Abstract
Background
Cardiac progenitor cells (CPCs) possess the IGF-1-IGF-1 receptor system and IGF-1 can be tethered to self-assembling peptide nanofibers (NF-IGF-1) leading to prolonged release of this growth factor to the myocardium. Therefore, we tested whether local injection of clonogenic CPCs and NF-IGF-1 potentiate the activation and differentiation of delivered and resident CPCs enhancing cardiac repair after infarction.
Methods and Results
Myocardial infarction was induced in rats, and untreated infarcts and infarcts treated with CPCs or NF-IGF-1 only, and CPCs and NF-IGF-1 together were analyzed. With respect to infarcts exposed to CPCs or NF-IGF-1 alone, combination therapy resulted in a greater increase in left ventricular mass-to-chamber volume ratio, and a better preservation of +dP/dt, −dP/dt, ejection fraction and diastolic wall stress. Myocardial regeneration was detected in all treated infarcts, but the number of newly formed myocytes with combination therapy was 32% and 230% higher than with CPCs and NF-IGF-1, respectively. Corresponding differences in the volume of regenerated myocytes were 48% and 115%. Similarly, the length density of newly formed coronary arterioles with both CPCs and NF-IGF-1 was 73% and 83% greater than with CPCs and NF-IGF-1 alone, respectively. Importantly, activation of resident CPCs by paracrine effects contributed to cardiomyogenesis and vasculogenesis. Collectively, CPCs and NF-IGF-1 therapy reduced infarct size more than CPCs and NF-IGF-1 alone.
Conclusions
The addition of nanofiber-mediated IGF-1-delivery to CPC therapy improved partly the recovery of myocardial structure and function after infarction.
Keywords: Myocardial Infarction, Heart Failure, Remodeling
Attempts made to introduce cell therapy in the management of the acutely infarcted heart in patients1,2 have raised critical questions concerning the fate of the delivered cells.3 The unfavorable microenvironment of the necrotic myocardium together with diffuse inflammatory infiltrates interferes with homing, survival and growth of the administered cells which are critical variables of successful myocardial repair. Additionally, these factors condition the number of cells to be injected since only a small fraction of the implanted cells are able to lodge in proximity and within the damaged myocardium.4 These limitations in the various protocols employed so far have emphasized an apparent discrepancy between the degree of functional recovery after infarction and the actual possibility of myocardial regeneration promoted by engraftment, proliferation and differentiation of the administered cells.5 The possibility has been advanced that a variety of cytokines is released by the delivered cells activating resident progenitors which are responsible for the partial recovery of structure and function of the infarcted heart.5,6 This information underscores the need to develop strategies which protect the viability of the injected cells and enhance the growth reserve of the surviving myocardium.
Self-assembling peptide hydrogels consist of individual interwoven nanofibers which can be engineered to deliver specific proteins to the myocardium.7 We have designed a method to tether factors to the peptide nanofibers and shown that this approach leads to prolonged delivery of IGF-1 to the heart favoring the integration of neonatal myocytes implanted with the tethered peptide.8 This therapeutic intervention positively affects post-infarction ventricular remodeling; it attenuates chamber dilation and improves cardiac performance. Similarly, administration of cardiac progenitor cells (CPCs) to the infarcted heart reconstitutes in part the lost myocardium and has a beneficial anatomical and functional outcome.9,10 Importantly, CPCs possess the IGF-1-IGF-1 receptor system which potentiates their survival and growth.11 Thus, local injection of CPCs together with the prolonged release of IGF-1 by self-assembling peptide nanofibers (NF-IGF-1) may enhance myocardial reconstitution after infarction. This strategy may improve cardiac repair by potentiating the regenerative response of the delivered and resident CPCs.
Methods
CPCs and NF-IGF-1
Clonogenic CPCs from the heart of female Fischer 344 rat were infected with a retrovirus carrying enhanced green fluorescence protein (EGFP).9 Biotinylated IGF-1 and self-assembling peptides were prepared as previously described.8 Immediately before injection, to initiate self-assembly, peptides were dissolved in sterile sucrose (295 mM) at 1% (weight/vol) and sonicated for 10 minutes. In all cases, this volume was injected in the border zone.
In Vitro Studies
Clonogenic CPCs were cultured in serum free medium (SFM) and stimulated with NF-IGF-1. Bromodeoxyuridine (BrdU) was added to the medium at 8 hour intervals. Cells were fixed and BrdU incorporation was measured.9,11 In a similar manner, CPCs were exposed to xanthine (0.5 mM)-xanthine oxidase (100 mU/ml) alone or in the presence of NF-IGF-1 for 24 hours. Cells were fixed and apoptosis was determined by TdT.9, 11
Myocardial Infarction
Under ketamine (100 mg/kg b.w., i.p.) and acepromazine (1 mg/kg b.w., i.p.) anesthesia, myocardial infarction was produced in female Fischer 344 rats at 3 months of age by permanent occlusion of the left coronary artery.9,10 Shortly after infarction, rats were treated with CPCs, NF-IGF-1 and CPCs-NF-IGF-1. Multiple injections were performed to deliver a total of 100,000 CPCs in the border zone; 1 ng of NF-IGF-1 was administered in the same region. The volume of injected CPCs and NF-IGF-1 was 5 µl each. Sham-operated and infarcted rats injected with PBS were used as controls. BrdU was given twice a day (50 mg/kg b.w., i.p.) throughout the experiment.9–11 Rats were killed 1 month later.
Hemodynamics
Under ketamine (100 mg/kg b.w., i.p.) anesthesia, echocardiograms were recorded before sacrifice to calculate ejection fraction by the area-length method.9–11 At sacrifice, animals were anesthetized with chloral hydrate (300 mg/kg b.w., i.p.) and left ventricular (LV) pressures and + and − dP/dt were measured in the closed-chest preparation.4,9–11
Fixation of the Heart
The heart was fixed by perfusion with formalin and cardiac weights were determined. The LV longitudinal axis was obtained and 5 sections from the apex to base were collected. Wall thickness and chamber diameter were assessed and chamber volume was computed. Tissue samples were embedded in paraffin and sections were employed for immunocytochemistry.4,9–11 The antibodies utilized and the methodology of immunolabeling are indicated in Table 1 (online-only Data Supplement).
Infarct Size, Myocyte Volume and Number
Infarct size was determined by the number of myocytes lost by the LV. Additionally, the size and number of spared and regenerated myocytes was determined.4,9,10
DNA Content
DNA content was measured by propidium iodide labeling of nuclei and confocal microscopy. Lymphocytes were used as reference cells for 2n values. Moreover, samples were stained with Ki67 to distinguish cycling and non-cycling cells.9,10
In Situ Hybridization
For the detection of X-chromosome, sections were exposed to Rat X-Chromosome Paint probe, denatured and hybridized for 14 hours. Nuclei were stained with DAPI.4,9,10
Statistical Analysis
The sample size is listed in Table 2 (online-only Data Supplement). Results are means±standard deviation (SD). The normality of distribution of data points was determined using the Kolmogorov-Smirnov test. P values of normal distribution were calculated by the Dallal and Wilkinson approximation to Lilliefor’s method. The homogeneity of variance was evaluated by the F-test for two-group comparisons and by Bartlett’s method for multiple-group comparisons. Student’s t test was employed to calculate the statistical significance between two independent groups. ANOVA with Tukey-Kramer post-test was performed to identify statistical significance in multiple-group comparisons. The significance level was corrected to reflect multiple comparisons. All tests were performed using GraphPad Prism version 3.00 for Windows, GraphPad Software, San Diego, CA.12,13 Multiple-comparison-adjusted P<0.05 was considered significant.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
NF-IGF-1 and CPC Growth and Apoptosis
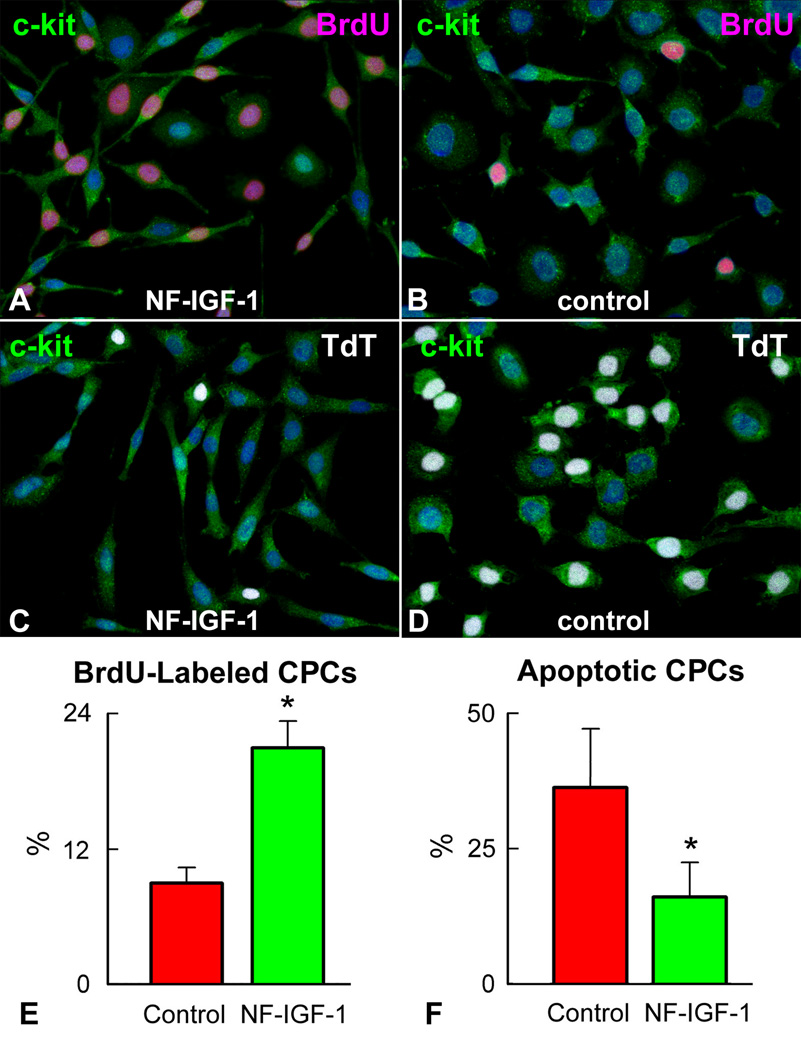
An important premise of this work was the documentation that NF-IGF-1 promotes CPC division and protects CPCs from the death signal triggered by the formation of reactive oxygen species. Oxidative stress is high in the ischemic myocardium14 and interventions preventing this effect would increase the pool of CPCs available for cardiac repair. Single cell-derived clonogenic CPCs9 were cultured in SFM in the presence and absence of NF-IGF-1 to detect the percentage of dividing cells. Conversely, xanthine-xanthine oxidase was employed to generate superoxide anion and activate apoptosis. In 24 hours, CPCs exposed to NF-IGF-1 showed a 2.4-fold higher level of cell replication and a 52% decrease in cell death (Figure 1), emphasizing the role that prolonged release of IGF-1 has in enhancing CPC proliferation and survival.
Figure 1.
IGF-1 and CPC growth and survival. CPCs (green) labeled by BrdU (magenta) are more frequent with (A) than without (B) NF-IGF-1. Conversely, TdT-labeling (white) is lower in CPCs exposed to NF-IGF-1 (C and D). CPCs incorporating BrdU (E) and undergoing apoptosis (F) in SFM and with NF-IGF-1. *Indicates P<0.05.
Cardiac Anatomy and Ventricular Function
The question was then whether the therapeutic efficacy of CPCs for the infarcted heart in vivo was increased by delivery of NF-IGF-1. Shortly after coronary ligation in syngeneic rats, CPCs expressing EGFP were injected in the viable myocardium of the border zone and a few minutes later NF-IGF-1 was delivered to the same region. Control groups included infarcted hearts treated only with CPCs, NF-IGF-1 or PBS. All animals received daily injections of BrdU so that EGFP and/or BrdU were employed to recognize newly formed structures within the infarct and the surviving myocardium.
All animals were sacrificed one month after surgery and infarct dimension determined. Two processes that vary with time occur in the infarcted heart: 1. Shrinkage of the infarcted region with healing and scar formation; and 2. Myocyte growth in the unaffected portion of the ventricle which expands chronically the surviving myocardium. These phenomena cannot be independently measured complicating the assessment of infarct size at distinct time points after coronary occlusion.15 Moreover, scattered myocyte death and regeneration occurs within the viable myocardium altering further the number of contracting cells.10 Thus, the number of myocytes lost and remaining within the LV provide an appropriate characterization of infarct dimension and extent of tissue recovery. These variables are the determinants of cardiac function.4,9,10
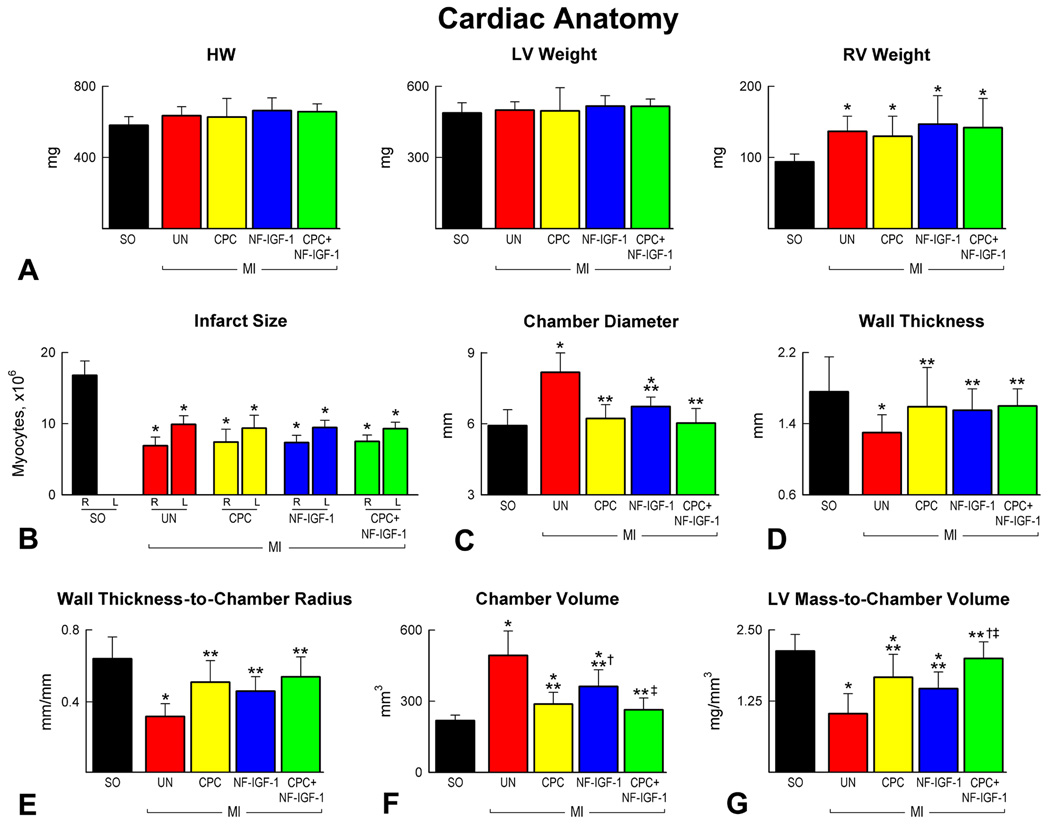
Following the collection of cardiac weights (Figure 2A), the percentage of myocytes lost and present in the LV wall was measured.15 Infarct size varied from 55% to 59%, averaging 57% in the four groups (Figure 2B). In comparison with untreated-infarcts, treatment with CPCs, NF-IGF-1 and CPCs-NF-IGF-1 attenuated chamber dilation and the decrease in LV mass-to-chamber volume ratio of the infarcted heart (Figure 2C–2F). Combination therapy (CPCs-NF-IGF-1), however, appeared to have a more consistent positive influence on cardiac size and shape (Figure 2G).
Figure 2.
Cardiac anatomy. (A and B) Cardiac weights and infarct size. R and L correspond, respectively, to the number of myocytes remaining and lost after infarction. (C–G) LV dimensions. Sham-operated: SO. *Indicates P<0.05 vs SO; **vs untreated infarcts (UN); †vs infarcts treated with CPCs; ‡vs infarcts treated with NF-IGF-1.
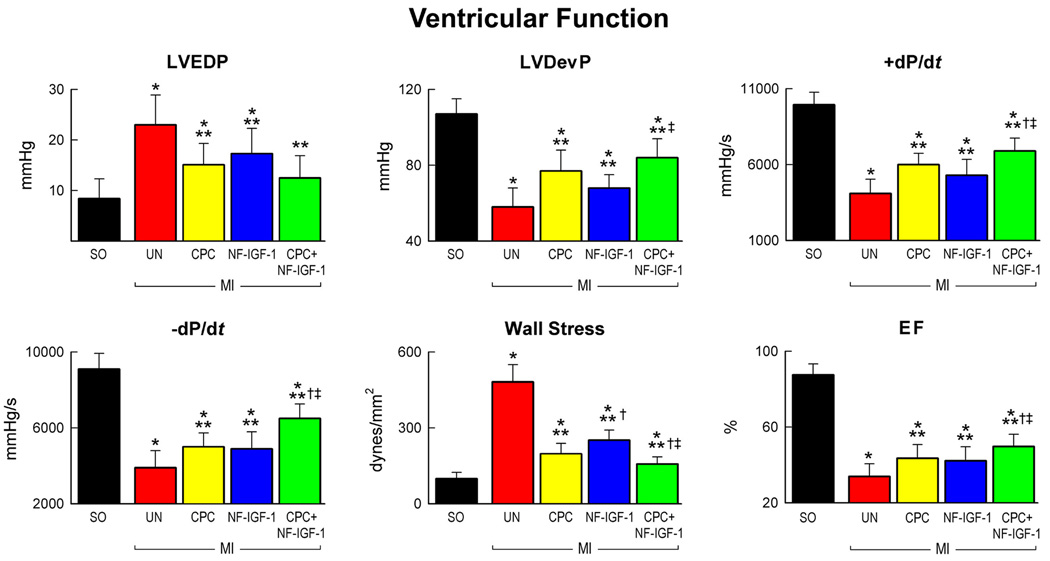
The effects of CPCs, NF-IGF-1 and CPCs-NF-IGF-1 on ventricular function mimicked those on cardiac anatomy. Hemodynamically, treated-infarcts showed a lower increase in LV end-diastolic pressure (LVEDP), and a lower decrease in LV systolic pressure, LV developed pressure (LVDevP) and dP/dt. These factors, together with the attenuation in ventricular dilation, resulted in a reduction in diastolic wall stress and an increase in ejection fraction (EF) of the infarcted heart (Figure 3). Combination therapy, however, led to a better preservation of +dP/dt, −dP/dt, diastolic wall stress and EF than CPCs or NF-IGF-1 alone. CPCs were superior to NF-IGF-1; CPC-treated infarcts had higher LVDevP and lower diastolic wall stress (Figure 3).
Figure 3.
Ventricular function. Combination therapy (CPC-NF-IGF-1) attenuated the most the negative impact of myocardial infarction on cardiac performance. See Figure 2 for symbols.
Cardiomyocyte Regeneration
Four critical variables have to be considered in the analysis of cardiac repair: a) Amount of reconstituted myocyte mass and coronary vasculature; b) Number and size of restored myocytes and vessels; c) Integration of newly formed myocytes and vessels with the surrounding myocardium; and d) Origin of the rebuilt myocardial structures.4,9–11,16
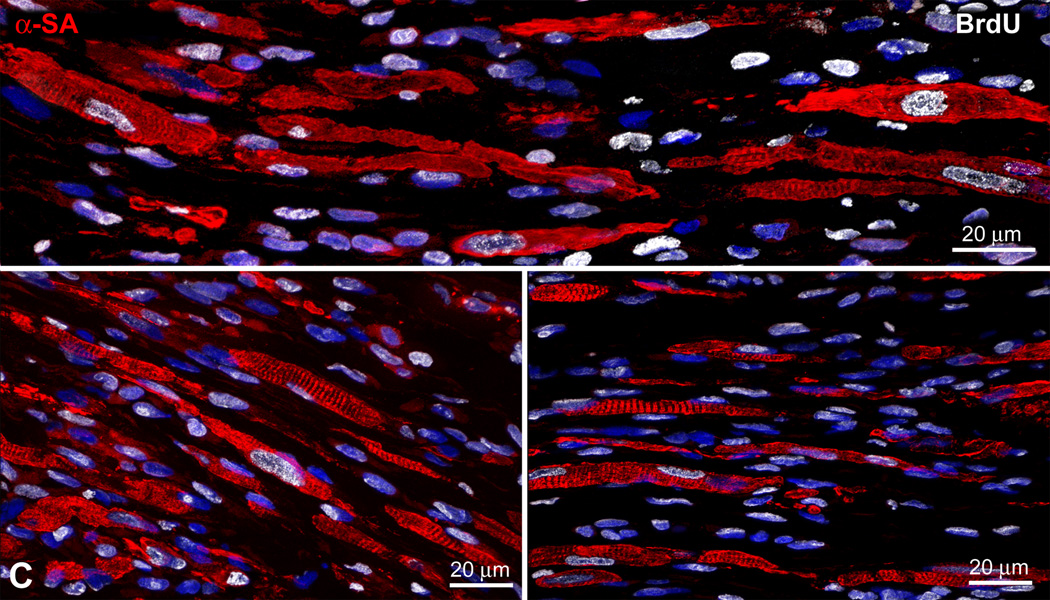
A band of regenerated myocardium was found within the infarcted region of the wall following injection of CPCs, NF-IGF-1 and CPCs-NF-IGF-1 (Figure 4A). Regeneration was not detected in untreated infarcted animals. In rats treated with EGFP-positive CPCs only or together with NF-IGF-1, the majority of newly formed cardiomyocytes expressed EGFP and was labeled by BrdU. However, EGFP-negative BrdU-positive myocytes were also detected with CPCs-NF-IGF-1 suggesting that some regenerated myocytes derived from activation, commitment and differentiation of resident CPCs (Figure 4B). Similarly, myocytes formed by delivery of NF-IGF-1 only were BrdU-positive and represented the progeny of resident CPCs (Figure 4C).
Figure 4.
Myocardial repair. (A) Band of regenerated myocardium (dashed-line) in infarcts treated with CPCs (upper panel), NF-IGF-1 (central panel), and CPCs-NF-IGF-1 (lower panel). In the inset, new myocytes (α-sarcomeric actin, α-SA; red) express EGFP (upper and lower panels, green) or are labeled by BrdU (central panel, white). (B) The majority of regenerated myocytes (upper left panel, red) are EGFP-positive (upper right panel: green, dashed white line) but some are EGFP-negative (dashed yellow line). All new myocytes shown at higher magnification in the lower panel are BrdU-positive (white). (C and D) Differentiating newly-formed myocytes (C: BrdU, white; D: EGFP, green) show sarcomere striation.
The regenerated EGFP-positive and EGFP-negative myocytes present in animals injected with CPCs-NF-IGF-1 reached a more advanced level of cell differentiation than in the other two conditions showing often sarcomere striation (Figure 4D). In this case, the frequency distribution of the volume of new myocytes was shifted to the right towards greater values (Figure 5A); average myocyte volume in CPC-NF-IGF-1 treated infarcts was 48% and 115% larger than in hearts exposed to CPCs and NF-IGF-1 alone, respectively (Figure 5B). The presence of IGF-1 most likely promoted cell differentiation mimicking observations in skeletal muscle.17 Additionally, the number of regenerated myocytes was 32% and 230% higher following combination therapy than after CPCs and NF-IGF-1, respectively. With CPCs-NF-IGF-1 treatment, there was a significantly larger formation of myocyte mass that resulted in a 53% reduction of infarct size. Infarct size decreased 33% with CPCs and 9% with NF-IGF-1 (Figure 5B). In infarct treated with CPCs-NF-IGF-1, the contribution of myocytes derived from activation of resident CPCs to infarct reduction accounted for nearly 18% of the regenerated tissue. This value was slightly higher than that produced by the injection of NF-IGF-1 alone, 14%, but this difference was not significant.
Figure 5.
Myocyte regeneration. (A) Size distribution of newly-formed myocytes. (B) Average volume, number and aggregate mass of regenerated myocytes. The latter was employed to compute infarct size. *Indicates P<0.05 vs infarcts treated with CPCs only; **vs infarcts treated with NF-IGF-1 only. See text for detail.
Vessel Regeneration
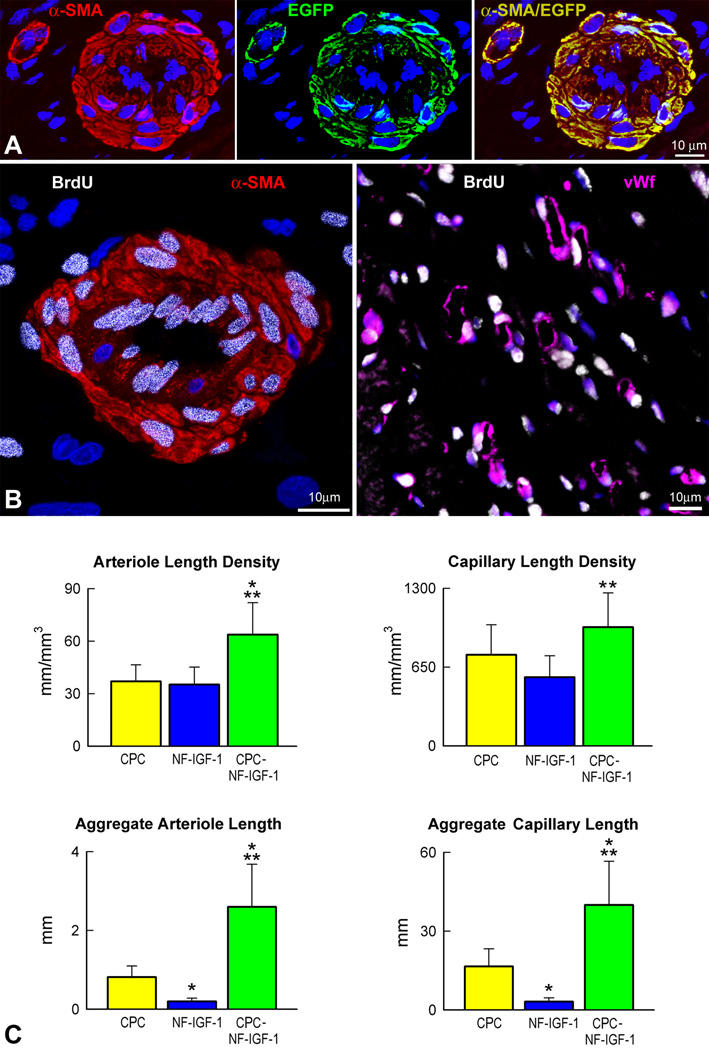
An important aspect of effective cardiac repair involves the formation of resistance arterioles and capillary profiles within the regenerated myocardium (Figure 6A). An analysis comparable to that performed for cardiomyocytes was conducted here for the newly formed coronary microcirculation. In infarcts treated with CPCs, NF-IGF-1 or CPCs-NF-IGF-1, the length density of capillaries and arterioles within the reconstituted myocardium was measured by evaluating separately the fraction of EGFP-positive-BrdU-positive and EGFP-negative-BrdU-positive vascular profiles. In EGFP-positive coronary vessels, all endothelial cells (ECs) and smooth muscle cells (SMCs) expressed the reporter gene. However, some BrdU-positive vascular structures in hearts injected with CPCs-NF-IGF-1 were constituted by ECs and SMCs which were all EGFP-negative (Figure 6B). BrdU-labeled vessel profiles were also detected in animals which received only NF-IGF-1. These findings suggest that CPCs resulted in a vasculogenic response which formed EGFP-positive coronary vessels independently from activation of resident CPCs or growth of ECs and SMCs from preexisting vascular structures. However, EGFP-negative-BrdU-positive vessel profiles with NF-IGF-1 or CPCs-NF-IGF-1 represented the product of growth and differentiation of resident CPCs, proliferation of ECs and SMCs from the spared coronary microcirculation, or both.
Figure 6.
Vascular regeneration. (A and B) New arterioles express α-smooth muscle actin (α-SMA, red) and EGFP (A, green) or are labeled by BrdU (B, white). New capillaries are positive for von Willebrand factor (B: vWf, magenta) and BrdU (B, white). (C) *Indicates P<0.05 vs infarcts treated with CPCs only; **vs infarcts treated with NF-IGF-1 only. (D and E) Border zone (BZ: α-SA, red) and regenerated myocardium (RM: EGFP, green) following CPCs-NF-IGF-1 treatment; areas in rectangles (left panels) are illustrated at higher magnification (right panels). Connexin 43 (D: Cx43, white) and N-cadherin (E: N-cadh, white) are expressed between preexisting EGFP-negative and newly-formed EGFP-positive myocytes (right panels). (F) Within an area of regenerated EGFP-positive myocardium, new arterioles (α-SMA, red) and capillaries (vWf, magenta) express EGFP (green) and contain red blood cells (TER-119, white; arrows).
The length density of EGFP-positive and/or BrdU-positive coronary arterioles per mm3 of regenerated muscle mass was 73% and 83% higher with combination therapy than following the injection of CPCs and NF-IGF-1, respectively (Figure 6C). Also, capillary length density was greater with CPCs-NF-IGF-1 than with the other two conditions but reached statistical significance only with respect to NF-IGF-1 treatment. Because of the larger amount of total myocyte mass obtained with the administration of CPCs-NF-IGF-1, the aggregate length of arterioles and capillaries with combination therapy was significantly higher than that with CPCs or NF-IGF-1 only (Figure 6C). In infarcts treated with CPCs-NF-IGF-1, endogenous vessel formation by activation of resident CPCs and/or preexisting ECs and SMCs comprised 16% and 12% of capillary and arteriolar length, respectively.
Myocyte and Vessel Integration
A relevant issue to be resolved concerned whether the newly formed myocytes incorporated structurally forming specific connections with the non-infarcted myocardium, which would strengthen the possibility that the developing cardiomyocytes were functionally-competent and contributed to ventricular performance. Importantly, connexin 43 and N-cadherin were detected between the viable and repaired myocardium (Figure 6D and 6E); these junctional proteins, which are responsible for electrical and mechanical coupling, were shared by preexisting EGFP-negative and new EGFP-positive cardiomyocytes, documenting the integration between resident and regenerated myocytes. Similarly, EGFP-positive coronary arterioles and capillary profiles frequently contained red blood cells within the lumen (Figure 6F), strongly suggesting that the restored coronary microcirculation was functionally connected with the original coronary vasculature and participated in flow regulation and oxygenation of the repaired expanding myocardium.
Cell Fusion
We then addressed the question whether the regenerated myocytes following the administration of CPCs, NF-IGF-1 or CPCs-NF-IGF-1 were the product of fusion events between the delivered cells and preexisting myocytes, rather than representing the progeny of differentiated CPCs. By necessity, this analysis involved EGFP-positive cardiomyocytes. BrdU labeling, however, was employed as a marker of newly formed myocytes associated with the injection of NF-IGF-1 and the activation and commitment of resident CPCs. In all cases, DNA content per nucleus and the number of X-chromosomes in myocyte nuclei were determined.
Nuclear fusion increases DNA content but changes in DNA may occur by other mechanisms. DNA replication is present during the S phase but this process does not necessarily indicate new cell formation. DNA synthesis can be followed by cytokinesis, giving rise to two daughter cells, nuclear division, giving rise to a multinucleated cell, or endoreplication, giving rise to a cell with extra copies of genomic DNA. In the latter case, the cell cycle proceeds through anaphase but lacks both nuclear division and cytokinesis.18 In polyploid cells, the chromosome number is increased in multiples of 2n. Polyploidy is consistently associated with an increase in the size of the nucleus that is proportional to the number of chromosome sets. Cell volume also increases. Once endocycles are initiated, mitotic division is unlikely to occur or, if it occurs, is extremely slow because of the bulky burden of DNA.
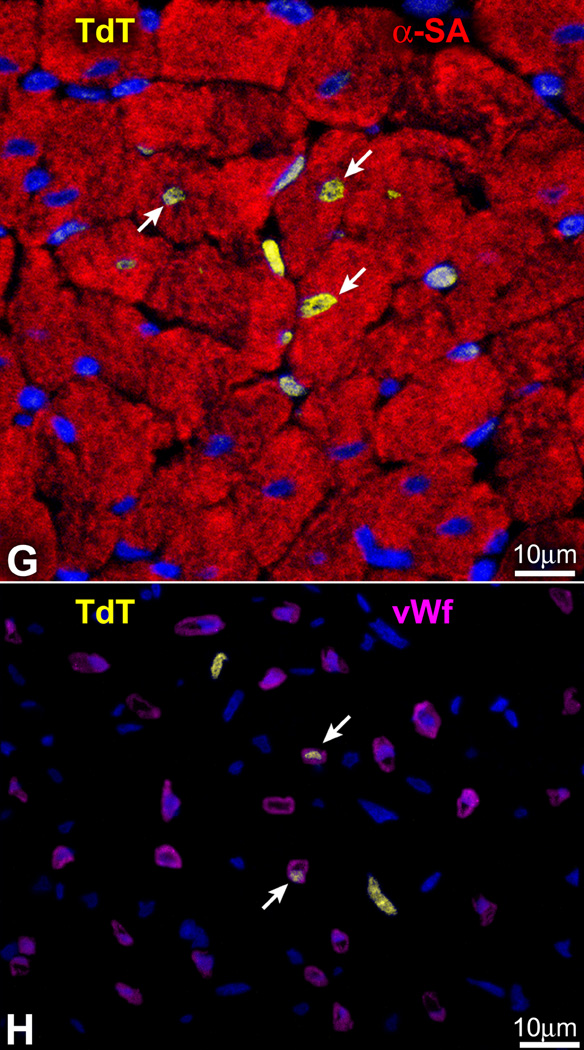
Measurements of DNA content in mononucleated and binucleated EGFP-positive and BrdU-positive myocytes showed diploid DNA content per nucleus (Figure 7A) excluding polyploidization or cell fusion. Higher DNA values were found only in cycling cells positive for Ki67. Another assay was then implemented to document the presence or absence of cell fusion. Since female rats were used and female CPCs were locally implanted within the myocardium, the number of X-chromosomes was measured by FISH. In all cases, at most two X-chromosomes were found in EGFP-positive cardiomyocytes and vessels (Figure 7B–7D). Collectively, these observations exclude that cell fusion played a major role in myocardial regeneration after infarction.
Figure 7.
Cell fusion. (A) DNA content in newly formed cardiomyocytes. Lymphocytes were used as control. Green part of each bar corresponds to cycling, Ki67-labeled cells. (B–D) Regenerated myocytes (B and C: EGFP-α-SA-positive, yellow-green) and arteriole (D: EGFP-α-SMA-positive SMCs, yellow-green) show at most two X-chromosomes.
Surviving Myocardium
To determine the adaptation of the surviving myocardium, BrdU-labeling of myocyte nuclei, myocyte cell volume and apoptosis of myocytes and ECs were measured together with capillary density. Reactive myocyte hypertrophy after infarction was attenuated in a comparable manner with each treatment. In the absence of therapy, myocyte volume increased nearly 75% while each intervention reduced this hypertrophic response by approximately 30% (Figure 8A–8C). Similarly, the decrease in capillary length density in the hypertrophic spared myocardium was attenuated by nearly 25% with these three forms of treatment (Figure 8D). Additionally, BrdU labeling of myocytes was higher in the presence of NF-IGF-1 alone and in combination with CPCs while infarcts treated with CPCs only or untreated showed similar values (Figure 8E and 8F). Myocyte and EC apoptosis was largely prevented by the administration of NF-IGF-1 and CPCs-NF-IGF-1 (Figure 8G–8I). These conditions were characterized by high levels of phospho-Akt labeling in myocytes and vascular ECs (Figure 8J and 8K), which may have accounted for the enhanced cell survival in the myocardium. Although IGF-1 had a more powerful effect on the spared myocardium than CPCs, the latter promoted a more intense form of cardiac repair resulting in a better preservation of ventricular function.
Figure 8.
Surviving myocardium. Spared myocytes of untreated infarcts (A) are larger than in treated infarcts (B). (C and D) *Indicates P<0.05 vs SO; **vs UN. (E) BrdU-labeled myocyte nuclei (green, arrows) in the border zone following CPCs-NF-IGF-1. (F) See C for symbols. †Indicates P<0.05 vs infarcts treated with CPCs. (G and H) TdT-labeled (yellow, arrows) myocyte (G: α-SA, red) and EC (H: vWf, magenta) nuclei in untreated infarcts. (I) See C and F for symbols. (J) Infarct treated with CPCs-NF-IGF-1: myocyte and EC nuclei are positive for phospho-Akt (bright blue, arrows). (K) See C and F for symbols.
Discussion
In recent years, several protocols have been developed experimentally in an attempt to identify novel therapeutic interventions aiming at the reduction of infarct size and prevention of short and long term negative ventricular remodeling following ischemic myocardial injury.19 Three main strategies have been employed and a significant amount of work is being conducted to determine the most effective form of action for acute ischemic heart failure. The delivery of bone marrow progenitor cells (BMCs) has been highly controversial20,21 but recent clinical data have shown improvement in ventricular performance and clinical outcome.1,22 These observations have not changed the nature of the debate concerning the efficacy of this cell category for the human disease and the mechanisms involved in the impact of BMCs on cardiac structure and function. Whether BMCs transdifferentiate and acquire the cardiomyocyte lineage has faced strong opposition and data in favor and against this possibility have been reported.4,20,21 However, this is the only cell class which has been introduced in the treatment of heart failure in patients and large clinical trials are in progress.
Human embryonic stem cells (ESCs) have repeatedly been utilized in animal models to restore the acutely infarcted myocardium, but limited cell engraftment, modest ability to generate vascular structures, teratoma formation and the apparent transient beneficial effects on cardiac hemodynamics23,24 have questioned the current feasibility of this approach clinically. Tremendous efforts are being performed to reduce the malignant tumorigenic potential of ESCs and promote their differentiation into cardiomyocytes23,24 with the expectation that these extremely powerful cells may be applied to human beings in the future. Additionally, the study of ESCs may provide unique understanding of the mechanisms of embryonic development that may lead to therapeutic interventions in utero and the correction of congenital malformations.25
The recognition that a pool of primitive cells with the characteristics of stem cells resides in the myocardium and that these cells form myocytes, ECs and SMCs has provided a different perspective of the biology of the heart and mechanisms of cardiac homeostasis and tissue repair.9,26–29 Regeneration implies that dead cells are replaced by newly formed cells restoring the original structure of the organ. In adulthood, this process occurs during physiological cell turnover, in the absence of injury. However, myocardial damage interferes with recapitulation of cell turnover and restitutio ad integrum of the organ.25 Because of the inability of the adult heart to regenerate itself after infarction, previous studies have promoted tissue repair by injecting exogenously expanded CPCs in proximity of the necrotic myocardium9,10,28,29 or by activating resident CPCs through the delivery of growth factors known to induce cell migration and differentiation.5,10,30 These strategies have attenuated ventricular dilation and the impairment in cardiac function and in some cases have decreased animal mortality.
Although various subsets of CPCs have been used to reconstitute the infarcted myocardium and different degrees of muscle mass regeneration have been obtained, in all cases the newly formed cardiomyocytes possessed fetal-neonatal characteristics and failed to acquire the adult cell phenotype. In the current study, to enhance myocyte growth and differentiation, we have introduced cell therapy together with the delivery of self-assembly peptide nanofibers to provide a specific and prolonged local myocardial release of IGF-1.7,8 IGF-1 increases CPC growth and survival in vitro and in vivo14,18 and this effect resulted here in a major increase in the formation of cardiomyocytes and coronary vessels, decreasing infarct size and restoring partly cardiac performance. This therapeutic approach was superior to the administration of CPCs or NF-IGF-1 only. Combination therapy appeared to be additive; it promoted myocardial regeneration through the activation and differentiation of resident and exogenously delivered CPCs.
Additionally, the strategy implemented here may be superior to the utilization of BMCs for cardiac repair.4,31 CPCs are destined to form myocytes, and vascular SMCs and ECs and, in contrast to BMCs, do not have to transdifferentiate to acquire cardiac cell lineages. Transdifferentiation involves chromatin reorganization with activation and silencing of transcription factors and epigenetic modifications.32 However, control studies addressing this question have yet to be performed, emphasizing the need to resolve this critical issue, before easily accessible BMCs are replaced and complex protocols of CPC collection, isolation and expansion are introduced clinically.
Data in the present study demonstrate that delivered and resident CPCs undergo lineage commitment and give rise to myocytes and coronary vessels across the infarcted myocardium in the absence of cell fusion. Heart homeostasis is modulated by CPCs that continuously differentiate into new younger cell replacing old dying cells. This mechanism of cardiac cell turnover is operative in animals9,26,27,30 and humans33 and does not involve cell fusion. However, the generation of cardiomyocytes by CPCs26 has been postulated to be largely the product of fusion events. If this were to occur, the process of cells fusion would require the merging of a CPC with a terminally differentiated, binucleated myocyte, ~25,000 µm3 in volume or larger. Thus, a trinucleated heterokaryon, a binucleated hyperploid synkaryon, restricted to one of the two nuclei or with a proportional partition of the DNA to each of the myocyte nuclei, would be formed. The unusual trinucleated myocyte heterokaryon will be no longer terminally differentiated; it will reenter the cell cycle, become ~50,000 µm3 in volume and divide, creating two trinucleated daughter cells, ~25,000 µm3 each. When cell fusion is accompanied by nuclear fusion, the high DNA content leads to genetic instability and minimal or null replicative potential.32 However, the replicating and non-replicating myocytes originated here from CPCs alone and together with NF-IGF-1 are predominantly mononucleated, at times binucleated and never trinucleated. Nearly 80% of these cells are less than 3,000 µm3 in volume and ~2% reach 6,000 µm3. All cells have a 2n karyotype and possess two sex chromosomes.
To date, the mechanism of cardiac repair after infarction mediated by the administration of CPCs or progenitor cells of bone marrow origin may involve paracrine effects resulting in the recruitment of endogenous primitive cells with the formation of cardiomyocytes and coronary vessels. The injected cells may release a variety of peptides which may promote the translocation of progenitors to the infarcted area and their differentiation in myocardial structures, accounting for the improvement in ventricular function reported in several studies.5,19 Endogenous cardiomyogenesis and attenuation in myocyte apoptosis have been demonstrated here following the injection of NF-IGF-1 alone and in combination with CPCs supporting the notion that this therapeutic strategy leads to myocyte and vessels regeneration within the infarcted tissue and potentiate the growth reserve of the surviving myocardium. Additionally, these paracrine effects appear to be mediated by activation of the IP3K/Akt effector pathway a distal event of IGF-1 receptor signaling. Collectively, these observations point to the potential therapeutic import of CPCs together with NF-IGF-1 for cardiac diseases in humans. This possibility is strengthened by the local delivery of the growth factor within the myocardium.
Clinical Perspective.
Myocardial infarction is characterized by an extensive loss of cardiomyocytes and vascular structures, and the size of the initial insult is a critical determinant of the evolution of the post-infarcted heart and negative ventricular remodeling. Resident cardiac progenitor cells (CPCs) do not migrate spontaneously to the area of damage and healing is associated with scar formation and alterations in cardiac structure and function. Recent clinical trials have suggested that the intracoronary delivery or intramyocardial injection of adult autologous progenitor cells may have a beneficial effect on the treatment of acute and chronic heart failure in patients. At least three possibilities have been advanced: a) myocardial regeneration mediated by differentiation of the delivered cells; b) paracrine effects triggered by activation of resident progenitor cells; and c) a combination of both processes. In the current study, we tested whether the local administration of CPCs together with IGF-1 tethered to self-assembling peptide nanofibers enhanced the activation and differentiation of exogenous and endogenous CPCs potentiating cardiac repair after infarction. By this strategy, the growth and differentiation of the delivered CPCs was markedly increased and these positive aspects of myocardial regeneration were accompanied by intense recruitment of resident CPCs. This pool of tissue-specific progenitor cells rapidly acquired the adult cardiomyocyte phenotype. In comparison with infarcts treated with CPCs alone, combination therapy resulted in a greater recovery of myocardial structure and ventricular performance. Collectively, our observations point to the potential therapeutic import of CPCs and nanofibers engineered to deliver growth factors for the management of ischemic cardiomyopathy in humans.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by NIH grants and grant EB003805.
Footnotes
Subject Codes: [4], [11], [130], [147], [154]
Conflicts of Interest Disclosures
There are no conflicts to disclose.
References
- 1.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 2.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 4.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 6.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 10.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Müller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 12.Berenson ML, Levine DM, Rindskopf D. Applied Statistics. Englewood Cliffs, NJ: Prentice Hall; 1988. [Google Scholar]
- 13.Motulsky HJ. Analyzing data with GraphPad Prism. San Diego, CA: GraphPad Software Inc.; 1999. [Google Scholar]
- 14.Levonen AL, Vähäkangas E, Koponen JK, Ylä-Herttuala S. Antioxidant gene therapy for cardiovascular disease: current status and future perspectives. Circulation. 2008;117:2142–2150. doi: 10.1161/CIRCULATIONAHA.107.718585. [DOI] [PubMed] [Google Scholar]
- 15.Anversa P, Olivetti G. Handbook of Physiology. The Cardiovascular System. The Heart. Bethesda, MD: Am. Physiol. Soc.; 2002. Cellular basis of physiological and pathological myocardial growth; pp. 75–144. [Google Scholar]
- 16.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musaro’ A, McCullagh K, Paul A, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 18.Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: more or less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 19.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 20.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, Kajstura J, Bolli R. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 21.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 23.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 24.Swijnenburg RJ, Tanaka MVH, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–I172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 25.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 26.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 28.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 29.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro Y. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linke A, Müller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Böhm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 32.Pomerantz J, Blau HM. Nuclear reprogramming: a key to stem cell function in regenerative medicine. Nat Cell Biol. 2004;6:810–816. doi: 10.1038/ncb0904-810. [DOI] [PubMed] [Google Scholar]
- 33.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.