Abstract
The precise molecular mechanism underlying arsenic trioxide (As2O3)-induced apoptosis is a subject of extensive study. Here, we show that clinically relevant doses of As2O3 can induce typical apoptosis in IM-9, a multiple myeloma cell line, in a Bcl-2 inhibitable manner. We confirmed that As2O3 directly induced cytochrome c (cyto c) release from isolated mouse liver mitochondria via the mitochondrial permeability transition pore, and we further identified the voltage-dependent anion channel (VDAC) as a biological target of As2O3 responsible for eliciting cyto c release in apoptosis. First, pretreatment of the isolated mitochondria with an anti-VDAC antibody specifically prevented As2O3-induced cyto c release. Second, in proteoliposome experiments, VDAC by itself was sufficient to mediate As2O3-induced cyto c release, which could be specifically inhibited by Bcl-XL. Third, As2O3 induced mitochondria membrane potential (ΔΨm) reduction and cyto c release only in the VDAC-expressing, but not in the VDAC-deficient yeast strain. Finally, we found that As2O3 induced the increased expression and homodimerization of VDAC in IM-9 cells, but not in Bcl-2 overexpressing cells, suggesting that VDAC homodimerization could potentially determine its gating capacity to cyto c, and Bcl-2 blockage of VDAC homodimerization represents a novel mechanism for its inhibition of apoptosis.
Keywords: apoptosis, arsenic trioxide, Bcl-2 proteins, cytochrome c, VDAC
Introduction
Apoptosis or programmed cell death is a genetically regulated process that plays an important role in tissue homeostasis and development in multicellular metazoans (Vander Heiden and Thompson, 1999; Green and Evan, 2002). Defects in apoptosis are often associated with diseases, such as neuronal degenerative diseases, tumorigenesis, autoimmune disorders, and viral infections (Hickman, 2002; Johnstone et al., 2002). Not only are mitochondria the cellular powerhouse for ATP generation but also they play an essential role in regulating apoptosis (Kroemer, 1999; Vander Heiden and Thompson, 1999; Gottlieb, 2000). Mitochondria-dependent apoptosis involves the permeabilization of mitochondrial membranes, which appears to mediate the release from the intermembrane space into the cytosol of apoptogenic factors such as cytochrome c (cyto c), AIF, Smac/DIABLO, and Endonuclease G (Wang, 2001). Cyto c, a soluble protein, normally resides between the inner and outer mitochondrial membrane and participates in oxidative phosphorylation required for energy production (Skulachev, 1998). Once released, it acts as a cofactor to induce the aggregation of Apaf-1 and apoptosome to activate caspases and subsequently the execution of programmed cell death (Wang, 2001).
The mechanisms of how cyto c and other apoptogenic factors are released from mitochondria remain elusive. It is generally acknowledged that opening of the permeability transition pore (PTP) located at the contact site of the inner and outer membrane of a mitochondrion could be involved (De Pinto and Palmieri, 1992; Marzo et al., 1998a; Desagher and Martinou, 2000). PTP is comprised of the voltage-dependent anion channel (VDAC) and the adenine nucleotide translocator (ANT) in association with other proteins (Colombini, 1989; De Pinto and Palmieri, 1992; Marzo et al., 1998c). ANT is an inner membrane channel that plays a role in ADP/ATP exchanges between the mitochondrial matrix and the intermembranous space (Graham et al., 1997; Fiore et al., 1998). On the other hand, VDAC is a Ca2+-sensitive channel, which functions as the major pathway for metabolite diffusion across the mitochondrial outer membrane (Colombini, 1989; De Pinto and Palmieri, 1992). VDAC was suggested to form a dimer (Krause et al., 1986; Szabo et al., 1993; Szabo and Zoratti, 1993), which could represent the PTP-forming component. It is believed that VDAC undergoes extensive conformational changes in response to a variety of stimuli (Szabo et al., 1992; Mannella, 1998; Song et al., 1998), and facilitates cyto c release following some apoptotic insults (Crompton, 1999; Martinou and Green, 2001). Interestingly, Bcl-2 and its family proteins, key regulators of apoptosis and cyto c release, are thought to interact physically with VDAC and/or ANT to regulate the opening of PTP during apoptosis (Kroemer, 1997; Shimizu et al., 1999). Bcl-2 could modulate the configuration of VDAC and thus the transport of metabolites and permeability transition, although the molecular details of how these proteins are involved in mediating cyto c release are a subject of intense debate. Our recent results indicate that, at least in vitro, VDAC interacts with both Bax and Bcl-XL to form a tertiary complex and that the function of VDAC in mediating cyto c release could depend on the ratio between Bax and Bcl-XL (Shi et al., 2003a).
Arsenic trioxide (As2O3) is a traditional drug that has been widely used for over 2000 years in China (Chen et al., 1996; Aposhian, 1997; Miller Jr et al., 2002). Recently, clinical data have shown that As2O3 induces complete remission of acute promyelocytic leukemia (APL) without any significant side effects (Shen et al., 1997; Soignet et al., 1998; Zhang et al., 2001). There are numerous reports on the proapoptotic effects of As2O3 in malignant cell lines through complex signaling pathways, with several clinic trials being conducted on hematopoietic malignancies and solid tumors (Shen et al., 1997; Wang et al., 1998; Bazarbachi et al., 1999; Jing et al., 1999; Rousselot et al., 1999; Perkins et al., 2000; Anderson et al., 2002; Miller Jr et al., 2002). Remarkably, it has been suggested that arsenite might directly target mitochondrial PTP to induce apoptosis in cancerous cells (Petronilli et al., 1994; Costantini et al., 1996; Larochette et al., 1999), although the precise molecular mechanism is still elusive. Here, we provide for the first time the genetic and biochemical evidence that VDAC is one of the biological targets responsible for induction of cyto c release and apoptosis by As2O3. A full understanding of the molecular mechanism of this ancient remedy may be useful to develop better therapeutic drugs for fighting cancer.
Results
As2O3 induces apoptosis, reduction of ΔΨm, and release of cyto c from mitochondria in a Bcl-2 inhibitable manner
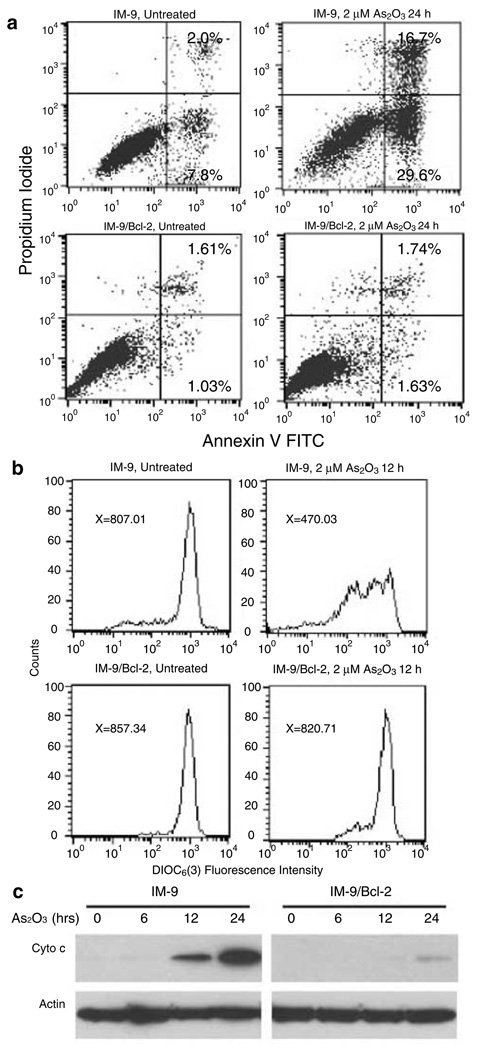
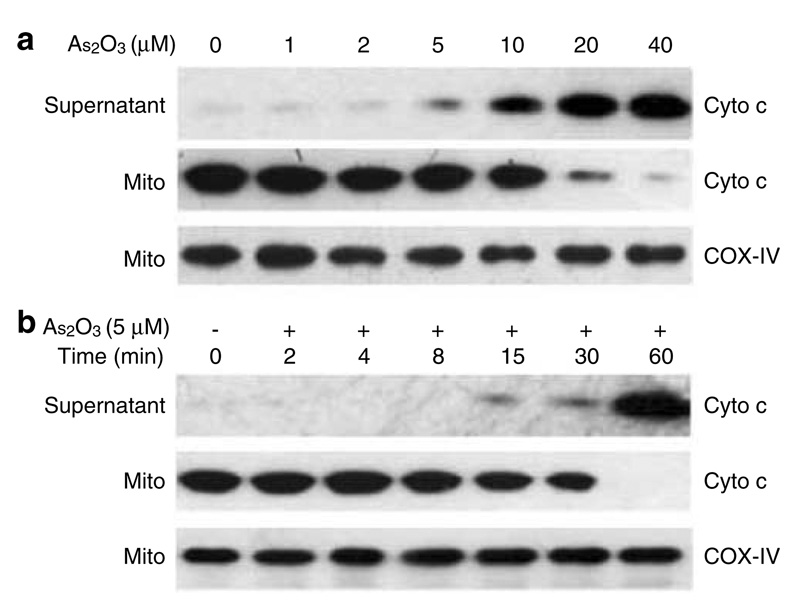
We used a clinically relevant concentration of As2O3 to treat a multiple myeloma cell line IM-9 for 24 h and assayed for the exposure of phosphotydyl-serine on the plasma membrane, indicative of apoptosis. FACS analysis showed that As2O3 induced a significant increase of the population of Annexin Vhigh/PIlow IM-9 cells (Figure 1a). This result clearly demonstrated that As2O3 could effectively induce apoptosis in multiple myeloma cells. Overexpression of Bcl-2 in IM-9/Bcl-2 cells, which we have shown previously to suppress ionizing radiation-induced apoptosis (Chen et al., 2000, 2003), also inhibited As2O3-induced apoptosis (Figure 1a). Moreover, the detection of cytoplasmic cyto c from IM-9 cells treated with 2 µM As2O3 showed the appearance of cyto c starting 12 h following treatment (Figure 1c), concomitant with the reduction of ΔΨm (Figure 1b), the onset of caspase 3 activity (data not shown), and Annexin V positivity. On the other hand, As2O3-induced release of cyto c in the IM-9/Bcl-2 cell line was significantly reduced and the reduction of ΔΨm was prevented (Figure 1b, c), although higher doses of As2O3 abrogated the ability of Bcl-2 to suppress apoptosis (data not shown). These results suggest that As2O3 evokes a specific apoptotic pathway related to mitochondrial functions. To test this directly, we incubated isolated mouse liver mitochondria with clinically relevant concentrations of As2O3 and measured the release of cyto c from mitochondria. Indeed, As2O3 induced the release of cyto c in a dose-(Figure 2a) and time-(Figure 2b) dependent manner. The release of cyto c from isolated mitochondria upon treatment with 5 µM As2O3 started at 15 min, with mitochondrial cyto c being exhausted at 60 min (Figure 2b). These results indicate that As2O3 could directly target mitochondria to induce cyto c release and apoptosis.
Figure 1.
As2O3 induces apoptosis, ΔΨm reduction, and cyto c release in IM-9 cells in a Bcl-2 inhibitable manner. (a) Flow cytometric analysis of apoptosis in IM-9 and IM-9/Bcl-2 cells treated in the absence or presence of 2µm As2O3 for 24 h as determined by binding of Annexin V and uptake of PI; (b) IM-9 and IM-9/Bcl-2 cells were treated with or without 2µm As2O3 for 12 h, and the ΔΨm was measured using DiOC6(3) by FACS analysis. X represents the mean value of green fluorescence [DiOC6(3)] from the subpopulation of cells that were negative for red fluorescence (PI); (c) The levels of cyto c released into cytosol were determined by differential centrifugation followed by Western blotting as described in ‘Materials and methods’ and actin was used as a loading control. All data shown are representative of three separate experiments
Figure 2.
As2O3 induces cyto c release from isolated mitochondria. Isolated mitochondria (5mg protein/ml) were incubated at 25°C for 30 min with different concentrations of As2O3 (a), or were incubated at 25°C with 5 µm As2O3 for different times (b). The levels of cyto c and COX-IV as a mitochondrial-specific marker were measured by Western blotting as described in ‘Materials and methods’. Data are representative of at least three independent experiments
As2O3-induced PTP opening and cyto c release is inhibited by PTP-specific inhibitors
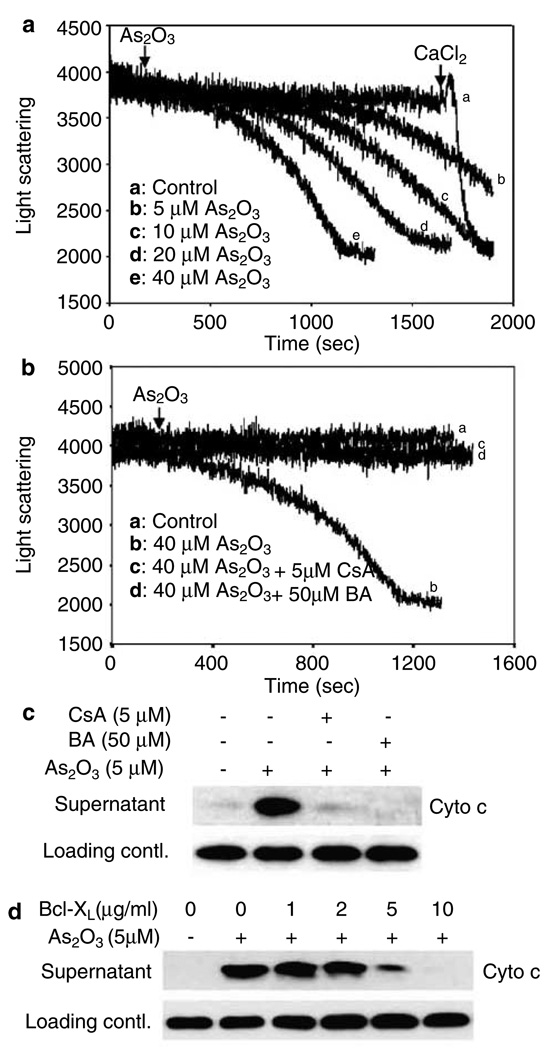
To understand the mechanism of cyto c release and to determine if PTP is targeted by As2O3, we exposed isolated mouse liver mitochondria to various concentrations of As2O3 and found that mitochondria swelling, indicative of PTP opening, was evoked in a dose-dependent manner (Figure 3a). A clinically relevant dose of As2O3 (5 µM) was sufficient to induce the opening of PTP, starting at about 12 min, while higher doses of As2O3 shortened the time required to cause PTP opening. Our results reveal that cyto c release occurs concomitantly with the PTP opening and precedes the disruption of ΔΨm (data not shown). Therefore, it is reasonable to propose that As2O3 might target the outer mitochondrial membrane (OMM) to induce cyto c release, while mitochondrial depolarization and the disruption of oxidative respiratory chain could be secondary to PTP opening and the loss of cyto c.
Figure 3.
PTP-specific inhibitors prevent As2O3-induced PTP opening and cyto c release, (a, b) PTP opening was monitored as described in ‘Materials and methods’. Isolated mitochondria were pretreated with 5 µm CsA, or 50 µm BA (c) or preincubated with different concentrations of Bcl-XL as indicated (d) in 50 µl PT buffer for 5 min before As2O3 (5 µm) being added, and further incubated at 25°C for 60 min. A volume of 100 µm CaCl2 was added at the end of experiments as a positive control for PTP opening. Released cyto c was detected as described in ‘Materials and methods’. Data are representative of at least three independent experiments
Cyclosporin A (CsA) and bongkrekic acid (BA) are very potent inhibitors of PTP (Marzo et al., 1998b). The mitochondrial swelling (Figure 3b) and the efflux of cyto c (Figure 3c) induced by As2O3 were completely inhibited by pretreatment of isolated mitochondria with either CsA or BA. Bcl-2 and Bcl-XL have been reported to prevent cyto c release and PTP opening induced by various stimuli (Kroemer, 1997; Marzo et al., 1998b; Narita et al., 1998). As shown in Figure 3d, the addition of Bcl-XL to isolated mitochondria prevented As2O3-induced cyto c release in a dose-dependent manner, with higher doses completely preventing cyto c release. These results strongly suggest that As2O3 might function through PTP to regulate mitochondrial swelling and the associated cyto c release.
VDAC is necessary and sufficient to mediate cyto c release induced by As2O3
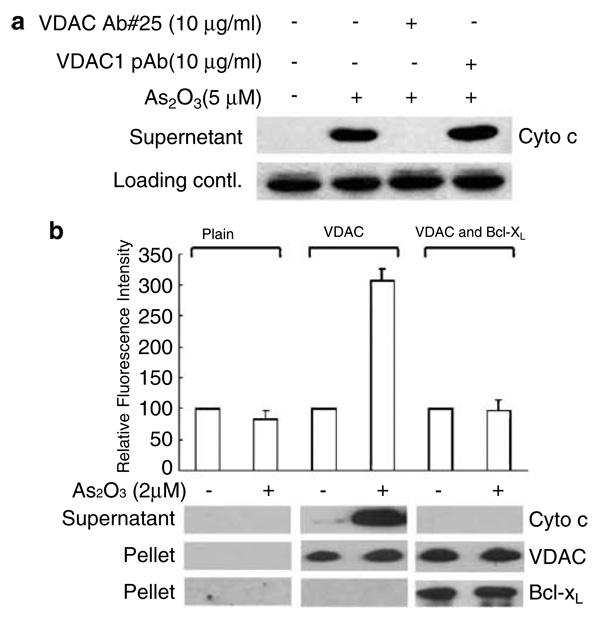
VDAC is an abundant outer membrane protein and a critical component of the PTP complex (Colombini et al., 1996). To determine whether the function of VDAC is required for As2O3-induced cyto c release, isolated mitochondria were preincubated with an anti-VDAC polyclonal antibody (Ab#25) reported to be a specific steric inhibitor of VDAC-mediated cyto c release induced by Bax and Bak (Shimizu et al., 2001). As shown in Figure 4a, the antibody could completely prevent the cyto c release induced by As2O3. In contrast, under identical conditions, the polyclonal antibody raised against the N-terminal peptide of VDAC had no such effect. These data demonstrate unambiguously that VDAC is involved in As2O3-induced cyto c release.
Figure 4.
VDAC is necessary and sufficient to mediate As2O3-induced cyto c release. (a) As 2O3-induced cyto c release was specifically inhibited by the anti-VDAC antibody (#25). Isolated mitochondria (5mg protein/ml) were preincubated with 10 µg/ml anti-VDAC Ab#25 or a polyclonal antibody against the N terminus of VDAC1 for 15 min in 50 µl PT buffer before the addition of 5 µm As2O3. The cyto c released into the supernatant was measured by Western blotting as described in ‘Materials and methods’ (b) As 2O3 induced hVDAC-mediated cyto c release from reconstituted liposomes, which was inhibited by Bcl-XL. Liposomes were reconstituted as described in ‘Materials and methods’ and incubated in the presence or absence of 2 µm As2O3 for 1 h at 25°C. After centrifugation, the FITC fluorescence in the supernatants was determined using a spectrofluorimeter. The data, normalized against the fluorescence of the untreated group as a control, represent the mean values of three independent experiments. Cyto c released in the supernatants was also detected by Western blotting using an anti-cyto c monoclonal antibody. Incorporation of the VDAC and Bcl-XL proteins in liposomes was confirmed by immunoblots with anti-VDAC1 and anti-Bcl-XL polyclonal antibodies
To investigate whether VDAC is sufficient to mediate As2O3-induced cyto c release, proteoliposomes encapsulated with FITC-cyto c were reconstituted in the presence or absence of VDAC. The reconstituted liposomes were then exposed to 2 µM As2O3 for 1 h. The liposomes were not leaky to cyto c since there was no detectable FITC fluorescence or cyto c protein in the plain liposome preparation. The release of cyto c-FITC to the supernatant was found to be strictly dependent on the presence of VDAC (Figure 4b) and such dependence could be abrogated by the presence of Bcl-XL in the VDAC liposomes, further supporting the notion that a direct interaction between Bcl-XL and VDAC provides a general yet robust antiapoptotic mechanism in response to As2O3. This and the above-mentioned antibody blocking experiment clearly demonstrate that VDAC is necessary and sufficient to mediate the proapoptotic function of As2O3, while Bcl-XL functions to block this effect, possibly by direct interaction with VDAC.
VDAC is required for As2O3-induced ΔΨm reduction and cyto c release in yeast
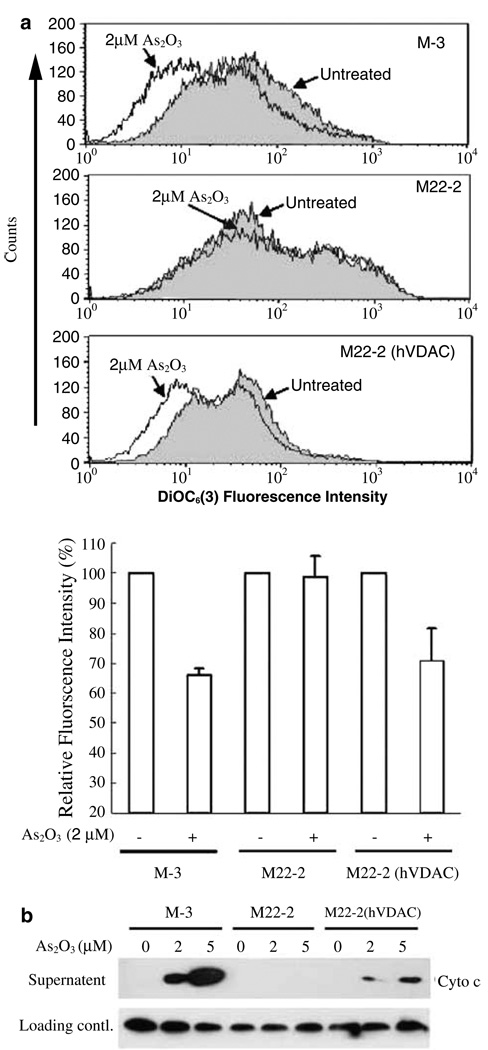
Saccharomyces cerevisiae has been used as a simple model for apoptosis studies (Matsuyama et al., 1999; Gross et al., 2000). To analyse directly whether VDAC is a target for As2O3-triggered apoptosis in intact cells, wild-type (M-3), a VDAC1-deficient (M22-2), or the M22-2 yeast strain supplemented with a human VDAC1 gene (M22-2/pBDL-VDAC) was treated with 2 µM As2O3 for 12 h and ΔΨm was then examined by flow cytometry. As shown in Figure 5a, As2O3 significantly decreased ΔΨm in M-3 cells (P < 0.01), but had no effect on M22-2 cells. The basal level of ΔΨm in VDAC-deficient yeast cells seemed to be higher than that of wild-type M-3 cells. Interestingly, reintroduction of human VDAC1 into M22-2 cells could partially restore its sensitivity of ΔΨm reduction to As2O3 and its basal level of ΔΨm to that of the wild-type yeast M-3 strain. To prove directly that VDAC is responsible for As2O3 induced cyto c release, we treated isolated mitochondria from the above yeast cells directly with As2O3. We found that mitochondria from M-3 and M22-2 (hVDAC1) were permeated by As2O3 to release cyto c in a dose-dependent manner. Remarkably, the release of cyto c was completely blocked in the mitochondria from the VDAC1-deficient yeast M22-2 strain (Figure 5b). These results further support the observation that VDAC is a target of As2O3 responsible for eliciting mitochondrial apoptotic changes in intact cells.
Figure 5.
VDAC is required for As2O3-induced ΔΨm reduction in yeast and cyto c release from isolated yeast mitochondria. (a) Wild-type (M-3), VDAC-1-deficient mutant (M22-2), or M22-2 mutant S. cerevisiae reconstituted with human VDAC-1, were incubated with or without 2 µm As2O3 at 30°C for 12 h before being subjected to ΔΨm analysis as described in ‘Materials and methods’. PI-negative yeast cells were analysed with a Cell Quest software (BD, CA) and the data were normalized against the untreated control. The upper panel histogram data are representative of three independent experiments. (b) Isolated mitochondria (5 mg protein/ml) from the three yeast strains were incubated for 1 h at 30°C in 50 µl YPT buffer (0.6m mannitol, 2mm HEPES, pH 7.4, 0.5mm KH2PO4, and 4.2mm potassium succinate) with different concentrations of As2O3, as indicated. The samples were then centrifuged at 12 000 g for 15 min at 4°C. The levels of cyto c in the supernatants were determined by Western blotting with an anti-cyto c antibody. The nonspecific bands that appeared in cyto c Western blotting were used as an internal loading control. Data are representative of three independent experiments
As2O3 induces VDAC upregulation and dimerization
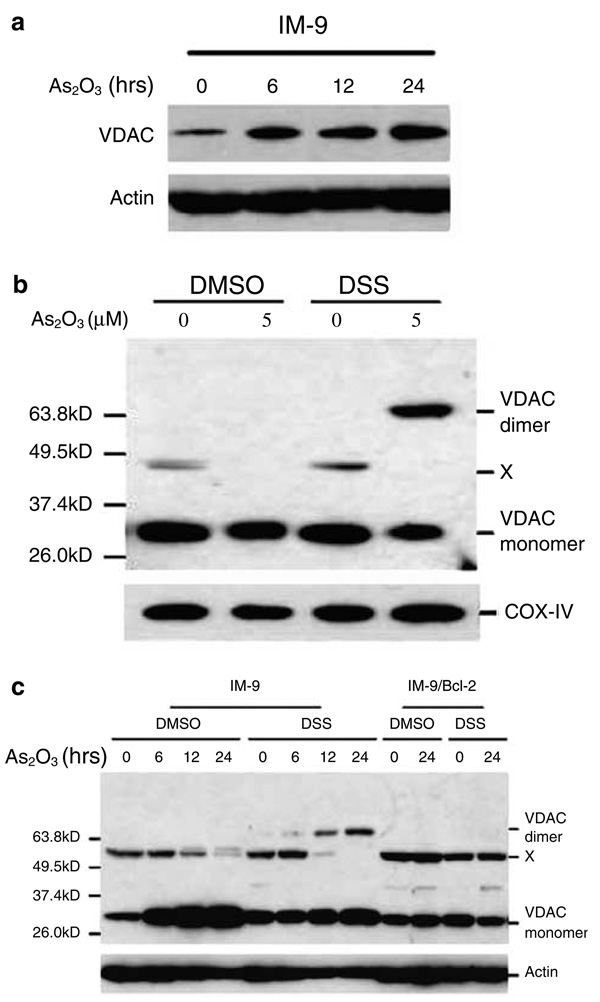
To further understand the functional aspects of VDAC in mediating As2O3-induced cyto c release, we examined the expression levels of VDAC following As2O3 treatment in IM-9 cells. To our surprise, we found that As2O3 upregulated the expression of VDAC in IM-9 cells (Figure 6a). It was suggested, although evidence remains to be provided, that VDAC could potentially form a higher-order complex that participates in gating the efflux of cyto c through OMM (Szabo and Zoratti, 1991; Szabo and Zoratti, 1992). To test directly whether As2O3 could induce the higher-order complexing of VDAC molecules, we used a cross-linking assay, a commonly used approach to detect the interactions between molecules, to examine the VDAC profile in isolated mouse liver mitochondria and intact multiple myeloma cells. First, incubation of isolated mitochondria with the cross-linking reagent DSS after As2O3 treatment indeed induced VDAC homodimerization (Figure 6b). We next examined homodimerization of VDAC following As2O3 treatment in the multiple myeloma cells. As expected, we found that As2O3 induced the homodimerization of VDAC in IM-9 cells (Figure 6c). In contrast, there was no detectable VDAC homodimer present in untreated or in DMSO-treated cells. Also, we did not detect a higher order of oligomerization.
Figure 6.
As2O3 induces VDAC upregulation and homodimerization (a) IM-9 cells were treated with 2 µm As2O3 for various times and subjected to SDS–PAGE/immunoblot analysis with the indicated antibodies and β-actin was used as a loading control. (b) Isolated mitochondria were treated with 5 µm As2O3 for 1 h or (c) IM-9 cells were treated with 2 µm As2O3 for the indicated times before treatment with the cross-linking agent DSS. VDAC proteins were resolved by SDS–PAGE and detected by Western blotting using a VDAC1 polyclonal antibody. A 32-kDa band represents VDAC monomers, with bands at 64 kDa, detected only in the presence of DSS, representing VDAC homodimers. X represents a nonspecific band. DMSO was used as the vehicle control. Results shown are representative of three independent experiments
Interestingly, ectopic overexpression of Bcl-2 blocked As2O3-induced VDAC upregulation and homodimerization. These results further suggest that VDAC could be a biological stress sensor to As2O3, and its homodimerization induced by As2O3 could potentially determine its gating capacity to efflux cyto c, as Bcl-2 effectively inhibited such a pathway.
Discussion
The present study provides genetic and biochemical evidence that VDAC might play an essential role in As2O3-induced apoptotic changes of the mitochondria. First, the anti-VDAC antibody (Ab#25) specifically and effectively prevented As2O3-induced cyto c release from isolated mitochondria. The same antibody was used as a specific VDAC channel blocker both in vitro and in vivo (Shimizu et al., 2001). Second, liposome and yeast experiments showed that VDAC was necessary and sufficient to mediate cyto c efflux caused by As2O3. Our results are in good agreement with earlier reports that arsenite might induce mitochondrial PTP opening (Petronilli et al., 1994; Costantini et al., 1996; Larochette et al., 1999). These results are in sharp contrast with a previous report that ANT, rather than VDAC, mediates the mitochondrial membrane permeabilization induced by arsenite (Belzacq et al., 2001). The participation of ANT in As2O3-mediated stress signal cannot be ruled out, since CsA and BA could inhibit As2O3-induced PTP opening and cyto c release. Third, Bcl-XL/Bcl-2 could potently inhibit VDAC-mediated cyto c release and its dimerization, possibly through a mechanism of protein–protein interactions (Tsujimoto and Shimizu, 2000; Shi et al., 2003a).
Considering the finding that the Bcl-2/Bcl-XL-VDAC interaction alleviated the apoptotic effect of As2O3, we favor the model by which VDAC might be directly targeted by As2O3 to mediate cyto c release. Our data do not rule out the participation of Bax and/or Bak, commonly acknowledged mediators for cyto c release, in As2O3-induced cyto c release and apoptosis. Further studies are required to understand the exact mechanisms of VDAC in regulating cyto c release and how its interactions with Bcl-2 family proteins may determine the outcome of cell fate.
As2O3 has been widely used to treat APL and other types of malignant leukemia. Caspase activation (Chen et al., 1998b; Soignet et al., 1998) and enhanced generation of reactive oxygen species (ROS)(Chen et al., 1998b) were suggested to be responsible for the specific cell death in cancer lesions. Our findings could offer an explanation for As2O3 apoptotic events, since cyto c release from mitochondria into cytosol is causally linked to caspase activation and disruption of mitochondrial respiratory chain, and subsequently the enhanced generation of ROS from the mitochondria. What is puzzling is how clinically relevant doses of As2O3 induce apoptosis, leading to tumor cell-specific killing in the clinic. It remains to be investigated whether there exists any tumor-specific target that determines the differential metabolism or distinct responses to As2O3-mediated stress between neoplastic and normal cells. Indeed, As2O3 induces degradation of the PML/RAR fusion protein in APL patients (Shen et al., 1997; Dai et al., 1999) or selectively downregulates the Bcl-2 protein via caspase-3 cleavage (Chen et al., 1996).
There are conflicting reports with regard to the Bcl-2 suppression of As2O3-induced apoptosis. It was reported that As2O3-induced apoptosis of multidrug-resistant acute myelocytic leukemia cells, regardless of whether Bcl-2 and Bcl-XL were overexpressed (Perkins et al., 2000), while other reports suggest that both Bcl-2 and its homologue Bcl-XL could confer resistance against apoptosis by inhibiting the reduction of ΔΨm, cyto c release, and caspase activation (Green and Reed, 1998; Cory and Adams, 2002). Our data indicate that overexpression of Bcl-2 could attenuate or delay apoptosis, cyto c release, and ΔΨm reduction induced by As2O3 in IM-9 cells, with Bcl-XL potently inhibiting As2O3-induced cyto c release from isolated mitochondria.
A rather surprising finding was that As2O3 upregulated the expression levels of VDAC. This suggests that VDAC could potentially serve as a biological stress sensor to As2O3, either directly or indirectly. It is known that radiation could also induce the upregulation of VDAC in LYas cells (Voehringer et al., 2000) and increased expression of VDAC is correlated with uterine epithelial apoptosis after estrogen deprivation (Takagi-Morishita et al., 2003).
Questions still remain as to how VDAC mediates cyto c release induced by As2O3. As2O3 is a selective dithiol cross-linker (Petronilli et al., 1994) that can modulate the levels of the redox modulators of the PT pore, such as GSH and NADH. As2O3 cross-linking of cysteine residues within the transmembrane domain of VDAC, if accessible, could lead to the changes of conformation, thus the channel activities, of VDAC and PTP (Jing et al., 1999). Alternatively, as we observed, As2O3 could induce homodimerization of VDAC molecules, and therefore the VDAC pore activity to mediate cyto c release. Previous biochemical and electrophysiological evidence indicates that VDAC tends to form a dimer or oligomer, at least in yeast and in artificial biolipid membranes (Krause et al., 1986; Szabo et al., 1993; Szabo and Zoratti, 1993). However, we do not observe either the dimers of VDAC in nonapoptotic cells or the oligomers in apoptotic cells in our cross-linking assay. There could be sequential events consisting in As2O3 inducing conformational changes of VDAC, which brings the different VDAC subunits in closer proximity to form a dimer to facilitate cyto c release. To the best of our knowledge, this is the first report indicating that VDAC forms a homodimer during As2O3-induced apoptosis in mammalian cells and that Bcl-2 prevents the homodimerization and apoptotic responses. Based on these data and our recent observation that Bcl-XL interacts with VDAC via the putative loop region (Shi et al., 2003a), we propose that Bcl-2/Bcl-XL may interact with VDAC to block its dimerization sterically, which may be a prerequisite for cyto c release. This may represent a novel mechanism for the inhibition of apoptosis by Bcl-2. Further studies are required to investigate the functional significance of VDAC homodimerization and its regulation by Bcl-2 family proteins.
Materials and methods
Chemicals
Bongkrekic acid (BA) was from BioMol Research Laboratories (Plymouth Meeting, PA, USA); 3,3′-dihexyloxacarbo-cyanineiodide [DiOC6(3)] and anti-cytochrome c oxidase monoclonal antibody (COX-IV)(A-21348) were from Molecular Probes (Eugene, OR, USA). The Annexin V apoptosis detection kit and purified anti-cytochrome c antibody (65981A) were from PharMingen (San Jose, CA, USA). Disuccinimidyl suberate (DSS) was from Pierce (Rockford, IL, USA). The VDAC1 (N-18) goat polyclonal antibody (sc-8828) was from Santa Cruz Biotechnology (CA, USA), the Bcl-x mouse monoclonal antibody (AHO0222) was from Biosource International (Camarillo, CA, USA). All other reagents were obtained from Sigma (St Louis, MO, USA).
Cell culture
Human IM-9 multiple myeloma cells were routinely maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (Hyclone) and penicillin/streptomycin at 37°C and 5% CO2. pSFFV-neo FLAG.Bcl-2 was used for stable transfection of IM-9 cells. Cell clones overexpressing Bcl-2 were selected in 1 mg/ml G418 as described previously (Chen et al., 2000). Exponentially growing cells were subjected to the various treatments as indicated.
M-3 and M22-2 yeast cells were grown in YPD medium containing 1% yeast extract, 2% peptone, and 2% dextrose (Gross et al., 2000). A human vdac1-expressing ΔVDAC S. cerevisiae strain [M22-2 (hVDAC1)] was produced by transfecting human vdac1 cDNA using standard lithium acetate method with tryptophan selection. The transformed yeast cells were grown in the minimal SD base (yeast nitrogen base, dextrose, and ammonium sulfate) plus TRP DO (dropout) supplement (Clontech, Palo Alto, CA, USA).
Flow cytometric assay for Annexin V positivity
Apoptosis was measured using the Annexin V detection kit according to the manufacturer’s instruction. Flow cytometric analysis was performed to monitor the green fluorescence of FITC-conjugated Annexin V (530 ± 30nm) and the red fluorescence of DNA bound propidium iodide (PI, 630 ± 22 nm) (Chen et al., 1998a). All data were analysed with a Cell Quest software (BD, CA, USA).
Measurement of mitochondrial membrane potential
This assay was performed as described previously (Chen et al., 1998a). Briefly, cells were collected after being treated with As2O3. DiOC6(3)[2 µl of 2µm stock solution in dimethyl sulfoxide (DMSO)] was added to 0.4 ml cell suspension (4 × 105 cells/ml) in PBS (pH 7.2) and incubated at 37°C for 5 min. PI (5 µl of 500 µg/ml stock) was added 30 s before analysis. ΔΨm was analysed by flow cytometry with excitation at 488 nm. DiOC6(3) data were validated by addition of 1 µm carbonyl cyanide m-chlorophenylhydrazone (CCCP) after 5 min of DiOC6(3) loading.
Cell fractionation and immunoblot analysis
As2O3-treated cells were fractionated by differential centrifugation as previously described (Chen et al., 1997, 2000). Briefly, cells were homogenized with a Dounce homogenizer and the homogenate was centrifuged at 800 g for 5 min to remove unbroken cells and nuclei, and the cytosolic fractions were obtained by further centrifugation at 100 000 g for 30 min. For immunoblots, proteins were separated by SDS–PAGE, transferred onto nitrocellulose membranes (Schleicher and Schull), and probed with specific antibodies as indicated. Immunoreactive bands were visualized using enhanced chemiluminescence (Pierce).
Isolation of mouse liver mitochondria and measurement of PTP opening and cyto c release
Liver mitochondria from Balb/c mice were isolated by routine methods as previously described (Bernardi, 1984; Xia et al., 2002a, b). The quality of isolated mitochondria was examined by oxygen consumption. The protein content of mitochondria was determined by the micro biuret method using BSA as a standard. Isolated mitochondria (5 mg protein/ml) were kept in a PT buffer containing 250mm sucrose, 2mm HEPES (pH 7.4), 0.5mm KH2PO4, 2µm rotenone, and 4.2mm potassium succinate. The PTP opening was monitored by the decrease of 90° light scattering at 520nm using a Jobin-Yvon FluoroMax-2 spectrofluorimeter as previously described (Petronilli et al., 1993; Xia et al., 2002a, b). For cyto c release, different concentrations of As2O3 were added at the indicated time. Various PTP blockers, if needed, were usually added 5 min before As2O3 treatment. The samples were then centrifuged at 12 000 g at 4°C for 15 min. Cyto c in the supernatant and mitochondrial pellets was detected by Western blotting using anti-cyto c monoclonal antibody. Protein loading was verified by immunodetection of COX-IV in the mitochondrial pellets. In certain cases, the nonspecific bands that appeared in cyto c Western blotting were used as an internal loading control. In most cases, 1–5 µm As2O3 were used, but in certain experiments, whereas indicated, to avoid potential damage to the mitochondria due to the prolonged exposure, higher doses, and shorter duration of treatments were applied.
Isolation of yeast mitochondria and measurement of yeast mitochondria membrane potential
For isolation of yeast mitochondria (Gross et al., 2000), 500 ml yeast cells (OD600 = 1.0) were collected, washed, and incubated with 1.2m, sorbitol–K2HPO4–KH2PO4 (pH 7.4) buffer containing Zymolyase-20 T [20 000 U/g (ICN, CA, USA); 2mg per g of cells] for 1 h at 30°C. The spheroplasts were homogenized in a tight glass homogenizer with about 20 strokes. Yeast mitochondria were obtained by differential centrifugation and were resuspended in buffer containing 0.6m mannitol, 20mm HEPES (pH 7.4), and 0.1% fatty acid-free BSA before use.
Flow cytometric analysis of ΔΨm in yeast was performed as described previously (Gross et al., 2000). Cells (1 × 106) of different yeast strains were treated with As2O3, washed twice in ice-cold PBS, and stained with 40 nm DiOC6(3) at 30°C for 15 min in the dark. PI (10 µg/ml) was added 30 s before analysis for detecting the dead cells. ΔΨm of yeast mitochondria was analysed using FACS with excitation at 488nm and the data were validated by addition of 1 µm CCCP after 15 min of loading of DiOC6(3).
Expression and purification of recombinant proteins
Human Bcl-XL was expressed as a GST fusion protein in E. coli, purified on a glutathione-Sepharose column, and concentrated by ultrafiltration to remove GSH. His-tagged human VDAC1 was purified by immobilized affinity chromatography (IMAC, Qiagen) to near homogeneity under denaturing conditions (Shi et al., 2003b).
Fluorescence measurement of cyto c release in VDAC liposomes
Liposomes were prepared by a standard method as described previously (Madesh and Hajnoczky, 2001; Shi et al., 2003a). Briefly, 500mg l-α-phosphatidyl choline was dissolved in 5ml chloroform, and the solvent was then evaporated under nitrogen. A phospholipid mixture was reconstructed in 10 ml liposome buffer containing 50mm KCl, 20mm KH2PO4, 20mm HEPES (pH 7.0) and 1mm EDTA. After sonication, purified VDAC (0.1 mg/ml, final concentration), and/or Bcl-XL (0.1 mg/ml, final concentration) was then mixed with liposomes and incubated for 20 min at 25°C. The resulting proteoliposomes were dialysed overnight at 4°C to remove excessive detergent. FITC-conjugated cyto c was loaded into the proteoliposomes by three freeze–thaw cycles, and then the proteoliposomes were washed three times with the liposome buffer to remove the cyto c-FITC present outside of the vesicles. Aliquots of the three types of liposomes were mixed with 2 µm As2O3 and incubated for 1 h at 25°C and the reactions were terminated by centrifugation (18 000 g, 30 min at 4°C). The cyto c-FITC released in the supernatant was quantified by a fluorometric method (490nm excitation/510nm emission) and was also immunodetected using a specific cyto c monoclonal antibody. Equal loading of cyto c in the proteoliposomes was determined by both fluorometric and immunodetection of cyto c levels. The incorporation of proteins in proteoliposomes was verified by Western blotting using VDAC or Bcl-XL-Specific antibodies.
Cross-linking for VDAC
Following treatments with As2O3, cells or isolated mitochondria were washed with conjugating buffer containing 150 µm NaCl, 20mm HEPES (pH 7.2), 1.5mm MgCl2, and 10mm glucose. DSS in DMSO was added to a final concentration of 2mm (Gross et al., 1998; Makin et al., 2001). After reaction at room temperature for 30 min, the cross-linker was quenched by the addition of 1m Tris-HCl (pH 7.5) to a final concentration of 20mm. Samples were then solubilized in 1% NP-40 and centrifuged at 12 000 g for 10 min. VDAC was detected by Western blotting using an anti-VDAC1 polyclonal antibody (sc-8828).
Statistical analysis
Significant differences between values under different experimental conditions were determined by paired Student’s t-test analyses. A value of P < 0.05 was considered to be significant.
Acknowledgements
We are grateful to Professor Y Tsujimoto (Osaka University, Japan) for providing the Bcl-XL expression vector and VDAC antibodies, Dr M Forte (Vollum Institute, Oregon Health Sciences University, Portland, USA) for wild-type and VDAC-deficient yeast strains. We wish to thank Mrs J Wang and Mr XD Liao for their technical assistance in flow cytometry. We would like to thank our colleagues for helpful discussions. This work was supported by grants of ‘One hundred Elite Scholars Project’, ‘Knowledge Innovation Key Project’ of Chinese Academy of Sciences, and the National Proprietary Research Program (973 program project, No. 2002CB513100 and 2002CB513001) awarded to QC and HT, National Outstanding Young Investigator Fellowship of NSFC to HT (30025010), QC and National Institutes of Health (CA81504 and CA82858) to AA.
References
- Anderson KC, Boise LH, Louie R, Waxman S. Cancer J. 2002;8:12–25. doi: 10.1097/00130404-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Aposhian HV. Annu. Rev. Pharmacol. Toxicol. 1997;37:397–419. doi: 10.1146/annurev.pharmtox.37.1.397. [DOI] [PubMed] [Google Scholar]
- Bazarbachi A, El Sabban ME, Nasr R, Quignon F, Awaraji C, Kersual J, Dianoux L, Zermati Y, Haidar JH, Hermine O, de The H. Blood. 1999;93:278–283. [PubMed] [Google Scholar]
- Belzacq AS, El Hamel C, Vieira HL, Cohen I, Haouzi D, Metivier D, Marchetti P, Brenner C, Kroemer G. Oncogene. 2001;20:7579–7587. doi: 10.1038/sj.onc.1204953. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Biochim. Biophys. Acta. 1984;766:277–282. doi: 10.1016/0005-2728(84)90242-1. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, Chisolm GM, Almasan A. Cell Death Differ. 2003;10:323–334. doi: 10.1038/sj.cdd.4401148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Gong B, Almasan A. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Takeyama N, Brady G, Watson AJ, Dive C. Blood. 1998a;92:4545–4553. [PubMed] [Google Scholar]
- Chen Q, Turner J, Watson AJ, Dive C. Oncogene. 1997;15:2249–2254. doi: 10.1038/sj.onc.1201371. [DOI] [PubMed] [Google Scholar]
- Chen YC, Lin-Shiau SY, Lin JK. J. Cell Physiol. 1998b;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Colombini M. J. Membr. Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- Colombini M, Blachly-Dyson E, Forte M. Ion Channels. 1996;4:169–202. doi: 10.1007/978-1-4899-1775-1_5. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Costantini P, Chernyak BV, Petronilli V, Bernardi P. J. Biol. Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- Crompton M. Biochem. J. 1999;341(Part 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- Dai J, Weinberg RS, Waxman S, Jing Y. Blood. 1999;93:268–277. [PubMed] [Google Scholar]
- De Pinto VD, Palmieri F. J. Bioenerg. Biomembr. 1992;24:21–26. doi: 10.1007/BF00769526. [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou JC. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Fiore C, Trezeguet V, Le Saux A, Roux P, Schwimmer C, Dianoux AC, Noel F, Lauquin GJ, Brandolin G, Vignais PV. Biochimie. 1998;80:137–150. doi: 10.1016/s0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- Gottlieb RA. FEBS Lett. 2000;482:6–12. doi: 10.1016/s0014-5793(00)02010-x. [DOI] [PubMed] [Google Scholar]
- Graham BH, Waymire KG, Cottrell B, Trounce IA, Mac-Gregor GR, Wallace DC. Nat. Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- Green DR, Evan GI. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Pilcher K, Blachly-Dyson E, Basso E, Jockel J, Bassik MC, Korsmeyer SJ, Forte M. Mol. Cell Biol. 2000;20:3125–3136. doi: 10.1128/mcb.20.9.3125-3136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman JA. Curr. Opin. Genet. Dev. 2002;12:67–72. doi: 10.1016/s0959-437x(01)00266-0. [DOI] [PubMed] [Google Scholar]
- Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Krause J, Hay R, Kowollik C, Brdiczka D. Biochim. Biophys. Acta. 1986;860:690–698. doi: 10.1016/0005-2736(86)90568-7. [DOI] [PubMed] [Google Scholar]
- Kroemer G. Nat. Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- Kroemer G. Biochem. Soc. Symp. 1999;66:1–15. doi: 10.1042/bss0660001. [DOI] [PubMed] [Google Scholar]
- Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G. Exp. Cell Res. 1999;249:413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]
- Madesh M, Hajnoczky G. J. Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin GW, Corfe BM, Griffiths GJ, Thistlethwaite A, Hickman JA, Dive C. EMBO J. 2001;20:6306–6315. doi: 10.1093/emboj/20.22.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA. J. Struct. Biol. 1998;121:207–218. doi: 10.1006/jsbi.1997.3954. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Green DR. Nat. Rev. Mol. Cell Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Kroemer G. Biomed. Pharmacother. 1998a;52:248–251. doi: 10.1016/S0753-3322(98)80009-7. [DOI] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G. Science. 1998b;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G. J. Exp. Med. 1998c;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Nouraini S, Reed JC. Curr. Opin. Microbiol. 1999;2:618–623. doi: 10.1016/s1369-5274(99)00031-4. [DOI] [PubMed] [Google Scholar]
- Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y. Proc. Natl. Acad. Sci. USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins C, Kim CN, Fang G, Bhalla KN. Blood. 2000;95:1014–1022. [PubMed] [Google Scholar]
- Petronilli V, Cola C, Massari S, Colonna R, Bernardi P. J. Biol. Chem. 1993;268:21939–21945. [PubMed] [Google Scholar]
- Petronilli V, Costantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P. J. Biol. Chem. 1994;269:16638–16642. [PubMed] [Google Scholar]
- Rousselot P, Labaume S, Marolleau JP, Larghero J, Noguera MH, Brouet JC, Fermand JP. Cancer Res. 1999;59:1041–1048. [PubMed] [Google Scholar]
- Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- Shi Y, Chen JJ, Chen R, Weng CJ, Zheng YH, Chen Q, Tang H. Biochem. Biophys. Res. Commun. 2003a;305:989–996. doi: 10.1016/s0006-291x(03)00871-4. [DOI] [PubMed] [Google Scholar]
- Shi Y, Jiang C, Chen Q, Tang H. Biochem. Biophys. Res. Commun. 2003b;303:475–482. doi: 10.1016/s0006-291x(03)00359-0. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y, Tsujimoto Y. J. Cell Biol. 2001;152:237–250. doi: 10.1083/jcb.152.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. FEBS Lett. 1998;423:275–280. doi: 10.1016/s0014-5793(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP., Jr N. Engl. J. Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- Song J, Midson C, Blachly-Dyson E, Forte M, Colombini M. J. Biol. Chem. 1998;273:24406–24413. doi: 10.1074/jbc.273.38.24406. [DOI] [PubMed] [Google Scholar]
- Szabo I, Bernardi P, Zoratti M. J. Biol. Chem. 1992;267:2940–2946. [PubMed] [Google Scholar]
- Szabo I, De P, Zoratti M. FEBS Lett. 1993;330:206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- Szabo I, Zoratti M. J. Biol. Chem. 1991;266:3376–3379. [PubMed] [Google Scholar]
- Szabo I, Zoratti M. J. Bioenerg. Biomembr. 1992;24:111–117. doi: 10.1007/BF00769537. [DOI] [PubMed] [Google Scholar]
- Szabo I, Zoratti M. FEBS Lett. 1993;330:201–205. doi: 10.1016/0014-5793(93)80273-w. [DOI] [PubMed] [Google Scholar]
- Takagi-Morishita Y, Yamada N, Sugihara A, Iwasaki T, Tsujimura T, Terada N. Biol. Reprod. 2003;68:1178–1184. doi: 10.1095/biolreprod.102.007997. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Cell Death Differ. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Thompson CB. Nat. Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, Herzenberg LA, Steinman L, Herzenberg LA. Proc. Natl. Acad. Sci. USA. 2000;97:2680–2685. doi: 10.1073/pnas.97.6.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Wang ZG, Rivi R, Delva L, Konig A, Scheinberg DA, Gambacorti-Passerini C, Gabrilove JL, Warrell RP, Jr, Pandolfi PP. Blood. 1998;92:1497–1504. [PubMed] [Google Scholar]
- Xia T, Jiang C, Li L, Wu C, Chen Q, Liu SS. FEBS Lett. 2002a;510:62–66. doi: 10.1016/s0014-5793(01)03228-8. [DOI] [PubMed] [Google Scholar]
- Xia T, Jiang CS, Li LJ, Zhang Y, Jin HJ, Liu SS, Wu CH, Chen Q. Chinese Sci. Bull. 2002b;47:553–557. [Google Scholar]
- Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen SJ, Chen Z. Oncogene. 2001;20:7146–7153. doi: 10.1038/sj.onc.1204762. [DOI] [PubMed] [Google Scholar]