Abstract
The germinal centre (GC) is a specialized microenvironment where high-affinity antibodies are produced through hypermutation and isotype switching. Follicular dendritic cells (FDCs) are the stromal cells of the GC. The timely expansion and establishment of an FDC network is essential for a protective GC reaction; however, only a few factors modulating FDC development have been recognized. In this study, we report that interleukin-15 (IL-15) enhances human primary FDC proliferation and regulates cytokine secretion. The FDCs express IL-15 receptor complexes for IL-15 signal transduction as well as for specific binding. Moreover, the secretion of chemokines CCL-2, CCL-5, CXCL-5 and CXCL-8 was reduced by blocking IL-15 signalling while the secretion of other cytokines, and the expression of CD14, CD44, CD54 (ICAM-1) and CD106 (VCAM-1) proteins remained unchanged. These results suggest that IL-15 plays a crucial role in the development of FDC networks during GC reaction, offering a new target for immune modulation.
Keywords: chemokine, follicular dendritic cell, germinal centre, germinal centre B cells, interleukin-15
Introduction
The germinal centre (GC) is a dynamic microenvironment where protective high-affinity antibodies are produced through extremely rapid B-cell proliferation and extensive modification of their immunoglobulin genes.1–4 The follicular dendritic cells (FDCs) are the stromal cells of the GC.5–7 The major function of FDCs is to retain intact antigen–antibody complexes to provide selective signals to GC-B cells expressing the highest affinity antigen receptor.8,9 The FDCs also provide other crucial microenvironmental factors for GC development. They prevent apoptosis of GC-B cells by cellular interaction and stimulate proliferation by providing adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1);9 the anti-apoptotic molecules BAFF/BLys;10 and a number of growth factors, such as 8D6, interleukin-6 (IL-6) and IL-15.11–13 In addition, FDCs secrete chemokines such as CXCL13, to direct the migration of lymphocytes and other bone-marrow-derived cells.14,15 While the functions of FDCs have been investigated, the factors that control FDC development have begun to be identified recently. B cells and T cells are essential for the development of the FDC network.16,17 Mice deficient in tumour necrosis factor-α (TNF-α) or lymphotoxins (LTs) reveal profound defects in FDC development.15,18,19 In addition, other cytokines including IL-4 and IL-6 appear to be associated with FDC development.20,21 In this report, we present evidence that IL-15 enhances the proliferation of human FDCs and regulates chemokine secretion of human FDCs.
Interleukin-15 is an IL-2-like T-cell proliferation factor that is required for the generation of cytotoxic T lymphocytes and natural killer cells.22–24 It is also important in humoral immunity.25–27 Interleukin-15 enhances the proliferation and immunoglobulin secretion of human peripheral B cells and is involved in B-cell lymphomagenesis.28–34 The heterotrimeric IL-15 receptor (IL-15R) specifically binds IL-15. The IL-15 receptor α-chain (IL-15Rα) is the distinctive component for this specific binding, whereas the IL-15 receptor β-chain (IL-2Rβ) and IL-15 receptor γ-chain (IL-2γ) chains in the receptor complex, which are shared with the IL-2 receptor, are involved in signal transduction.35 Unlike IL-2, however, IL-15 is expressed in various cell types including dendritic cells, keratinocytes,36 monocytes,37,38 thymic epithelial stromal cells,39 bone marrow stromal cells40 and fibroblasts.41 The membrane-bound form of IL-15 plays an essential role in proliferation, or apoptosis of various kinds of cells in an autocrine fashion.37,42–44 Previously, we showed that IL-15 is produced by human FDCs and presented on the surface in a membrane-bound form.13 The IL-15 enhances GC-B-cell proliferation rather than protecting GC-B cells from apoptosis. Furthermore, the level of IL-15 on the surface of FDCs increased following the cellular interaction with GC-B cells. However, the functional role of IL-15 in FDCs has not been investigated.
In this study, we show that IL-15 augments the proliferation of human primary FDCs in vitro. The FDCs express the IL-15R complex that is functional because anti-IL-15 or anti-IL-15R antibodies that block IL-15 signalling reduced FDC proliferation. In addition, blocking of FDC IL-15 signalling reduced FDC secretion of CCL-2, CCL-5, CXCL-5 and CXCL-8, suggesting potentially important roles for recruitment of other cellular components required for GC reaction. Because IL-15 is expressed by FDCs within the GC microenvironment and enhances the proliferation of both GC-B cells and FDCs, IL-15 may contribute to the rapid expansion and formation of the GC structure, suggesting an important role of IL-15 in the humoral immune response.
Materials and methods
Antibodies
Anti-IL-15 monoclonal antibodies (mAbs) [M110, M111 and M112: immunoglobulin G1 (IgG1)] were kindly provided by Dr R. Armitage (Amgen Inc., Seattle, WA). Anti-IL-2Rβ (Mik-β2) was purchased from BD Biosciences, (San Jose, CA). Mouse IgG1 (MOPC 21; used as an isotype control) was purchased from Sigma (St Louis, MO). All mAbs for fluorescence-activated cell sorting (FACS) staining were purchased from BD Biosciences; these were phycoerythrin (PE) -conjugated anti-CD14, anti-CD44, anti-CD54 (ICAM-1), and anti-CD106 (VCAM-1); allophycocyanin (APC) -conjugated anti-CD38; fluorescein isothiocyanate-conjugated anti-IgD; PE-conjugated anti-CD3; and peridinin chlorophyll protein (PerCP) -Cy5.5-conjugated anti-CD20. Blocking and the corresponding control mAbs contained < 0·00002% [weight/volume (w/v)] sodium azide at working concentration. This is 100-fold lower than the concentration of sodium azide that started to show toxicity in our in vitro culture experiments (data not shown).
Cytokines and reagents
The culture media used were Iscove’s modified Dulbecco’s medium (Irvine Scientific, Santa Ana, CA) and RPMI-1640 (Sigma) supplemented with 10% (v/v) fetal calf serum (CFS; Life Technologies, Inc., Grand Island, NY), 2 mm glutamine, 100 U/ml penicillin G and 100 μg/ml streptomycin (Irvine Scientific). Recombinant human IL-15 and recombinant trimeric human CD40 ligand (CD40L) were provided by Dr R. Armitage. Interleukin-2 was obtained from Hoffmann-La Roche (Nutley, NJ). Recombinant IL-4 was kindly provided by Dr Y Choi (Ochsner Clinic Foundation, New Orleans, LA). Percoll and Ficoll were purchased from Pharmacia LKB Biotechnology (Uppsala, Sweden) and bovine serum albumin was obtained from Sigma. The TNF-α was purchased from PeproTech, Inc. (Rocky Hill, NJ).
Preparation and culture of human tonsillar FDCs and GC-B cells
Primary human FDCs were established as described previously.45 Briefly, tonsils freshly obtained from routine tonsillectomies were cut into small pieces and subjected to enzymatic digestion. The released cells were pooled and subjected to Percoll gradient centrifugation for 10 min at 1200 g. Cells with densities < 1·050 g/ml were collected and washed with Hanks’ buffered salt solution (HBSS). Cells were re-suspended in RPMI solution and centrifuged at 300 g for 10 min at 4° over a discontinuous gradient of 1·05 and 1·03 g/ml bovine serum albumin. FDC-enriched fractions were collected from the interface. The cells were washed with HBSS and cultured on tissue culture dishes. Cells isolated and cultured after these procedures initially contained large adherent cells with attached lymphocytes. Non-adherent cells were removed and adherent cells were replenished with fresh medium every 3–4 days. Adherent cells were trypsinized when confluence was attained. The cultured cells were morphologically homogeneous non-phagocytic cells. Purity of FDCs was > 95% as assessed by the expression of 8D6 antigen.11
GC-B cells were purified from tonsillar B cells by MACS® procedure (Miltenyi Biotec Inc., Auburn, CA), as described previously.46 GC-B-cell purity was greater than 95% as assessed by the expression of CD20 and CD38. All samples were obtained with written informed consent in accordance with the guidelines set forth by the Institutional Review Board of the Clinical Research Institute, the Asan Medical Center.
Reverse transcription–polymerase chain reaction
RNA extraction and reverse transcription–polymerase chain reaction (RT-PCR) were performed as described previously.13 Briefly, total RNA was extracted from cells using the RNeasy kit (Qiagen, Valencia, CA). RNA was reverse-transcribed using Moloney murine leukaemia virus reverse transcriptase (Invitrogen Corporation, Carlsbad, CA). Complementary DNA was amplified as follows: denaturation at 94° for 50 seconds, annealing at 57° for 50 seconds, and extension at 72° for 50 seconds. Human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control to ensure equal sample loading. Primers used were as follows: for IL-15Rα, 5′-GTCAAGAGCTACAGCTTGTAC-3′ and 5′-CATAGGTGGTGAGAGCAGTTTTC-3′; for IL-2Rα, 5′-AAGCTCTGCCACTCGGAACACAAC-3′ and 5′-TGATCAGCAGGAAAACACAGC-3′; for IL-2Rβ, 5′-ACCTCTTGGGCATCTGCAGC-3′ and 5′-CTCTCCAGCACTTCTAGTGG-3′; for IL-2Rγ, 5′-CCAGAAGTGCAGCCACTATC-3′ and 5′-GTGGATTGGGTGGCTCCAT-3′; and for GAPDH, 5′-CCCTCCAAAATCAAGTGGGG-3′ and 5′-CGCCACAGTTTCCCGGAGGG-3′.
CFSE labelling
For cell division experiments, FDCs (1 × 107 cells/ml) were labelled with carboxyfluorescein succinimidyl ester (CFSE; Sigma, 0·2 μm in phosphate-buffered saline) and incubated at 37° for 10 min. Cold CFS was added to stop staining, and labelled cells were next washed twice with culture media. After 3 days of culture, CFSE intensity was measured using a FACSCalibur™ flow cytometer and analysed using flowjo software (Ashland, OR).
Apoptosis assay
The apoptosis assay employed staining with Annexin V and 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3); Molecular Probes, Eugene, OR]. The FDCs (1 × 106 cells/ml) suspended in 100 μl of Annexin V binding buffer [0·1 m HEPES/NaOH (pH 7·4), 1·4 m NaCl, 25 mm CaCl3] were stained with 5 μl Annexin V-APC and 5 μl propidium iodide (BD Biosciences). Cells were incubated for 15 min at 25° in the dark. The same number of cells was employed for DiOC6(3) staining; 20 μl 8 μm DiOC6(3) was added, followed by incubation for 10 min. Samples were analysed on a FACSCalibur™ running cellquest-pro® programs (BD Biosciences).
Flow cytometric analysis
Follicular DCs at passages 4–9 were used in experiments. For FACS analysis, FDCs were collected using Enzyme-free Cell Dissociation Solution (Specialty Media, Philipsburg, NJ). All FACS staining for surface CD14, CD44, CD54 and CD106 detection was performed as follows. Briefly, cells were washed in cold FACS buffer [0·05% (v/v) FCS, 0·01% (w/v) NaN3 in phosphate-buffered saline] and subsequently incubated with the appropriate concentration of anti-CD14, anti-CD44, anti-CD54 or anti-CD106 mAbs for 15 min at 4°. After washing with cold FACS buffer, cells were fixed in 1% (v/v) paraformaldehyde. Subsequently, samples were analysed on a FACSCalibur™ running cellquest-pro® program (BD Biosciences).
LUMINEX assay
Follicular DCs at passages 4–9 were seeded at 2 × 104 cells/well in 24-well plates. The next day, the medium was changed and a combination of reagents was added as indicated in the legend to Fig. 4. The concentration of each reagent was as follows: anti-IL-15 mAb (100 ng/ml), mouse IgG1 (100 ng/ml), GC-B cells (2 × 105 per well), TNF-α (10 ng/ml), IL-2 (30 U/ml), IL-4 (50 U/ml) and CD40L (100 ng/ml). GC-B cells were removed after 12 hr by gentle pipetting until most of the GC-B cells disappeared under inverted microscopy. The efficiency of the removal was validated by comparing the total cell number of collected GC-B cells with that of GC-B cells in the control culture. After removing GC-B cells by centrifugation, the supernatant was returned to the original wells. Then cells were cultured for an additional 24 hr, supernatants were harvested by centrifugation at 16 000 g for 5 min and stored at −70° for LUMINEX analysis (Rules Based Medicine, Austin, TX).
Figure 4.
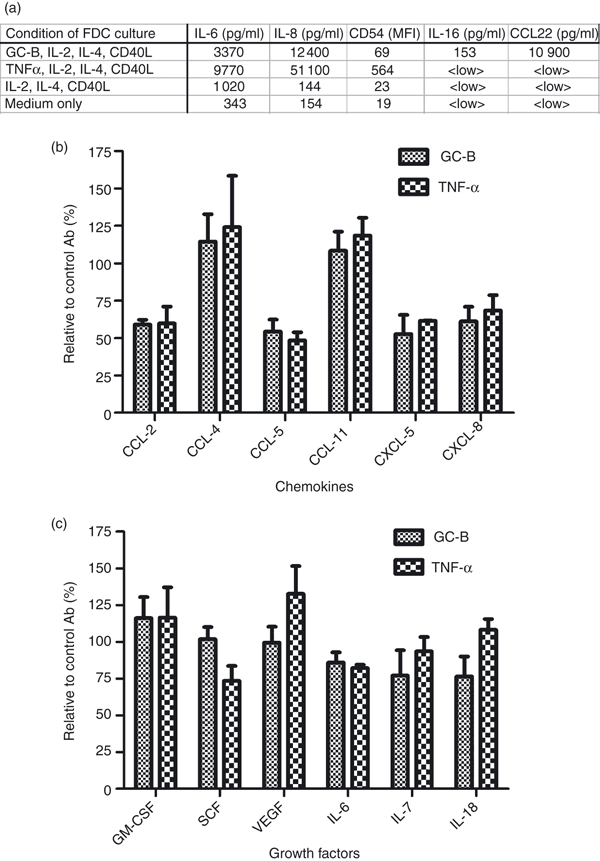
Anti-interleukin-15 (IL-15) monoclonal antibody (mAb) -mediated changes in cytokine secretion levels in media from follicular dendritic cells (FDCs). The FDCs were seeded in 24-well plates (2 × 104 cells/well) and anti-IL-15 mAb, or control immunoglobulin G (IgG), with GC-B cells or tumour necrosis factor-α (TNF-α), was added. GC-B cells (if present) were removed after 12 hr and the supernatants were returned to the original wells. Supernatants were harvested after an additional 24 hr and analysed by LUMINEX assay. (a) Expression levels of IL-6, IL-8, IL-16 and CCL22 in supernatants and CD54 (ICAM-1) on the surface of FDCs cultured with GC-B, and controls. IL-2, IL-4, and sCD40L were added to maintain GC-B-cell survival in the culture system. The cytokine-only (IL-2, IL-4 and sCD40L) control was included to exclude possible cytokine effects on FDCs. The amounts of IL-6 IL-8, IL-16 and CCL22 were measured with the LUMINEX assay. Values represent the concentration of each cytokine (pg/ml) in the culture media. Expression of CD54 (ICAM-1) was measured by fluorescence-activated cell sorting analysis and is represented by the mean fluorescence intensity (MFI). <LOW> values reflect samples below the range measurable by the LUMINEX assay. (b, c) Relative amount of secreted chemokines (b) and cytokines (c) when IL-15 antibody was present, shown as a percentage of the amount when the corresponding control IgG was present. Each experiment with GC-B cells or TNF-α was duplicated. Data are presented at the mean of relative cell numbers ± SD.
Results
IL-15 enhances FDC recovery
In the previous report, we showed that IL-15 on the surface of FDCs strongly enhanced the proliferation of GC-B cells.13 We also suggested a possible autocrine effect of IL-15 on FDCs per se. To evaluate the effect of IL-15 on FDCs, we first examined the FDC recovery in the presence of the exogenous IL-15 by counting viable cell numbers in the culture for 3 days. The number of FDCs cultured with 100 ng/ml of IL-15 increased approximately two-fold compared with the control (Fig. 1a). In addition, the number of recovered cells decreased, in a dose-dependent manner, when three different anti-IL-15 blocking antibodies (M110, M111, M112)13,30,47 were added to the FDC culture (Fig. 1b). These results strongly suggest that IL-15 increased cell recovery of cultured FDCs in an autocrine fashion.
Figure 1.
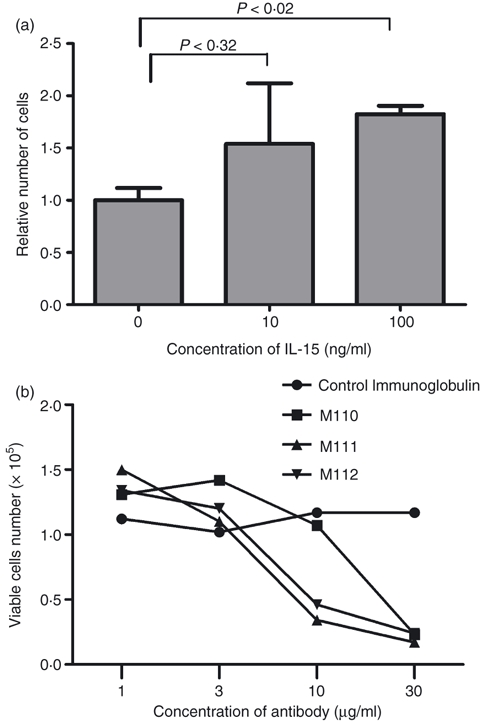
Interleukin-15 (IL-15) increases follicular dendritic cell (FDC) recovery. (a) 1 × 104 FDCs were cultured for 4 days in 24-well plates either with or without additional IL-15. Cells were harvested and viable cells were counted by the trypan blue exclusion assay. Numbers of cells are represented as a relative value with the control sample set to 1. Results are expressed as the mean of relative cell numbers ± standard deviation (SD) of two independent experiments. (b) 1 × 104 FDCs were cultured for 3 days with 1, 3, 10 or 30 μg of anti-IL-15 monoclonal antibody (M110, M111, or M112), or control immunoglobulin G. Cells were harvested and counted on day 3. Absolute cell numbers from each antibody treatment are indicated on the graph at each concentration. Each antibody is represented by a different symbol.
FDCs express IL-15R components for signal transduction
As IL-15 enhanced the FDCs proliferation, we examined whether FDCs had the components necessary for IL-15 signal transduction. The IL-15 binds strongly to IL-15R through IL-15Rα, a component for the specific binding,48 and transmits signals through IL-2Rβ49 and IL-2Rγ.50 Although FDCs express the high-affinity receptor component, IL-15Rα,13 it is not known whether FDC express the signal transduction components of IL-15Rs. Hence, we determined the expression of the other receptor components, IL-2Rβ and IL-2Rγ by RT-PCR. The transcripts for IL-2Rβ and IL-2Rγ were detected in the three human primary FDCs as well as in GC-B cells, which were included as a positive control. In agreement with previous reports,13 messenger RNA for IL-15Rα was not detected in GC-B cells (Fig. 2a).
Figure 2.
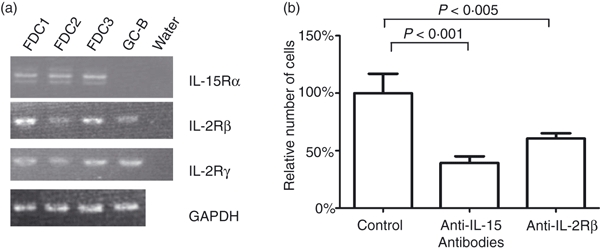
Expression of interleukin-15 (IL-15) receptors in primary follicular dendritic cells (FDCs) and reduction in FDC recovery by anti-IL-15 and anti-IL-2Rβ treatment. (a) Expression of three components of the IL-15 receptor (IL-15Rα, IL-2Rβ and IL-2Rγ) was examined by reverse transcription–polymerase chain reaction of messenger RNA from three different human primary FDCs (FDC1, FDC2, FDC3) and freshly isolated GC-B cells. (b) 0·5 × 104 FDCs were cultured for 3 days in 24-well plates with 10 μg/ml of anti-IL-15, anti-IL-2Rβ, or control immunoglobulin G (IgG). Cells were counted on day 3 by the trypan blue exclusion assay. Data are presented as the mean of relative cell numbers ± SD of four independent experiments.
The signal transduction function of IL-15R was further determined by the blocking experiments as follow. After FDCs were cultured with anti-IL-2Rβ mAb for 3 days, the number of recovered cells was 40% less than the number of cells obtained after culture with control IgG (Fig. 2b). Under the same conditions, the number of recovered cells in the presence of anti-IL-15 antibody, decreased by 60%. These results suggest that human FDCs contain all IL-15R components required for the IL-15 signalling.
IL-15 enhances FDC proliferation
To identify the mechanism involved in the IL-15-mediated increase in cultured FDC recovery, we analysed cell division profiles by CFSE labelling. When CFSE-stained FDCs were cultured with anti-IL-15 mAb, the number of recovered cells decreased by 60% compared with that of control IgG-treated cells, which was comparable to that of the unstained blocking experiment (Fig. 3b). In cell division analysis by CFSE labelling, CFSE intensity was reduced as cell division progressed at day 3. However, the downshift of CFSE intensity was evidently reduced in FDCs cultured with anti-IL-15 mAb rather than in FDCs cultured with control IgG (Fig. 3a). This result suggests that blocking of the IL-15 signal retards cell division. There was no significant difference in apoptosis between cells cultured with anti-IL-15 antibody or control IgG as determined by Annexin V and DiOC6(3) (Fig. 3c,d). These results imply that the increase in recovery of cultured FDCs by IL-15 is mainly through enhancement of cell proliferation, although contribution of proapoptotic mechanism cannot be excluded entirely.
Figure 3.
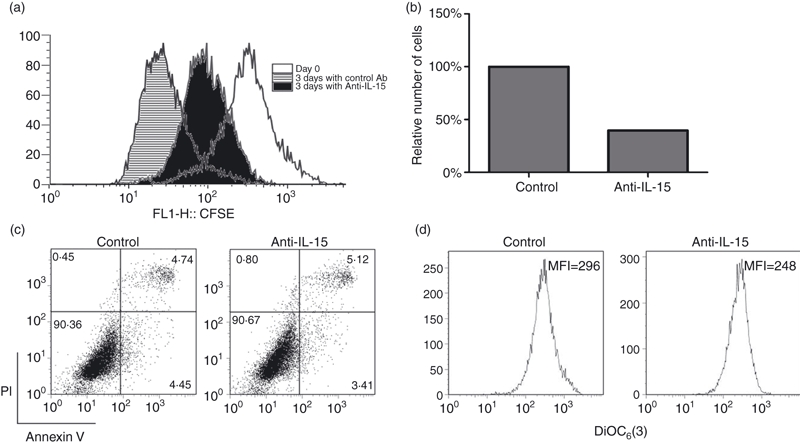
Anti-interleukin-15 (IL-15) monoclonal antibody suppresses the proliferation of follicular dendritic cells (FDCs). (a) The FDCs were labelled with carboxyfluorescein succinimidyl ester (CFSE). After 3 days, the CFSE intensity was measured using a FACSCalibur™ and analysed on flowjo software. (b) FDC recovery was diminished by anti-IL-15 administration when cells were stained with CFSE. (c, d) FDCs cultured with anti-IL-15 or control immunoglobulin G (IgG) were stained with allophycocyanin (APC) -conjugated Annexin V and propidium iodide (PI) (c) or DiOC6(3) (d). Apoptotic cells were analysed using a FACSCalibur™. There was no significant difference between the control and anti-IL-15-treated groups.
Anti-IL-15 decreases FDC secretion of chemokines
To investigate whether IL-15 had effects on FDC function other than the cellular proliferation, we examined the amounts of secreted cytokines in FDC culture medium in the presence or absence of IL-15 signalling using the LUMINEX assay. We designed a co-culture system whereby FDCs were grown with GC-B cells.5,16 We included various controls (as indicated in Fig. 4a) to focus exclusively on the effect of IL-15 on FDCs under stimulation by GC-B cells. The FDCs and GC-B cells were co-cultured overnight (12 hr) to permit cell–cell interaction. Next, GC-B cells were removed, to minimize possible consumption of FDC factors by GC-B cells, TNF-α instead of GC-B cells were added in one control experiment set. This control was used to ascertain the factors produced by FDCs, and to distinguish such components from any contaminating factors secreted by GC-B cells. An additional control, with cytokines IL-2, IL-4 and CD40L, was included to eliminate possible direct effects attributable to these cytokines. These cytokines are essential for GC-B-cell co-culture because they are required for survival of cultured GC-B cells. The TNF-α control contained the same amount of IL-2, IL-4 and CD40L cytokines, to permit a direct comparison. The ‘medium-only’ control set baseline values for the experiment. The TNF-α, produced from B cells, is known to induce changes in both cytokine and surface molecule expression in FDCs.51–53 Both the FDC and GC-B-cell co-culture, and the TNF-α control, showed an increase in the concentrations of IL-6 and IL-8 cytokines in the culture medium, and an enhanced surface expression of CD54 (ICAM-1), when compared with the cytokine-only or medium-only controls (Fig. 4a). Of note, the amount of IL-16 and CCL21 was increased only by the GC-B-cell co-culture, but not by the additional TNF-α (Fig. 4a), which showed that there are other factors affecting the secretion of cytokines from FDCs than TNF-α in GC-B co-culture. These results suggested that the co-cultured GC-B cells appeared to be more physiological than additional TNF-α alone and provide sufficient FDC-stimulating factors Hence, co-culture of FDCs and GC-B cells is useful for the study of FDC function in vitro.
We employed this system to investigate the effect of anti-IL-15 antibody on the secretion of cytokines from FDCs. Secretion of some chemokines, such as CCL-2, CCL-5, CXCL-5 and CXCL-8, was significantly reduced when anti-IL-15 mAb was added to the culture medium (Fig. 4b). However, other cytokines, including CCL-4, CCL-11, granulocyte–macrophage colony stimulating factor and vascular endothelial growth factor were not affected. These data suggest that blocking by anti-IL-15 antibodies has a selective effect on secretion, of particular chemokines, rather than causing a general non-specific suppression of FDC function (Fig. 4c).
Anti-IL-15 did not alter the FDC surface expression levels of CD14, CD44, CD54 (ICAM-1) or CD106 (VCAM-1)
CD14, CD44, CD54 (ICAM-1) and CD106 (VCAM-1) are some of the major surface molecules that play important roles in the cellular interactions between GC-B cells and FDCs.6 We therefore investigated the effect of blocking of the IL-15 signal on FDC surface expression of CD14, CD44, CD54 (ICAM-1) and CD106 (VCAM-1) via FACS analysis. However, the expression of these surface proteins was not altered by anti-IL-15 mAb treatment (Fig. 5).
Figure 5.
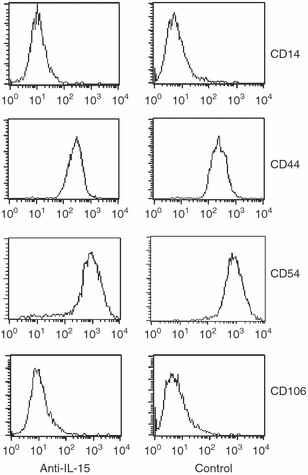
The surface expression levels of CD14, CD44, CD54 (ICAM-1) and CD106 (VCAM-1) are unchanged by anti-interleukin-15 (IL-15) monoclonal antibody (mAb) addition. Follicular dendritic cells (FDCs) were stained with the corresponding phycoerythrin-conjugated antibodies and analysed by fluorescence-activated cell sorting.
Discussion
During GC formation, stromal cells in primary follicles proliferate rapidly and differentiate into FDCs.6 Both TNF-α and LT from GC-B cells have been considered essential soluble factors for FDC development because genetically engineered TNF-α-knockout and LT-knockout mice are defective in GC formation. However, a number of gene-knockout mouse studies do not distinguish between FDC development in primary B-cell follicles rather than in the GC.6 Therefore, a proliferation assay with in vitro culture of human primary FDCs could be a plausible system with which to investigate the FDC development during the mature GC formation. Although in vitro culture of human primary FDCs has been established, and studied for decades, only a few proliferation factors, including TNF-α and IL-1β, have been identified.54,55
Previously, we demonstrated that IL-15 expressed in human tonsillar FDCs enhanced the proliferation of GC-B cells.13 The function of IL-15 has not been extensively studied in FDCs because there is little difference in the humoral immune response of genetically modified mice.25–27 We therefore investigated the biological function of IL-15 on human FDCs. In the present study, we examined the functional role of IL-15 in FDCs using human primary FDCs. First, we found that the addition of IL-15 enhanced recovery of the FDC proliferation in cultures and that the addition of anti-IL-15 antibody reduced the recovery of cultured FDCs. The FDCs have the IL-15R components necessary for signal transduction by IL-15, as well as IL-15 binding. These observations strongly suggest that IL-15 plays a functional role in FDCs.
Interestingly, the effect of IL-15 in increasing the recovery of cultured FDCs is mainly attributed to enhanced proliferation rather than protection from apoptosis, as determined by CFSE labelling. Previously, we demonstrated that IL-15 enhanced GC-B-cell proliferation and that the amount of IL-15 on the surface of FDCs was increased by co-culture with GC-B cells. Together, these results suggest the existence of a strong positive-feedback loop, using IL-15 as a common trophic signal, in early GC development. Once IL-15 signalling is induced, proliferation of GC-B cells and FDCs is augmented, and the amount of IL-15 per se will be dramatically amplified by reciprocal signalling between the cells. Given the urgency of generation and production of protective high-affinity antibodies in case of infection, this sharing of common pro-proliferative cytokines, by both functional GC-B cells and microenvironmental stromal cells, FDCs, may be advantageous for the timely development of the GC reaction. Moreover, proliferation of FDCs is thereby coupled to antigen-specific proliferation of GC-B cells, augmenting the selective generation of GC-B cells with high-affinity B-cell receptors for antigen.
Interleukin-15 does not have a significant effect on the apoptosis of FDC in our in vitro culture model (Fig. 3c) in contrast to previous reports on the anti-apoptotic effects of IL-15 in various cells.44,56,57 The reported anti-apoptotic effects were measured in the presence of strong apoptotic signals, including stimulation of other surface molecules by anti-Fas, TNF-α, anti-CD3 and IgM, or use of toxic chemicals. In contrast, we examined the effect of IL-15 in the absence of apoptotic inducers, which may be more relevant to the early GC reaction in vivo. We attempted to induce apoptosis of FDCs using anti-Fas antibody or TNF-α to investigate an anti-apoptotic function of IL-15 on FDCs; however, apoptosis was not detected in freshly isolated FDCs (C-S. Park, unpublished data). Therefore, although an anti-apoptotic effect of IL-15 on FDCs undergoing apoptosis during the GC response54 cannot be excluded, the major role of IL-15 in the developing GC is to enhance proliferation of both FDCs and GC-B cells.
Another important question regarding the function of IL-15 on FDCs is whether IL-15 is involved in FDC differentiation. One of the major obstacles in FDC research has been the lack of a reliable, functional, experimental system. For instance, it is difficult to distinguish between any changes in FDCs from those of other cellular components of the GC reaction, using a genetically modified mouse model. Immunohistochemical analysis has limitations because such analysis cannot be used to measure functional changes. In vitro culture experiments are a plausible alternative. However, the culture experiments also have limitations, including the possible loss of functional competency during in vitro culture. The FDCs needs various factors from GC-B cells to develop and to maintain their function. To compensate for these problems, we designed a culture protocol to mimic in vivo functional FDCs by co-culturing primary human FDCs with GC-B cells. Hence, signals from GC-B cells essential for FDC function16,58 are provided in our experimental model. The TNF-α control set is included for two purposes. First, to validate the efficacy of in vitro cultured GC-B cells as FDC stimulators by comparing them with that of TNF-α. GC-B cells stimulated FDCs to enhance the expression of the cytokines and the adhesion molecules as much as TNF-α did (Fig. 4a). The enhanced secretion of IL-6 and IL-8 and elevated surface expression of ICAM-1 by TNF-α treatment in our experiment (Fig. 4a) is consistent with previous reports.51,52 In addition, GC-B cells can induce secretion of IL-16 and CCL22, which were not increased by the TNF-α. This suggests that GC-B cells produced more factors stimulating the FDCs other than TNF-α. Together, the results in Fig. 4(a) indicate that our co-culture system is a useful in vitro model to investigate the function of FDCs. The second purpose is to ensure that the change of IL-15 blocking originated from FDC not from GC-B cells. The co-culture experiment has its own limitations. Testing anti-IL-15 can affect stimulator GC-B cells not only FDCs, resulting in the alteration of cytokine profiles in the culture supernatant as the result of contaminating GC-B cell factors, and because of FDC factor consumption by GC-B cells. We can determine the exclusive effect of the change of the cytokine profile of IL-15 on FDC in the co-culture experiment by comparing the result with that of the TNF-α set because FDC is the only cellular component in the TNF-α set. For this reason, we only included the secreted factors augmented by both GC-B co-culture and TNF-α addition for the analysis in Fig. 4(b,c).
In Fig. 4(b), we suggest that IL-15 signalling is necessary for the increased production of some chemokines. However, it is not definite whether IL-15 alone is sufficient to the increased production of those cytokines. Interleukin-15 can be a co-factor of GC-B-cell factors because there are other GC-B-cell factors including TNF-α in our co-culture experiments. Alternatively, increased amounts of surface IL-15 per se can be sufficient for augmented production of the cytokines because IL-15 expression on the surface of FDCs is increased remarkably upon co-culturing with GC-B cells or addition of TNF-α.13 The effect of IL-15 blocking without GC-B-cell factors cannot be determined effectively in our system because very low or undetectable amounts of cytokines are produced in cultured FDCs without stimulation.
Interestingly, the altered production of CCL-2, CCL-5 and CXCL-8 by blocking of IL-15 signalling corresponds well with findings from earlier studies, which reported that IL-15 increased production of these chemokines from human T cells and monocytes.59,60 There are also reports that IL-15 is a potent inducer of chemokines involved in chemotaxis in other cellular systems.25,61–63 Further investigation of the functional roles of these chemokines produced by FDCs with IL-15 may provide important clues regarding development of the GC reaction.
Protective immune responses against an invading pathogen are a race against time.64 The rapid expansion of GC should be accompanied by a comparable fast development of FDCs, as well as timely recruitment of other cellular constituents. In this report, we have demonstrated that IL-15 plays an important role in supporting FDC proliferation and in the production of certain chemokines by FDCs. These findings suggest that IL-15 is one of the key factors in the production of protective antibodies by stimulating rapid GC formation, offering a potential target for immune modulation.
Acknowledgments
This study was initiated at the Laboratory of Cellular Immunology (Ochsner Clinic Foundation, New Orleans, LA) and completed at the Asan Institute for Life Science, Seoul. The reagents IL-15 and CD40L were the generous gift of Dr Richard Armitage (Amgen, Seattle, WA). The study was supported by a grant W06-408 from the Asan Institute for Life Science, Seoul, and by a National Research Foundation grant from the Korean government A (R13-2008-023-01003).
Glossary
Abbreviations:
- Ab
antibody
- CD40L
CD40 ligand
- FACS
flow cytometric analysis
- FDC
follicular dendritic cell
- GC
germinal centre
- ICAM-1
intercellular adhesion molecule 1
- IL-15R
interleukin-15 receptor
- IL-15Rα
interleukin-15 receptor α chain
- IL-15Rβ
interleukin-15 receptor β chain
- IL-15Rγ
interleukin-15 receptor γ chain
- LT
lymphotoxin
- mAb
monoclonal antibody
- VCAM-1
vascular cell adhesion molecule 1
Disclosures
None of the authors have any potencial financial conflict of interest related to this work.
References
- 1.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 2.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–26. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 3.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 5.Park CS, Choi YS. How do follicular dendritic cells interact intimately with B cells in the germinal centre? Immunology. 2005;114:2–10. doi: 10.1111/j.1365-2567.2004.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YJ, Grouard G, de Bouteiller O, Banchereau J. Follicular dendritic cells and germinal centers. Int Rev Cytol. 1996;166:139–79. doi: 10.1016/s0074-7696(08)62508-5. [DOI] [PubMed] [Google Scholar]
- 8.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 9.Qin D, Wu J, Carroll MC, Burton GF, Szakal AK, Tew JG. Evidence for an important interaction between a complement-derived CD21 ligand on follicular dendritic cells and CD21 on B cells in the initiation of IgG responses. J Immunol. 1998;161:4549–54. [PubMed] [Google Scholar]
- 10.Hase H, Kanno Y, Kojima M, et al. BAFF/BLyS can potentiate B-cell selection with the B-cell coreceptor complex. Blood. 2004;103:2257–65. doi: 10.1182/blood-2003-08-2694. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Zhang X, Kovacic S, Long AJ, Bourque K, Wood CR, Choi YS. Identification of a human follicular dendritic cell molecule that stimulates germinal center B cell growth. J Exp Med. 2000;191:1077–84. doi: 10.1084/jem.191.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jego G, Bataille R, Pellat-Deceunynck C. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood. 2001;97:1817–22. doi: 10.1182/blood.v97.6.1817. [DOI] [PubMed] [Google Scholar]
- 13.Park CS, Yoon SO, Armitage RJ, Choi YS. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J Immunol. 2004;173:6676–83. doi: 10.4049/jimmunol.173.11.6676. [DOI] [PubMed] [Google Scholar]
- 14.Ngo VN, Korner H, Gunn MD, et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–12. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–93. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 16.Cerny A, Zinkernagel RM, Groscurth P. Development of follicular dendritic cells in lymph nodes of B-cell-depleted mice. Cell Tissue Res. 1988;254:449–54. doi: 10.1007/BF00225818. [DOI] [PubMed] [Google Scholar]
- 17.Kapasi ZF, Burton GF, Shultz LD, Tew JG, Szakal AK. Induction of functional follicular dendritic cell development in severe combined immunodeficiency mice. Influence of B and T cells. J Immunol. 1993;150:2648–58. [PubMed] [Google Scholar]
- 18.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 19.Tumanov AV, Kuprash DV, Nedospasov SA. The role of lymphotoxin in development and maintenance of secondary lymphoid tissues. Cytokine Growth Factor Rev. 2003;14:275–88. doi: 10.1016/s1359-6101(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 20.Yamada K, Yamakawa M, Imai Y, Tsukamoto M. Expression of cytokine receptors on follicular dendritic cells. Blood. 1997;90:4832–41. [PubMed] [Google Scholar]
- 21.Deng C, Goluszko E, Tuzun E, Yang H, Christadoss P. Resistance to experimental autoimmune myasthenia gravis in IL-6-deficient mice is associated with reduced germinal center formation and C3 production. J Immunol. 2002;169:1077–83. doi: 10.4049/jimmunol.169.2.1077. [DOI] [PubMed] [Google Scholar]
- 22.Burton JD, Bamford RN, Peters C, et al. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4935–9. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 24.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:11445–50. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–90. [PubMed] [Google Scholar]
- 29.Trentin L, Zambello R, Facco M, Sancetta R, Agostini C, Semenzato G. Interleukin-15: a novel cytokine with regulatory properties on normal and neoplastic B lymphocytes. Leuk Lymphoma. 1997;27:35–42. doi: 10.3109/10428199709068269. [DOI] [PubMed] [Google Scholar]
- 30.Tinhofer I, Marschitz I, Henn T, Egle A, Greil R. Expression of functional interleukin-15 receptor and autocrine production of interleukin-15 as mechanisms of tumor propagation in multiple myeloma. Blood. 2000;95:610–8. [PubMed] [Google Scholar]
- 31.Kacani L, Sprinzl GM, Erdei A, Dierich MP. Interleukin-15 enhances HIV-1-driven polyclonal B-cell response in vitro. Exp Clin Immunogenet. 1999;16:162–72. doi: 10.1159/000019108. [DOI] [PubMed] [Google Scholar]
- 32.Bulanova E, Budagian V, Pohl T, Krause H, Durkop H, Paus R, Bulfone-Paus S. The IL-15R alpha chain signals through association with Syk in human B cells. J Immunol. 2001;167:6292–302. doi: 10.4049/jimmunol.167.11.6292. [DOI] [PubMed] [Google Scholar]
- 33.Kumaki S, Armitage R, Ahdieh M, Park L, Cosman D. Interleukin-15 up-regulates interleukin-2 receptor alpha chain but down-regulates its own high-affinity binding sites on human T and B cells. Eur J Immunol. 1996;26:1235–9. doi: 10.1002/eji.1830260608. [DOI] [PubMed] [Google Scholar]
- 34.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 35.Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4:329–36. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 36.Blauvelt A, Asada H, Klaus-Kovtun V, Altman DJ, Lucey DR, Katz SI. Interleukin-15 mRNA is expressed by human keratinocytes Langerhans cells, and blood-derived dendritic cells and is downregulated by ultraviolet B radiation. J Invest Dermatol. 1996;106:1047–52. doi: 10.1111/1523-1747.ep12338641. [DOI] [PubMed] [Google Scholar]
- 37.Neely GG, Epelman S, Ma LL, et al. Monocyte surface-bound IL-15 can function as an activating receptor and participate in reverse signaling. J Immunol. 2004;172:4225–34. doi: 10.4049/jimmunol.172.7.4225. [DOI] [PubMed] [Google Scholar]
- 38.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 39.Leclercq G, Debacker V, de Smedt M, Plum J. Differential effects of interleukin-15 and interleukin-2 on differentiation of bipotential T/natural killer progenitor cells. J Exp Med. 1996;184:325–36. doi: 10.1084/jem.184.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–40. [PubMed] [Google Scholar]
- 41.Briard D, Brouty-Boye D, Azzarone B, Jasmin C. Fibroblasts from human spleen regulate NK cell differentiation from blood CD34+ progenitors via cell surface IL-15. J Immunol. 2002;168:4326–32. doi: 10.4049/jimmunol.168.9.4326. [DOI] [PubMed] [Google Scholar]
- 42.Kurowska M, Rudnicka W, Kontny E, et al. Fibroblast-like synoviocytes from rheumatoid arthritis patients express functional IL-15 receptor complex: endogenous IL-15 in autocrine fashion enhances cell proliferation and expression of Bcl-x(L) and Bcl-2. J Immunol. 2002;169:1760–7. doi: 10.4049/jimmunol.169.4.1760. [DOI] [PubMed] [Google Scholar]
- 43.Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173:1463–76. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- 44.Ruckert R, Asadullah K, Seifert M, Budagian VM, Arnold R, Trombotto C, Paus R, Bulfone-Paus S. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J Immunol. 2000;165:2240–50. doi: 10.4049/jimmunol.165.4.2240. [DOI] [PubMed] [Google Scholar]
- 45.Kim HS, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994;153:2951–61. [PubMed] [Google Scholar]
- 46.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, Choi YS. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int Immunol. 2005;17:779–88. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 47.Musso T, Calosso L, Zucca M, et al. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999;93:3531–9. [PubMed] [Google Scholar]
- 48.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–63. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bamford RN, Grant AJ, Burton JD, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4940–4. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giri JG, Ahdieh M, Eisenman J, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–30. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Husson H, Lugli SM, Ghia P, et al. Functional effects of TNF and lymphotoxin alpha1beta2 on FDC-like cells. Cell Immunol. 2000;203:134–43. doi: 10.1006/cimm.2000.1688. [DOI] [PubMed] [Google Scholar]
- 52.Skibinski G, Skibinska A, Deckers M, James K. Tonsil stromal-cell lines expressing FDC-like properties: isolation, characterization, and interaction with B lymphocytes. Dev Immunol. 1998;6:273–84. doi: 10.1155/1998/81637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park SM, Kim HS, Choe J, Lee TH. Differential induction of cytokine genes and activation of mitogen-activated protein kinase family by soluble CD40 ligand and TNF in a human follicular dendritic cell line. J Immunol. 1999;163:631–8. [PubMed] [Google Scholar]
- 54.Park SM, Park HY, Lee TH. Functional effects of TNF-alpha on a human follicular dendritic cell line: persistent NF-kappa B activation and sensitization for Fas-mediated apoptosis. J Immunol. 2003;171:3955–62. doi: 10.4049/jimmunol.171.8.3955. [DOI] [PubMed] [Google Scholar]
- 55.Kim Y, Kim J, Park J, Bang S, Jung Y, Choe J, Song K, Lee I. TC1(C8orf4) is upregulated by IL-1beta/TNF-alpha and enhances proliferation of human follicular dendritic cells. FEBS Lett. 2006;580:3519–24. doi: 10.1016/j.febslet.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 56.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H, Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo. Nat Med. 1997;3:1124–8. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 57.Bulfone-Pau SS, Bulanova E, Pohl T, et al. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. FASEB J. 1999;13:1575–85. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 58.MacLennan IC, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 59.Perera LP, Goldman CK, Waldmann TA. IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J Immunol. 1999;162:2606–12. [PubMed] [Google Scholar]
- 60.Badolato R, Ponzi AN, Millesimo M, Notarangelo LD, Musso T. Interleukin-15 (IL-15) induces IL-8 and monocyte chemotactic protein 1 production in human monocytes. Blood. 1997;90:2804–9. [PubMed] [Google Scholar]
- 61.McInnes IB, al-Mughales J, Field M, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–82. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 62.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–9. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barlic J, Sechler JM, Murphy PM. IL-15 and IL-2 oppositely regulate expression of the chemokine receptor CX3CR1. Blood. 2003;102:3494–503. doi: 10.1182/blood-2003-03-0946. [DOI] [PubMed] [Google Scholar]
- 64.Junt T, Scandella E, Ludewig B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol. 2008;8:764–75. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]


