Abstract
Natural killer (NK) cells can destroy xenogeneic tissues by antibody-dependent cell cytotoxicity (ADCC) and direct lysis. Unlike ADCC, activating interactions between human NK receptors and their cognate ligands in pigs are not fully elucidated. We set up this study to identify human NK activating receptors recognizing porcine cells isolated from distinct organs, e.g., aorta, cornea and liver, and to provide a molecular basis for effective immunosuppressive regimens. Among the array of NK receptors tested, NKp46, 2B4, CD49d, CD48, CD2 and NKG2D, only CD2 and NKG2D were shown to be involved in both cytotoxicity and cytokine (interferon-γ and tumour necrosis factor-α) production against porcine targets. Simultaneous blocking of CD2 and NKG2D by combining its monoclonal antibodies further suppressed xenogeneic NK responses. Moreover, addition of a suboptimal dose of PD98059, an extracellular signal-regulated kinase (ERK) kinase inhibitor, to those cells maximally reduced NK cytotoxicity, suggesting that ERK plays an important role in NK-mediated xenoreactivity. These impairments in NK cells were tightly associated with defective intracellular calcium mobilization and the subsequent degranulation process. Therefore, our data demonstrate a distinct role of CD2 and NKG2D on human NK cells in recognizing porcine grafts and further provide a potentially efficacious combinational regimen using anti-CD2 and anti-NKG2D monoclonal antibodies with PD98059 in a pig-to-human transplantation model.
Keywords: extracellular signal-regulated kinase kinase inhibitor, human natural killer cells, immunosuppression, monoclonal antibodies, xenotransplantation
Introduction
Xenotransplantation represents a life-saving technique to treat end-stage organ failure. Its success is largely dependent on an optional immunosuppressive regimen. The pig is among the most promising organ sources for xenotransplantation because of its similarities with human counterparts. However, following xenotransplantation, early hyperacute rejection and acute humoral xenograft rejection later limit the use of these organs. These rejections are mainly mediated by natural antibodies and the complement system directed at terminal carbohydrate Galα1-3Galβ1-4GlcNAc.1 In this context, natural killer (NK) cells along with macrophages and neutrophils can destroy the xenogeneic tissue by antibody-dependent cell cytotoxicity, which is mediated by CD16 (FcγRIIIa and FcγRIIIb), and contribute to acute rejection.2–4 Recent development of strategies to deplete natural antibodies or to produce α1,3galatosyltransferase-deficient pigs5–7 may afford longer survival of transplanted organs.
The NK cells mediate endothelial injury via direct cytotoxicity against surface antigens and contribute to the cellular rejection process.8 Although the role of cytokines and chemokines produced by NK cells is less understood in the context of xenotransplantation, these cells are likely to be involved in promoting cellular rejection either directly or indirectly by activating other cells in the immune system. Natural killer cells recognize ‘missing self’ via inhibitory receptors such as killer cell immunoglobulin-like receptors in humans.9,10‘Missing self’ ligands could be down-regulated, allogeneic or xenogeneic major histocompatibility complex (MHC) class I molecules. Consequently, introducing the human counterpart of MHC class I molecules and their variants into pigs has provided a promising strategy to prevent rejection of porcine grafts.11–17
In addition to inhibitory receptors, NK cells express multiple activating receptors, e.g. CD2, 2B4, CD48, CD16, NKG2D, NKp46, NKp30 and NKp44. Upon target recognition and cross-linking of individual NK activating receptors listed above, NK cells have been shown to transmit intracellular signals via phosphatidyl inositol 3-kinase–Ras-related C3 botulinum toxin substrate 1–P21 activated kinase–mitogen-activated protein kinase/extracellular signal-regulated kinase–extracellular signal-regulated kinase (PI3K-Rac1-PAK-MEK-ERK) pathways, leading to exocytosis and granule release.18–20 It is therefore logical to presume that these receptors play a role in NK-mediated xenogeneic cytotoxicity. The NK cells also express cell adhesion receptors, CD11a, CD18, CD162 and CD49d.21 Among these molecules, CD49d has been shown to play a crucial role in both rolling and firm adhesion of human NK cells to porcine endothelial cells via binding to its ligand CD106 (vascular cell adhesion molecule 1; VCAM-1).21 Along with VCAM-1 (CD106), porcine cardiac and aortic endothelial cells expressed fibronectin and mucosal vascular addressin cell adhesion molecule 1,21–23 providing potential therapeutic targets for suppressing xenogeneic NK activity. Therefore, these activating and adhesion receptors on NK cells may potentially become important in lysing porcine grafts depending on the level of their cognate ligand recognition. It was shown recently that porcine aortic endothelial cells expressed CD58 (LFA-3), a ligand for CD2, and UL16-binding protein 1 (ULBP1), a ligand for NKG2D, on their surface.24,25 Therefore, the role of CD2 and/or NKG2D may become critical in NK-medated xenoreactivity against porcine targets. In line with this idea, blocking NKG2D in a pig-to-human model has been shown to suppress NK-mediated cytotoxicity.26 Unlike these receptors, the ligand of 2B4 is CD48, both of which are constitutively expressed on NK cells, but not on porcine cells, which allows homotypic NK-to-NK cell interaction.27 Ligands for NKp30, NKp44 and NKp46 are not yet known, but the role of NKp44 has been reported in xenogeneic NK cytotoxicity.26
As the activation status of NK cells in the MHC class I-mismatched transplant setting is determined by the strength of NK receptor/ligand interactions, identification of the cognate ligand/receptor pairs would be critical to control the NK-mediated xenogeneic rejection process. Therefore, we set up this study to dissect the role of various NK activating and adhesion receptors in xenogeneic responses and consequently to provide an efficient therapeutic regimen via evaluating combined use of NK receptor-specific monoclonal antibodies (mAbs) and a small molecule inhibitor of ERK kinases. Our data suggest that each NK receptor, CD2 or NKG2D, plays a partial role in lysing porcine cells freshly isolated from distinct organs and that inhibition of relevant receptors using their specific mAbs in combination with an ERK kinase inhibitor, PD98059, provides a promising immunosuppressive regimen following pig-to-human xenotransplantation.
Materials and methods
Human primary NK cell isolation and culture
Human primary NK cells were isolated from whole blood from a total of 56 healthy volunteers as previously described28 (CD3− CD56+ NK cells > 90%). All procedures were approved by the Korea University Institutional Review Board (IRB No. HU-IRB-08017-A-2) and the donors gave informed consents. Isolated NK cells were cultured in RPMI-1640 (Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum (Lonza Walkersville Inc. Walkersville, MD), 100 U/ml penicillin (Lonza Walkersville Inc.), 100 U/ml streptomycin (Lonza Walkersville Inc.) and 300 U/ml human recombinant interleukin-2 (hrIL-2; Chiron, Charlotte, NC). The IL-2-activated NK cells at culture day 10–14 were used for assays. K562 (human Caucasian chronic myelogenous leukaemia cell line) and P815 (mouse lymphoblast-like mastocytoma cell line; both from American Type Culture Collection, Manassas, VA) were cultured in RPMI-1640 (Welgene) supplemented with 10% fetal bovine serum (Lonza Walkersville Inc.), 100 U/ml penicillin (Lonza Walkersville Inc.) and 100 U/ml streptomycin (Lonza Walkersville Inc.); referred to as complete RPMI.
Porcine cell isolation and culture
An simian virus 40-immortalized porcine aortic endothelial cell (PAEC) line, MPN3, was cultured in complete RPMI.29 Primary PAEC were isolated ex vivo from Minnesota miniature pigs30 maintained in specific pathogen-free facilities in Seoul National University Hospital as previously described.29 Porcine primary liver cells (PLC) and porcine primary cornea endothelial cells (PCEC) were kindly provided by Xenotransplantation Research Center in Seoul National University Medical School (Seoul, Korea). Cells were cultured in complete RPMI. All procedures were approved by Seoul National University Hospital Institutional Animal Care and Use Committee (SNUH-IACUC No. 06225).
Reverse transcription–polymerase chain reaction
Total RNA was isolated from MPN3 cells, and primary PAEC using Trizol (Life Technologies, Carlsbad, CA,), and transcribed to complementary DNA using the Moloney murine leukaemia virus reverse transcriptase (Bioneer, Daejeon, Korea) and following the manufacturer’s instructions. Polymerase chain reaction (PCR) was performed using primers (Bioneer) for the porcine ULBP1 (forward 5′-GCGGCCTGCGATACTCACTCTCTTTGC-3′ and reverse 5′-GGAAGCTGGTCACAATCCGGTCACTCTCCC-3′), for porcine CD58 (forward 5′-CTCTTCCAGAGAGCCAGAACTA-3′ and reverse 5′-CTGCGACCAGCACATATCTA-3′), and porcine glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward 5′ GAAGGACTCATGACCACAGT-3′ and reverse 5′-GCTGTAGCCAAATTCATTATTGT-3′)24,25 in 50 μl of reaction mixture containing 1 μg complementary DNA, 1·2 nm of each primer, 0·1 mm of dNTP and 2·5 unit of Taq I DNA polymerase (Bioneer). Reaction mixture without DNA was used as a negative control. The PCR protocols were as follows: amplification in a My Cycler™ (Bio-Rad, Hercules, CA) for 25–30 cycles with a denaturing step at 94° for 45 seconds, an annealing step at 54–57° for 30 seconds and an amplification step at 72° for 45 seconds. The PCR product was analysed by gel electrophoresis and photographed using LabWork (BioImaging Systems, UVP, Inc., Cambridge, UK).
Flow cytometry
Anti-human CD3 (Clone UCHT-1), CD56 (MEM188), CD107a (eBio H4A3), interferon-γ (IFN-γ; 4S.B3) and tumour necrosis factor-α (TNF-α; MAb11) mAbs conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC) and isotype controls were purchased from eBioscience (San Diego, CA). Isotype controls (eBioscience) were used as controls. Flow cytometry was performed with a FACScalibur (BD Bioscience, San Diego, CA) and the data were analysed with CellQuest software (BD Pharmingen, San Jose, CA). Lymphocyte populations were gated by forward scatter/side scatter and NK cells were defined as CD3− CD56+.
Cytotoxicity assays
Cytotoxic activity of IL-2-activated human NK cells against porcine cells was tested in a standard 4-hr 51Cr-release assay.31 For blocking NK receptor interaction with their cognate ligands, a saturating dose (10 μg/ml) of mAbs was used in each experiment. Briefly, NK cells were pre-incubated for 30 min at 4° with 10 μg/ml of anti-human NKG2D mAb (Clone 149810; R&D Systems, Minneapolis, MN), anti-human CD2 mAb (RPA-2·10; e-Bioscience), anti-human NKp44 mAb (253415; R&D Systems) alone or in combination. Anti-2B4 (C1.7; Beckman Coulter, Brea, CA), anti-CD48 (TU145; BD Pharmingen), anti-CD49d (44H6; Serotec), and anti-NKp46 (195314; R&D Systems) mAbs were also used at 10 μg/ml. In some experiments, NK cells were pre-incubated with an ERK inhibitor, PD98059 (Sigma, St Louis, MO) or ethylene glycol tetraacetic acid (EGTA), a calcium chelator at designated concentrations at 37° for 30 min. Dimethylsulphoxide and phosphate-buffered saline were used as vehicle controls, respectively. After 4 hr of reactions, supernatant was collected and 51Cr-release was assessed with a gamma-counter. The percentage of specific lysis was calculated as follows: (experimental release – spontaneous release)/(maximum release – spontaneous release) × 100.
Measurement of intracellular calcium mobilization
Interleukin-2-activated human NK cells were pre-incubated with 10 μg/ml anti-CD2 or anti-NKG2D mAb and loaded with 5 μm Fura 2-AM (Molecular Probes; Invitrogen, Carlsbad, CA) in Hanks’ balanced salt solution (HBSS) supplemented with 1 mg/ml bovine serum albumin and 0·025% pluronic F-127. The cells were incubated in HBSS for another 30 min. The cells were then placed on poly-l-lysine-coated 25-mm coverslips and incubated for 20 min. A coverslip was mounted and the slide was placed on the stage of a Nikon Diaphot inverted epifluorescence microscope (Nikon, Garden City, NJ). MPN3 and ionomycin were inoculated at time intervals and changes of intracellular calcium concentrations were measured by fluorescence ratio, which was monitored using a Metafluor Imaging System (Molecular Devices).
CD107a degranulation assay and intracellular cytokine analysis
CD107a assay was performed following Alter et al.32 with minor modifications. Briefly, IL-2-activated NK cells were co-cultured with MPN3 cells in the presence of anti-CD2 mAb or anti-NKG2D mAb alone or in combination at an E : T ratio of 5 : 1. After 1 hr, anti-CD107a mAb conjugated with FITC was treated at 10 μl/well and Golgistop (BD Pharmingen) was added to the culture for the last 5 hr. After the incubation, samples were harvested and incubated for 20 min with CD56 conjugated with APC. The samples were fixed and permeabilized using Cytofix/Cytoperm Intracellular Staining kits (BD Pharmingen). Then NK cells were intracellularly stained with anti-IFN-γ-PE or anti-TNF-α-PE mAb for an additional 30 min. Flow cytometry was performed with FACScalibur (BD Pharmingen) and the data were analysed with CellQuest software (BD Pharmingen).
Western blotting
Western blotting to assess phosphorylated ERK and pan-ERK in NK cells was performed as previously described.18 In brief, human NK cells and MPN3 target cells were serum-starved for 15 hr. MPN3 cells were fixed in 1·6% formaldehyde for 10 min at room temperature and washed twice with phosphate-buffered saline. Then, IL-2-activated NK cells and fixed MPN3 cells were co-incubated at an E : T ratio of 2 : 1 for designated times and lysed. Immunoblotting was performed with primary antibody for phosphorylated ERK1/2 (Cell Signaling Technology, Danvers, MA). After stripping the blot, total ERK1/2 immunoblotting (Cell Signaling Technology) was also performed. The band intensities were quantified using Labwork (BioImaging Systems).
Statistics
For statistical analysis, Student’s t-test was performed using microsoft excel (Microsoft, Seattle, WA). Where P-value was < 0·05, the result was considered significant.
Results
Immortalized porcine aortic endothelial cells retained sensitivity to xenogeneic NK-mediated lysis
Porcine primary aortic endothelial cells freshly isolated ex vivo from Minnesota miniature pigs were used as a xenogeneic target to investigate NK cell-mediated immune responses. The characteristics and comparability of previously established PAEC were also explored as a substitute for primary cells. The MPN3 cell line, simian virus 40-immortalized PAEC, expresses anti-platelet endothelial cell adhesion molecule 1, the von Willebrand factor, swine leucocyte antigen class I, E-selectin and inducible swine leucocyte class II molecules, featuring similar surface phenotypes as PAEC.29 To validate the use of MPN3 cells as a target in this study, we first examined whether they retained sensitivity to NK cell-mediated lysis. Standard 51Cr-release assay using IL-2-activated human NK cells and MPN3 or primary PAEC as their targets demonstrated that both cells were efficiently lysed by human NK cells to a similar level (Fig. 1a).
Figure 1.
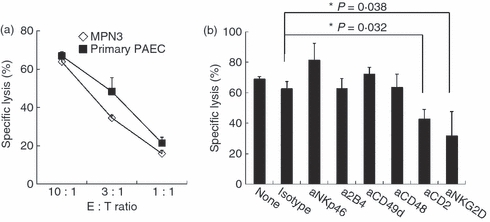
Immortalized porcine aortic endothelial cells (PAEC), MPN3 cells, retained sensitivity to xenogeneic natural killer (NK) -mediated lysis. (a) A standard 51Cr-release assay was performed using interleukin-2 (IL-2) -activated human primary NK cells as effector cells and MPN3 cells and primary PAEC as target cells. Specific lysis (%) was presented as mean + SD obtained from three to six replicates. The data shown are representatives of three independent experiments. (b) A standard 51Cr-release assay was performed with IL-2-activated human primary NK cells as effector cells and MPN3 as target cells at an effector to target (E : T) ratio of 3 : 1. NK cells were pre-incubated with 10 μg/ml designated blocking monoclonal antibodies (mAbs) or with their appropriate isotype control. Specific lysis (%) was presented as mean + SD of triplicates. The data are representatives of minimum three independent experiments. Students’t-test was performed. *P < 0·05.
To evaluate the NK-specific receptors engaged in lysing MPN3 cells, we performed a series of standard 51Cr-release assays in the presence or absence of blocking antibodies to a panel of NK surface activating receptors (Fig 1b). Among the antibodies tested (NKp46, 2B4, CD49d, CD48, CD2 and NKG2D), only anti-CD2 and anti-NKG2D antibodies were found to suppress NK-mediated lysis of MPN3 cells. Addition of anti-CD2 or anti-NKG2D mAb inhibited lysis of MPN3 cells by approximately 34% (P = 0·032) or 51% (P = 0·038), respectively. These data demonstrate that lysis of PAEC by IL-2-activated human NK cells was partially mediated by activation of CD2 and NKG2D on NK cells.
Combination of anti-CD2 and NKG2D mAbs resulted in additive suppression of xenogeneic NK cell responses
Our data provide the therapeutic potential of anti-CD2 or anti-NKG2D mAbs monotherapy following xenotransplantation, hence we propose that a combined use of both mAbs may provide better blockade of xenogeneic NK cytotoxicity. As expected, the addition of anti-CD2 and anti-NKG2D mAbs to the assay led to a synergistic decrease of NK cytotoxicity against MPN3 cells at all the ratios tested (Fig. 2a). Anti-CD2 or anti-NKG2D mAb alone at an effector to target (E : T) ratio of 10 : 1 led to 10·8% and 36·5% decrease in MPN3 lysis, respectively, whereas a combination of the two mAbs led to more than 63% reduction in NK-mediated lysis (Fig. 2a). Accordingly, MPN3 cells as well as primary PAEC expressed significant levels of messenger RNA of porcine CD58 and ULBP1, ligands of CD2 and NKG2D, respectively (Fig. 2b).
Figure 2.
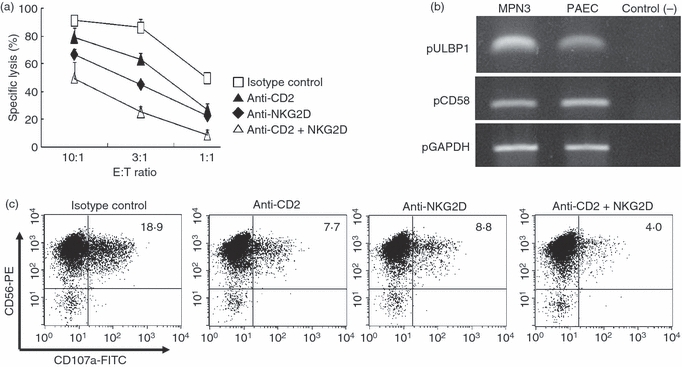
Anti-CD2 and anti-NKG2D monoclonal antibodies (mAbs) blocked natural killer (NK) cytotoxicity against MPN3. (a) A standard 51Cr-release assay was performed with interleukin-2 (IL-2) -activated human primary NK cells as effector cells and MPN3 as target cells. NK cells were pre-incubated with 10 μg/ml of designated blocking mAbs or with their isotype control. (b) Reverse transcription–polymerase chain reaction for porcine CD58 and ULBP1 genes was performed with RNAs isolated from MPN3 and primary porcine aortic endothelial cells (PAEC). The expression of messenger RNA of porcine GAPDH genes was shown as an internal control for house-keeping genes. Reaction mixture without complementary DNAs was used as negative control. (c) CD107a assay was performed to assess degranulation events, as described in the Materials and methods. IL-2-activated human primary NK cells were pre-incubated with designated mAbs before incubation with MPN3 cells. Six hours later, CD107a expression was detected using anti-CD107a mAb by fluorescence-activated cell sorting. Degranulation was measured by expression of CD107a on CD56+ NK cells. The values represent the percentages of CD56+ CD107a+ cells, which are representatives of a minimum of three independent experiments.
To clarify the mechanism for the inhibition of NK cytotoxicity by anti-CD2 and/or anti-NKG2D mAbs, we examined whether degranulation events, as measured by LAMP1 (CD107a) expression,32 were affected by antibody treatment. In the absence of blocking mAbs and in the presence of isotype controls, degranulation levels were found to be 18·9% in this particular experiment (Fig. 2c). Addition of anti-CD2 mAbs inhibited degranulation by 59% (from 18·9% to 7·7%) whereas that of anti-NKG2D mAbs reduced by 53% (to 8·8%). The combination of both mAbs led to maximal inhibition of NK degranulation down to 4·0%, a total of 79% inhibition in degranulation events (Fig. 2c). Taken together, these data demonstrate that combined treatment of anti-CD2 and anti-NKG2D mAbs conferred additive suppression of degranulation events and further contributed to impaired cytotoxicity as seen above.
Production of IFN-γ and TNF-α was inhibited by anti-CD2 and anti-NKG2D mAbs
Activated NK cells mount a dichotomous response for their effector functions; cytolysis and cytokine production. However, cytokine expression by NK cells in anti-porcine xenogeneic responses has not been clearly elucidated. We therefore examined whether NK cells secreted cytokines upon recognition of MPN3 cells and if so, the ability of anti-CD2 and anti-NKG2D mAbs to inhibit the secretion of IFN-γ and TNF-α was investigated. As shown in Fig. 3, NK cells stimulated both IFN-γ and TNF-α production upon incubation with MPN3 cells. Secretion of both cytokines was inhibited by addition of anti-CD2 or anti-NKG2D mAbs and this inhibitory effect was found to be additive. For IFN-γ, approximately 38% inhibition was achieved by anti-CD2 or anti-NKG2D mAb alone (from 12·1% to 7·5% for CD2 and to 7·4% for NKG2D) while 58% inhibition was observed for a combination of both mAbs (from 12·1% to 5·1%). By comparison, addition of anti-CD2 or anti-NKG2D mAbs reduced TNF-α production by 30% or 48%, respectively, and combined treatment of both mAbs reduced it by 70%. It is noteworthy that the effects of anti-CD2 or anti-NKG2D mAbs on cytokine secretion in activated NK cells are directly correlated with the findings observed on CD107a expression as shown in Fig. 2(c). Together, these data provide a potential benefit of combined use of anti-CD2 and anti-NKG2D mAbs for inhibiting two arms of xenogeneic NK effector functions; cytotoxicity and cytokine production.
Figure 3.
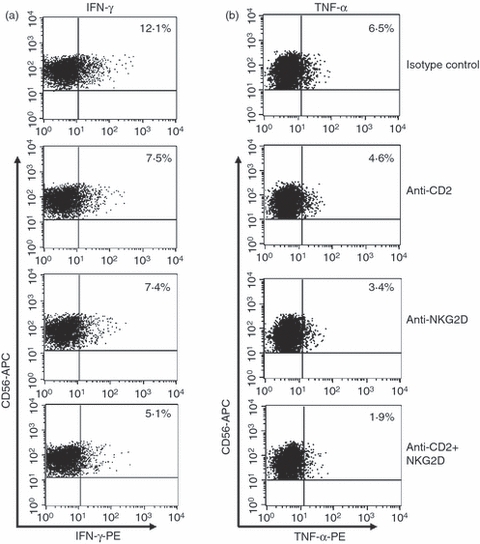
Expression level of intracellular interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) were reduced by treatment with anti-CD2 and anti-NKG2D monoclonal antibodies (mAbs). Interleukin-2 (IL-2)-activated human primary natural killer (NK) cells were pre-incubated with designated mAbs as shown on the right and then co-incubated with MPN3 cells. Intracellular IFN-γ (a) and TNF-α (b) were detected in CD56+ NK cells. The values represent percentages of CD56+ IFN-γ+ or CD56+ TNF-α+ cells, which are representative of five independent experiments.
Blocking CD2 and NKG2D inhibited NK cytotoxicity against an array of porcine primary cells
We next evaluated if the inhibitory effect observed by anti-CD2 and anti-NKG2D mAbs, alone or in combination, suppressed xenogeneic NK activity against a variety of porcine cells isolated from distinct organs. For this, PCEC and PLC along with PAEC were freshly isolated from miniature pigs. As shown in Fig. 4, single mAbs alone or the combined use of anti-CD2 and anti-NKG2D mAbs inhibited NK cytotoxicity against PCEC (Fig. 4b) and PLC (Fig. 4c) in a similar fashion as had been observed with PAEC (Fig. 4a). The inhibitory effect of these mAbs was variable among the cell types tested. Anti-NKG2D mAb was more effective in blocking PAEC killing whereas both mAbs were comparable in their ability to inhibit the lysis of PCEC and PLC. Nevertheless, the combination of both mAbs resulted in the greatest suppression of NK cytotoxicity against all targets. These data imply that the combined treatment of anti-CD2 and anti-NKG2D mAbs could be used to manage a rejection process in a variety of xenogeneic organ transplantation models.
Figure 4.
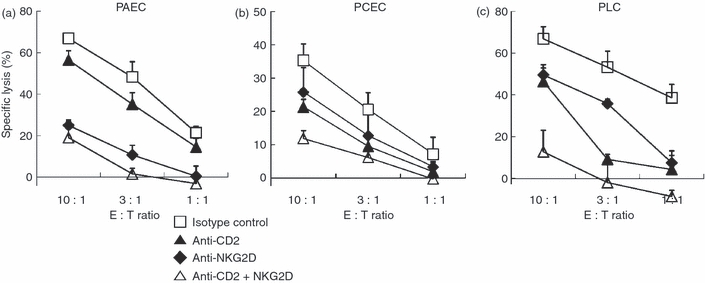
Combined use of anti-CD2 and anti-NKG2D monoclonal antibodies (mAbs) inhibited natural killer (NK) cytotoxicity against a range of porcine primary cells. A standard 51Cr-release assay was performed with interleukin-2 (IL-2) -activated human primary NK cells against porcine primary aortic endothelial cells (PAEC) (a), porcine primary cornea endothelial cells (PCEC) (b), or porcine primary liver cells (PLC) (c). Specific lysis (%) was presented as mean + SD of triplicates. The data are representatives of minimum three independent experiments.
Intracellular calcium mobilization was involved in signal transduction by CD2 and NKG2D
Defective cytotoxicity and cytokine secretion in anti-CD2 or anti-NKG2D mAb-treated NK cells imply blockade of signalling pathways following target cell recognition. As calcium flux is one of the early events leading to perforin-dependent cytolysis and degranulation,19 intracellular calcium mobilization was measured in NK cells treated with isotype control, anti-CD2, or anti-NKG2D mAb upon stimulation with MPN3 cells. As shown in Fig. 5(a), addition of mAbs against CD2 or NKG2D inhibited the elevation of intracellular calcium in NK cells exposed to MPN3 cells. Impaired calcium release in anti-CD2 or anti-NKG2D mAb-treated cells was not the result of an intrinsic defect because these cells show normal intracellular calcium concentrations, which could be released by the addition of the calcium ionophore, ionomycin. Decreased intracellular calcium in NK cells was found to be directly correlated with impaired cytotoxicity as chelating extracellular calcium with EGTA mounted dose-dependent inhibition of NK cytotoxicity against MPN3 cells (Fig. 5b). These data suggest that blocking NK activating receptors, CD2 and NKG2D, inhibits signals leading to intracellular calcium mobilization and subsequent degranulation of cytolytic molecules and cytokine production.
Figure 5.
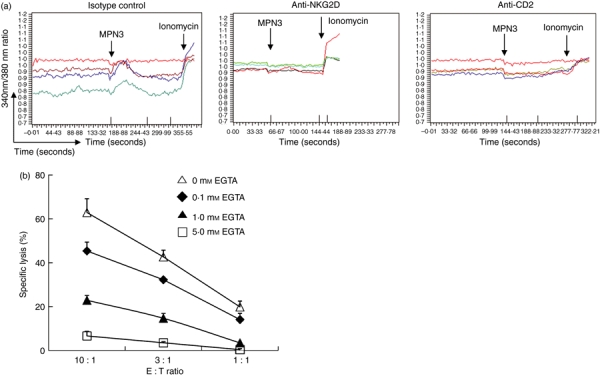
Intracellular calcium mobilization and extracellular signal-regulated kinase (ERK) activation events were involved in naturla killer (NK) cell-mediated xenoreactive cytotoxicity. (a) Interleukin-2 (IL-2) -activated human primary NK cells were pre-incubated with designated monoclonal antibodies (mAbs). Calcium flux was measured as described in the Materials and methods. MPN3 cells and ionomycin were inoculated at the times indicated by arrows. Each line represents intracellular calcium concentration from a single cell in a given microscopic field. The data are representatives of five independent experiments. (b) A standard 51Cr-release assay was performed with IL-2-activated human primary NK cells as effector cells and MPN3 cells as target cells. EGTA at designated concentrations was added to NK cells during cytotoxicity assay. Specific lysis (%) was presented as mean + SD of triplicates. The data are representatives of minimum three independent experiments.
Combination of an ERK kinase inhibitor and mAbs to CD2 and NKG2D efficiently suppresses NK cytotoxicity
Combined use of anti-CD2 and anti-NKG2D mAbs provided substantial, but not complete, inhibition of NK cytotoxicity. In NK cells, ERK have been shown to play an important role in multiple signalling pathways.18 As shown in Fig. 6(a), we found that ERK in human NK cells underwent time-dependent phosphorylation upon stimulation by MPN3 cells, reaching a plateau within 10 min. This pathway has been documented to be downstream of both NKG2D33 and CD2;34 however, it remains possible that other unidentified receptors might also use this pathway to activate NK cells to lyse xenogeneic targets. Hence, we speculate that blocking ERK activation may synergize with CD2 and NKG2D blockade to maximally inhibit NK cell responses. An ERK kinase inhibitor, PD98059, has been widely used to suppress activation of ERK downstream of various signalling receptors in NK cells.35–37 We found that PD98059 reduced NK cytotoxicity against MPN3 in a dose-dependent manner (Fig. 6b). At an E : T ratio of 10 : 1, 50 μmPD98059 inhibited NK cytotoxicity by 24% whereas 100 μmPD98059 inhibited it by 46%. These results demonstrated that ERK was involved in signalling upon stimulation by xenogeneic porcine cells and a blockade of ERK activation by PD98059 might provide additional NK suppression in xenogeneic settings. As 50 μm was shown to be a concentration for 50% ERK inhibition (IC50), and was often used in experiments in vitro, this concentration was chosen for further experiments. Cellular toxicity was observed beyond 100 μmPD98059 (data not shown).
Figure 6.
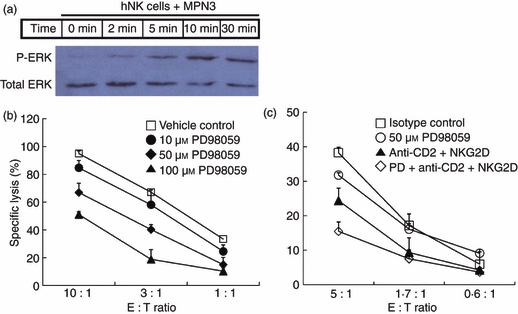
Combined use of anti-CD2 and anti-NKG2D monoclonal antibodies (mAbs) in the presence of a sub-optimal concentration of PD98059 additively suppressed natural killer (NK) cytotoxicity. (a) Western blotting was performed to assess extracellular signal-regulated kinase (ERK) phosphorylation upon stimulation with MPN3. Interleukin-2 (IL-2) -activated human primary NK cells were co-incubated with fixed MPN3 and cells were harvested at designated times for lysis. Upper bands represent phosphorylated ERK1/2 and lower bands represent total ERK1/2 proteins. (b) A standard 51Cr-release assay was performed with IL-2-activated human primary NK cells as effector cells and MPN3 as target cells. NK cells were pre-incubated with PD98059 at designated concentrations for 30 min before the assay. Specific lysis (%) was presented as mean + SD of triplicates. The data are representative of five independent experiments. (c) IL-2-activated human primary NK cells were pre-incubated with 50 μmPD98059 and/or 10 μg/ml designated blocking mAb(s) for 30 min before the assay. A standard 51Cr-release assay was performed with the cells against MPN3. Specific lysis (%) was presented as mean + SD of triplicates. The data are representatives of minimum three independent experiments.
We next examined whether a combinatorial regimen including suboptimal doses of PD98059 in the presence of anti-CD2 and anti-NKG2D mAbs could provide maximum suppression of NK cytotoxicity (Fig. 6c). Blocking CD2 and NKG2D in combination resulted in 36% suppression of NK cytotoxicity. Addition of PD98059 increased the level of suppression by 60% (at an E : T ratio of 5 : 1) under conditions where single use of a suboptimal concentration of PD98059 reduced NK cytotoxicity by only 17%. It should be pointed out that there still remained residual NK cytotoxicity even after triple treatment, suggesting that other ERK-independent NK cell receptors might be involved in xenoreactivity of NK cells. These data suggest that PD98059 may target residual ERK activation following blockade of CD2 and NKG2D signalling to lead to maximal suppression of xenogeneic NK responses.
Discussion
Porcine xenogeneic grafts in humans that survive beyond the period of hyperacute rejection are subject to NK cell-mediated immune destruction. The NK cells mediate acute rejection processes by directly recognizing and destroying the xenogeneic graft. This study was designed to understand the role of each NK surface receptor in lysing porcine grafts and to provide a molecular basis to develop an effective and safe combinatorial immunosuppressive regimen targeted at human NK cells. As expected, human NK cells exhibited significant reactivity against porcine cells, supporting the crucial role of NK cells as a potent mediator of organ rejection in xenotransplantation. Adhesion of human NK cells to porcine endothelial cells was shown to be mainly mediated by the binding of CD49d on NK cells with CD106 on porcine cells;21 however, our data showed that this adhesion did not appear to be critical for NK cytotoxicity. Neither blocking homotypic 2B4/CD48 interactions among NK cells by anti-2B4 or anti-CD48 mAbs nor blocking NKp46 activation by anti-NKp46 mAbs affected NK cytotoxicity, suggesting that these receptors were not involved in lysing porcine cells. In contrast, CD2 and NKG2D were found to play an important role in lysing porcine cells. Combined use of anti-CD2 and anti-NKG2D mAbs profoundly blocked NK cytotoxicity against porcine primary cells from various organs: aorta, cornea and liver. Interestingly, the effect of anti-CD2 or anti-NKG2D mAb alone varied depending on target cell types; for PAEC, anti-NKG2D mAb was more effective but for PCEC and PLC, both anti-CD2 and anti-NKG2D mAbs exhibited comparable levels of inhibition. This difference might be the result of varying levels of NK ligands on different cell types, some of which could be subject to up-regulation upon stimulation. For instance, porcine CD80 expression was induced in mixed culture with human antigen-presenting cells.38 Our data imply that expression of NK ligands may be screened for each tissue and/or organ type to be transplanted, allowing the optimal combination of blocking mAbs to prevent the xenogeneic NK-mediated rejection process.
An ERK kinase inhibitor, PD98059 was also a potent inhibitor of NK cytotoxicity against PAEC. In human NK cells, the NKG2D signal was shown to be transduced to phospholipase Cγ2 and PI3K via DAP10, an adaptor molecule.39,40 Phospholipase Cγ2 mediates signals for calcium mobilization and subsequent degranulation events19 that were shown to be reduced by blocking NKG2D in this study. The PI3K signalling leads to the MEK-ERK activation pathway.18 Of note, CD2 signalling has been shown to lead to linker for activation of T-cell (LAT) phosphorylation in T cells, which is required for ERK activation.34 Although it is not known whether such signalling occurs in NK cells, it is tempting to speculate that CD2 signalling activates ERK. Taken together, the signalling pathway from NKG2D and CD2 appears to employ activation of phospholipase Cγ2 and be converged at the site of ERK activation. Considering that PD98059 had an additive effect to the combination of anti-CD2 and anti-NKG2D mAbs, other NK cell receptors leading to ERK activation might also be involved in xenoreactivity of NK cells.
At present, there have been no published reports suggesting the use of an ERK kinase inhibitor as an immunosuppressant following xenotransplantation, although a recent report showed that allograft survival was prolonged by treatment of mice with PD98059.41 Because ERK is involved in a variety of essential cellular functions, e.g. proliferation, growth, migration, cell death,42 one of the concerns is severe side-effects. However, a recently developed highly selective inhibitor of ERK kinases, CI-1040 (PD184352), has been shown to be well tolerated in both phase I and II clinical trials in cancer patients.43 Although CI-1040 did not significantly exert sufficient anti-tumour activity in these patients, we speculate that it might serve as an effective immunosuppressant, especially in combination with other NK-blocking mAbs. In addition, blocking CD2–CD58 interaction by Alefacept, a human CD58-immunoglobulin G1 fusion protein, is already being administered in clinics for the treatment of psoriasis. Interestingly, Alefacept has been shown to delay rejection in non-human primate cardiac and renal transplantation and a phase II clinical trial is currently underway in kidney transplant recipients.44,45 Therefore, combinational therapy of an ERK inhibitor with Alefacept and other blocking mAbs could certainly provide a promising and readily applicable immunosuppressive regimen for pig-to-human graft transplantation.
The role of cytokines produced by NK cells in a xenogeneic transplantation model has not been clearly elucidated. The fact that blocking CD2 and/or NKG2D signalling by their specific mAbs reduced cytokine production suggests that NK cells might be even more effective in preventing rejection in vivo as less cytokine, e.g. IFN-γ, could inefficiently stimulate other cell types, for example dendritic cells (DCs) and T cells. NK cells can kill immature DCs to promote cross-presentation of antigens by mature DCs.46 Although the role of DCs in xenotransplantation is poorly investigated, T cells were shown to play an important role in the rejection of porcine pancreatic islet xenotransplantation in primates.47,48 Because NK cells could influence T-cell activation and differentiation processes via cytokine production and activating DCs, blocking NK cell activation would lead to dampening T-cell responses and, if any, possibly preventing Th1 immune responses. Inhibition of ERK has also been shown to promote Th2 differentiation by up-regulating IL-4 in a cardiac allograft model.41 In this context, in vivo administration of an ERK inhibitor in the presence of anti-CD2 and anti-NKG2D mAbs may not only inhibit antigen-specific T-cell activation but also diminish Th1 responses by promoting Th2 responses.
Recently combination of anti-NKG2D and anti-NKp44 mAbs has been shown to offer a great therapeutic regimen for NK suppression in xenotransplantation.26 We also found that anti-NKp44 mAbs suppressed IL-2-activated NK cell cytotoxicity against porcine cells (Supplementary Fig. S1). However, resting NK cells do not express NKp44, whose expression undergoes up-regulation upon activation.49 Therefore, blocking NKp44 signalling at the beginning of transplantation may not be relevant for inhibiting activation of resting NK cells. In contrast, both CD2 and NKG2D are expressed on all resting NK cells, so blocking CD2 and NKG2D may provide better outcome in transplantation. It is important to point out that CD2 and NKG2D are also expressed in other cell types, notably in T cells, so the effect of these mAbs can be extended to inhibit T-cell activation as well. In conclusion, our data reveal the critical role of CD2 and NKG2D on human NK cells reacting against porcine cells. Therefore, combinatorial treatment with anti-CD2 mAb, anti-NKG2D mAb and an ERK inhibitor is likely to provide a potent and safe immunosuppressive regimen to prevent acute and cellular xenogeneic graft rejection in vivo by blocking NK cell functions and their subsequent cellular interactions.
Acknowledgments
The authors are thankful to Professor Chung-Gyu Park at Seoul National University College of Medicine for providing primary porcine cells. This study was supported by a grant from KICOS through the Korean Ministry of Science & Technology (K20704000007-09A0500-00710 and K20601000002-09E0100-00210), the Innovative Research Institute for Cell Therapy (A062260) and Ministry of Health & Welfare (A050331), and the National Nuclear R&D program of the Ministry of Science and Technology of Korea (Grant BAERI).
Glossary
Abbreviations:
- APC
allophycocyanin
- ERK
extracellular signal-regulated kinase
- FITC
fluorescein isothiocyanate
- IFN
interferon
- IL-2
interleukin-2
- mAb
monoclonal antibody
- MEK
mitogen-activated protein kinase/extracellular signal-regulated kinase
- MHC
major histocompatibility complex
- NK
natural killer
- PAEC
porcine aortic endothelial cells
- PCEC
porcine cornea endothelial cells
- PCR
polymerase chain reaction
- PE
phycoerythrin
- PI3K
phosphatidyl inositol 3 kinase
- PLC
porcine liver cells
- TNF
tumour necrosis factor
- ULBP1
UL16-binding protein 1
- VCAM
vascular cell adhesion molecule
Disclosures
The authors have no financial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Combined use of anti-CD2, anti-NKG2D, and anti-NKp46 mAbs in the presence of suboptimal concentration of PD98059 efficiently suppressed NK cytotoxicity. IL-2-activated human primary NK cells were pre-incubated with 50 mM PD98059 and/or 10 mg/ml designated blocking mAb(s) as shown in the right for 30 min before assays. A standard Cr release assay was performed with the cells against MPN3. Specific lysis (%) was presented as mean + SD of triplicates. The data are representatives of minimum three independent experiments.
Figure S2. PD98059 reduced natural cytotoxicity. IL-2 activated human primary NK cells were pre-incubated with designated concentrations of PD98059 or ethanol as vehicle control. A standard Cr release assay was performed with K562 (human caucasian chronic myelogenous leukaemia cell line; ATCC, Manassas, VA) as target cells at an E:T ratio of 3:1. Specific Lysis (%) was presented as mean + SD of triplicates. The data are representatives of minimum three independent experiments.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–31. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]
- 2.Gourlay WA, Chambers WH, Monaco AP, Maki T. Importance of natural killer cells in the rejection of hamster skin xenografts. Transplantation. 1998;65:727–34. doi: 10.1097/00007890-199803150-00021. [DOI] [PubMed] [Google Scholar]
- 3.Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12:181–8. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mohanna F, Collison K, Parhar R, et al. Activation of naive xenogeneic but not allogeneic endothelial cells by human naive neutrophils: a potential occult barrier to xenotransplantation. Am J Pathol. 1997;151:111–20. [PMC free article] [PubMed] [Google Scholar]
- 5.Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–5. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 6.Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–92. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–47. doi: 10.1146/annurev.med.53.082901.103900. [DOI] [PubMed] [Google Scholar]
- 8.Watier H, Guillaumin JM, Vallee I, Thibault G, Gruel Y, Lebranchu Y, Bardos P, et al. Human NK cell-mediated direct and IgG-dependent cytotoxicity against xenogeneic porcine endothelial cells. Transpl Immunol. 1996;4:293–9. doi: 10.1016/s0966-3274(96)80050-5. [DOI] [PubMed] [Google Scholar]
- 9.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 10.Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–8. doi: 10.1126/science.7716543. [DOI] [PubMed] [Google Scholar]
- 11.Seebach JD, Comrack C, Germana S, Leguern C, Sachs DH, Dersimonian H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997;159:3655–61. [PubMed] [Google Scholar]
- 12.Crew MD, Cannon MJ, Phanavanh B, Garcia-Borges CN. An HLA-E single chain trimer inhibits human NK cell reactivity towards porcine cells. Mol Immunol. 2005;42:1205–14. doi: 10.1016/j.molimm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Forte P, Baumann BC, Weiss EH, Seebach JD. HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am J Transplant. 2005;5:2085–93. doi: 10.1111/j.1600-6143.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 14.Lilienfeld BG, Crew MD, Forte P, Baumann BC, Seebach JD. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation. 2007;14:126–34. doi: 10.1111/j.1399-3089.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 15.Zeng MH, Fang CY, Wang SS, et al. A study of soluble HLA-G1 protecting porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Transplant Proc. 2006;38:3312–4. doi: 10.1016/j.transproceed.2006.10.179. [DOI] [PubMed] [Google Scholar]
- 16.Weiss EH, Lilienfeld BG, Muller S, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87:35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 17.Forte P, Baumann BC, Schneider MK, Seebach JD. HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a+ human NK cells. Xenotransplantation. 2009;16:19–26. doi: 10.1111/j.1399-3089.2009.00510.x. [DOI] [PubMed] [Google Scholar]
- 18.Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–25. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 19.Caraux A, Kim N, Bell SE, et al. Phospholipase C-gamma2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood. 2006;107:994–1002. doi: 10.1182/blood-2005-06-2428. [DOI] [PubMed] [Google Scholar]
- 20.Suck G, Branch DR, Smyth MJ, Miller RG, Vergidis J, Fahim S, Keating A. KHYG-1, a model for the study of enhanced natural killer cell cytotoxicity. Exp Hematol. 2005;33:1160–71. doi: 10.1016/j.exphem.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Schneider MK, Strasser M, Gilli UO, Kocher M, Moser R, Seebach JD. Rolling adhesion of human NK cells to porcine endothelial cells mainly relies on CD49d–CD106 interactions. Transplantation. 2002;73:789–96. doi: 10.1097/00007890-200203150-00023. [DOI] [PubMed] [Google Scholar]
- 22.Johnson CM, Helgeson SC. Glycoproteins synthesized by cultured cardiac valve endothelial cells: unique absence of fibronectin production. Biochem Biophys Res Commun. 1988;153:46–50. doi: 10.1016/s0006-291x(88)81187-2. [DOI] [PubMed] [Google Scholar]
- 23.Bourges D, Meurens F, Berri M, et al. New insights into the dual recruitment of IgA+ B cells in the developing mammary gland. Mol Immunol. 2008;45:3354–62. doi: 10.1016/j.molimm.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Brossay A, Hube F, Moreau T, Bardos P, Watier H. Porcine CD58: cDNA cloning and molecular dissection of the porcine CD58–human CD2 interface. Biochem Biophys Res Commun. 2003;309:992–8. doi: 10.1016/j.bbrc.2003.08.099. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Borges CN, Phanavanh B, Saraswati S, Dennis RA, Crew MD. Molecular cloning and characterization of a porcine UL16 binding protein (ULBP)-like cDNA. Mol Immunol. 2005;42:665–71. doi: 10.1016/j.molimm.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Forte P, Lilienfeld BG, Baumann BC, Seebach JD. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J Immunol. 2005;175:5463–70. doi: 10.4049/jimmunol.175.8.5463. [DOI] [PubMed] [Google Scholar]
- 27.Mcnerney ME, Lee KM, Kumar V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol Immunol. 2005;42:489–94. doi: 10.1016/j.molimm.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Poggi A, Massaro AM, Negrini S, Contini P, Zocchi MR. Tumor-induced apoptosis of human IL-2-activated NK cells: role of natural cytotoxicity receptors. J Immunol. 2005;174:2653–60. doi: 10.4049/jimmunol.174.5.2653. [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Kim JY, Koh HS, et al. Establishment and characterization of endothelial cell lines from the aorta of miniature pig for the study of xenotransplantation. Cell Biol Int. 2005;29:638–46. doi: 10.1016/j.cellbi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Dettmers A, Rempel WE. Minnesota’s miniature pigs. Lab Anim Care. 1968;18:104–9. [PubMed] [Google Scholar]
- 31.Yoon SH, Yun SO, Park JY, Won HY, Kim EK, Sohn HJ, Cho HI, Kim TG, et al. Selective addition of CXCR3+ CCR4− CD4+ Th1 cells enhances generation of cytotoxic T cells by dendritic cells in vitro. Exp Mol Med. 2009;41:161–70. doi: 10.3858/emm.2009.41.3.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martelli MP, Lin H, Zhang W, Samelson LE, Bierer BE. Signaling via LAT (linker for T-cell activation) and Syk/ZAP70 is required for ERK activation and NFAT transcriptional activation following CD2 stimulation. Blood. 2000;96:2181–90. [PubMed] [Google Scholar]
- 35.Trotta R, Puorro KA, Paroli M, Azzoni L, Abebe B, Eisenlohr LC, Perussia B. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases. J Immunol. 1998;161:6648–56. [PubMed] [Google Scholar]
- 36.Mainiero F, Gismondi A, Soriani A, et al. Integrin-mediated ras-extracellular regulated kinase (ERK) signaling regulates interferon gamma production in human natural killer cells. J Exp Med. 1998;188:1267–75. doi: 10.1084/jem.188.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milella M, Gismondi A, Roncaioli P, Bisogno L, Palmieri G, Frati L, Cifone MG, Santoni A. CD16 cross-linking induces both secretory and extracellular signal-regulated kinase (ERK)-dependent cytosolic phospholipase A2 (PLA2) activity in human natural killer cells: involvement of ERK, but not PLA2, in CD16-triggered granule exocytosis. J Immunol. 1997;158:3148–54. [PubMed] [Google Scholar]
- 38.Brossay A, Harang S, Herault O, Bardos P, Watier H. The active role played by xenogeneic endothelial cells in the indirect presentation pathway is not lymphocyte trans-co-stimulation. Transpl Int. 2005;17:787–94. doi: 10.1007/s00147-004-0773-9. [DOI] [PubMed] [Google Scholar]
- 39.Segovis CM, Schoon RA, Dick CJ, Nacusi LP, Leibson PJ, Billadeau DD. PI3K links NKG2D signaling to a CrkL pathway involved in natural killer cell adhesion, polarity, and granule secretion. J Immunol. 2009;182:6933–42. doi: 10.4049/jimmunol.0803840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upshaw JL, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. The isoforms of phospholipase C-gamma are differentially used by distinct human NK activating receptors. J Immunol. 2005;175:213–8. doi: 10.4049/jimmunol.175.1.213. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Guan Q, Diao H, Lian D, Zhong R, Jevnikar AM, Du C. Prolongation of cardiac allograft survival by inhibition of ERK1/2 signaling in a mouse model. Transplantation. 2007;83:323–32. doi: 10.1097/01.tp.0000251374.49225.19. [DOI] [PubMed] [Google Scholar]
- 42.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009;8:1168–75. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–62. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 44.Vincenti F, Kirk AD. What’s next in the pipeline. Am J Transplant. 2008;8:1972–81. doi: 10.1111/j.1600-6143.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 45.Weaver TA, Charafeddine AH, Agarwal A, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15:746–9. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 47.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 48.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–3. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 49.Vitale M, Bottino C, Sivori S, et al. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–72. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


