Abstract
Rationale: Studies have shown that reducing sedation of critically ill patients shortens time on the ventilator and in the intensive care unit (ICU). Little is known, however, of how such strategies affect long-term cognitive, psychological, and functional outcomes.
Objectives: To determine the long-term effects of a wake up and breathe protocol that interrupts and reduces sedative exposure in the ICU.
Methods: In this a priori planned substudy conducted at one tertiary care hospital during the Awakening and Breathing Controlled Trial, a multicenter randomized controlled trial, we assessed cognitive, psychological, and functional/quality-of-life outcomes 3 and 12 months postdischarge among 180 medical ICU patients randomized to paired daily spontaneous awakening trials with spontaneous breathing trials (SBTs) or to sedation per usual care plus daily SBTs.
Measurements and Main Results: Cognitive impairment was less common in the intervention group at 3-month follow-up (absolute risk reduction, 20.2%; 95% confidence interval, 1.5–36.1%; P = 0.03) but not at 12-month follow-up (absolute risk reduction, −1.9%; 95% CI, −21.3 to 27.1%; P = 0.89). Composite cognitive scores, alternatively, were similar in the two groups at 3-month and 12-month follow-up (P = 0.80 and 0.61, respectively), as were symptoms of depression (P = 0.59 and 0.82) and posttraumatic stress disorder (P = 0.59 and 0.97). Activities of daily living, functional status, and mental and physical quality of life were similar between groups throughout follow-up.
Conclusions: In this trial, management of mechanically ventilated medical ICU patients with a wake up and breathe protocol resulted in similar cognitive, psychological, and functional outcomes among patients tested 3 and 12 months post-ICU. The proven benefits of this protocol, including improved 1-year survival, were not offset by adverse long-term outcomes.
Clinical trial registered with www.clinicaltrials.gov (NCT 00097630).
Keywords: sedation, cognitive impairment, depression, PTSD
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Much debate exists over the effects of strategies that reduce sedation in critically ill patients, particularly as they relate to cognitive and mental health outcomes. There are some concerns related to possible negative psychological consequences that could develop in individuals who receive decreased sedation.
What This Study Adds to the Field
Our study adds to current knowledge by demonstrating that individuals receiving decreased sedation do not experience any more adverse long-term consequences than their counterparts.
Hundreds of thousands of critically ill patients around the world each year are treated with mechanical ventilation and other invasive therapies that can induce pain and anxiety. To prevent or relieve these symptoms, intensive care unit (ICU) practitioners treat more than 80% of such patients with sedative and analgesic medications (1). In fact, the usual practice in many ICUs has been to moderately or heavily sedate patients (2, 3), perhaps to ensure that there will be little or no recall of events because of concerns that patients who remember their ICU experience may have adverse psychological sequelae (4). Deviation from this usual care approach is viewed by some as inherently “risky” and dangerous to the overall health and safety of patients with critical illness (5).
Despite the unquestioned short-term usefulness of sedatives, these medications can delay extubation and ICU discharge unless delivered in a judicious way. Several randomized clinical trials have clearly elucidated the short-term benefits of approaches to lighter sedation, including protocols that promote daily interruption of sedatives or intermittent boluses rather than continuous infusions (6–8), but key questions remain unanswered regarding the long-term consequences of such approaches, specifically regarding the long-term psychological effects of daily interruption of sedatives (4, 5). Data from recent observational studies and one small nonrandomized clinical trial suggest that, contrary to traditional thinking, sedative medications may contribute to adverse psychological outcomes rather than prevent them. Jones and colleagues demonstrated that patients who experience sedative-induced delusions while in the ICU, for example, are more likely to develop posttraumatic stress disorder (PTSD) than patients who have factual memories of their ICU stay (9), and higher doses of benzodiazepines have been associated with PTSD symptoms months after discharge (10). Patients in another study who were managed with daily sedative interruption had fewer PTSD symptoms than did controls (11). Additionally, sedative exposure may be one factor that leads to the adverse cognitive outcomes now recognized to occur in a significant portion of ICU survivors (12). Recent evidence suggests that up to half of those who survive a critical illness have cognitive impairment a year or more after discharge (13), but no study to date has assessed the relationship between sedative exposure and long-term cognitive outcomes.
In contrast to the widely held view that decreasing sedative exposure might be harmful, we hypothesized that interrupting sedation and reducing sedation exposure via a wake up and breathe protocol would improve long-term cognitive, psychological, and functional outcomes for mechanically ventilated ICU patients. To test our hypotheses, we prospectively studied the long-term outcomes of patients enrolled at the largest site of the Awakening and Breathing Controlled (ABC) Trial (8), a randomized controlled trial that evaluated the efficacy and safety of a wake up and breathe approach designed to interrupt and reduce sedative exposure during management of critically ill patients.
METHODS
Design Overview
In this a priori–planned substudy of a multicenter randomized controlled trial, we assessed long-term cognitive, psychological, and functional outcomes at 3 and 12 months after discharge among patients enrolled in the ABC Trial at Saint Thomas Hospital. Because of limited personnel and funding, patients enrolled in the ABC Trial at other medical centers were not enrolled in the long-term outcomes component of the trial. The long-term outcomes component was designed to be conducted as a single-center randomized trial nested within the larger multicenter trial, maintaining balance between treatment groups at the single study center via use of a stratified randomization scheme. All study methods used at this study center before hospital discharge were identical to those used at the other ABC Trial study centers.
Setting and Participants
We recruited study participants at a large, private tertiary care medical center, Saint Thomas Hospital (Nashville, TN), between October 2003 and March 2006. Vanderbilt Coordinating Center (Nashville, TN) supervised the trial, and the Vanderbilt University and Saint Thomas Hospital institutional review boards approved the study protocol. Informed consent was initially obtained from authorized surrogates and later from participants themselves.
Study personnel screened all medical ICU patients each day to identify adult patients (>18 yr of age) who required mechanical ventilation for more than 12 hours. Exclusion criteria were admission after cardiopulmonary arrest, continuous mechanical ventilation more than 2 weeks before potential enrollment, moribund state and/or withdrawal of life support, profound neurological deficits (e.g., large stroke or severe dementia) that prevented patients from living independently, and enrollment in another clinical trial. Additionally, we excluded patients who underwent cardiac surgery or neurosurgery or had a stroke before or during the trial from the long-term component of the trial.
Randomization and Intervention
Patients were randomly assigned in a 1:1 manner to management with a wake up and breathe protocol that paired daily spontaneous awakening trials (SATs) with spontaneous breathing trials (SBTs) (the intervention group) or to usual care, consisting of patient-targeted sedation and an SBT protocol (the control group). We used a unique computer-generated, permuted-block randomization scheme at each study center (i.e., a stratified randomization scheme); thus, patients enrolled in the long-term component of the trial represent a single population randomized to treatment assignment. After informed consent was obtained, before data were collected, local study personnel opened a consecutively numbered, sealed opaque envelope containing a tri-folded piece of paper with the treatment assignment. The investigator conducting all follow-up patient evaluations (J.C.J.) was blinded to treatment group allocation.
A detailed description of the trial protocol is provided elsewhere (8, 14). Throughout the trial, patients in both treatment groups were managed with patient-targeted sedation per the study center's usual practice; sedative and analgesic doses were titrated to maintain the level of arousal and comfort deemed clinically appropriate for each patient. Additionally, patients in the intervention group were assessed each morning with an SAT safety screen to determine whether or not an SAT was safe. Those passing the screen underwent cessation of all sedative medications as well as analgesics as long as pain was judged adequately treated. Patients who passed the SAT (i.e., opened their eyes to verbal stimulus without meeting any failure criteria or tolerated the SAT more than 4 hours despite not opening their eyes) were immediately managed with the SBT protocol, which began with an SBT safety screen to determine whether or not an SBT was appropriate. Patients who passed the SBT safety screen underwent an SBT, during which ventilatory support was discontinued; patients breathed through a T-tube circuit or a ventilatory circuit with continuous positive airway pressure of 5 cm H2O or pressure support ventilation of less than 7 cm H2O. If the patient tolerated the SBT for 2 hours without signs of distress, the patient's physician was verbally notified. Otherwise, full ventilatory support was restarted. If sedatives or analgesics were judged necessary at any time during or after the SAT or SBT, they were restarted at half the previous dose and titrated to achieve patient comfort.
As previously described, patients in the control group received sedation according to usual care (i.e., patient-targeted sedation), and they were managed with the aforementioned SBT protocol. Every patient was monitored using a validated sedation scale (Richmond Agitation and Sedation Scale) to assess depth of sedation (15).
Cognitive, Psychological, and Functional Outcomes
We assessed each patient's cognitive, psychological, and functional status at 3 and 12 months postdischarge. In addition, at study enrollment, a surrogate provided information on each patient's premorbid cognitive, psychological, and functional status. When surrogates reported that a patient had baseline cognitive impairment (which was not severe enough to prevent them from living independently and therefore did not exclude them from trial enrollment) and for all patients older than 60 years of age, we used a validated surrogate questionnaire, the Short Informant Questionnaire of Cognitive Decline in the Elderly (Short IQCODE), to determine the presence or absence of preexisting cognitive impairment (16–18). Per our a priori plan, patients with a Short IQCODE score greater than or equal to four were considered to have preexisting cognitive impairment (19), presumably of mild to moderate severity, because we excluded patients from study enrollment with dementia that prevented them from living independently. Patients were not excluded from study enrollment on the basis of Short IQCODE scores.
At 3 and 12 months postdischarge, a neuropsychologist (J.C.J.) assessed each patient in person with a comprehensive battery of cognitive, psychological, and functional/quality-of-life measures (Table 1). These assessments were primarily done in patients' homes, although a small number were done at Saint Thomas Hospital. Testing was conducted in a quiet environment and in a standardized fashion. Patients who were delirious at the time of their scheduled assessment—as determined by the Confusion Assessment Method for the ICU (20, 21)—were not evaluated at that time but were tested at a later date when this assessment was negative.
TABLE 1.
COGNITIVE, PSYCHOLOGICAL, AND FUNCTIONAL ASSESSMENTS
| Test (Ref. no.) | Description | Area Measured | Scoring |
|---|---|---|---|
| Cognitive Assessments | |||
| Digit Span (57) | Subject listens to and then immediately repeats a progressively longer sequence of digits forward and backward | Attention/concentration, working memory | Age-adjusted T-scores obtained using Wechsler Adult Intelligence Scale-Third Edition (57) |
| Digit Symbol Coding (57) | Subject copies symbols into a series of empty numbered boxes using an answer key | Information processing speed | Age-adjusted T-scores obtained using Wechsler Adult Intelligence Scale-Third Edition (57) |
| MMSE (58) | Briefly surveys a wide range of cognitive domains | Global mental status | Age- and education-adjusted T-scores obtained using Crum et al. (59) |
| RAVLT (60) | Tests immediate and delayed memory and learning using a 15-item word list, which is repeated to the subject across numerous trials | Immediate and delayed memory, learning | Age-adjusted T-scores obtained using Spreen and Strauss (61) |
| Rey-Osterreith Complex Figure – Copy (62) | Subject copies a picture of a complex geometric figure | Visual-spatial construction | Age-adjusted T-scores obtained using Spreen and Strauss (61) |
| Rey-Osterreith Complex Figure – Delayed Recall (62) | Subject draws a picture of a complex geometric figure 30 min after viewing the figure | Visual memory | Age-adjusted T-scores obtained using Spreen and Strauss (61) |
| Trailmaking Test A (63) | Subject draws a line between a series of consecutive numbers during a timed test | Attention | T-scores adjusted for age, education, and sex obtained using the Heaton manual (64) |
| Trailmaking Test B (63) | Subject draws a line between a series of alternating numbers and letters according to a specified sequence during a timed test | Executive functioning | T-scores adjusted for age, education, and sex obtained using the Heaton manual (64) |
| Verbal Fluency Test (65) | During three 1-min trials, subject generates as many words as possible beginning with the letters F, A, and S | Language (verbal fluency), executive functioning | T-scores adjusted for age, education, and sex obtained using the Heaton manual (64) |
| Psychological assessments | |||
| Awareness Questionnaire (66) | Subject and surrogate rate the patient's physical, mental and social functioning | Patient self-awareness, accuracy of patient perceptions | 34 questions (17 for patient/17 for surrogate) with a range of 0-5 each, with low scores indicating impairment |
| BDI-II (67) | Brief screening tool assesses the presence and severity of depressive symptoms | Depression | 21 questions with a range of 0–3 each (total range, 0–63), with scores > 10 suggesting depression |
| PTSS-10 for the ICU (68) | Brief screening tool assesses the presence and severity of PTSD-related symptoms | PTSD Symptoms | 10 questions with a score of 0–7 each (total range, 0–70), with scores > 35 suggesting PTSD |
| Functional assessments | |||
| FAQ (69) | Assesses higher order functioning (e.g., managing finances, medications, etc.) | Independent activities of daily livings | 10 questions with a range of 0–3 each (total range, 0–30), with scores ≥ 9 indicating impaired functioning |
| Katz ADL (70) | Assesses basic abilities such as bathing, feeding, and transferring | Basic functional abilities | 6 questions (total range, 0–18), with high scores indicating dependence |
| SF-36 (71) | Assesses quality of life and overall functioning across a range of physical and mental health domains | Generic quality of life | 36 questions across 8 domains, each with a range of 0–100, with low scores indicating poor quality of life |
Definition of abbreviations: ADL = activities of daily living; BDI-II = Beck Depression Inventory-II; FAQ = Functional Activities Questionnaire; MMSE = Mini-mental state examination; PTSD = posttraumatic stress disorder; PTSS-10 = Posttraumatic Stress Scale-10; RAVLT = Rey Auditory Verbal Learning Test; SF-36 = Short Form-36.
Using normative population data, all cognitive test results were converted to T-scores, (i.e., equivalent standard scores in a normal distribution with a mean of 50 and a standard deviation of 10). To identify cognitive impairment, we compared each patient's cognitive test scores to the normative population data and adhered to widely used psychometric definitions (22–24), categorizing patients as having cognitive impairment if they (1) scored 1.5 SDs or more below the mean on two or more of the nine cognitive tests, or (2) scored 2 or more SDs below the mean on one or more of the nine cognitive tests. By using this conservative definition of cognitive impairment, we ensured that patients identified as impaired had clinically significant deficits that were outside the range of normal cognitive functioning. This approach contrasts with less restrictive approaches that have been used in other studies; for example, a study of cognitive functioning in patients with liver disease diagnosed cognitive impairment if patients scored 1 or more SDs below the mean on any one of the cognitive tests administered (22). To further quantify each patient's cognitive performance using a continuous scale, we also calculated a composite score by averaging the T-scores of all nine cognitive tests. Use of such a composite cognitive score, which has been used in a variety of investigations (25–27), minimizes floor and ceiling effects and reduces the risk of type I error when multiple cognitive tests are used (28, 29).
Results from psychological, functional, and quality-of-life assessments were examined as raw scores and were used to identify dysfunction dichotomously using widely-used validated cutoffs on individual tests (Table 1).
Patients were followed from enrollment until 1-year follow-up or death via monthly telephone calls. Additionally, electronic medical records and a commercial version of the Social Security Death Master File (30) were used to confirm vital status for patients who were not tested during the follow-up period.
Statistical Analysis
Sample size was determined based on calculations using the trial's primary outcome, ventilator-free days (8). A separate power analysis regarding the outcomes examined in this long-term substudy was not performed. Instead, long-term outcomes were measured as previously stated in patients enrolled at Saint Thomas Hospital because of limitations in funding available to hire personnel with expertise in the measurement of cognitive outcomes. Data were analyzed with an intention-to-treat approach; patients with follow-up data were analyzed in the group to which they were randomized. We used Pearson chi-square test to compare categorical variables between the study groups and the Wilcoxon-Mann-Whitney two-sample rank-sum test to compare continuous variables. A two-sided α of 0.05 was used to indicate statistical significance. We used R (version 2.4 patched) for all statistical analyses (31).
RESULTS
Patient Characteristics and Management
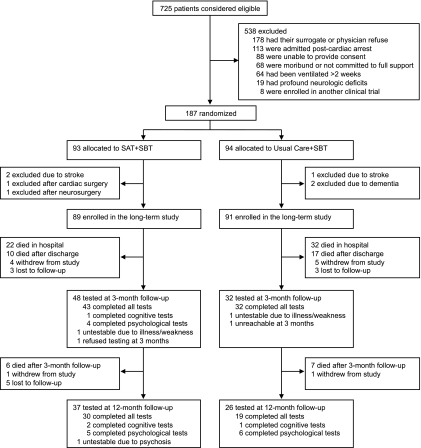
Figure 1 shows the trial follow-up. Between October 2003 and March 2006, 725 patients were identified as eligible at Saint Thomas Hospital. Of the 187 patients randomized in the ICU, 180 were eligible for and included in the long-term study (89 in the intervention group and 91 in the control group). Follow-up was completed in March 2007. These two groups were similar according to all baseline variables (Table 2). According to surrogates, patients' baseline activity of daily living and instrumental activity of daily living abilities did not differ between treatment groups. Using the Short IQCODE, preexisting cognitive impairment was identified in 10% of patients in the control group and 10% in the intervention group.
Figure 1.
Enrollment, randomization, and follow-up. Patients were assessed 3 and 12 months after discharge. From screening to the completion of follow-up, horizontal arrows indicate those patients who were not evaluated during the remainder of follow-up due to exclusion, death, study withdrawal, or loss to follow-up. Vertical arrows indicate patients (even if they were not assessed at one time point) for whom follow-up at a later time point was achieved. SAT = spontaneous awakening trial; SBT = spontaneous breathing trial.
TABLE 2.
BASELINE AND IN-HOSPITAL CHARACTERISTICS
| Characteristic | Intervention (n = 89) | Control (n = 91) | P Value |
|---|---|---|---|
| Age, yr | 65 (53–73) | 68 (56–76) | 0.13 |
| Female, % (n/total) | 46 (41/89) | 55 (50/91) | 0.16 |
| APACHE II | 28 (22–34) | 28 (21–33) | 0.74 |
| SOFA | 9 (7–12) | 9 (6–12) | 0.55 |
| Admission diagnoses, % (n/total) | 0.13 | ||
| Sepsis/ARDS | 39 (35/89) | 45 (41/91) | |
| Myocardial infarction/CHF | 17 (15/89) | 27 (25/91) | |
| COPD/asthma | 12 (11/89) | 8 (7/91) | |
| Altered mental status | 10 (9/89) | 8 (7/91) | |
| Hepatic or renal failure | 8 (7/89) | 3 (3/91) | |
| Malignancy | 1 (1/89) | 0 (0/91) | |
| Alcohol withdrawal | 1 (1/89) | 0 (0/91) | |
| Other | 11 (10/89) | 9 (8/91) | |
| Preexisting cognitive impairment, % (n/total) | 10 (9/89) | 10 (9/91) | 0.84 |
| Baseline ADL category, % (n/total) | 0.26 | ||
| Fully independent | 88 (68/77) | 83 (58/70) | |
| Partially dependent | 5 (4/77) | 13 (9/70) | |
| Totally dependent | 6 (5/77) | 4 (3/70) | |
| Baseline IADL impairment, % (n/total) | 17 (12/69) | 21 (14/68) | 0.47 |
| Preenrollment sedative exposure | |||
| Lorazepam equivalents, mg | 6 (2–22) | 10 (2–39) | 0.42 |
| Fentanyl equivalents, μg | 300 (100–1,400) | 252 (75–2,850) | 0.69 |
| Propofol | 5,070 (2,290–8,825) | 2,600 (1,310–7,395) | 0.04 |
| Sedative exposure during trial | |||
| Lorazepam equivalents, mg | 21 (5–83) | 42 (10– 296) | 0.04 |
| Fentanyl equivalents, μg | 1,800 (210–9,800) | 3,300 (210–22,360) | 0.19 |
| Propofol | 7,900 (3,300–15,300) | 11,390 (3,780–19,210) | 0.55 |
Definition of abbreviations: ADL = activities of daily living; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; IADL = instrumental activities of daily living. SOFA = Sequential Organ Failure Assessment.
Data are presented as median (interquartile range) unless otherwise noted.
Preenrollment exposure to benzodiazepines and opiates was similar between study groups (Table 2). Before enrollment, patients in the intervention group received more propofol than patients in the control group (P = 0.04), but preenrollment propofol dose was not associated with long-term outcomes. Management with the wake up and breathe protocol was associated with a significant reduction in total exposure to benzodiazepines while in the ICU (P = 0.04), whereas total doses of opiates and propofol administered in the ICU were not significantly different between treatment groups.
Success in Obtaining Long-Term Follow-up
The percentage of eligible patients (excluding those who died or met exclusion criteria but including patients who withdrew or were lost to follow-up) receiving follow-up assessments at 3-month and 12-month follow-up was 81% (80 of 99 eligible patients were tested) and 73% (63 of 86 eligible patients were tested), respectively.
Cognitive Outcomes
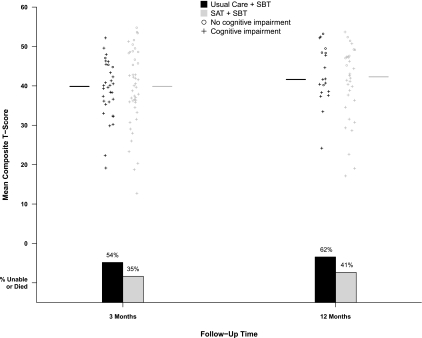
Cognitive impairment was pervasive at 3-month follow-up (Table 3 and Figure 2), occurring in 79% of all patients evaluated. Although fewer patients in the intervention group than in the control group were cognitively impaired at 3-month follow-up (absolute risk reduction [ARR], 20.2%; 95% confidence interval [CI], 1.5–36.1%; P = 0.03), composite cognitive scores were similar in the two groups (P = 0.80). Additionally, the prevalence of cognitive impairment was no longer different between groups 12 months after enrollment, with cognitive impairment remaining common (ARR, −1.9%; 95% CI, −21.3 to 27.1%; P = 0.89). Similarly, composite cognitive scores at 12-month follow-up were again similar between groups (P = 0.61).
TABLE 3.
LONG-TERM OUTCOMES
| 3-mo Follow-up |
12-mo Follow-up |
|||||
|---|---|---|---|---|---|---|
| Outcome | Intervention | Control | P Value | Intervention | Control | P Value |
| Cognitive | ||||||
| Composite T-score | 40 (36–47) | 40 (36–45) | 0.80 | 42 (36–47) | 42 (38–48) | 0.61 |
| Impaired, % | 70 | 91 | 0.03 | 72 | 70 | 0.89 |
| Psychological | ||||||
| Depression | ||||||
| BDI-II score | 13 (7–20) | 11 (7–17) | 0.43 | 12 (5–20) | 14 (6–20) | 0.59 |
| Prevalence, % | 64 | 58 | 0.59 | 59 | 62 | 0.82 |
| Posttraumatic stress disorder | ||||||
| PTSS-10 score | 22 (12–29) | 20 (14–26) | 0.83 | 23 (16–31) | 22 (15–34) | 0.60 |
| Prevalence, % | 14 | 10 | 0.59 | 24 | 24 | 0.97 |
| Functional | ||||||
| ADL impairment, % | 19 | 15 | 0.36 | 11 | 8 | 0.30 |
| Functional disability, % | 18 | 10 | 0.32 | 6 | 4 | 0.76 |
| Quality of life | ||||||
| SF-36 scores | ||||||
| Mental component | 53 (42–57) | 53 (43–57) | 0.93 | 54 (46–60) | 51 (41–62) | 0.78 |
| Physical component | 27 (19–32) | 28 (23–36) | 0.50 | 25 (21–41) | 29 (22–36) | 0.87 |
| Worse than baseline, %* | 72 | 74 | 0.84 | 64 | 87 | 0.05 |
Definition of abbreviations: ADL = activities of daily living; BDI-II = Beck Depression Inventory-II; PTSS-10 = Posttraumatic Stress Scale-10; SF-36 = Short Form-36.
Median (interquartile range) unless otherwise specified.
According to the Awareness Questionnaire.
Figure 2.
Cognitive outcomes and mortality at 3-month and 12-month follow-up according to treatment group. At each period of follow-up, every patient who was able to undergo cognitive testing is represented by a single symbol, which displays their cognitive outcome in two ways: (1) A plus symbol indicates the patient had cognitive impairment according to a priori criteria (see text), and a circle symbol indicates the patient did not have cognitive impairment. (2) Position along the Y-axis shows the patient's mean composite T-score, which is an average of their T-scores on nine individual cognitive tests. Normal performance on one of the nine individual tests would be a T-score between 40 and 60, with higher scores reflecting better performance. Medians of the composite T-scores according to treatment group are displayed as horizontal lines beside each scatter plot. Because the scatterplots (and lines representing medians) do not account for potential confounding due to death, bar graphs show the percentages of patients who had died or were too ill to undergo testing (only two patients at 3 months and no patients at 12 months). This graph shows that composite cognitive scores among those tested (summarized by horizontal lines) were similar between treatment groups at 3- and 12-month follow-up, but more patients in the intervention group survived without cognitive impairment (shown as circles vs. plus symbols) than in the control group.
Psychological Outcomes (Depression and PTSD Symptoms)
At 3-month follow-up, clinically significant symptoms of depression were observed in 64% of patients in the intervention group and 58% of those in the control group (Table 3). Symptoms remained largely the same at 12-month follow-up, with 59% of patients in the intervention group and 62% of patients in the control group reporting the presence of significant depression. Significant PTSD symptoms were less common than symptoms of depression, occurring in 14% of patients in the intervention group and 10% of those in the control group at 3-month follow-up and in 24% of patients in each group at 12-month follow-up.
Functional Outcomes
A large percentage of patients in both groups were discharged to long-term care facilities (global P = 0.21); 8% of patients in the intervention group were discharged to nursing homes compared with 15% of patients in the control group and 2% versus 5% were discharged to assisted living facilities. Alternatively, 62% of patients in the intervention group were discharged home compared with 51% of patients in the control group.
At both 3- and 12-month follow-up, functional status and quality of life were similar in the two treatment groups (Table 3). According to the Awareness Questionnaire, three of every four patients in both groups reported worse overall functional outcomes at 3-month follow-up compared with premorbid functioning. The percentage of patients, however, who reported their overall functional status was worse at 12-month follow-up than before their critical illness was lower in the intervention group than in the control group (absolute risk reduction, 23.3%; 95% CI, 0–42.3%; P = 0.05).
DISCUSSION
In this randomized controlled trial of mechanically ventilated medical ICU patients, patients managed with a wake up and breathe protocol, which paired daily spontaneous awakening trials (i.e., interruption of sedatives) with spontaneous breathing trials, experienced similar long-term cognitive, psychological, functional, and quality-of-life outcomes as those managed with usual care. Additionally, patients in the intervention group were less likely to report significant functional decline 1 year after ICU discharge than patients in the control group. Our results, along with those of a recent randomized trial of light versus deep sedation (32), challenge the suspicion that decreasing sedation—even temporarily each morning—might be harmful to patients' long-term psychological and overall well-being. This concern likely fuels resistance to daily interruption of sedation and drives the common global practice of heavy and prolonged sedation in the ICU. In light of the previously published benefits of the wake up and breathe protocol, including a 14% absolute survival advantage and 4-day reductions in ICU and hospital lengths of stay (8), our study's findings should usher in a new approach to the management of mechanically ventilated patients.
Cognitive impairment was pervasive among survivors in our trial, affecting 79% and 71% of patients able to undergo testing at 3- and 12-month follow-up, respectively. Symptoms of depression and PTSD, impaired functional status, and reduced quality of life were also common, although not as prevalent as cognitive impairment. These results are consistent with previous research showing that survivors of critical illness are at high risk for long-term cognitive, psychological, and functional disability (13, 33, 34). To date, at least a dozen prospective cohort publications have assessed cognitive and/or psychological outcomes in patients who survived critical illness managed in medical, surgical, or trauma ICUs (35–47). The prevalence of cognitive impairment reported in these studies has varied considerably, with deficits observed in 20 to 75% of patients up to a year or more after discharge. The rates of impairment observed in the ABC Trial are in the upper end of these ranges, possibly due to the advanced age of many patients included in this investigation. Whereas earlier studies included populations with average ages between 45 and 55 years, the median age among patients enrolled in the long-term component of the ABC Trial was 66 years, with one-quarter of these patients 75 years of age or older. Although not yet shown in ICU cohorts, human and animal studies suggest that advanced age is a risk factor for cognitive impairment after traumatic brain injury, coronary artery bypass graft surgery, and hypoxemia for a variety of reasons, including decreased cognitive or brain reserve (48–52). Based on the Short IQCODE, only 10% of our patients had preexisting cognitive impairment, implying that the high prevalence of cognitive impairment during follow-up is due to development of an ICU-acquired cognitive insult. Our conservative definition of cognitive impairment—designed to ensure that patients classified with long-term cognitive impairment had clinically significant deficits—required a degree of cognitive deficits that would be detectable to patients in their daily life as well as to their surrogates. These effects may be amplified for those patients with concomitant depression or PTSD.
Notable strengths of our trial include a randomized study design, the breadth of outcomes assessed, higher follow-up rates than those achieved in earlier similar studies, and blinding of the investigator who conducted all follow-up evaluations. Randomization in a clinical trial facilitates equal distribution of potential confounders, both measured and unmeasured. Thus, potential confounding of the trial results due to preexisting cognitive impairment—which was balanced between treatment groups, according to a validated surrogate instrument—should be reduced through appropriate randomization. Similar to the comprehensive methods used in some cardiac bypass surgery and stroke trials (48, 53, 54), the cognitive battery used in our trial tested a broad range of cognitive domains. Individual instruments in the battery were selected because they are psychometrically robust and yet tolerable to patients, giving the battery a balance of methodological rigor and feasibility (54).
Because the wake up and breathe protocol reduced the likelihood of death, which was a common outcome in this critical care trial, our analyses of outcomes among patients tested could be significantly confounded by the differential mortality in the two groups. If risk factors for death (e.g., older age and ICU delirium) also increased the risk of long-term cognitive impairment, patients who might have died if not managed with the intervention were likely at high risk for poor cognitive outcomes; the survival benefit increased the number of such patients in the intervention group, potentially biasing the trial toward the null and against our finding improved cognitive outcomes in the intervention group. Other limitations include the single-center design (which limits generalizability to populations similar to those we enrolled and reduces sample size), incomplete follow-up, the use of self-report questionnaires for some outcomes rather than formal diagnostic instruments (which were too time-consuming to administer to patients who also completed an extensive battery of cognitive tests), and our inability to directly assess premorbid cognitive or psychological function. In studies of ICU survivors, investigators have used the Posttraumatic Stress Scale-10 more often than any other measurement of PTSD symptoms (33), but this self-report questionnaire was based on the PTSD criteria outlined in the now-outdated third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III) (55) rather than the more expansive criteria included in the current DSM-IV-TR (56). As with most critical care trials, patients could not be identified before their acute critical illness and thus could not be directly assessed for preexisting cognitive or psychological dysfunction. Thus, a surrogate questionnaire rather than direct testing was used to assess for baseline cognitive impairment according to previously published ICU methodology (54). The results of the validated Short IQCODE questionnaire (16–18) indicate that baseline cognitive impairment was evenly distributed between treatment groups, as would be expected in a randomized trial. Unfortunately, no comparable surrogate-based method exists to reliably assess for preexisting depression or PTSD. Finally, the current study was not powered to prove differences or equivalence for some outcomes; sample size was based on estimated improvements in short-term outcomes, and no power calculations were performed regarding potential effects on the long-term outcomes presented herein. Nevertheless, the results do not allude to any long-term harm associated with the intervention, and a very large trial would be needed to demonstrate noninferiority for any psychological outcomes, one that would likely be deemed unethical as patients in the control group would be exposed to increased risk for worse in-hospital outcomes as well as for death (8).
In conclusion, our trial found that, compared with usual care sedation and ventilation weaning practices, a wake up and breathe protocol that pairs daily spontaneous awakening trials (i.e., interruption of sedatives) with spontaneous breathing trials for the management of mechanically ventilated medical ICU patients resulted in similar cognitive, psychological, functional, and quality-of-life outcomes among patients tested 3 and 12 months after their ICU stay. Despite widespread concerns regarding the potential long-term risks of interrupting or reducing sedation in the ICU, the wake up and breathe approach results in improved short- and long-term outcomes and does not increase the risk of adverse cognitive, psychological, or other outcomes.
Supported by the Saint Thomas Foundation (Nashville, TN); National Institutes of Health grants AG001023, HL007123, and RR024975; Veterans Affairs Tennessee Valley Geriatric Research, Education, and Clinical Center (GRECC); Hartford Geriatrics Health Outcomes Research Scholars Award Program; and Vanderbilt Physician Scientist Development Program.
Originally Published in Press as DOI: 10.1164/rccm.200903-0442OC on March 18, 2010
Conflict of Interest Statement: J.C.J. received $10,001–$50,000 from MedImmune in consultancy fees and up to $1,000 from Rhode Island Hospital in lecture fees. T.D.G. received $1,001–$5,000 from Hospira Inc. in lecture fees for presentation of research findings and holds $1,001–$5,000 in stock ownership or options in Pfizer Inc. S.M.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.L.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.K.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.W.W.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.T.P. received $10,001–$50,000 from Hospira, Inc. in lecture fees. A.E.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.G.D. is employed by Saint Thomas Health Services as a Research Coordinator and received up to $1,000 from the Academy for Continued Healthcare Learning in lecture fees. G.R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.W.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Arroliga AC, Thompson BT, Ancukiewicz M, Gonzales JP, Guntupalli KK, Park PK, Wiedemann HP, Anzueto A. Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Crit Care Med 2008;36:1083–1088. [DOI] [PubMed] [Google Scholar]
- 2.Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit. Crit Care Med 2007;35:393–401. [DOI] [PubMed] [Google Scholar]
- 3.Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, Binhas M, Genty C, Rolland C, Bosson JL. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology 2007;106:687–695. [DOI] [PubMed] [Google Scholar]
- 4.Heffner JE. A wake-up call in the intensive care unit. N Engl J Med 2000;342:1520–1522. [DOI] [PubMed] [Google Scholar]
- 5.Brochard L. Sedation in the intensive-care unit: good and bad? Lancet 2008;371:95–97. [DOI] [PubMed] [Google Scholar]
- 6.Brook AD, Ahrens TS, Schaiff R, Prentice D, Sherman G, Shannon W, Kollef MH. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 1999;27:2609–2615. [DOI] [PubMed] [Google Scholar]
- 7.Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471–1477. [DOI] [PubMed] [Google Scholar]
- 8.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126–134. [DOI] [PubMed] [Google Scholar]
- 9.Jones C, Griffiths RD, Humphris G, Skirrow PM. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 2001;29:573–580. [DOI] [PubMed] [Google Scholar]
- 10.Girard TD, Shintani AK, Jackson JC, Gordon SM, Pun BT, Henderson MS, Dittus RS, Bernard GR, Ely EW. Risk factors for posttraumatic stress disorder symptoms following critical illness requiring mechanical ventilation: a prospective cohort study. Crit Care 2007;11:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med 2003;168:1457–1461. [DOI] [PubMed] [Google Scholar]
- 12.Herridge MS, Batt J, Hopkins RO. The pathophysiology of long-term neuromuscular and cognitive outcomes following critical illness. Crit Care Clin 2008;24:179–199. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest 2006;130:869–878. [DOI] [PubMed] [Google Scholar]
- 14.Girard TD. Wake up and breathe flowchart. (Accessed June 14, 2010.) Available from: http://www.icudelirium.org/docs/WakeUpAndBreathe.pdf
- 15.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–1344. [DOI] [PubMed] [Google Scholar]
- 16.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994;24:145–153. [DOI] [PubMed] [Google Scholar]
- 17.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med 1989;19:1015–1022. [DOI] [PubMed] [Google Scholar]
- 18.Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med 1991;21:785–790. [DOI] [PubMed] [Google Scholar]
- 19.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr 2004;16:275–293. [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–2710. [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001;29:1370–1379. [DOI] [PubMed] [Google Scholar]
- 22.Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology 2002;35:440–446. [DOI] [PubMed] [Google Scholar]
- 23.Vardy J, Wong K, Yi QL, Park A, Maruff P, Wagner L, Tannock IF. Assessing cognitive function in cancer patients. Support Care Cancer 2006;14:1111–1118. [DOI] [PubMed] [Google Scholar]
- 24.Mathiesen HK, Jonsson A, Tscherning T, Hanson LG, Andresen J, Blinkenberg M, Paulson OB, Sorensen PS. Correlation of global N-acetyl aspartate with cognitive impairment in multiple sclerosis. Arch Neurol 2006;63:533–536. [DOI] [PubMed] [Google Scholar]
- 25.Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer's disease. J Neurol Neurosurg Psychiatry 2006;77:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samra SK, Giordani B, Caveney AF, Clarke WR, Scott PA, Anderson S, Thompson BG, Todd MM. Recovery of cognitive function after surgery for aneurysmal subarachnoid hemorrhage. Stroke 2007;38:1864–1872. [DOI] [PubMed] [Google Scholar]
- 27.Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol 2009;66:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand 2001;45:275–289. [DOI] [PubMed] [Google Scholar]
- 29.Mollica CM, Maruff P, Vance A. Development of a statistical approach to classifying treatment response in individual children with ADHD. Hum Psychopharmacol 2004;19:445–456. [DOI] [PubMed] [Google Scholar]
- 30.Social Security Death Index. Available at: http://ssdi.rootsweb.com
- 31.Ihaka R, Gentleman RR. a language for data analysis and graphics. J Comput Graph Statist 1996;5:299–314. [Google Scholar]
- 32.Treggiari MM, Romand JA, Yanez ND, Deem SA, Goldberg J, Hudson L, Heidegger CP, Weiss NS. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med 2009;37:2527–2534. [DOI] [PubMed] [Google Scholar]
- 33.Jackson JC, Hart RP, Gordon SM, Hopkins RO, Girard TD, Ely EW. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Crit Care 2007;11:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med 2009;35:796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;160:50–56. [DOI] [PubMed] [Google Scholar]
- 36.Marquis KA, Curtis JR, Caldwell ES, Davidson TA, Davis JM, Sanchez P, Rosenbaum G, Hudson LD, Steinberg KP. Neuropsychological sequelae in survivors of ARDS compared with critically ill control patients [abstract]. Am J Respir Crit Care Med 2000;161:A383. [Google Scholar]
- 37.Rothenhausler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry 2001;23:90–96. [DOI] [PubMed] [Google Scholar]
- 38.Al-Saidi F, McAndrews MP, Cheung AM, Tansey CM, Matté-Martyn A, Diaz-Granados N, Herridge MS. Neuropsychological sequelae in ARDS survivors [abstract]. Am J Respir Crit Care Med 2003;167:A737. [Google Scholar]
- 39.Jackson JC, Hart RP, Gordon SM, Shintani A, Truman B, May L, Ely EW. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med 2003;31:1226–1234. [DOI] [PubMed] [Google Scholar]
- 40.Hopkins RO, Weaver LK, Chan KJ, Orme JF Jr. Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc 2004;10:1005–1017. [DOI] [PubMed] [Google Scholar]
- 41.Suchyta MR, Hopkins RO, White J, Jephson A, Morris AH. The incidence of cognitive dysfunction after ARDS [abstract]. Am J Respir Crit Care Med 2004;169:A18. [Google Scholar]
- 42.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005;171:340–347. [DOI] [PubMed] [Google Scholar]
- 43.Hopkins RO, Jackson JC, Wallace C. Neurocognitive impairments in ICU patients with prolonged mechanical ventilation [abstract]. J Int Neuropsychol Soc 2005;11:60. [Google Scholar]
- 44.Sukantarat KT, Burgess PW, Williamson RC, Brett SJ. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia 2005;60:847–853. [DOI] [PubMed] [Google Scholar]
- 45.Christie JD, Biester RC, Taichman DB, Shull WH Jr, Hansen-Flaschen J, Shea JA, Hopkins RO. Formation and validation of a telephone battery to assess cognitive function in acute respiratory distress syndrome survivors. J Crit Care 2006;21:125–132. [DOI] [PubMed] [Google Scholar]
- 46.Jones C, Griffiths RD, Slater T, Benjamin KS, Wilson S. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med 2006;32:923–926. [DOI] [PubMed] [Google Scholar]
- 47.Jackson JC, Obremskey W, Bauer R, Greevy R, Cotton BA, Anderson V, Song Y, Ely EW. Long-term cognitive, emotional, and functional outcomes in trauma intensive care unit survivors without intracranial hemorrhage. J Trauma 2007;62:80–88. [DOI] [PubMed] [Google Scholar]
- 48.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001;344:395–402. [DOI] [PubMed] [Google Scholar]
- 49.Newman MF, Croughwell ND, Blumenthal JA, White WD, Lewis JB, Smith LR, Frasco P, Towner EA, Schell RM, Hurwitz BJ. Effect of aging on cerebral autoregulation during cardiopulmonary bypass. Association with postoperative cognitive dysfunction. Circulation 1994;90:II243–II249. [PubMed] [Google Scholar]
- 50.Hammon JW Jr, Stump DA, Kon ND, Cordell AR, Hudspeth AS, Oaks TE, Brooker RF, Rogers AT, Hilbawi R, Coker LH, et al. Risk factors and solutions for the development of neurobehavioral changes after coronary artery bypass grafting. Ann Thorac Surg 1997;63:1613–1618. [DOI] [PubMed] [Google Scholar]
- 51.Testa JA, Malec JF, Moessner AM, Brown AW. Outcome after traumatic brain injury: effects of aging on recovery. Arch Phys Med Rehabil 2005;86:1815–1823. [DOI] [PubMed] [Google Scholar]
- 52.Marquez de la Plata CD, Hart T, Hammond FM, Frol AB, Hudak A, Harper CR, O'Neil-Pirozzi TM, Whyte J, Carlile M. az-Arrastia R. Impact of age on long-term recovery from traumatic brain injury. Arch Phys Med Rehabil 2008;89:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg 1995;59:1289–1295. [DOI] [PubMed] [Google Scholar]
- 54.Jackson JC, Gordon SM, Ely EW, Burger C, Hopkins RO. Research issues in the evaluation of cognitive impairment in intensive care unit survivors. Intensive Care Med 2004;30:2009–2016. [DOI] [PubMed] [Google Scholar]
- 55.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington, DC: American Psychiatric Association; 1980.
- 56.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text revision. Washington, DC: American Psychiatric Association; 2000.
- 57.Wechsler D. Wechsler Adult Intelligence Scale, 3rd ed. San Antonio: The Psychological Corporation; 1997.
- 58.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 59.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993;269:2386–2391. [PubMed] [Google Scholar]
- 60.Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Arch Psychol 1941;28:286–340. [Google Scholar]
- 61.Spreen O, Strauss E. A Compendium of neuropsychological tests: administration, norms, and commentary, 2nd ed. New York: Oxford University Press; 1998.
- 62.Rey A. L'examen clinique en psychologie. Paris: Presses Universitaites de France; 1964.
- 63.Reitan RM, Wolfson D. The Halstead Reitan neuropsychological test battery Tuscon, AZ: Neuropsychology Press; 1985.
- 64.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan battery: demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources; 1991.
- 65.Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasis (NCCEA) Victoria: University of Victoria Neuropsychological Laboratory; 1969.
- 66.Sherer M, Bergloff P, Boake C, High W Jr, Levin E. The Awareness Questionnaire: factor structure and internal consistency. Brain Inj 1998;12:63–68. [DOI] [PubMed] [Google Scholar]
- 67.Beck AT. BDI-II depression inventory manual, 2nd ed. New York: Harcourt Brace; 1996.
- 68.Stoll C, Kapfhammer HP, Rothenhausler HB, Haller M, Briegel J, Schmidt M, Krauseneck T, Durst K, Schelling G. Sensitivity and specificity of a screening test to document traumatic experiences and to diagnose post-traumatic stress disorder in ARDS patients after intensive care treatment. Intensive Care Med 1999;25:697–704. [DOI] [PubMed] [Google Scholar]
- 69.Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 70.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 71.Ware JE. SF-36 physical and mental health summary scales: a user's manual, 5th ed. Boston: Health Assessment Lab, New England Medical Center; 1994.