Abstract
Natural killer T (NKT) cells are innate T lymphocytes that are restricted by CD1d antigen-presenting molecules and recognize lipids and glycolipids as antigens. NKT cells have attracted attention for their potent immunoregulatory effects. Like other types of regulatory lymphocytes, a high proportion of NKT cells appear to be autoreactive to self antigens. Thus, as myeloid antigen-presenting cells (APCs) such as monocytes, dendritic cells (DCs) and myeloid-derived suppressor cells (MDSCs) constitutively express CD1d, NKT cells are able to interact with these APCs not only during times of immune activation but also in immunologically quiescent periods. The interactions of NKT cells with myeloid APCs can have either pro-inflammatory or tolerizing outcomes, and a central question is how the ensuing response is determined. Here we bring together published results from a variety of model systems to highlight three critical factors that influence the outcome of the NKT–APC interaction: (i) the strength of the antigenic signal delivered to the NKT cell, as determined by antigen abundance and/or T-cell receptor (TCR) affinity; (ii) the presence or absence of cytokines that costimulate NKT cells [e.g. interleukin (IL)-12, IL-18 and interferon (IFN)-α]; (iii) APC intrinsic factors such as differentiation state (e.g. monocyte versus DC) and Toll-like receptor (TLR) stimulation. Together with recent findings that demonstrate new links between NKT cell activation and endogenous lipid metabolism, these results outline a picture in which the functions of NKT cells are closely attuned to the existing biological context. Thus, NKT cells may actively promote tolerance until a critical level of danger signals arises, at which point they switch to activating pro-inflammatory immune responses.
Keywords: autoreactive, CD1, innate T cell, myeloid-derived suppressor cell, myeloid dendritic cell, natural killer T cell, α-GalCer
Introduction
Natural killer T (NKT) cells were first identified as a small population of T cells in naïve mice that express CD161 (also called NK1.1 or NKR-P1A), a marker that is characteristic of natural killer (NK) cells.1 It subsequently became clear that most of these T cells are restricted by CD1d, a non-classical type of antigen-presenting molecule with structural similarity to major histocompatibility complex (MHC) class I proteins.2,3 Further studies have revealed that, while NKT cells often express NK receptors, these are not specific lineage markers for CD1d-restricted T cells.4,5 Moreover, while NKT cells share some functional and gene expression patterns with NK cells and cytotoxic T lymphocytes (CTLs), they also have many prominent features that are more frequently associated with helper T cells.6–8 Thus, while NKT cells are an innate T lymphocyte population, the implication from their name that they function predominantly as cytolytic effectors is not entirely accurate.
Instead, a number of observations suggest that a major role of NKT cells is to serve as a type of regulatory T cell that can drive downstream immune responses along either pro-inflammatory or silencing pathways. Support for this view comes from findings that NKT cells produce a wide variety of cytokines, including both T helper type 1 (Th1) and Th2 types; that mice genetically deficient in NKT cells show defects not only in resistance to microbial infections and in tumour immunosurveillance but also in establishing peripheral tolerance and preventing autoimmunity; and that specific activation of NKT cells in vivo can inhibit the onset of autoimmune diseases as well as promote microbial clearance or tumour rejection.9–11 This evidence suggests that, despite their small population size, NKT cells have potent effects on immune responses, and they facilitate different outcomes in different contexts. These properties are probably in large part a result of the ability of NKT cells to influence the functions of critical antigen-presenting cell (APC) types. In the following sections we will briefly review the CD1 antigen-presenting system and consider the activation mechanisms underlying NKT cell functional responses, and then we will discuss the pro- and anti-inflammatory interactions of NKT cells with myeloid APCs and the mechanisms by which these are mediated.
CD1 molecules and CD1-restricted T cells
CD1 glycoproteins are a family of antigen-presenting molecules that bind hydrophobic ligands such as lipids, glycolipids and lipopeptides.12 Five CD1 genes have been identified, called CD1A–E, with the corresponding protein products denoted CD1a–e.13 CD1a–d molecules have been shown to present lipidic antigens at the cell surface to T cells, while CD1e remains intracellularly localized and aids in glycolipid processing and loading by other types of CD1.14–18 Like MHC class I molecules, CD1 molecules are synthesized in the endoplasmic reticulum (ER) and then follow the secretory pathway through the Golgi aparatus to the cell surface.19 However, like MHC class II molecules, they then become re-internalized from the plasma membrane and traffic through the endosomal vesicular system and back out again to the cell surface in a recycling loop.20 CD1 molecules are thus able to bind lipid ligands within the secretory system, at the cell surface, or within the endosomal system.
A striking commonality among the CD1-restricted T cells that have been identified thus far is that, although some of them show highly specific recognition of particular microbial antigens,14,21,22 there also seems to be a high frequency of T cells displaying functional autoreactivity to CD1+ APCs without the need for the addition of foreign lipids.23–25 Hence, T cells that are restricted by CD1a, CD1b or CD1c, may resemble CD1d-restricted NKT cells in having innate-like properties that are regulated by recognition of self antigens. However, an important difference between CD1d and the other CD1 antigen-presenting molecules is that CD1d is constitutively expressed on most types of myeloid APC, whereas APC expression of CD1a, CD1b or CD1c molecules is markedly up-regulated by exposure to Toll-like receptor (TLR) agonists or other pro-inflammatory stimuli. Therefore, while CD1d-restricted T cells may be active during periods of relative immune quiescence as well as during immunological challenge, T cells that are restricted by CD1a, CD1b or CD1c may mainly function during periods of immune activation by danger signals.
NKT cell antigen recognition and activation
The CD1d-restricted T-cell compartment includes an evolutionarily conserved population that is characterized by the usage of a nearly invariant T-cell receptor (TCR)-α chain rearrangement,26,27 and also includes other T cells that do not seem to have such highly restricted TCR structures.28–30 The first population is often referred to as ‘invariant’ (iNKT) or ‘type I’ NKT cells, while the second type is called ‘non-invariant’, ‘diverse’ or ‘type II’ NKT cells. There are data suggesting that, like type I NKT cells, the type II subset may perform beneficial regulatory functions,31–33 although this subset has also been associated with pathological outcomes in a number of systems.34–36 As the functions of type II NKT cells are not yet well defined and little is known about their interactions with APCs, in this review we focus only on the type I subset (iNKT cells).
One of the foremost mysteries about iNKT cells is how they are able to mediate such contrasting immunological effects as promoting tumour rejection or clearance of microbial infections, and preventing or ameliorating autoimmune diseases. Previous studies have established that the iNKT cell population contains functionally distinct subsets; for example, CD4− iNKT cells appear to be biased towards production of Th1 cytokines and expression of perforin, whereas CD4+ iNKT cells produce both Th1 and Th2 cytokines and are more notable for up-regulating FAS-ligand after stimulation.37 Thus, it is possible that different iNKT cell subsets become activated in different situations, and mediate distinct effects. This could be a result of differential anatomical localization of iNKT subsets, or of different costimulation requirements. However, as described in the next paragraph, it is not clear that different iNKT cell subsets recognize distinct antigens.
Because of their canonical TCR rearrangements, all iNKT cells share the ability to recognize a specific molecular ‘pattern’ in which a galactose or glucose sugar is attached in an α-anomeric conformation to the polar head group of a lipid.38,39 The prototypical synthetic lipid of this type, α-galactosylceramide (α-GalCer), is a highly potent agonist for iNKT cells.15 Lipids with structural similarity to α-GalCer have been identified from several microbial sources, including a pathogenic Borrelia species.40–43 However, these microbial analogues of α-GalCer generally appear to be substantially weaker TCR agonists than α-GalCer itself. Importantly, mammalian cells do not seem to produce glycolipids in which the first sugar is attached to the lipid via an α-linkage, and thus the self antigens recognized by iNKT cells apparently do not contain this molecular pattern. The nature of the self antigens recognized by iNKT cells will be discussed at the end of the review; suffice it to note here that there is also as yet no clear evidence that iNKT self-antigen specificities differ according to subset.
Another possibility (not mutually exclusive with the subset model) is that the same iNKT cell can mediate distinct functional effects as a result of variations in the activation stimuli in different contexts. We have recently shown that iNKT cells produce cytokines hierarchically in response to increasing TCR signal strength: granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-13 are activated by exposure to low doses of α-GalCer, higher levels of α-GalCer increase secretion of these cytokines and also induce IFN-γ and IL-4, and production of IL-2 requires the highest amounts of antigen.44 This hierarchical response pattern is a consequence of differences in the dependence of each cytokine on calcium signalling, with GM-CSF less dependent than either IFN-γ or IL-4, and IL-2 the most dependent of all. Thus, exposure of iNKT cells to an increasing density of CD1d molecules presenting a strong TCR agonist such as α-GalCer results in greater and greater intracellular calcium flux, which is translated into a quantitatively and qualitatively graded functional output.
Interestingly, self-antigenic stimulation of iNKT cells appears to provide relatively weak TCR signalling, as it failed to induce detectable cytoplasmic calcium flux and led mainly to secretion of GM-CSF and IL-13, with little IFN-γ or IL-4, and generally undetectable IL-2.44 Hence, under normal circumstances, iNKT cell autoreactive recognition of self antigens probably elicits only a partial functional response that is not highly pro-inflammatory. However, in the presence of cytokines such as IL-12p70 and IL-18, iNKT cells are able to produce IFN-γ in response to self-antigenic stimulation.41,45,46 This is a consequence of complementation of the calcium-deficient self-antigenic TCR signalling by the janus kinase-signal transducers and activators of transcription (JAK-STAT) signalling that results from cytokine receptor engagement on the iNKT cells.44 Thus, the nature of the functional response produced by an individual iNKT cell is determined both by the strength of TCR signalling during activation and by the presence or absence of costimulating signalling pathways such as JAK-STAT activation resulting from cytokine receptor engagement.
Promotion of inflammatory responses by iNKT cells
The ability of iNKT cells to potently initiate downstream immune activation was established by two early observations: (i) that injection of α-GalCer into experimental mice results in widespread polyclonal up-regulation of CD69 on other lymphocytes, including B cells, T cells and NK cells;47 and (ii) that the marked elevation of serum IFN-γ levels that follows α-GalCer injection results mainly from iNKT cell-mediated activation of NK cells, rather than coming directly from the iNKT cells themselves.48,49 Subsequently, this pharmacological pathway of iNKT cell activation has been found to enhance protective immunity in a variety of model systems, including bacterial, protozoal, fungal and viral infections (reviewed in Ref. 50). Additionally, administration of α-GalCer has powerful antitumour effects in vivo.51,52 Thus, it is now abundantly clear that iNKT cell activation by a strong agonist such as α-GalCer can dramatically enhance pro-inflammatory protective immune responses in vivo. But what about the pro-inflammatory effects of iNKT cells in the absence of such pharmacological activation?
By using fluorescent tetramers of CD1d to specifically identify iNKT cells, it has been shown that they are among the first lymphocytes to produce IFN-γ during a bacterial infection.45 To specifically assess their contributions during the natural course of microbial infections, extensive use has been made of knockout mice that are deficient in either CD1d or the Jα281 gene segment that is a central part of the iNKT canonical TCR-α chain. Studies of this type have demonstrated that mice deficient in iNKT cells show increased susceptibility to bacterial,53,54 protozoal,55,56 fungal57 and viral infections,58,59 suggesting a role for iNKT cells in natural defence against a variety of pathogens. Similarly, studies using knockout mice and adoptive transfer of iNKT cells have demonstrated that they play a critical role in protection against the development of spontaneous tumours, and have further clarified that the effects of iNKT cells in antitumour responses depend in large part on the involvement of NK cells and CTLs.60–63 Thus, it seems clear that there are physiological pathways by which iNKT cells contribute to protective immune responses. In the next sections we will compare and contrast the mechanisms involved in these pathways.
α-GalCer induced dendritic cell (DC) maturation
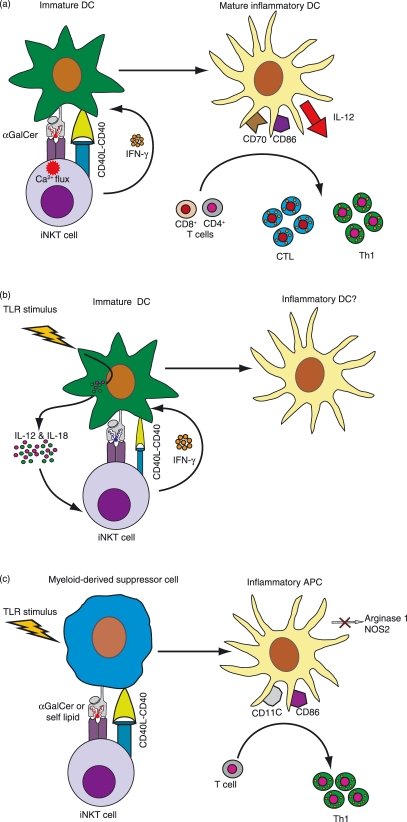
A series of studies have now established that presentation of α-GalCer by DCs to iNKT cells initiates a sequential interaction involving the following steps (see Fig. 1a): (i) the TCR stimulation from recognition of α-GalCer activates iNKT cells to produce cytokines such as IFN-γ and IL-4, and also causes them to strongly up-regulate their cell surface CD40L; (ii) exposure to these factors induces the DCs to mature into a highly stimulatory phenotype that produces sustained IL-12p70 and has high levels of activating ligands such as CD40, CD80, CD86 and CD70; (iii) MHC-restricted T cells that encounter these DCs are efficiently stimulated to produce IFN-γ and are licensed to become effective killers.64–68 While it is not clear whether physiological iNKT cell antigens exist that recapitulate these α-GalCer-induced DC maturation effects, this pathway is nevertheless of clear therapeutic interest. For example, it has been shown that labelling tumour cells with α-GalCer before feeding them to DCs results in efficient priming of CD4- and CD8-mediated T-cell responses and produces tumour regression in vivo.69,70 Similarly, immunizing animals with soluble ovalbumin along with α-Galcer leads to enhanced ovalbumin-specific CD4 and CD8 T-cell memory responses, suggesting that this pathway could provide a valuable vaccine adjuvant strategy.71
Figure 1.
Interactions between invariant natural killer T (iNKT) cells and myeloid antigen-presenting cells (APCs) that produce pro-inflammatory outcomes. (a) Activation of iNKT cells by the strong agonist α-galactosylceramide (α-GalCer). Presentation of α-GalCer by CD1d molecules on immature dendritic cells (DCs) induces robust iNKT cell activation that is associated with both calcium and mitogen-activated protein (MAP) kinase signalling, and results in their secretion of interferon (IFN)-γ and in strong up-regulation of cell surface CD40L. These signals induce maturation of the DCs into an inflammatory phenotype that shows sustained production of interleukin (IL)-12p70 and increased expression of costimulatory molecules such as CD86, as well as the cytotoxic T lymphocyte (CTL)-licensing molecule CD70. Naïve T cells that interact with these DCs are efficiently stimulated to proliferate, and CD4+ T cells acquire a T helper type 1 (Th1) phenotype, while CD8+ T cells are licensed to become efficient killers. References: 44,64–68. (b) The indirect pathway of natural killer T (NKT) cell activation in microbial infection. In response to Toll-like receptor (TLR)-mediated recognition of microbial compounds, immature DCs produce pro-inflammatory cytokines such as IL-12 and IL-18. Although the NKT cells that interact with these DCs may receive only relatively weak T-cell receptor (TCR) stimulation from self antigen recognition, costimulation by the DC cytokines activates them to secrete IFN-γ. The effect of this interaction on DC maturation is not known. However, it is possible that the combination of a microbial TLR stimulus along with CD40L and IFN-γ from NKT cells might induce the DCs to mature into a pro-inflammatory phenotype that could further participate in downstream immune responses. References: 41,44–46. (c) Conversion of myeloid-derived suppressor cells (MDSCs) into pro-inflammatory APCs. NKT cells that are activated by recognition of either α-GalCer or self antigen on immune-suppressive MDSCs up-regulate their cell surface CD40L. In conjunction with other inflammatory signals (e.g. from pathogens or possibly tumours), the resulting CD40 stimulation of the MDSCs converts them into pro-inflammatory APCs that no longer produce the immunosuppressive enzymes arginase 1 and nitric oxide synthase 2 (NOS2) and have increased expression of costimulatory molecules such as CD86. References: 59,77.
The direct and indirect pathways of iNKT cell activation
Two models have been proposed for the mechanism of iNKT cell activation during microbial infection. The first model, called the ‘direct’ pathway of activation, involves iNKT cell recognition of specific microbial lipids as foreign antigens. In contrast, in the second model, the ‘indirect’ pathway, iNKT cells are activated by recognition of self antigens in the presence of costimulation by cytokines such as IL-12 and IL-18 that are produced by DCs upon TLR stimulation by microbial compounds (Fig. 1b). An important difference between the two models is that the direct pathway would be expected to induce iNKT cell secretion of both IFN-γ and IL-4, whereas the indirect pathway would promote IFN-γ production with little or no IL-4. Support for the direct pathway comes from the identification of specific microbial lipids that bind to CD1d and activate iNKT cells.40,41,43 The indirect pathway is supported by observations that in many cases there is no evidence of a specific microbial antigen, and the iNKT cell response involves IFN-γ but not IL-4 production and appears to be completely dependent on costimulation by cytokines such as IL-12p70.41,45
However, because it is difficult to rule out the possibility that microbes for which no iNKT cell antigen has been identified nevertheless do contain cryptic antigens, while microbes that do contain such antigens will also concurrently provide TLR-mediated stimulation that activates DC cytokine production, it is not clear that these two pathways are actually separate during most physiological infections. For example, it has recently been shown that CD1d-mediated presentation of a lipo-peptido-phosphatidylinositol from Entamoeba histolytica is necessary for secretion of IFN-γ by iNKT cells, but that the response requires simultaneous TLR-induced IL-12 secretion.72 Similarly, in a mouse model of tuberculosis it has recently been shown that iNKT cells have a protective effect through recognition of infected macrophages, and that macrophage production of IL-12 and IL-18 is critical for this effect.73 It is not clear whether recognition of mycobacterial antigens is required for the iNKT cell-mediated protection; however, a previous study has identified mycobacterial lipids that may serve as iNKT antigens.74 Thus, it seems likely that the two pathways of iNKT cell activation are not mutually exclusive, and that they occur simultaneously in many systems.
Notably, it is not yet clear whether either the direct or indirect pathways of iNKT cell activation during microbial infection result in the maturation of pro-inflammatory DCs, such as those that are observed after administration of α-GalCer. Induction of a pro-inflammatory DC phenotype was shown in one system to depend on the up-regulation of CD40L expression by iNKT cells as well as their secretion of cytokines such as IFN-γ, both of which are induced by a strong TCR stimulus.65 While self-antigen recognition in the presence of IL-12 and IL-18 is sufficient to induce iNKT cell IFN-γ secretion, the extent to which this form of stimulation also induces cell surface CD40L up-regulation remains unclear. Nevertheless, it is possible that, when combined with a TLR stimulus and IFN-γ, even weak CD40L stimulation from iNKT cells is sufficient to induce the maturation of pro-inflammatory DCs (Fig. 1b).
Conversion of regulatory APCs
Although mature DCs have the capability to potently activate naïve T cells, it is well established that immature DCs have tolerizing effects.75 Thus, by inducing maturation of immature DCs, iNKT cells may tend to promote pro-inflammatory responses simply by shifting the balance away from the more tolerizing stage of DC differentiation. However, it has recently also been shown that iNKT cells can reverse the suppressive phenotype of a type of regulatory APC known as myeloid-derived suppressor cells (MDSCs). MDSCs were first identified as tumour-associated APCs that have highly suppressive effects on T-cell responses via their production of enzymes such as arginase and inducible nitric oxide synthase (iNOS),76 but this type of regulatory APC may also play an important role in immune responses during infection. De Santo et al.59 found that infection of Jα281 knockout mice with influenza virus resulted in the appearance of an increased frequency of MDSCs compared with wild-type mice. The suppressive effects of MDSCs diminished after adoptive transfer of iNKT cells, and this conversion was mediated through the interaction of CD40 and CD40L.59 Similarly, Ko et al.77 used a tumour model system to demonstrate that iNKT cells can induce the differentiation of MDSCs into a mature DC-like cell that can mediate protective antitumour responses. These studies suggest that another pro-inflammatory pathway mediated by iNKT cells is the conversion of tolerogenic APCs into DCs that stimulate Th1 T-cell responses (Fig. 1c).
Promotion of immune tolerance by iNKT cells
Evidence for a role of iNKT cells in promoting tolerance in vivo comes from studies in several different systems, including models of: (1) autoimmune disorders; (2) transplant tolerance; (3) burn injury-induced immune suppression; and (4) antigen-specific tolerance. The following is a brief review of the primary findings in these areas.
Autoimmune disorders. Initial indications of the involvement of iNKT cells in immune tolerance came from observations that the frequency and functional responses of iNKT cells are diminished in non-obese diabetic (NOD) mice, which are highly susceptible to developing autoimmune diseases,78 and that depletion of iNKT cells leads to the development of autoimmunity in MRL/lpr mice, a model with similarity to human systemic lupus erythematosus.79 There also appear to be selective reductions in iNKT cell frequency and function in human patients with a variety of autoimmune diseases.80–83 Adoptive transfer of iNKT cells, or over-expression of either iNKT cells or CD1d molecules, prevents the onset of diabetes in NOD mice.84–86 Moreover, administration of α-GalCer or similar lipids results in amelioration of autoimmune disease in many systems, including models of multiple sclerosis,87–89 type I diabetes,90–92 and myasthenia gravis.93
Transplant tolerance. Because transplantation is associated with a certain amount of unavoidable wounding and also introduces foreign immunogens, survival of transplanted allogeneic tissue requires the successful engagement of at least one pathway of immunological tolerance. Studies in a number of different transplant models have indicated that iNKT cells can promote graft acceptance. It has been observed that tissues or organs are more rapidly rejected when they are transplanted into iNKT cell-deficient mice (Jα281 or CD1d knockouts) than when they are transplanted into wild-type controls, and adoptive transfer of iNKT cells can restore graft acceptance.94–98 Furthermore, activation of iNKT cells by administration of α-GalCer significantly increased graft survival in wild-type mice.97
Burn injury-induced immune suppression. Burn injury is known to result in marked suppression of immune function, which leads to susceptibility to life-threatening systemic infections. Blocking CD1d by administration of anti-CD1d antibodies immediately prior to a burn injury has been shown to prevent immunosuppressive effects.99 Further analysis revealed that the suppressive effect required both CD1d+ APCs and iNKT cells.100 Moreover, administration of α-GalCer prevented the burn-induced suppression of antigen-specific T cells, and restored the expression levels of MHC class II and CD40 on the APCs of burn-injured mice to the levels observed in sham-treated mice.101
Antigen-specific tolerance. A role for iNKT cells in the induction of antigen-specific tolerance has been established in two different model systems. In the first, called anterior chamber-associated immune deviation (ACAID), injection of an antigen into the anterior chamber of the eye (an immunologically privileged site) results in the subsequent development of systemic tolerance to the antigen. This effect was found to be dependent on NKT cells, as it was not observed in CD1d-deficient mice,102 and involves the interaction of NKT cells with CD1d+ tolerogenic APCs.103 In a second type of tolerance model, oral tolerance, mice lacking either iNKT cells or CD1d failed to acquire systemic tolerance after being fed a low dose of ovalbumin, and adoptive transfer of iNKT cells restored the ability to induce tolerance by this method.104 In this system, the presence of iNKT cells was associated with reduced expression of B7 molecules on Peyer’s patch DCs, suggesting the iNKT cells may be involved in the induction of the tolerogenic DC phenotype.104
Immunoregulatory mechanisms
The studies described above clearly establish that iNKT cells play a role in inducing and/or maintaining peripheral tolerance, yet the mechanisms by which they mediate their tolerogenic effects are not well resolved. As iNKT cells are known to produce a wide variety of cytokines, one possibility is that they provide an essential source of immunoregulatory cytokines such as IL-10, or that they can shift the balance away from pro-inflammatory processes by producing Th2 cytokines such as IL-4. Indeed, iNKT cell production of IL-10 has been shown to be required for their tolerance-promoting effects in the ACAID model.105 However, studies using IL-4- and IL-10-deficient mice have demonstrated that the secretion of these cytokines is dispensable for iNKT cells to mediate regulatory effects in many systems. For example, activation of iNKT cells by administration of α-GalCer has been shown to protect against autoimmune diseases in IL-4- or IL-10-deficient mice.106,107 It has also been demonstrated that iNKT cells can prevent type I diabetes without driving a Th2 shift in autopathogenic T cells.108 Thus, attention has focused on the role of iNKT cells in the induction of tolerizing or non-inflammatory DCs. At least three different pathways have been identified by which iNKT cells may promote the generation of regulatory DCs. These are illustrated in Fig. 2, and described in detail below.
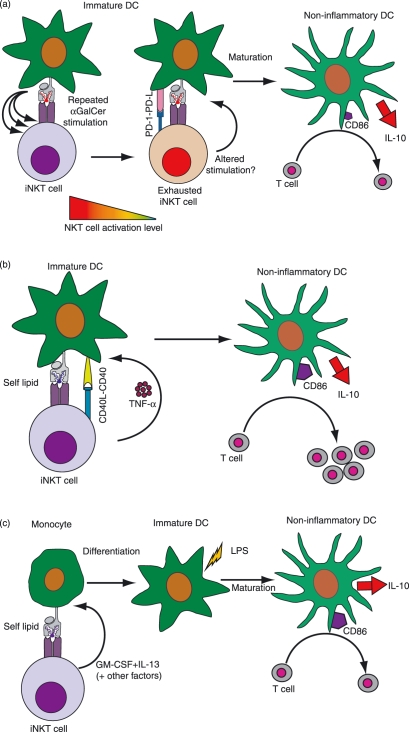
Figure 2.
Interactions between iNKT cells and myeloid ACPs that produce non-inflammatory or tolerizing outcomes. (a) Instruction by exhausted NKT cells. Repeated activation of iNKT cells by α-GalCer results in an anergic or ‘exhausted’ phenotype characterized by the expression of programmed death (PD)-1. Although the precise mechanism is not yet resolved, the exhausted NKT cells may provide altered signals to the DCs. What is clear is that NKT cell exhaustion is associated with the development of a non-inflammatory type of APC that shows low expression of the costimulatory marker CD86, and high production of the anti-inflammatory cytokine IL-10. These DCs fail to promote T-cell proliferation or Th1 responses. References: 109–111. (b) DC instruction by autoantigen-activated NKT cells. In the absence of pro-inflammatory costimulation, self antigenic activation of NKT cells results in little or no production of effector cytokines such as IFN-γ. However, the tumour necrosis factor (TNF)-α secretion and up-regulation of cell surface CD40L that are induced by this pathway are sufficient to stimulate DC maturation. The resulting DCs show elevated levels of CD86 and stimulate T-cell proliferation, but produce high levels of IL-10 and do not efficiently promote T-cell IFN-γ secretion. Reference: 65. (c) Induction of monocyte differentiation into non-inflammatory DCs. NKT cell recognition of self antigens or α-GalCer presented by CD1d molecules on monocytes causes them to secrete GM-CSF and IL-13 along with other factors. These induce the monocytes to differentiate into immature DCs. Further maturation of these DCs induced by exposure to lipopolysaccharide (LPS) results in a phenotype that is characterized by high cell surface expression of MHC class II molecules and costimulatory molecules such as CD86, and production of IL-10 but little or no IL-12. Despite expressing high levels of costimulatory markers, these APCs fail to induce T-cell proliferation or IFN-γ secretion. References: 118,119.
Repeated administration of α-GalCer
Repeated administration of cognate antigens can lead to an ‘exhaustion’ phenotype in MHC-restricted T cells, and a similar effect appears to occur for iNKT cells with α-GalCer (Fig. 2a): after multiple exposures to α-GalCer in vivo, iNKT cells develop a functionally anergic phenotype that is associated with expression of the inhibitory receptor programmed death (PD)-1.109 When iNKT cells become exhausted in this way, their interactions with DCs change and instead of promoting the maturation of pro-inflammatory DCs, they induce a regulatory DC phenotype that is characterized by lower expression levels of CD80, CD86 and CD40, with reduced IL-12 and increased IL-10 secretion.110,111 In autoimmune disease models, regulatory DCs that are generated through this pathway prevent the onset of autoimmunity and silence autopathogenic T cells.91,111
Conversion of immature DCs into a mature regulatory phenotype
It is difficult to fully gauge the effects of self antigen-activated iNKT cells on DC phenotype in vivo; however, in vitro studies have suggested that this pathway can provide a maturation stimulus to immature DCs, but that the resulting DC phenotype is a comparatively non-inflammatory one (Fig. 2b). Vincent et al.65 showed that, in contrast to DCs that matured in response to α-GalCer-stimulated iNKT cells, those that matured in response to self antigen-activated iNKT cells showed up-regulation of costimulatory molecules such as CD86 but produced more IL-10 than IL-12. These DCs efficiently promoted T-cell proliferation, but did not stimulate marked T-cell IFN-γ production.65
Recruitment of monocytes into a regulatory DC lineage
DCs are known to develop from haematopoietic stem cells via multiple distinct differentiation pathways. Some develop directly into precursor DCs in the bone marrow, which then enter the bloodstream and continuously renew immature DC populations within the tissues.112 Other myeloid DCs arise from progenitors that reside in the periphery. Monocytes constitute one such precursor population. Every day about one-third of the blood monocytes are estimated to leave the bloodstream and enter the tissues.113,114 There, they can remain monocytic, become macrophages, or become DCs. Thus, understanding the types of signals that determine their choice of fate is an area of great interest.
Monocytes constitutively express CD1d and have a similar chemokine receptor expression pattern as iNKT cells, suggesting that they may co-localize in vivo.115–117 Recently, we have shown that human iNKT cells direct peripheral blood monocytes to differentiate into immature DCs.118 This process is initiated by NKT cell recognition of CD1d expressed by the monocytes, which activates the NKT cells to secrete GM-CSF and IL-13, cytokines that stimulate the monocytes to follow a DC differentiation pathway (Fig. 2c). The resulting DCs acquired a phenotype resembling immature DCs, and were capable of differentiating into cells that resembled mature DCs upon exposure to lipopolysaccharide (LPS).118 Interestingly, although the mature DCs expressed high levels of costimulatory molecules and MHC class II, they failed to stimulate T-cell proliferation or IFN-γ production and had a highly non-inflammatory phenotype in vivo.119 In contrast to similar model systems in which iNKT cells interact with immature DCs to promote their differentiation to mature DCs,64–68 the DCs that resulted from iNKT cell interactions with monocytes had a non-inflammatory phenotype regardless of whether the iNKT cells were activated by self antigens or by α-GalCer.119 These results suggest that, in addition to converting the phenotype of existing DCs, iNKT cells can also expand the tolerogenic DC population by recruiting monocytic progenitors into the DC lineage.
Deciding between pro- and anti-inflammatory effects
Thus far, we have discussed how the interactions of iNKT cells with DCs can promote either pro- or anti-inflammatory effects, but the question that remains is how it is determined when one pathway will predominate over the other. The short answer to this question is that it is not yet known how this decision is made. However, recent results provide some new insights into physiological mechanisms that control iNKT cell responses.
Antigenic changes during inflammation
Our analysis of the cellular processes involved in iNKT cell activation demonstrated that the intensity of TCR stimulation is a major mechanism governing the qualitative and quantitative nature of their cytokine responses.44 Given that a large number of the lipids presented by CD1d molecules at the cell surface are probably non-antigenic, and only a comparatively small proportion are agonists for iNKT cells, the intensity of iNKT cell TCR stimulation could be modulated either by the relative affinity or the relative abundance of antigenic lipids. Recent studies have suggested that both of these types of changes may occur as a result of myeloid APC activation.
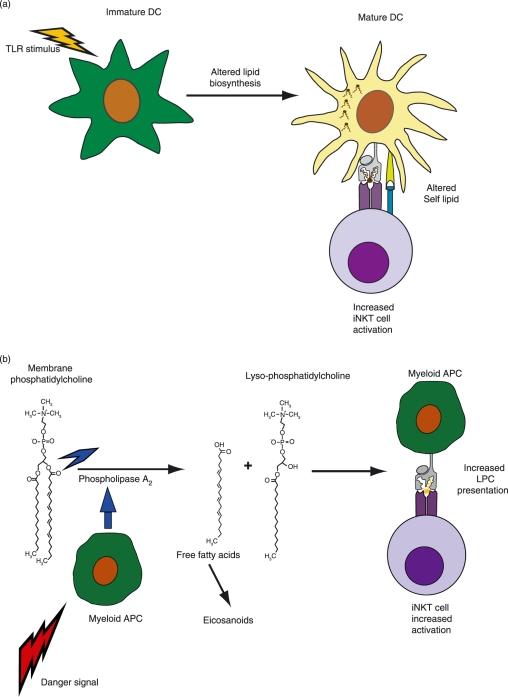
Stimulation of monocytic cells or myeloid DCs by exposure to TLR ligands has been found to result in modifications to glycolipid biosynthesis pathways, including the induction of de novo synthesis of new types of glycosphingolipids, and to concomitantly result in enhanced activation of iNKT cells.120,121 As noted in previous studies, cytokines produced by DCs in response to TLR stimulation were important for activation of the iNKT cells, however, it also appeared that there were antigenic changes that resulted in increased TCR binding, as TLR stimulation of the APCs resulted in stronger staining by a soluble recombinant iNKT TCR even though CD1d expression levels were not increased.121 Thus, activation of myeloid APCs via exposure to certain types of TLR ligands may result in the biosynthesis of different self lipids that are not yet identified but that may be stronger agonists for iNKT cells than the lipids presented by non-activated APCs (Fig. 3a).
Figure 3.
Antigenic changes during inflammation. (a) Altered lipid biosynthesis in myeloid APCs in response to TLR stimuli. Exposure of myeloid APCs to a variety of different TLR ligands appears to cause alterations in endogenous glycolipid biosynthesis. This may result in the de novo presentation of self antigens that are not normally expressed. Recent results suggest that TCR-mediated activation of NKT cells is enhanced as a result of the alterations in self lipid biosynthesis. References: 120,121. (b) Recognition of a self lipid that becomes specifically up-regulated during inflammation. Upon encountering a variety of danger signals, myeloid APCs rapidly activate phospholipase A2 enzymes. This results in increased cleavage of membrane phosphatidylcholine molecules, releasing free fatty acids that are used for eicosanoid (i.e. leukotriene, prostaglandin and lipoxin) biosynthesis. The other product from this reaction is lyso-phosphatidylcholine (LPC), which is able to be bound and presented by CD1d molecules. A fraction of human NKT cells have been found to recognize LPC as an antigen. Thus, the increased levels of LPC produced during inflammation may promote increased NKT cell activation. References: 128–130.
Our recent discovery that a substantial fraction of human iNKT cells recognize lyso-phosphatidylcholine (LPC) as a self antigen suggests a mechanism by which antigen abundance may be connected to endogenous signalling pathways.122 One of the first things to happen upon stimulation of myeloid cells by growth factors, cytokines, neurotransmitters, hormones, and danger signals such as TLR ligands is the activation of phospholipase A2 (PLA2) enzymes.123,124 PLA2 cleaves the sn-2 acyl chain bond of phosphatidylcholine (PC), one of the most abundant membrane lipids in eukaryotic cells, releasing LPC and a free fatty acid (Fig. 3b). The free fatty acids produced by this process are the biochemical substrates for the synthesis of lipid mediators such as leukotrienes, prostaglandins and lipoxins which are critical elements in the regulation of inflammation.125,126 LPC can itself serve as an intercellular lipid messenger or it may be further chemically modified, for example by an acetylation reaction that produces platelet-activating factor.125,127 Thus, the finding that many iNKT cells recognize LPC as a CD1d-presented antigen provides a novel molecular link between these innate regulatory T cells and the initiation point of the biosynthesis of lipid mediators that have key roles in inflammation.
As LPC is generated during the course of normal cellular growth processes, it is probably constitutively presented by CD1d molecules on APCs. Indeed, recent analyses have identified LPC as one of the types of cellular lipids bound to human CD1d molecules.128,129 However, it is also known that during acute and chronic inflammatory states the levels of both LPC and secreted PLA2 enzymes can rise dramatically in serum and other extracellular fluids, and therefore it is reasonable to suppose that the amount of LPC presented by CD1d might increase under inflamed conditions, and that this might cause enhanced iNKT cell activation (Fig. 3b). A further possibility suggested by our data, however, is that at some point the LPC concentrations may become inhibitory and may fail to induce iNKT cell activation, suggesting that this pathway may shut down under conditions of very strong or prolonged inflammation.122 It is also interesting to note that another report has described the expansion of LPC-reactive CD1d-restricted T cells that are not iNKT cells (i.e. a population of type II NKT cells) in blood of human multiple myeloma patients.130 However, it is not yet clear whether these LPC-reactive type II NKT cells have a protective or pathogenic effect. Together, these results suggest that there may be a complex relationship between the homeostatic and inflammation-associated production of LPC by APCs and the resulting activation of iNKT cells and other CD1d-restricted subsets.
Up-regulation of CD1d expression levels
Another mechanism by which iNKT cell responses may be physiologically modulated is via the regulation of CD1d cell surface expression levels. It has been shown that CD1d is up-regulated on murine macrophages following exposure to IFN-γ and one other signal, which can come from inflammation-associated cytokines such as tumour necrosis factor (TNF)-α, or from microbial infection of the macrophage, or simply from exposure to microbial products.131 As the up-regulated CD1d expression was associated with enhanced iNKT cell activation, this observation suggests that infected and non-infected bystander macrophages might similarly stimulate increased iNKT cell responses.
Expression levels of CD1d on human myeloid DCs have been found to be regulated by a type of nuclear hormone receptor called peroxisome proliferator-activated receptor γ (PPAR-γ). Receptors of this family are known to regulate the expression of genes involved in energy management (e.g. genes relating to lipid storage, metabolism and transport), as well as genes involved in inflammatory processes and wound healing.132 Like other receptors of this type, PPAR-γ resides in the cytoplasm in an inactivated state until it binds a specific ligand, generally a hydrophobic or lipidic molecule, whereupon it translocates to the nucleus and acts as a transcription factor for genes that include the appropriate response element sequences.132 Szatmari et al.133 have shown that exposure of DCs to oxidized low-density lipoprotein (LDL) results in the activation of PPAR-γ and transactivation of genes that turn on the retinoic acid synthesis pathway. The resulting production of all-trans retinoic acid eventually leads to activation of retinoic acid receptor-α (RAR-α), which in turn transactivates CD1d mRNA synthesis.133 Thus, CD1d expression levels are directly modulated by RAR-α, but this pathway can be indirectly activated by exposure to PPAR-γ ligands, including lipids associated with oxidized LDL. As oxidized LDL is an inflammation-associated danger signal that may be generated even in the absence of a pathogenic microbial challenge, these results suggest that CD1d expression by myeloid APCs, and consequently NKT cell activation, may be linked to broad pathways of endogenous inflammatory activation.
Conclusions and future directions
Investigations over the last 15 years have revealed a surprising complexity and variety to the range of interactions between iNKT cells and myeloid APCs. It seems that iNKT cells can induce DCs to become highly stimulatory, but they can also cause them to gain a more tolerizing phenotype. Moreover, they can convert suppressive APCs such as MDSCs into a pro-inflammatory phenotype, but can also recruit potentially inflammatory cell types such as monocytes into a tolerogenic DC lineage.
Although it is not yet well understood how it is ultimately determined which of these processes will assume the upper hand in any given situation, a few themes have emerged. Tolerance-promoting effects of iNKT cells appear to be clearly favoured when there is a lack of inflammatory stimuli in the local milieu, or when the level of antigenic stimulation is low. In contrast, exposure to an initial strong antigenic stimulus or to cytokine-mediated costimulation can favour the pro-inflammatory effects of iNKT cells. Questions that remain to be resolved include why in some cases iNKT cells nevertheless seem to contravene these ‘rules’, for example, by promoting tolerance in situations where there is substantial inflammatory immune activation (e.g. organ transplantation). Based on our current picture, one thing that is a reasonably safe bet is that gaining a handle on how iNKT cells mediate their contrasting effects will not only reveal novel insights into the workings of these remarkable lymphocytes, but will also produce new information on the biology of DCs and other myeloid APCs.
Disclosures
The authors were supported by National Institutes of Health (NIH) grants AI074940 and AI076707, and by the Pew Scholars in the Biomedical Sciences Program.
References
- 1.Ballas ZK, Rasmussen W. NK1.1+ thymocytes. Adult murine CD4−, CD8− thymocytes contain an NK1.1+, CD3+, CD5hi, CD44hi, TCR-V beta 8+ subset. J Immunol. 1990;145:1039–45. [PubMed] [Google Scholar]
- 2.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–8. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–5. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 4.Assarsson E, Kambayashi T, Sandberg JK, Hong S, Taniguchi M, Van Kaer L, Ljunggren HG, Chambers BJ. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J Immunol. 2000;165:3673–9. doi: 10.4049/jimmunol.165.7.3673. [DOI] [PubMed] [Google Scholar]
- 5.Kambayashi T, Assarsson E, Michaelsson J, Berglund P, Diehl AD, Chambers BJ, Ljunggren HG. Emergence of CD8+ T cells expressing NK cell receptors in influenza A virus-infected mice. J Immunol. 2000;165:4964–9. doi: 10.4049/jimmunol.165.9.4964. [DOI] [PubMed] [Google Scholar]
- 6.Niemeyer M, Darmoise A, Mollenkopf HJ, Hahnke K, Hurwitz R, Besra GS, Schaible UE, Kaufmann SH. Natural killer T-cell characterization through gene expression profiling: an account of versatility bridging T helper type 1 (Th1), Th2 and Th17 immune responses. Immunology. 2008;123:45–56. doi: 10.1111/j.1365-2567.2007.02701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson SB, Byrne MC. Gene expression in NKT cells: defining a functionally distinct CD1d-restricted T cell subset. Curr Opin Immunol. 2001;13:555–61. doi: 10.1016/s0952-7915(00)00258-2. [DOI] [PubMed] [Google Scholar]
- 8.Rolf J, Berntman E, Stenstrom M, et al. Molecular profiling reveals distinct functional attributes of CD1d-restricted natural killer (NK) T cell subsets. Mol Immunol. 2008;45:2607–20. doi: 10.1016/j.molimm.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 10.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 12.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 13.Martin LH, Calabi F, Milstein C. Isolation of CD1 genes: a family of major histocompatibility complex-related differentiation antigens. PNAS. 1986;83:9154–8. doi: 10.1073/pnas.83.23.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–4. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 15.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 16.Rosat JP, Grant EP, Beckman EM, et al. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. J Immunol. 1999;162:366–71. [PubMed] [Google Scholar]
- 17.Gumperz JE, Roy C, Makowska A, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–21. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 18.de la Salle H, Mariotti S, Angenieux C, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–4. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- 19.De Silva AD, Park JJ, Matsuki N, Stanic AK, Brutkiewicz RR, Medof ME, Joyce S. Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J Immunol. 2002;168:723–33. doi: 10.4049/jimmunol.168.2.723. [DOI] [PubMed] [Google Scholar]
- 20.Gumperz JE. The ins and outs of CD1 molecules: bringing lipids under immunological surveillance. Traffic. 2006;7:2–13. doi: 10.1111/j.1600-0854.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 21.Moody DB, Reinhold BB, Guy MR, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–6. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 22.Moody DB, Young DC, Cheng TY, et al. T cell activation by lipopeptide antigens. Science. 2004;303:527–31. doi: 10.1126/science.1089353. [DOI] [PubMed] [Google Scholar]
- 23.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989;341:447–50. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]
- 24.Spada FM, Grant EP, Peters PJ, et al. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191:937–48. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent MS, Xiong X, Grant EP, Peng W, Brenner MB. CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J Immunol. 2005;175:6344–51. doi: 10.4049/jimmunol.175.10.6344. [DOI] [PubMed] [Google Scholar]
- 26.Koseki H, Imai K, Nakayama F, Sado T, Moriwaki K, Taniguchi M. Homogenous junctional sequence of the V14+ T-cell antigen receptor alpha chain expanded in unprimed mice. Proc Natl Acad Sci U S A. 1990;87:5248–52. doi: 10.1073/pnas.87.14.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162:161–7. [PubMed] [Google Scholar]
- 30.Exley MA, Tahir SM, Cheng O, Shaulov A, Joyce R, Avigan D, Sackstein R, Balk SP. A major fraction of human bone marrow lymphocytes are Th2-like CD1d- reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167:5531–4. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 31.Duarte N, Stenstrom M, Campino S, Bergman ML, Lundholm M, Holmberg D, Cardell SL. Prevention of diabetes in nonobese diabetic mice mediated by CD1d-restricted nonclassical NKT cells. J Immunol. 2004;173:3112–8. doi: 10.4049/jimmunol.173.5.3112. [DOI] [PubMed] [Google Scholar]
- 32.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–57. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sfondrini L, Besusso D, Zoia MT, et al. Absence of the CD1 molecule up-regulates antitumor activity induced by CpG oligodeoxynucleotides in mice. J Immunol. 2002;169:151–8. doi: 10.4049/jimmunol.169.1.151. [DOI] [PubMed] [Google Scholar]
- 34.Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–33. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–94. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 36.Durante-Mangoni E, Wang R, Shaulov A, He Q, Nasser I, Afdhal N, Koziel MJ, Exley MA. Hepatic CD1d expression in hepatitis C virus infection and recognition by resident proinflammatory CD1d-reactive T cells. J Immunol. 2004;173:2159–66. doi: 10.4049/jimmunol.173.3.2159. [DOI] [PubMed] [Google Scholar]
- 37.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kjer-Nielsen L, Borg NA, Pellicci DG, et al. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J Exp Med. 2006;203:661–73. doi: 10.1084/jem.20051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott-Browne JP, Matsuda JL, Mallevaey T, et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–13. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 40.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 41.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 42.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 43.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–86. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Chen X, Rodenkirch L, et al. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112:4128–38. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 46.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–13. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 47.Burdin N, Brossay L, Kronenberg M. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014–25. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 48.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 49.Kitamura H, Ohta A, Sekimoto M, et al. Alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 50.Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–34. [PubMed] [Google Scholar]
- 52.Kawano T, Cui J, Koezuka Y, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A. 1998;95:5690–3. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawakami K, Yamamoto N, Kinjo Y, et al. Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol. 2003;33:3322–30. doi: 10.1002/eji.200324254. [DOI] [PubMed] [Google Scholar]
- 54.Nieuwenhuis EE, Matsumoto T, Exley M, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–93. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 55.Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, Porcelli SA, Spath GF. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004;200:895–904. doi: 10.1084/jem.20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73:181–92. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, Nakayama T, Taniguchi M, Saito A. Activation of Valpha14(+) natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun. 2001;69:213–20. doi: 10.1128/IAI.69.1.213-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J Immunol. 2003;170:1430–4. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- 59.De Santo C, Salio M, Masri SH, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–48. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–27. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–63. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 62.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–66. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 63.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–8. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–8. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 66.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–18. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taraban VY, Martin S, Attfield KE, et al. Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J Immunol. 2008;180:4615–20. doi: 10.4049/jimmunol.180.7.4615. [DOI] [PubMed] [Google Scholar]
- 69.Liu K, Idoyaga J, Charalambous A, et al. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–16. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung Y, Qin H, Kang CY, Kim S, Kwak LW, Dong C. An NKT-mediated autologous vaccine generates CD4 T-cell dependent potent antilymphoma immunity. Blood. 2007;110:2013–9. doi: 10.1182/blood-2006-12-061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hermans IF, Silk JD, Gileadi U, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 72.Lotter H, Gonzalez-Roldan N, Lindner B, et al. Natural killer T cells activated by a lipopeptidophosphoglycan from Entamoeba histolytica are critically important to control amebic liver abscess. PLoS Pathog. 2009;5:e1000434. doi: 10.1371/journal.ppat.1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 2008;4:e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fischer K, Scotet E, Niemeyer M, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. 2004;101:10685–90. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–28. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- 78.Baxter AG, Kinder SJ, Hammond KJ, Scollay R, Godfrey DI. Association between alphabetaTCR+CD4-CD8- T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 1997;46:572–82. doi: 10.2337/diab.46.4.572. [DOI] [PubMed] [Google Scholar]
- 79.Mieza MA, Itoh T, Cui JQ, et al. Selective reduction of Va14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–40. [PubMed] [Google Scholar]
- 80.Sumida T, Sakamoto A, Murata H, et al. Selective reduction of T cells bearing invariant Va24JaQ antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163–8. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Vliet HJ, von Blomberg BM, Nishi N, et al. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001;100:144–8. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 82.Wilson SB, Kent SC, Horton HF, Hill AA, Bollyky PL, Hafler DA, Strominger JL, Byrne MC. Multiple differences in gene expression in regulatory Valpha 24Jalpha Q T cells from identical twins discordant for type I diabetes. Proc Natl Acad Sci U S A. 2000;97:7411–6. doi: 10.1073/pnas.120161297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gausling R, Trollmo C, Hafler DA. Decreases in interleukin-4 secretion by invariant CD4(-)CD8(-)V alpha 24J alpha Q T cells in peripheral blood of patientswith relapsing-remitting multiple sclerosis. Clin Immunol. 2001;98:11–7. doi: 10.1006/clim.2000.4942. [DOI] [PubMed] [Google Scholar]
- 84.Falcone M, Facciotti F, Ghidoli N, et al. Up-regulation of CD1d expression restores the immunoregulatory function of NKT cells and prevents autoimmune diabetes in nonobese diabetic mice. J Immunol. 2004;172:5908–16. doi: 10.4049/jimmunol.172.10.5908. [DOI] [PubMed] [Google Scholar]
- 85.Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. Alpha/beta-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–56. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach JF, Monteiro RC. Overexpression of natural killer T cells protects Valpha14-Jalpha281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–9. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh AK, Wilson MT, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–11. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–4. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 90.Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–6. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 91.Naumov YN, Bahjat KS, Gausling R, et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc Natl Acad Sci U S A. 2001;98:13838–43. doi: 10.1073/pnas.251531798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharif S, Arreaza GA, Zucker P, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 93.Liu R, La Cava A, Bai XF, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175:7898–904. doi: 10.4049/jimmunol.175.12.7898. [DOI] [PubMed] [Google Scholar]
- 94.Ikehara Y, Yasunami Y, Kodama S, Maki T, Nakano M, Nakayama T, Taniguchi M, Ikeda S. CD4(+) Valpha14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest. 2000;105:1761–7. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seino KI, Fukao K, Muramoto K, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci U S A. 2001;98:2577–81. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sonoda KH, Taniguchi M, Stein-Streilein J. Long-term survival of corneal allografts is dependent on intact CD1d-reactive NKT cells. J Immunol. 2002;168:2028–34. doi: 10.4049/jimmunol.168.4.2028. [DOI] [PubMed] [Google Scholar]
- 97.Oh K, Kim S, Park SH, Gu H, Roopenian D, Chung DH, Kim YS, Lee DS. Direct regulatory role of NKT cells in allogeneic graft survival is dependent on the quantitative strength of antigenicity. J Immunol. 2005;174:2030–6. doi: 10.4049/jimmunol.174.4.2030. [DOI] [PubMed] [Google Scholar]
- 98.Yang SH, Jin JZ, Lee SH, et al. Role of NKT cells in allogeneic islet graft survival. Clin Immunol. 2007;124:258–66. doi: 10.1016/j.clim.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 99.Faunce DE, Gamelli RL, Choudhry MA, Kovacs EJ. A role for CD1d-restricted NKT cells in injury-associated T cell suppression. J Leukoc Biol. 2003;73:747–55. doi: 10.1189/jlb.1102540. [DOI] [PubMed] [Google Scholar]
- 100.Palmer JL, Tulley JM, Kovacs EJ, Gamelli RL, Taniguchi M, Faunce DE. Injury-induced suppression of effector T cell immunity requires CD1d-positive APCs and CD1d-restricted NKT cells. J Immunol. 2006;177:92–9. doi: 10.4049/jimmunol.177.1.92. [DOI] [PubMed] [Google Scholar]
- 101.Tulley JM, Palmer JL, Gamelli RL, Faunce DE. Prevention of injury-induced suppression of T-cell immunity by the CD1d/NKT cell-specific ligand alpha-galactosylceramide. Shock. 2008;29:269–77. doi: 10.1097/shk.0b013e31811ff60c. [DOI] [PubMed] [Google Scholar]
- 102.Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–26. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Faunce DE, Stein-Streilein J. NKT cell-derived RANTES recruits APCs and CD8+ T cells to the spleen during the generation of regulatory T cells in tolerance. J Immunol. 2002;169:31–8. doi: 10.4049/jimmunol.169.1.31. [DOI] [PubMed] [Google Scholar]
- 104.Kim HJ, Hwang SJ, Kim BK, Jung KC, Chung DH. NKT cells play critical roles in the induction of oral tolerance by inducing regulatory T cells producing IL-10 and transforming growth factor beta, and by clonally deleting antigen-specific T cells. Immunology. 2006;118:101–11. doi: 10.1111/j.1365-2567.2006.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sonoda KH, Faunce DE, Taniguchi M, Exley M, Balk S, Stein-Streilein J. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J Immunol. 2001;166:42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- 106.Chen YG, Choisy-Rossi CM, Holl TM, et al. Activated NKT cells inhibit autoimmune diabetes through tolerogenic recruitment of dendritic cells to pancreatic lymph nodes. J Immunol. 2005;174:1196–204. doi: 10.4049/jimmunol.174.3.1196. [DOI] [PubMed] [Google Scholar]
- 107.Mi QS, Ly D, Zucker P, McGarry M, Delovitch TL. Interleukin-4 but not interleukin-10 protects against spontaneous and recurrent type 1 diabetes by activated CD1d-restricted invariant natural killer T-cells. Diabetes. 2004;53:1303–10. doi: 10.2337/diabetes.53.5.1303. [DOI] [PubMed] [Google Scholar]
- 108.Beaudoin L, Laloux V, Novak J, Lucas B, Lehuen A. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic beta cells. Immunity. 2002;17:725–36. doi: 10.1016/s1074-7613(02)00473-9. [DOI] [PubMed] [Google Scholar]
- 109.Parekh VV, Lalani S, Kim S, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–26. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang J, Cho S, Ueno A, Cheng L, Xu BY, Desrosiers MD, Shi Y, Yang Y. Ligand-dependent induction of noninflammatory dendritic cells by anergic invariant NKT cells minimizes autoimmune inflammation. J Immunol. 2008;181:2438–45. doi: 10.4049/jimmunol.181.4.2438. [DOI] [PubMed] [Google Scholar]
- 111.Kojo S, Seino K, Harada M, Watarai H, Wakao H, Uchida T, Nakayama T, Taniguchi M. Induction of regulatory properties in dendritic cells by Valpha14 NKT cells. J Immunol. 2005;175:3648–55. doi: 10.4049/jimmunol.175.6.3648. [DOI] [PubMed] [Google Scholar]
- 112.Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80:448–62. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 113.Whitelaw DM. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet. 1972;5:311–7. doi: 10.1111/j.1365-2184.1972.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 114.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–18. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 115.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 116.Thomas SY, Hou R, Boyson JE, et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J Immunol. 2003;171:2571–80. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 117.Kim CH, Johnston B, Butcher EC. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24(+)V beta 11(+) NKT cell subsets with distinct cytokine-producing capacity. Blood. 2002;100:11–6. doi: 10.1182/blood-2001-12-0196. [DOI] [PubMed] [Google Scholar]
- 118.Hegde S, Chen X, Keaton JM, Reddington F, Besra GS, Gumperz JE. NKT cells direct monocytes into a DC differentiation pathway. J Leukoc Biol. 2007;81:1224–35. doi: 10.1189/jlb.1206718. [DOI] [PubMed] [Google Scholar]
- 119.Hegde S, Jankowska-Gan E, Roenneburg DA, Torrealba J, Burlingham WJ, Gumperz JE. Human NKT cells promote monocyte differentiation into suppressive myeloid antigen-presenting cells. J Leukoc Biol. 2009;86:757–68. doi: 10.1189/jlb.0209059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paget C, Mallevaey T, Speak AO, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 121.Salio M, Speak AO, Shepherd D, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007;104:20490–5. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fox LM, Cox DG, Lockridge JL, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 124.Touqui L, Alaoui-El-Azher M. Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases. Curr Mol Med. 2001;1:739–54. doi: 10.2174/1566524013363258. [DOI] [PubMed] [Google Scholar]
- 125.Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–58. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 126.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 127.Murakami M, Kudo I. Phospholipase A2. J Biochem. 2002;131:285–92. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 128.Cox D, Fox L, Tian R, Bardet W, Skaley M, Mojsilovic D, Gumperz J, Hildebrand W. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol. 2009;182:4784–91. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang DH, Deng H, Matthews P, et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–16. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Skold M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J Immunol. 2005;175:3584–93. doi: 10.4049/jimmunol.175.6.3584. [DOI] [PubMed] [Google Scholar]
- 132.Nunn AV, Bell J, Barter P. The integration of lipid-sensing and anti-inflammatory effects: how the PPARs play a role in metabolic balance. Nucl Recept. 2007;5:1. doi: 10.1186/1478-1336-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Szatmari I, Pap A, Ruhl R, et al. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–62. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]