen-induced increase in pulmonary IL-25 levels, suggesting that in this model system IL-25 acts downstream of activin to modulate airway remodeling and AHR.
IL-25 (IL-17E) is a member of the IL-17 cytokine family and is believed to play a role in the development of Th2-type immunity (39, 40). Transgenic expression of IL-25 in the bronchial epithelium results in mucus production and recruitment of macrophages and eosinophils to the airways (39). Similarly, intranasal instillation of recombinant IL-25 promotes AHR, eosinophilic inflammation, mucus hypersecretion, and production of Th2-type cytokines in the lung (41). In contrast, blockade of IL-25 reduces airway inflammation and Th2 cytokine production in an acute allergen-induced asthma model (39, 42). Interestingly, the latter study suggested that IL-25–mediated AHR occurred independently of the inflammatory response, because intranasal instillation of IL-25 caused AHR in naive il4−/−il5−/−il9−/−il13−/− mice (42). In our study, HDM-treated mice overexpressing Smad2 had significantly increased levels of IL-25 in the lungs after 1 week of allergen challenge and showed enhanced AHR but no increase in Th2 cell number or IL-13 levels compared with AdC mice. The lack of enhancement of Th2-type inflammation despite elevated levels of IL-25 in the lung may be due to the local microenvironment. We did not measure significant increases in the BAL, and all previous studies have relied on delivery of recombinant IL-25 protein directly to the airway lumen. Interestingly, the study by Ballantyne and colleagues (42) determined that IL-25–induced AHR occurred via either an IL-13–dependent or IL-13–independent pathway, implicating a key role for IL-25 in the changes in lung function that are indicative of allergen challenge. Previous studies have determined that although IL-13 is critical for the initiation of AHR, other mechanisms are important in maintenance of AHR (43). Our data implicate IL-25 via activin A in this process. It is possible that the early (after three HDM challenges) induction of IL-25 in Smad2-expressing mice promoted the AHR and remodeling pathology observed at later stages in disease. Previous studies suggest a role for IL-25 in acute Th2-type inflammation; however, the present study is the first to implicate IL-25 in the promotion of airway remodeling.
Immunohistochemical analysis of biopsies from subjects with asthma reveals that the IL-25 receptor is abundant in smooth muscle layers (44). Although IL-25 does not directly induce smooth muscle contraction, stimulation of cells with IL-25 increases their expression of components of the extracellular matrix, namely procollagen-α1 and lumican mRNA (44). Instillation of a single dose of IL-25 to the airways of mice has been shown to result in AHR, which is maintained for at least 16 days (41). Conversely, blocking IL-25 in an acute experimental model of allergic asthma has been demonstrated to prevent AHR (42). We have also demonstrated a reduction in AHR using a neutralizing antibody to activin A, which resulted in an inhibition of HDM-induced IL-25 release in mice overexpressing Smad2 in the airway epithelium. Importantly, the critical role of IL-25 in the induction of AHR has been shown to be independent of the inflammatory response because administration of IL-25 to naive IL-4/5/9/13 knockout mice results in significant AHR. These data provide compelling evidence for the involvement of IL-25 in the initiation and perpetuation of airway remodeling during allergic airways disease. It is interesting to note that although a causative link to asthma has not been established, the IL-25 gene maps to a postulated asthma susceptibility locus (45).
The use of transient adenovirus-mediated gene transfer to investigate lung inflammation has previously been described (46). Epithelial overexpression of IL-1β induces marked tissue injury and inflammation, whereas expression of active TGF-β induces severe and progressive fibrosis in rodent lung without apparent inflammation (47–49). In these experiments both vector treatments induced a fibrotic response in the absence of allergen challenge extending over days involving the entire lung and pleural surface. In the current study, airway remodeling changes were restricted to the peribronchiolar airways rather than lung parenchyma, making this a more relevant model to investigate aberrant epithelial mesenchymal cell interaction during allergic disease. In all these studies it is interesting to note that despite the transient nature of exogenous gene expression from adenoviral vectors the features of fibrosis and airway remodeling persist, suggesting that the remodeling process, once initiated, is self-perpetuating. These data reinforce the importance of the local microenvironment at the EMTU to the development of remodeling and support the hypothesis that the epithelium is central to the development of pathophysiological symptoms after inhalation of allergen.
This is the first report to describe how epithelial overexpression of a gene can specifically influence AHR and airway remodeling in response to an aeroallergen without affecting the inflammatory profile. The data therefore support the hypothesis that inflammation, AHR, and remodeling can be dissociated from each other and may be separately regulated. Moreover, we have identified novel roles for activin A and IL-25 in driving airway remodeling and AHR. We show that perturbations of the airway epithelium are capable of driving structural changes linked to airway remodeling. Thus, the model lends further support to the idea that the epithelium is central to asthma pathogenesis (2). This alternative view of the pathogenesis of asthma is important to open up new therapeutic avenues that focus on protection of the epithelium from external environmental stimuli rather than suppression of Th2-driven inflammation.
Supplementary Material
Acknowledgments
The authors thank Matthew Bell for assistance with the flow cytometric analysis and lung function measurements and Lorraine Lawrence for histological sectioning and staining.
Supported by the Wellcome Trust (Ref 05574). C.M.L. is a Welcome Senior Fellow. AdSmad2 was a gift from Paschalis Sideris and Evangelos Andreakos, Foundation for Biomedical Research, Academy of Athens.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200905-0725OC on March 25, 2010
Conflict of Interest Statement: L.G.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.P.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.M.L. received $1,001–$5,000 from MedImmune in consultancy fees, $1,001–$5,000 from GlaxoSmithKline in lecture fees, and more than $100,001 from Leti in industry-sponsored grants.
References
- 1.Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int 2008;57:1–10. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc 2009;6:655–659. [DOI] [PubMed] [Google Scholar]
- 3.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol 2003;111:215–225. [DOI] [PubMed] [Google Scholar]
- 4.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 2003;8:432–446. [DOI] [PubMed] [Google Scholar]
- 5.Knight D. Increased permeability of asthmatic epithelial cells to pollutants. Does this mean that they are intrinsically abnormal? Clin Exp Allergy 2002;32:1263–1265. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Yssel H, Vignola AM. Is allergic asthma associated with delayed fetal maturation or the persistence of conserved fetal genes? Allergy 2000;55:1194–1197. [DOI] [PubMed] [Google Scholar]
- 7.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc 2004;1:93–98. [DOI] [PubMed] [Google Scholar]
- 8.Gregory LG, Mathie SM, Lloyd CM. Epithelial overexpression of smad2 exacerbates airway hypereactivity and remodeling after aeroallergen challenge [abstract]. Proc Am Thor Soc 2008;177:A294. [Google Scholar]
- 9.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol 1988;64:2318–2323. [DOI] [PubMed] [Google Scholar]
- 10.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 1997;156:766–775. [DOI] [PubMed] [Google Scholar]
- 11.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-β antibody: effect on the smad signaling pathway. J Immunol 2005;174:5774–5780. [DOI] [PubMed] [Google Scholar]
- 12.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 2000;13:573–583. [DOI] [PubMed] [Google Scholar]
- 13.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory LG, Causton B, Murdoch JR, Mathie SA, O'Donnell V, Thomas CP, Priest FM, Quint DJ, Lloyd CM. Inhaled house dust mite induces pulmonary T helper 2 cytokine production. Clin Exp Allergy 2009;39:1597–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-β in airway remodeling in asthma. Immunol Cell Biol 2007;85:348–356. [DOI] [PubMed] [Google Scholar]
- 16.Sagara H, Okada T, Okumura K, Ogawa H, Ra C, Fukuda T, Nakao A. Activation of TGF-β/Smad2 signaling is associated with airway remodeling in asthma. J Allergy Clin Immunol 2002;110:249–254. [DOI] [PubMed] [Google Scholar]
- 17.Rosendahl A, Checchin D, Fehniger TE, ten Dijke P, Heldin CH, Sideras P. Activation of the TGF-β/activin-smad2 pathway during allergic airway inflammation. Am J Respir Cell Mol Biol 2001;25:60–68. [DOI] [PubMed] [Google Scholar]
- 18.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-β and the smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell 2005;16:1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meiler F, Zimmermann M, Blaser K, Akdis CA, Akdis M. T-cell subsets in the pathogenesis of human asthma. Curr Allergy Asthma Rep 2006;6:91–96. [DOI] [PubMed] [Google Scholar]
- 20.Kay AB. The role of T lymphocytes in asthma. Chem Immunol Allergy 2006;91:59–75. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Curr Allergy Asthma Rep 2004;4:123–131. [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, Karin M, Roberts AB. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol 1990;10:1492–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardoux C, Derynck R. JNK regulates expression and autocrine signalling of TGF-beta1. Mol Cell 2004;15:170–171. [DOI] [PubMed] [Google Scholar]
- 25.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Mol Cell Biol 1999;1:260–266. [DOI] [PubMed] [Google Scholar]
- 26.Karagiannidis C, Hense G, Martin C, Epstein M, Ruckert B, Mantel PY, Menz G, Uhlig S, Blaser K, Schmidt-Weber CB. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J Allergy Clin Immunol 2006;117:111–118. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Weber CB, Blaser K. Regulation and role of transforming growth factor-beta in immune tolerance induction and inflammation. Curr Opin Immunol 2004;16:709–716. [DOI] [PubMed] [Google Scholar]
- 28.Semitekolou M, Alissafi T, Aggelakopoulou M, Kourepini E, Kariyawasam HH, Kay AB, Robinson DS, Lloyd CM, Panoutsakopoulou V, Xanthou G. Activin-A induces regulatory T cells that suppress T helper cell immune responses and protect from allergic airway disease. J Exp Med 2009;206:1769–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fattouh R, Jordana M. TGF-beta, eosinophils and IL-13 in allergic airway remodeling: a critical appraisal with therapeutic considerations. Inflamm Allergy Drug Targets 2008;7:224–236. [DOI] [PubMed] [Google Scholar]
- 30.Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC Jr, Lonning S, Gauldie J, et al. Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling. Am J Respir Crit Care Med 2008;177:593–603. [DOI] [PubMed] [Google Scholar]
- 31.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R Jr., Castro M, Curran-Everett D, Fitzpatrick AM, et al. for the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med 2010;181:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojima I, Mogami H, Kawamura N, Yasuda H, Shibata H. Modulation of growth of vascular smooth muscle cells by activin A. Exp Cell Res 1993;206:152–156. [DOI] [PubMed] [Google Scholar]
- 33.Matsuse T, Ikegami A, Ohga E, Hosoi T, Oka T, Kida K, Fukayama M, Inoue S, Nagase T, Ouchi Y, et al. Expression of immunoreactive activin A protein in remodeling lesions associated with interstitial pulmonary fibrosis. Am J Pathol 1996;148:707–713. [PMC free article] [PubMed] [Google Scholar]
- 34.Sakurai T, Abe Y, Kasuya Y, Takuwa N, Shiba R, Yamashita T, Endo T, Goto K. Activin A stimulates mitogenesis in swiss 3T3 fibroblasts without activation of mitogen-activated protein kinases. J Biol Chem 1994;269:14118–14122. [PubMed] [Google Scholar]
- 35.Sugiyama M, Ichida T, Sato T, Ishikawa T, Matsuda Y, Asakura H. Expression of activin A is increased in cirrhotic and fibrotic rat livers. Gastroenterology 1998;114:550–558. [DOI] [PubMed] [Google Scholar]
- 36.Wada W, Kuwano H, Hasegawa Y, Kojima I. The dependence of transforming growth factor-beta-induced collagen production on autocrine factor activin A in hepatic stellate cells. Endocrinology 2004;145:2753–2759. [DOI] [PubMed] [Google Scholar]
- 37.Munz B, Hubner G, Tretter Y, Alzheimer C, Werner S. A novel role of activin in inflammation and repair. J Endocrinol 1999;161:187–193. [DOI] [PubMed] [Google Scholar]
- 38.Hubner G, Hu Q, Smola H, Werner S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev Biol 1996;173:490–498. [DOI] [PubMed] [Google Scholar]
- 39.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med 2007;204:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol 2006;118:606–614. [DOI] [PubMed] [Google Scholar]
- 41.Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, Foster PS. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy 2006;36:1575–1583. [DOI] [PubMed] [Google Scholar]
- 42.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol 2007;120:1324–1331. [DOI] [PubMed] [Google Scholar]
- 43.Leigh R, Ellis R, Wattie J, Donaldson DD, Inman MD. Is interleukin-13 critical in maintaining airway hyperresponsiveness in allergen-challenged mice? Am J Respir Crit Care Med 2004;170:851–856. [DOI] [PubMed] [Google Scholar]
- 44.Lajoie-Kadoch S, Joubert P, Letuve S, Halayko AJ, Martin JG, Soussi-Gounni A, Hamid Q. TNF-alpha and IFN-gamma inversely modulate expression of the IL-17E receptor in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L1238–L1246. [DOI] [PubMed] [Google Scholar]
- 45.A genome-wide search for asthma susceptibility loci in ethnically diverse populations. The Collaborative Study on the Genetics of Asthma (CSGA). Nat Genet 1997;15:389–392. [DOI] [PubMed] [Google Scholar]
- 46.Stampfli MR, Wiley RE, Neigh GS, Gajewska BU, Lei XF, Snider DP, Xing Z, Jordana M. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest 1998;102:1704–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans 2007;35:661–664. [DOI] [PubMed] [Google Scholar]
- 48.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 2001;107:1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997;100:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.