Abstract
Tumour necrosis factor-related apoptosis inducing ligand (TRAIL) is a death-inducing cytokine whose physiological function is not well understood. Here, we show that TRAIL has a role in programming human dendritic cell (DC) differentiation. TRAIL expression was strongly induced in DCs upon stimulation with lipopolysaccharide (LPS) or Polyinosine-polycytidylic acid (poly(I:C)) stimulation. Blockade of TRAIL with neutralizing antibody partially inhibited LPS-induced up-regulation of co-stimulatory molecules and the expression of inflammatory cytokines including interleukin-12 (IL-12) p70. In addition, neutralization of TRAIL in LPS-treated DCs inhibited the DC-driven differentiation of T cells into interferon-γ (IFN-γ) -producing effectors. The effects of TRAIL neutralization in poly(I:C)-treated DCs were similar, except that IL-12 production and the differentiation of effector T cells into IFN-γ producers were not inhibited. Strikingly, TRAIL stimulation alone was sufficient to induce morphological changes resembling DC maturation, up-regulation of co-stimulatory molecules, and enhancement of DC-driven allogeneic T-cell proliferation. However, TRAIL alone did not induce inflammatory cytokine production. We further show that the effects of TRAIL on DC maturation were not the result of the induction of apoptosis, but may involve p38 activation. Hence, our data demonstrate that TRAIL co-operates with other cytokines to facilitate DC functional maturation in response to Toll-like receptor activation.
Keywords: apoptosis, dendritic cells, tumour necrosis factor, tumour necrosis factor-related apoptosis-inducing ligand
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cells that drive the adaptive immune responses. Sentinel or immature DCs exhibit high phagocytic activity for antigen sampling. By contrast, mature DCs exhibit lower phagocytic capacity, but up-regulate co-stimulatory molecules to maximize the stimulation of lymphocytes.1 Many of the innate pattern recognition receptors (PRRs) are potent inducers of DC functional maturation.2 The PRRs mediate the functional maturation of DCs in part through the production of cytokines. Several TNF family cytokines, including CD40 ligand (CD40L), tumour necrosis factor (TNF) and receptor activator of nuclear factor-κB (RANKL)/TRANCE, are produced as a result of PRR activation and are important regulators of DC functions.3 Full DC activation probably involves the co-ordinated action of different cytokine factors because in vitro experiments revealed that many of these TNF-like cytokines exert overlapping and distinct effects on DCs.4
The TNF-related apoptosis-inducing ligand (TRAIL) belongs to a sub-class of TNF-like cytokines that preferentially induce apoptosis. In humans, TRAIL binds to five receptors, the membrane-anchored TRAIL-R1 (DR4), TRAIL-R2 (DR5/Killer/Trick), TRAIL-R3 (DcR1/LIT/TRID) and TRAIL-R4 (DcR2/TRUNDD), and the soluble receptor osteoprotegerin (OPG) (reviewed in ref. 5). TRAIL-R1 and TRAIL-R2 contain functional cytoplasmic death domains that recruit apoptotic signal adaptors upon ligation by TRAIL. Unlike the human receptors, a single receptor, TRAIL-R2/DR5/Killer, mediates TRAIL-induced apoptosis in mouse cells. In contrast, TRAIL-R3, TRAIL-R4 and OPG do not contain a functional cytoplasmic death domain and are thought to function as ‘decoys’ or ‘inhibitory receptors’ against TRAIL-induced apoptosis.6–8 In certain cell types, TRAIL-R4 inhibits apoptosis through complexing with the death receptor TRAIL-R2 in a ligand-independent manner.7,8 Studies in TRAIL−/− or TRAIL-R2−/− mice indicate that TRAIL has an important function in controlling tumour growth and metastasis.9–12 Interestingly, most normal untransformed cells are resistant to TRAIL-induced apoptosis. Combinatorial treatment with TRAIL and chemotherapeutic agents often led to synergistic killing of cancer cells (reviewed in ref. 13). For these reasons, there are intense interests in targeting TRAIL in cancer therapies.
Besides controlling tumour growth and metastasis, TRAIL is likely to play important roles in normal physiology. For instance, TRAIL expression is highly inducible by interferons,9–11,14–16 suggesting that TRAIL may participate in innate immune responses against infections. Indeed, TRAIL has been implicated in the control against influenza virus,17,18 Dengue virus,19 respiratory syncytial virus,20 murine cytomegalovirus 21 and human immunodeficiency virus 22 infections. Consistent with these observations, many immune effector cells such as DCs, natural killer cells and T cells have been shown to use TRAIL as a target cell killing mechanism.9,15,23,24 Further evidence of a normal physiological role of TRAIL in immunity comes from the observations that CD4+ T-cell-dependent memory CD8+ T-cell generation and homeostatic proliferation of functional memory CD8+ T cells requires an intact TRAIL signal.25–27
Consistent with a positive regulatory role for TRAIL in immunity, TRAIL has been shown to activate nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPK).28,29Here, we report that TRAIL positively promotes DC differentiation in response to lipopolysaccharide (LPS) or poly(I:C) stimulation. The LPS or poly(I:C) stimulation strongly induced the expression of TRAIL by DCs. Blockade of TRAIL partially suppressed the production of inflammatory cytokines by LPS-stimulated DCs. Using an allogeneic CD4+ T-cell stimulation assay, we show that the reduction of inflammatory cytokine production by TRAIL neutralizing antibody led to a reduced DC-driven differentiation of allogeneic CD4+ T-cells into interferon-γ (IFN-γ) -producing effectors. Strikingly, TRAIL stimulation alone, which activated p38, was sufficient to partially induce DC maturation. Our data show that TRAIL acts in concert with other TNF-like factors to facilitate the functional maturation of DCs.
Materials and methods
Reagents
Antibodies against the different TRAIL receptors, recombinant TRAIL, RANKL and CD40L were purchased from Axxora (San Diego, CA, USA). Neutralizing TNF antibody was from NCI (Bethesda, MD). Recombinant human TNF-α was purchased from Biosource (Rockville, MD). Antibodies for flow cytometry [interleukin-4 (IL-4), IFN-γ, CD1a, CD14, CD80, CD83, CD86, human leucocyte antigen (HLA) -ABC, HLA-DR] were purchased from BD Pharmingen (San Jose, CA, USA) or eBioscience (San Diego, CA, USA). Phenol-purified LPS was a kind gift from Everlyn Kurt-Jones (UMMS, Worcester, MA). Poly(I:C) was purchased from Invivogen (San Diego, CA, USA). CD3+ or naïve CD4+ CD45RO− T-cell isolation columns were purchased from R&D Systems (Minneapolis, MN, USA). Enzyme-linked immunosorbent assay (ELISA) kits for TRAIL, TNF-α, IL-6, IL-8 and IL-12 p70 were purchased from Biosource/Invitrogen (San Diego, CA, USA).
Dendritic cell culture
Monocytes were isolated from peripheral blood mononuclear cells from anonymous donors by negative selection using the Rosette Sep monocyte isolation reagent from Stem Cell Technologies (Vancouver, BC, Canada). Monocytes were cultured in the presence of 34·5 ng/ml IL-4 (eBioscience) and 50 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (eBioscience) for 6 days according to an established protocol.4 The phenotypes of the immature DCs (iDCs) were distinguished by high CD1a surface expression, absence of CD14 expression and low levels of CD80, CD83 and CD86. Maturation of DCs was induced with 0·5 μg/ml LPS, 10 μg/ml poly(I:C) (Invivogen), 10 ng/ml human TNF-α (Biosource) or 1 μg/ml Killer TRAIL (Axxora). Anti-TRAIL antibody (clone 2E5 from Axxora), anti-human TNF-α antibody (NCI) or TRAIL-R2-Fc fusion protein (R&D Systems) were used at 2 μg/ml. In some experiments, ultraviolet (UV) -treated syngeneic CD3+ lymphocytes were used to stimulate DCs at a 1 : 1 ratio. For confocal microscopy, cells were stained with Mitotracker Green, fixed, stained with anti-TRAIL-R2 antibody plus phycoerythrin-conjugated anti-mouse immunoglobulin G secondary antibody (HS201; Axxora), and counterstained with Hoeschst 33342. Images were captured with a TCS-NT confocal microscope (Leica) with a 63 X objective.
Allogeneic T-cell stimulation assays
CD3+ or CD4+ T cells were purified from peripheral blood mononuclear cells using purification columns from R&D Systems. For mixed lymphocyte reaction, 5 × 104 irradiated DCs (5000 rads) were washed twice with phosphate-buffered saline followed by co-culture with 2 × 105 purified allogeneic T cells in 96-well round-bottom plates. After 5 days, cells were pulsed with 1 μCi [3H]thymidine for 20 hr to measure cell proliferation. For CD4+ T-cell intracellular cytokine staining, after the initial 5 days of co-culture with DCs, the CD4+ T cells were washed with phosphate-buffered saline and cultured in 100 units/ml of recombinant human IL-2 (NCI) for an additional 9 days. The resulting T cells were restimulated with 10 ng/ml phorbol 12-myristate 13-acetate and 0·5 μm ionomycin for 5 hr before intracellular staining for IL-4 and IFN-γ.
Western blots
Monocyte-derived iDCs were stimulated with TRAIL, LPS or TNF for the indicated times and whole cell extracts [150 mm NaCl, 10 mm Tris–Cl (pH 7·4), 1 mm NaF, 1 mmβ-glycerophosphate, 1 mm Na3VO4 and 1% nonidet-40 supplemented with complete protease inhibitors (Roche, Indianapolis, IN, USA)] were analysed for MAPK and NF-κB activation by Western blots. Antibodies against phospho-p38, phospho-Jnk, phospho-Erk, total p38 (Cell Signaling, Danvers, MA), inhibitor of NF-κB (IκBα; Santa Cruz, CA, USA) and β-actin (BD Pharmingen) were used.
Quantitative polymerase chain reaction for TRAIL expression
Total RNA was extracted using RNeasy Kit (Qiagen, Valencia, CA, USA). Expression of TRAIL was determined by quantitative polymerase chain reaction (PCR) using the Biorad iCycler (Hercules, CA, USA) after first-strand complementary DNA synthesis. Primers for human TRAIL were: 5′-actgggaccagaggaagaagc-3′ and 5′-tgcaagttgctcaggaatgaa-3′. Control primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were: 5′-atgacatcaagaaggtggtg-3′ and 5′-cataccaggaaatgagcttg-3′. Relative TRAIL expression was normalized to the signal obtained from the GAPDH controls. Quantitative PCRs were performed using the Biorad iCycler.
Statistical analyses
Results shown are average of triplicates ± SEM unless stated otherwise. All statistical analyses were performed using Student’s t-test. One-way analysis of variance was used in Figs 4d, 5e, 6c,h, 7e.
Figure 4.
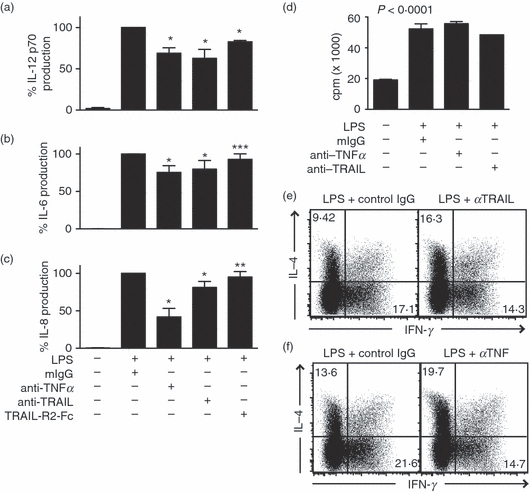
Tumour necrosis factor-related apoptosis inducing ligand (TRAIL) neutralization impairs lipopolysaccharide (LPS) -induced cytokine production and dendritic cell (DC) -driven T-cell differentiation into interferon-γ (IFN-γ) -producing effectors. TRAIL or tumour necrosis factor (TNF) neutralization partially inhibits LPS-induced production of (a) interleukin-12 (IL-12), (b) IL-6 and (c) IL-8 by DCs. Cytokine production was determined by enzyme-linked immunosorbent assay 24 hr after stimulation with LPS and the indicated immunoglobulin G antibodies. The samples treated with LPS and control IgG were normalized as 100%. Results represent the average from five experiments using DCs derived from different donors (mean ± SEM). The ranges of cytokine production from different donor cells were: IL-12 (6–32 pg/ml); TNF (349–992 pg/ml); IL-8 (87–216 pg/ml); IL-6 (398–695 pg/ml). *P < 0·0001, **P < 0·05 and ***P = 0·256 because of one outlier that exhibited 20% increase in IL-8 production compared with LPS + mIgG control. (d) DC-induced allogeneic T-cell proliferation was not affected by TRAIL or TNF neutralization. Five days after co-culture with DCs stimulated with the indicated treatments, T cells were pulsed with [3H]thymidine for 20 hr to measure T-cell proliferation (mean ± SEM). (e,f) Neutralization of (e) TRAIL or (f) TNF partially inhibits the DC-driven development of IFN-γ-producing effector T cells. DCs stimulated with LPS in the presence of neutralizing antibody against TRAIL, TNF or control IgG were washed and co-cultured with allogeneic CD4+ T cells as described in the Methods. IFN-γ and IL-4 production were determined by intracellular staining and fluorescence-activated cell sorting analysis. The numbers represent the percentages of cells in the respective quadrants. Results are representative of six experiments with cells derived from different donors.
Figure 5.
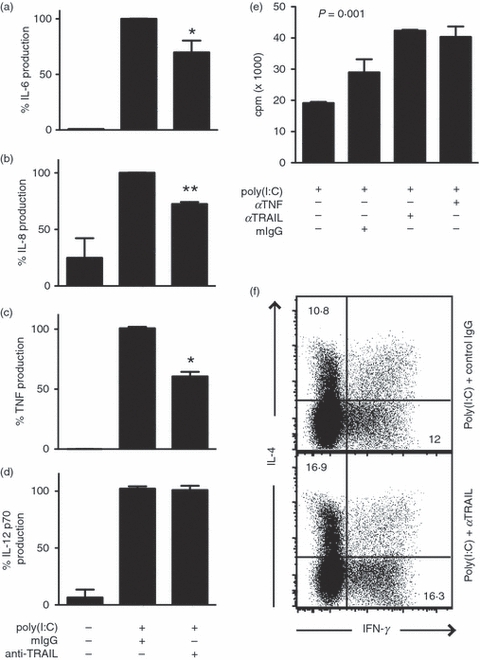
Tumour necrosis factor-related apoptosis inducing ligand (TRAIL) promotes poly(I:C)-induced inflammatory cytokine production by dendritic cells (DCs). TRAIL neutralization partially inhibits poly(I:C)-induced production of (a) interleukin-6 (IL-6), (b) IL-8 and (c) tumour necrosis factor (TNF), but not (d) IL-12 by DCs. Results represent the average from three experiments using DCs derived from different donors (mean ± SEM). The samples treated with poly(I:C) and control immunoglobulin G (IgG) were normalized as 100%. *P < 0·0001. **P < 0·005. (e) TRAIL neutralization did not inhibit allogeneic T-cell proliferation driven by poly(I:C)-treated DCs (mean ± SEM). (f) TRAIL neutralization did not skew allogeneic T-cell differentiation into IL-4 or interferon-γ (IFN-γ) -producing effectors. DCs stimulated with poly(I:C) and anti-TRAIL or control IgG were co-cultured with allogeneic CD4+ T cells as described in Methods. IL-4 and IFN-γ production by the DC-stimulated T cells was determined by intracellular cytokine staining. The numbers represent the percentages of cells in the quadrants.
Figure 6.
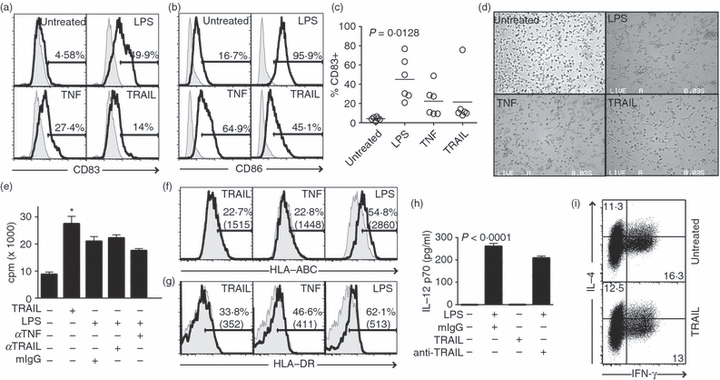
Tumour necrosis factor-related apoptosis inducing ligand (TRAIL) stimulates partial maturation of dendritic cells (DCs). (a,b) TRAIL induces expression of CD83 and CD86 on DCs. Monocyte-derived immature DCs (iDCs) were treated with lipopolysaccharide (LPS), tumour necrosis factor (TNF), recombinant TRAIL, or left untreated. Twenty-four hours later, the expression of (a) CD83 and (b) CD86 was determined by flow cytometry. Tinted curves: isotype control. The numbers represent the percentage of cells in the positive gate. (c) TRAIL-induced up-regulation of CD83 was observed in DCs derived from multiple donors. Each circle represents an individual donor (n = 6). The horizontal bars represent the mean values for each treatment group. (d) TRAIL induces morphological change associated with DC differentiation. DCs were treated with the indicated stimuli. Cell morphologies were imaged 24 hr post-stimulation. (e) TRAIL promotes DC-driven allogeneic T-cell proliferation (mean ± SEM). *P < 0·001. (f,g) TRAIL treatment did not up-regulate (f) Class I major histocompatibility complex (MHC) or (g) Class II MHC expression on DCs. The tinted curves represent isotype control staining. (h) TRAIL stimulation did not induce interleukin-12 (IL-12) p70 expression in DCs. Culture supernatants of DCs treated with the indicated stimuli were measured for IL-12 production by enzyme-linked immunosorbent assay (mean ± SEM). (i) TRAIL did not alter the DC-driven differentiation of T cells into IL-4 or interferon-γ (IFN-γ) -producing effectors. Allogeneic CD4+ T cells were co-cultured with untreated iDCs or TRAIL-treated DCs as described in Methods. Production of IL-4 and IFN-γ by the responding T cells was determined by intracellular staining and flow cytometry.
Figure 7.
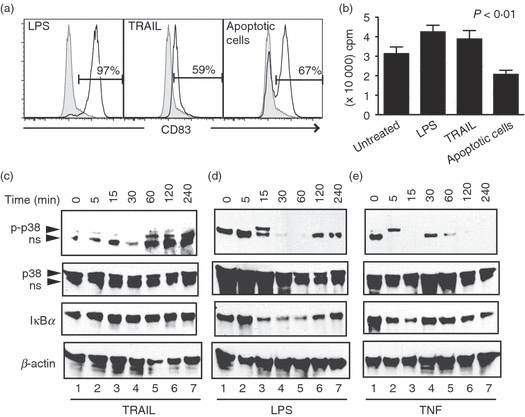
Tumour necrosis factor-related apoptosis inducing ligand (TRAIL) induces dendritic cell (DC) maturation independent of apoptosis induction. (a) Co-culture with apoptotic cells stimulates expression of CD83 on the surface of DCs. The DCs were treated with lipopolysaccharide (LPS), TRAIL or ultraviolet-treated apoptotic lymphocytes from the same donor. Expression of CD83 was evaluated by flow cytometry 24 hr later. (b) Co-culture of apoptotic cells inhibits rather than enhances DC-driven T-cell proliferation. Untreated DCs or DCs treated with the indicated stimuli were used to induce allogeneic CD4+ T-cell proliferation. Five days later, T-cell proliferation was measured by [3H]thymidine incorporation (mean ± SEM). (c–e) Monocyte-derived DCs were stimulated with (c) TRAIL, (d) LPS or (e) tumour necrosis factor (TNF) for the indicated times. Whole cell extracts were analysed for phospho-p38 (p-p38), total p38 and inhibitor of nuclear factor-κB (IκBα) degradation and β-actin by Western blot. ns = non-specific band.
Results
LPS induced TRAIL expression and resistance to TRAIL-induced apoptosis in DCs
Previous reports indicate that DCs employ TRAIL as an effector mechanism to mediate target cell killing.16,24 In this scenario, DCs are likely to be resistant to TRAIL-induced apoptosis. To investigate the mechanism of DC resistance to TRAIL-induced apoptosis, we examined the expression of TRAIL in monocyte-derived DCs. Purified monocytes (Fig. 1a, left panel) from peripheral blood were cultured in the presence of GM-CSF and IL-4.4 Seven days later, the majority (>; 95%) of cells were positive for the human DC marker CD1a (Fig. 1a, right panel). The DCs generated using this protocol exhibited an immature phenotype as determined by their low expression of CD80, CD83 and CD86 (see below). These iDCs expressed almost undetectable level of TRAIL as determined by real-time PCR and ELISA (Fig. 1b–c). However, within 2 hr after stimulation with the Toll-like receptor 4 (TLR-4) agonist LPS, DCs potently up-regulated the expression of TRAIL at the messenger RNA level (Fig. 1b). LPS-induced expression of soluble TRAIL ligand 20 hr post-stimulation was confirmed by ELISA (Fig. 1c). In addition, poly(I:C), a TLR-3 agonist, also strongly up-regulated TRAIL expression (Fig. 1c). Despite the strong induction of TRAIL, viability of the LPS-stimulated DCs as determined by exclusion of the propidium iodide was not compromised (Fig. 1d). In fact, while a low level of apoptosis could be induced by exogenous TRAIL in iDCs (Fig. 1e, < 20%), LPS-stimulated mature DCs were refractory to TRAIL-induced apoptosis (Fig. 1e, < 5%). Other stimuli including TNF, CD40L and RANKL that have been shown to induce DC maturation did not alter the sensitivity to TRAIL-induced apoptosis (Fig. 1e). Hence, TLR-4 stimulation induced TRAIL expression and resistance to TRAIL-induced apoptosis concomitantly.
Figure 1.
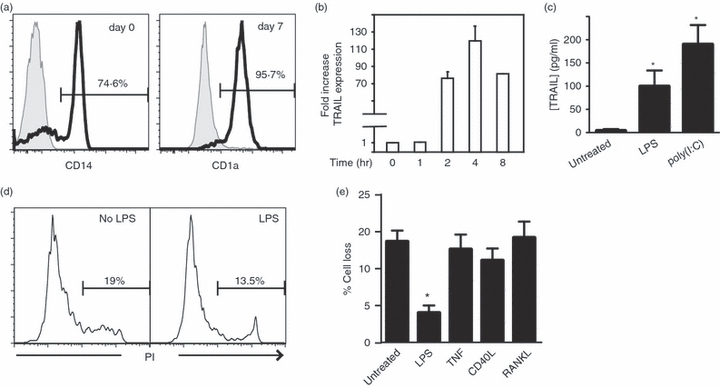
Lipopolysaccharide (LPS) -stimulated tumour necrosis factor-related apoptosis inducing ligand (TRAIL) expression in dendritic cells (DCs) without induction of cell death. (a) Purity of the isolated monocyte and immature DC (iDC) populations. Monocytes isolated from human peripheral blood lymphocytes were stained for CD14 (left panel). Cells were stained for the DC marker CD1a 7 days after culture with interleukin-4 and granulocyte–macrophage colony-stimulating factor. Shaded curves: isotype antibody control. (b) LPS-induced TRAIL expression in DCs. Total RNA was prepared from untreated iDCs or DCs treated with LPS for the indicated times. After reverse transcription, the expression of TRAIL was quantified by real-time quantitative polymerase chain reaction. TRAIL expression was normalized to that of GAPDH (c) Twenty hours after LPS or poly(I:C) stimulation, soluble TRAIL (mean ± SEM) in the culture supernatant was detected by enzyme-linked immunosorbent assay. *P < 0·005. (d) LPS stimulation did not trigger increased cell death in monocyte-derived DCs. Day 7 iDCs were treated with LPS or left untreated. Cell death determined by propidium iodide staining was determined 20 hr later by flow cytometry. (e) LPS stimulation rendered DCs resistant to TRAIL-induced apoptosis. The iDCs were treated with the indicated stimuli for 20 hr. The cells were washed and treated with TRAIL for another 24 hr. TRAIL-induced apoptosis was determined by Annexin V and propidium iodide staining on flow cytometry. Percentage cell loss was calculated as described (mean ± SEM).55*P = 0·005.
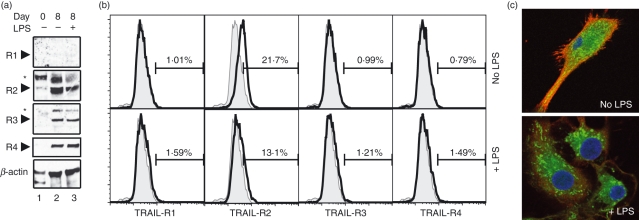
TRAIL binds to both apoptosis-inducing receptors (TRAIL-R1 and TRAIL-R2) and decoy/inhibitory receptors (TRAIL-R3 and TRAIL-R4). Hence, cellular sensitivity to TRAIL-induced apoptosis could be controlled at the level of expression of different TRAIL receptors.8 Western blot analyses reveal that monocytes expressed only TRAIL-R2 (Fig. 2a, lane 1). As has been reported before, differentiation of monocytes into DCs with IL-4 and GM-CSF was accompanied by an increase in β-actin expression.30 In addition, the decoy receptors TRAIL-R3 and TRAIL-R4, but not the death receptor TRAIL-R1, was induced in iDCs (Fig. 2a, lane 2) and mature DCs (Fig. 2a, lane 3). However, FACS analysis reveals that only TRAIL-R2 was detected on the cell surface of iDCs (Fig. 2b, top panels). This is consistent with previous observations that TRAIL-R3 and TRAIL-R4 could reside in intracellular compartments.31 The LPS stimulation led to a reduction in cell surface expression of TRAIL-R2 (Fig. 2b, bottom panels), which corresponded with a reduction in the total cellular TRAIL-R2 (Fig. 2a, second panel, compare lanes 2 and 3). The loss of cell surface TRAIL-R2 expression upon LPS stimulation was confirmed by confocal microscopy (Fig. 2c). These results show that TRAIL-R2 is the major receptor that mediates TRAIL signalling in monocyte-derived DCs. Our results also implicate that the loss of cell surface TRAIL-R2 expression likely contributes to the resistance of LPS-treated DCs to TRAIL-induced apoptosis.
Figure 2.
Tumour necrosis factor-related apoptosis inducing ligand (TRAIL) signalling in monocyte-derived dendritic cells (DCs) is mediated by TRAIL-R2. (a) Western blot analysis of TRAIL receptor expression in monocytes, immature DCs (iDCs) and DCs stimulated with lipopolysaccharide (LPS) for 20 hr. The arrows indicate the TRAIL receptors. The asterisks indicate the glycosylated receptors.63 (b) Fluorescence-activated cell sorting analyses of cell surface TRAIL receptor expression in day 8 iDCs or LPS-treated DCs. Tinted curves represented staining with isotype control immunoglobulin G. Percentages of cells in the positive gates are shown. (c) Confocal microscopy of TRAIL-R2 localization in untreated iDCs and LPS-treated DCs. Red: TRAIL-R2; Green: Mitotracker Red; Blue: Hoescht 33342.
TRAIL expression upon LPS stimulation facilitates DC maturation
Many TNF-like cytokines including TNF and CD40L are important for functional differentiation of DCs.32,33 Although TRAIL is an effector mechanism employed by DCs for target cell killing,24 its role in DC functional maturation has not been examined. We explored the role of TRAIL in DC maturation using neutralizing antibodies against TRAIL. Under these conditions, more than 80% of LPS-induced TRAIL was neutralized (Fig. 3a). As a control, we compared the response to that using anti-TNF antibody, which completely neutralized LPS-induced TNF production (Fig. 3b). Interestingly, anti-TRAIL antibody also modestly reduced LPS-induced TNF production (Fig. 3b), suggesting that any effects of TRAIL neutralization might be attributed to reduction in TNF production. As has been reported previously, LPS treatment led to DC maturation marked by an increase in CD80, CD83, CD86, Class I major histocompatibility complex (MHC) and Class II MHC expression (Fig. 3c–g). Neutralization of TRAIL with TRAIL-specific antibody led to a modest but consistent decrease in CD80 and CD83 in LPS-treated DCs (Fig. 3c,d). The inhibition of CD80 and CD83 expression was manifested by a reduction in the percentages of CD80+ and CD83+ cells and a decrease in the mean fluorescence intensities (CD80 MFI: from 1327 to 1144; CD83 MFI from 1589 to 1032). The TNF neutralizing antibody led to a stronger inhibition of LPS-induced expression of CD80, CD83 and CD86 (Fig. 3c–e). Neither TNF nor TRAIL antibodies had any effects on MHC class I and class II expression (Fig. 3f,g). The combination of the two neutralizing antibodies did not result in a further reduction in co-stimulatory molecule expression compared with samples treated with either LPS and anti-TNF or LPS and anti-TRAIL antibody (data not shown). These results suggest that TRAIL plays a role in DC maturation.
Figure 3.
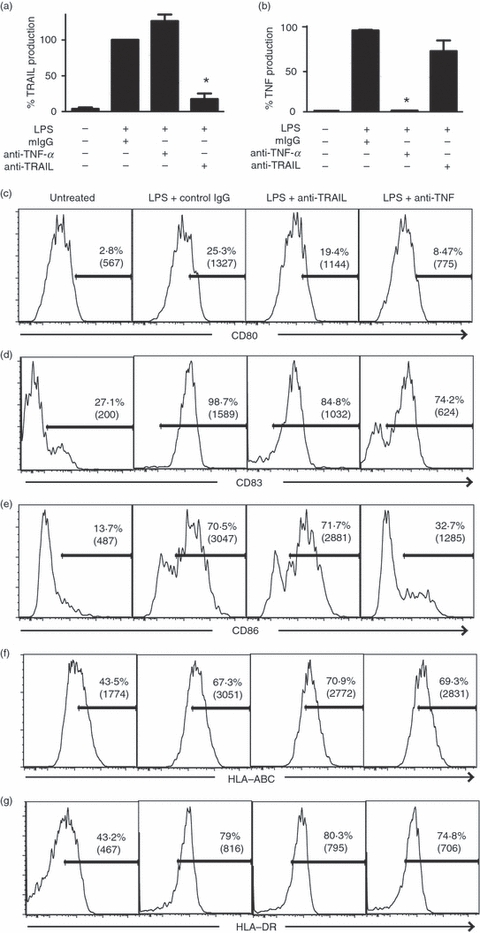
Neutralization tumour necrosis factor-related apoptosis inducing ligand (TRAIL) partially inhibited the expression of co-stimulation markers. (a) Effect of anti-TRAIL neutralizing antibody on lipopolysaccharide (LPS) -induced TRAIL expression. Immature dendritic cells (iDCs) were stimulated as indicated for 20 hr. Bioactive TRAIL in the culture supernatants was determined by enzyme-linked immunsorbent assay (ELISA; mean ± SEM). The samples treated with LPS and control immunoglobulin G (IgG) were normalized as 100%. *P < 0·0001. (b) Effect of tumour necrosis factor (TNF) neutralizing antibody on LPS-induced TNF expression. Production of TNF in culture supernatants was measured by ELISA as in (a) (mean ± SEM). *P < 0·0001. (c–g) TRAIL neutralization moderately inhibited up-regulation of co-stimulatory molecules on DCs. Monocyte-derived iDCs were left untreated, stimulated with LPS + control murine IgG, LPS + anti-TRAIL antibody or LPS + anti-TNF antibody for 24 hr. The expression of (c) CD80, (d) CD83, (e) CD86, (f) HLA-ABC and (g) HLA-DR was examined by flow cytometry. The percentages of cells in the positive gates and the mean fluorescence intensities (in parentheses) are shown.
LPS-induced TRAIL expression facilitates cytokine production by DCs
Consistent with the inhibition of co-stimulatory molecule expression, neutralization of TRAIL also led to a reduction in cytokine production by LPS-treated DCs. Because DCs derived from different donors produce cytokines at different levels, we normalized the amount of cytokine produced by LPS and control immunoglobulin G treatment as 100% to facilitate comparison among cell preparations from different donors. Using this method, IL-12 production as determined by ELISA was reduced by 20–30% with TRAIL neutralizing antibody or TRAIL-R2-Fc fusion protein in LPS-treated DCs (Fig. 4a, n = 5). Significantly, the reduction in IL-12 production was similar to that achieved by neutralizing antibody to TNF (Fig. 4a). TRAIL neutralization also modestly inhibited IL-6 (Fig. 4b), IL-8 (Fig. 4c) and TNF (Fig. 3b) production by LPS-treated DCs, although the inhibition in IL-8 production was less than that achieved by TNF neutralization (Fig. 4c).
Dendritic cells are potent inducers of T-cell proliferation and differentiation. We reasoned that the impaired cytokine production by DCs in the presence of TRAIL neutralization might hamper the ability of DCs to activate T cells. To test this notion, we stimulated DC maturation with LPS in the presence of neutralizing antibody to TRAIL or TNF. After 24 hr, the DCs were washed extensively and co-cultured with allogeneic CD4+ T cells. Five days later, the activated T cells were pulsed with [3H]thymidine to measure DC-driven T-cell proliferation. Consistent with previous results,34 LPS-treated DCs were more potent than untreated iDCs in promoting allogeneic T-cell proliferation (Fig. 4d). Neutralization of TRAIL or TNF did not alter DC-driven allogeneic T-cell proliferation (Fig. 4d). However, when the allogeneic T cells were tested for effector cytokine production after an additional 9 days of culture in IL-2, TRAIL neutralization during LPS stimulation of DCs led to reduced IFN-γ but enhanced IL-4 expression (Fig. 4e). Neutralization of TNF also led to a similar inhibition of T helper type 1 (Th1) differentiation and skewing towards a Th2 phenotype (Fig. 4f). Although the effects were modest, they nonetheless show that TRAIL signalling qualitatively alters the functional differentiation of DCs.35
The effect of TRAIL on DC cytokine production was not restricted to TLR-4. When iDCs were stimulated with poly(I:C), a TLR3 agonist, neutralization of TRAIL also led to 20–30% inhibition of IL-6, IL-8 and TNF production (Fig. 5a–c). Surprisingly, IL-12 production (Fig. 5d) or DC-driven allogeneic T-cell proliferation (Fig. 5e) was unaffected by neutralization of TRAIL. Moreover, allogeneic T-cell differentiation to IFN-γ producing Th1 effectors was not inhibited by TRAIL neutralization (Fig. 5f). Rather, TRAIL neutralization enhanced effector T-cell expression of both IL-4 and IFN-γ (Fig. 5f). These results show that the effects of TRAIL on DCs are TLR-specific.
TRAIL triggered partial DC maturation
Our results so far suggest that TRAIL is one of several TNF-like cytokines that influence DC functional differentiation in response to TLR stimulation. To determine if TRAIL alone might stimulate DC differentiation similar to that achieved by TNF or CD40L,32,33 we treated iDCs with recombinant TRAIL and examined the phenotypic changes associated with DC maturation. Indeed, TRAIL induced cell surface expression of the DC maturation markers CD83 and CD86 (Fig. 6a,b). Although the level of CD83 and CD86 up-regulation by TRAIL was modest compared with that achieved by LPS or TNF (Fig. 6a–b), it was consistently observed in DCs derived from multiple donors (Fig. 6c, n = 6). Moreover, TRAIL-treated DCs exhibited ‘dendritic’ morphology similar to that achieved by TNF or LPS (Fig. 6d). TRAIL was sufficient to enhance DC-driven T-cell proliferation (Fig. 6e), although TRAIL neutralization did not inhibit T-cell proliferation driven by LPS-treated DCs (Fig. 4d). However, TRAIL did not fully induce DC maturation. For instance, TRAIL failed to up-regulate expression of class I MHC (Fig. 6f), class II MHC (Fig. 6g), or IL-12 production (Fig. 6h). TRAIL treatment also did not alter the DC-driven differentiation of T cells into IL-4- or IFN-γ-producing effectors (Fig. 6i). Taken together, our results suggest that full activation of DCs requires the action of multiple cytokines.
TRAIL promotes DC maturation independent of apoptosis signalling
We next sought to investigate the mechanism by which TRAIL mediates DC maturation. The release of ‘endogenous adjuvants’ from dying cells stimulates DC maturation.36–40 Because TRAIL is an apoptosis-inducing agent, we asked whether pro-apoptotic signalling by TRAIL might drive DC maturation. When iDCs were co-cultured with UV-treated syngeneic apoptotic lymphocytes, CD83 expression was induced (Fig. 7a). However, unlike LPS or TRAIL stimulation, which enhanced DC-driven allogeneic T-cell proliferation, DCs stimulated with UV-treated apoptotic cells inhibited rather than enhanced DC-driven T-cell proliferation (Fig. 7b). The DC maturation effects of TRAIL are therefore unlikely to be the result of TRAIL-induced apoptosis.
In addition to apoptosis, TRAIL has been shown to activate MAPK and NF-κB.41,42 TRAIL stimulation led to a delayed induction of p38 phosphorylation in iDCs (Fig. 7c, lanes 5–7). In contrast, LPS (Fig. 7d, lane 3) and TNF (Fig. 7e, lane 2) led to a more rapid but transient increase in p38 phosphorylation. Unlike LPS or TNF, TRAIL did not activate NF-κB in DCs (Fig. 7c–e) or Jnk phosphorylation (data not shown). Collectively, our results suggest that TRAIL might promote DC differentiation through activating the p38 pathway.
Discussion
In this report, we show that TLR3- and TLR4-induced TRAIL expression promotes DC maturation. Lipopolysaccharide rapidly induced expression of TRAIL in DCs. TRAIL was required to fully activate cytokine production and co-stimulatory molecule expression in response to TLR-3 or TLR-4 stimulation. In addition, TRAIL promotes DC-driven differentiation of T cells into IFN-γ-producing effectors. The DC-produced TRAIL might act directly on T cells to promote their differentiation into Th1 effectors. However, this is unlikely because TRAIL produced in response to poly(I:C) stimulation did not have the same effect on IFN-γ and IL-4 production. The effects of TRAIL neutralization were similar to those achieved by TNF neutralizing antibody. Because TRAIL neutralization partially suppressed TNF production (Fig. 3b), the DC-stimulating effect of TRAIL during TLR stimulation might be partially the result of promoting TNF production.
Interestingly, TRAIL stimulation alone was sufficient to induce partial DC maturation as marked by morphological changes, increased expression of the co-stimulatory molecules CD83 and CD86, and enhanced the DC-driven proliferation of allogeneic T cells. However, TRAIL alone did not induce inflammatory cytokine production. The lack of inflammatory cytokine explains why TRAIL-treated DCs promoted T-cell proliferation whereas TRAIL neutralization had no effect on T-cell proliferation driven by LPS-treated DCs. Our results are consistent with the notion that full DC activation requires the co-ordinated action of multiple cytokine factors.
How might TRAIL facilitate DC maturation? The release of endogenous adjuvants such as HMGB1 or monosodium urate crystals from dying cells stimulates DC differentiation.43 Although iDCs were somewhat sensitive to apoptosis induction by exogenous TRAIL, the DC-stimulating effect of TRAIL was unlikely to be the result of induction of apoptosis. This is because DCs rapidly down-regulated the cell surface expression of TRAIL-R2 and became resistant to TRAIL-induced apoptosis upon LPS treatment. Moreover, while TRAIL-treated DCs enhanced the proliferation of the allogeneic T cells, co-culture with UV-treated apoptotic lymphocytes suppressed DC-driven allogeneic T-cell proliferation. In contrast, p38 was activated in response to TRAIL. Inhibition of p38 with pharmacological inhibitor was inconclusive because of extensive cell death induced by the inhibitor alone (data not shown). We conclude that TRAIL triggers DC maturation via non-apoptotic signalling mechanisms.
Emerging evidence reveals that death cytokines can also mediate non-apoptotic signals. In fact, the archetype member of the family TNF is well known to transduce both death and non-death-inducing signals.44 TRAIL has been reported to activate NF-κB and MAPK in certain cell types through downstream signal adaptors such as RIP1 and TRAF2.45–47 In DCs, the p38 pathway may play a crucial role in DC differentiation. The effect of TRAIL on DCs was reminiscent of that reported for another death cytokine, Fas ligand (FasL).48–50 FasL induces apoptosis in iDCs, while mature DCs are resistant to FasL-induced apoptosis.51–53 Instead, FasL triggers Erk activation, up-regulates co-stimulatory molecule expression, and induces IL-1β production.48,50 Intriguingly, the resistance of mature DCs to FasL-induced apoptosis is also partly attributed to a reduction in Fas expression.49 Induction of TNFR-2 expression also critically regulates cellular sensitivity to TNF-induced apoptosis and programmed necrosis.54–56 Hence, down-regulation of death receptor expression might be a key event in switching the apoptotic signal to one that drives cellular differentiation.
Besides having a positive effect on DC differentiation, TRAIL has also been implicated as a negative regulator in certain autoinflammatory conditions. In experimental autoimmune encephalitis,57,58 collagen-induced arthritis 59,60 and drug-induced autoimmune diabetes,60,61 TRAIL deficiency or blockade of TRAIL with neutralizing antibody or soluble TRAIL receptor decoys exacerbated the diseases. Similar to the findings reported here, the function of TRAIL in these situations appears to be independent of apoptosis induction.57–59,62 Hence, non-apoptotic TRAIL signalling might have additional roles in preventing autoinflammatory diseases.
The preferential cytotoxicity of TRAIL against tumour cells has led to intense investigation into the potential therapeutic value of targeting TRAIL–TRAIL receptor interaction in cancers. In light of our results and the emerging role of non-apoptotic TRAIL signalling in cellular differentiation, the effects of TRAIL blockade on normal immune functions should be carefully evaluated in TRAIL targeted therapies.
Acknowledgments
This work was supported by National Institutes of Health grant CA113786. Core resources supported by the Diabetes Endocrinology Research Center Grant DK32520 were also used. F.K.C. was a recipient of investigator awards from the Smith Family Foundation and the Cancer Research Institute. F.K.C. is a member of the UMass DERC (DK32520).
Disclosures
None.
References
- 1.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 2.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Zou GM, Tam YK. Cytokines in the generation and maturation of dendritic cells: recent advances. Eur Cytokine Netw. 2002;13:186–99. [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 6.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;4:337–48. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 7.Clancy L, Mruk K, Archer K, Woelfel M, Mongkolsapaya J, Screaton G, Lenardo MJ, Chan FK. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci U S A. 2005;102:18099–104. doi: 10.1073/pnas.0507329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan FK. Three is better than one: pre-ligand receptor assembly in the regulation of TNF receptor signaling. Cytokine. 2007;37:101–7. doi: 10.1016/j.cyto.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–54. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–70. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med. 2002;195:161–9. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–61. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 13.Cretney E, Shanker A, Yagita H, Smyth MJ, Sayers TJ. TNF-related apoptosis-inducing ligand as a therapeutic agent in autoimmunity and cancer. Immunol Cell Biol. 2006;84:87–98. doi: 10.1111/j.1440-1711.2005.01413.x. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Yamaguchi N, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cell Immunol. 2001;214:194–200. doi: 10.1006/cimm.2001.1896. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Hida S, Takayanagi H, et al. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol. 2001;31:3138–46. doi: 10.1002/1521-4141(200111)31:11<3138::aid-immu3138>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Yu Y, Zhang M, Wang W, Cao X. The involvement of TNF-alpha-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-beta-stimulated human dendritic cells to tumor cells. J Immunol. 2001;166:5407–15. doi: 10.4049/jimmunol.166.9.5407. [DOI] [PubMed] [Google Scholar]
- 17.Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–25. doi: 10.4049/jimmunol.181.7.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herold S, Steinmueller M, von Wulffen W, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–77. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warke RV, Martin KJ, Giaya K, Shaw SK, Rothman AL, Bosch I. TRAIL is a novel antiviral protein against dengue virus. J Virol. 2008;82:555–64. doi: 10.1128/JVI.01694-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotelkin A, Prikhod’ko EA, Cohen JI, Collins PL, Bukreyev A. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J Virol. 2003;77:9156–72. doi: 10.1128/JVI.77.17.9156-9172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diehl GE, Yue HH, Hsieh K, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–89. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Lum JJ, Pilon AA, Sanchez-Dardon J, et al. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l. J Virol. 2001;75:11128–36. doi: 10.1128/JVI.75.22.11128-11136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639–47. [PubMed] [Google Scholar]
- 24.Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Exp Med. 1999;190:1155–64. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 26.Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–81. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 28.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–20. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 29.Varfolomeev E, Maecker H, Sharp D, Lawrence D, Renz M, Vucic D, Ashkenazi A. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 2005;280:40599–608. doi: 10.1074/jbc.M509560200. [DOI] [PubMed] [Google Scholar]
- 30.Baltathakis I, Alcantara O, Boldt DH. Expression of different NF-kappaB pathway genes in dendritic cells (DCs) or macrophages assessed by gene expression profiling. J Cell Biochem. 2001;83:281–90. doi: 10.1002/jcb.1231. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XD, Franco AV, Nguyen T, Gray CP, Hersey P. Differential localization and regulation of death and decoy receptors for TNF-related apoptosis-inducing ligand (TRAIL) in human melanoma cells. J Immunol. 2000;164:3961–70. doi: 10.4049/jimmunol.164.8.3961. [DOI] [PubMed] [Google Scholar]
- 32.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuurhuis DH, Fu N, Ossendorp F, Melief CJ. Ins and outs of dendritic cells. Int Arch Allergy Immunol. 2006;140:53–72. doi: 10.1159/000092002. [DOI] [PubMed] [Google Scholar]
- 34.Chitta S, Santambrogio L, Stern LJ. GMCSF in the absence of other cytokines sustains human dendritic cell precursors with T cell regulatory activity and capacity to differentiate into functional dendritic cells. Immunol Lett. 2008;116:41–54. doi: 10.1016/j.imlet.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 36.Rovere P, Vallinoto C, Bondanza A, Crosti MC, Rescigno M, Ricciardi-Castagnoli P, Rugarli C, Manfredi AA. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–71. [PubMed] [Google Scholar]
- 37.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 39.Pietra G, Mortarini R, Parmiani G, Anichini A. Phases of apoptosis of melanoma cells, but not of normal melanocytes, differently affect maturation of myeloid dendritic cells. Cancer Res. 2001;61:8218–26. [PubMed] [Google Scholar]
- 40.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:90–8. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 41.Choi K, Song S, Choi C. Requirement of caspases and p38 MAPK for TRAIL-mediated ICAM-1 expression by human astroglial cells. Immunol Lett. 2008;117:168–73. doi: 10.1016/j.imlet.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Yen ML, Tsai HF, Wu YY, Hwa HL, Lee BH, Hsu PN. TNF-related apoptosis-inducing ligand (TRAIL) induces osteoclast differentiation from monocyte/macrophage lineage precursor cells. Mol Immunol. 2008;45:2205–13. doi: 10.1016/j.molimm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan KF, Siegel MR, Lenardo JM. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity. 2000;13:419–22. doi: 10.1016/s1074-7613(00)00041-8. [DOI] [PubMed] [Google Scholar]
- 45.Hu WH, Johnson H, Shu HB. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NF-kappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem. 1999;274:30603–10. doi: 10.1074/jbc.274.43.30603. [DOI] [PubMed] [Google Scholar]
- 46.Muhlenbeck F, Schneider P, Bodmer JL, et al. The tumor necrosis factor-related apoptosis-inducing ligand receptors TRAIL-R1 and TRAIL-R2 have distinct cross-linking requirements for initiation of apoptosis and are non-redundant in JNK activation. J Biol Chem. 2000;275:32208–13. doi: 10.1074/jbc.M000482200. [DOI] [PubMed] [Google Scholar]
- 47.Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, Liu ZG. The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol Cell Biol. 2000;20:6638–45. doi: 10.1128/mcb.20.18.6638-6645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rescigno M, Piguet V, Valzasina B, et al. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1beta, and the production of interferon gamma in the absence of IL-12 during DC-T cell cognate interaction: a new role for Fas ligand in inflammatory responses. J Exp Med. 2000;192:1661–8. doi: 10.1084/jem.192.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nat R, Radu E, Regalia T, Popescu LM. Apoptosis in the immune system: 1. Fas-induced apoptosis in monocytes-derived human dendritic cells. J Cell Mol Med. 2002;6:223–34. doi: 10.1111/j.1582-4934.2002.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Z, Zhang M, An H, Chen W, Liu S, Guo J, Yu Y, Cao X. Fas ligation induces IL-1beta-dependent maturation and IL-1beta-independent survival of dendritic cells: different roles of ERK and NF-kappaB signaling pathways. Blood. 2003;102:4441–7. doi: 10.1182/blood-2002-11-3420. [DOI] [PubMed] [Google Scholar]
- 51.McLellan AD, Terbeck G, Mengling T, et al. Differential susceptibility to CD95 (Apo-1/Fas) and MHC class II-induced apoptosis during murine dendritic cell development. Cell Death Differ. 2000;7:933–8. doi: 10.1038/sj.cdd.4400734. [DOI] [PubMed] [Google Scholar]
- 52.Lundqvist A, Nagata T, Kiessling R, Pisa P. Mature dendritic cells are protected from Fas/CD95-mediated apoptosis by upregulation of Bcl-X(L) Cancer Immunol Immunother. 2002;51:139–44. doi: 10.1007/s00262-002-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoves S, Krause SW, Halbritter D, Zhang HG, Mountz JD, Scholmerich J, Fleck M. Mature but not immature Fas ligand (CD95L)-transduced human monocyte-derived dendritic cells are protected from Fas-mediated apoptosis and can be used as killer APC. J Immunol. 2003;170:5406–13. doi: 10.4049/jimmunol.170.11.5406. [DOI] [PubMed] [Google Scholar]
- 54.Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–60. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 55.Chan FK, Shisler J, Bixby JG, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–21. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 56.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cretney E, McQualter JL, Kayagaki N, Yagita H, Bernard CC, Grewal IS, Ashkenazi A, Smyth MJ. TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L suppresses experimental autoimmune encephalomyelitis in mice. Immunol Cell Biol. 2005;83:511–9. doi: 10.1111/j.1440-1711.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- 58.Hilliard B, Wilmen A, Seidel C, Liu TS, Goke R, Chen Y. Roles of TNF-related apoptosis-inducing ligand in experimental autoimmune encephalomyelitis. J Immunol. 2001;166:1314–9. doi: 10.4049/jimmunol.166.2.1314. [DOI] [PubMed] [Google Scholar]
- 59.Song K, Chen Y, Goke R, Wilmen A, Seidel C, Goke A, Hilliard B, Chen Y. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095–104. doi: 10.1084/jem.191.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat Immunol. 2003;4:255–60. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- 61.Lamhamedi-Cherradi SE, Zheng S, Tisch RM, Chen YH. Critical roles of tumor necrosis factor-related apoptosis-inducing ligand in type 1 diabetes. Diabetes. 2003;52:2274–8. doi: 10.2337/diabetes.52.9.2274. [DOI] [PubMed] [Google Scholar]
- 62.Mi QS, Ly D, Lamhamedi-Cherradi SE, et al. Blockade of tumor necrosis factor-related apoptosis-inducing ligand exacerbates type 1 diabetes in NOD mice. Diabetes. 2003;52:1967–75. doi: 10.2337/diabetes.52.8.1967. [DOI] [PubMed] [Google Scholar]
- 63.Wagner KW, Punnoose EA, Januario T, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–7. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]