Abstract
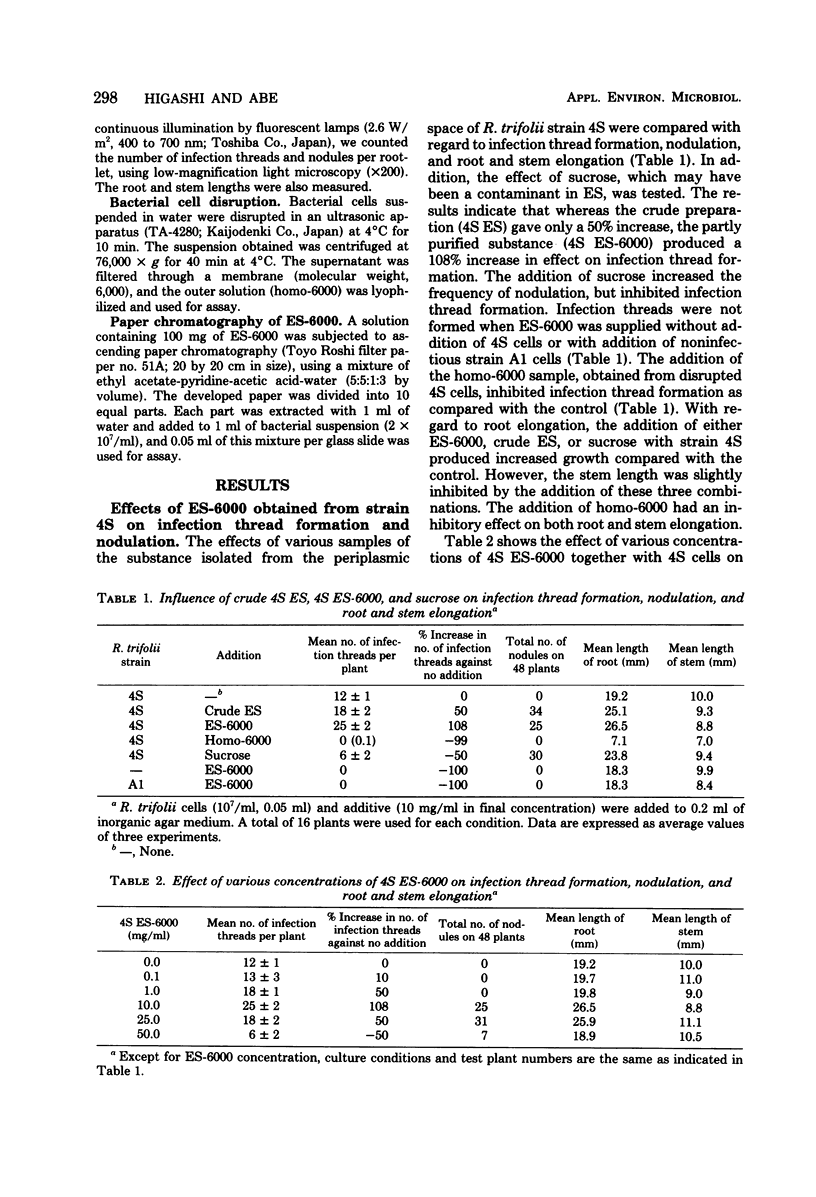
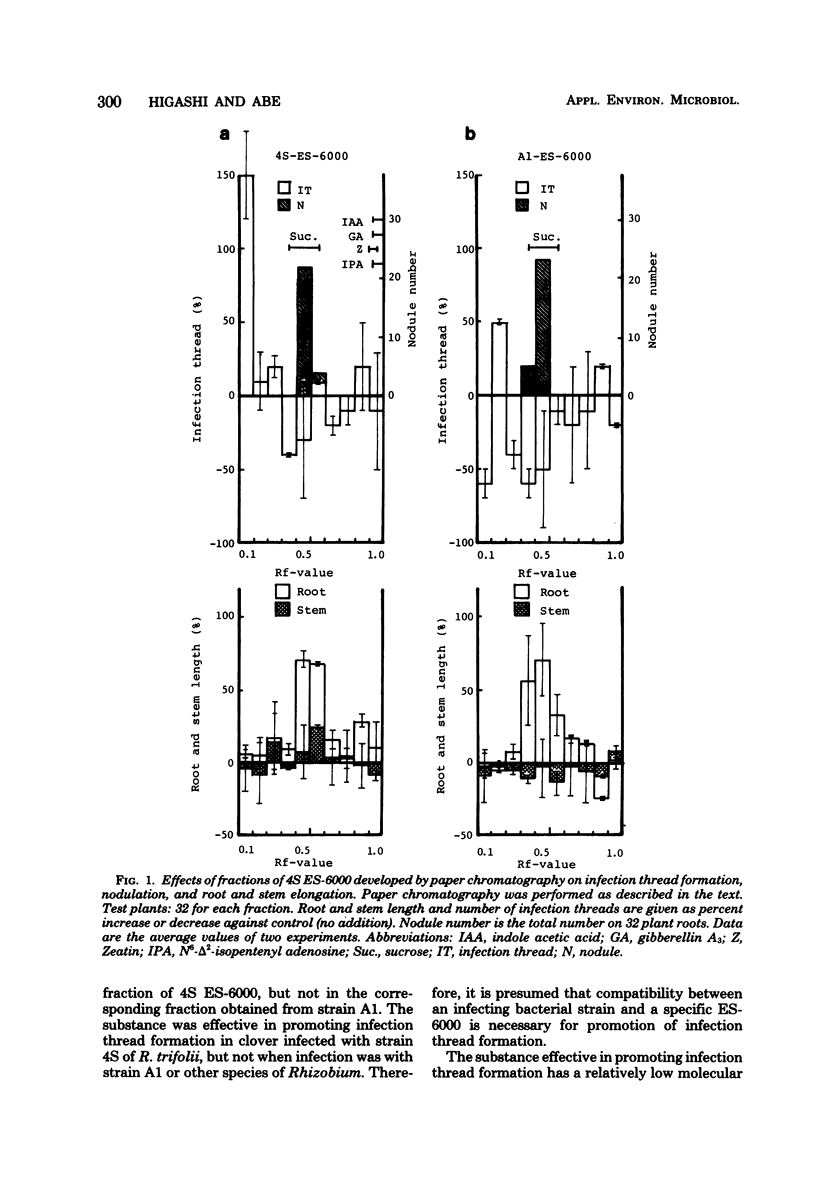
An extrinsic substance (ES-6000) was isolated from the periplasmic space of Rhizobium trifolii (strain 4S) cells by osmotic shock, using a high-density sucrose solution. This substance promoted infection thread formation in root hairs of white clover when inoculated together with the infectious strain (4S). However, ES-6000 obtained from another rhizobial species and from strain A1, which is a noninfectious mutant strain obtained from strain 4S, did not have this effect. The promoter in the ES-6000 from strain 4S is a relatively small molecule since it passed through a hollow-fiber membrane (molecular weight, 6,000). This substance was also recognized as an Rf 0.1 fraction by paper chromatography. Sucrose was effective in promoting nodulation and root elongation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y., Heppel L. A. On the nature of the changes induced in Escherichia coli by osmotic shock. J Biol Chem. 1967 May 25;242(10):2561–2569. [PubMed] [Google Scholar]
- Dazzo F. B., Hubbell D. H. Cross-reactive antigens and lectin as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975 Dec;30(6):1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Goodchild D. J., Bergersen F. J. Electron microscopy of the infection and subsequent development of soybean nodule cells. J Bacteriol. 1966 Jul;92(1):204–213. doi: 10.1128/jb.92.1.204-213.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourret J. P., Fernandez-Arias H. Etude ultrastructurale et cytochimique de la différenciation des bactéroïdes de Rhizobium trifolii Dangeard dans les nodules de Trifolium repens L. Can J Microbiol. 1974 Aug;20(8):1169–1181. [PubMed] [Google Scholar]
- Keele B. B., Jr, Hamilton P. B., Elkan G. H. Glucose catabolism in Rhizobium japonicum. J Bacteriol. 1969 Mar;97(3):1184–1191. doi: 10.1128/jb.97.3.1184-1191.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LJUNGGREN H., FAHRAEUS G. The role of polygalacturonase in root-hair invasion by nodule bacteria. J Gen Microbiol. 1961 Nov;26:521–528. doi: 10.1099/00221287-26-3-521. [DOI] [PubMed] [Google Scholar]
- Phillips D. A., Torrey J. G. Studies on cytokinin production by Rhizobium. Plant Physiol. 1972 Jan;49(1):11–15. doi: 10.1104/pp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syõno K., Torrey J. G. Identification of Cytokinins of Root Nodules of the Garden Pea, Pisum sativum L. Plant Physiol. 1976 Apr;57(4):602–606. doi: 10.1104/pp.57.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]



