Abstract
Histamine controls the function of dendritic cells (DCs). It appears to be required for the normal development of DCs. It also induces the chemotaxis of immature DCs and promotes the differentiation of CD4+ T cells into cells with a T helper type 2 (Th2) profile. Moreover, we have recently shown that histamine stimulates both the uptake and the cross-presentation of antigens by DCs, supporting the theory that histamine promotes activation of CD8+ T cells during the development of allergic pathologies. Here, we investigated whether the course of an allergic response, in a well-defined model of ovalbumin (OVA)-induced allergic airway inflammation, could be modulated by intratracheal injection of OVA-pulsed DCs previously treated with histamine (DCHISs). Compared with control DCs, DCHISs induced: (i) greater recruitment of CD8+ T cells in the lung, (ii) greater stimulation of the production of interleukin (IL)-5 by lung CD8+ T cells, and (iii) increased recruitment of CD11c/CD8 double-positive DCs in the lungs of allergic mice. Moreover, mice treated with DCHISs showed increased levels of serum-specific immunoglobulin E (IgE) antibodies directed to OVA, and a higher proportion of eosinophils in bronchoalveolar lavage (BAL) compared with mice treated with OVA-pulsed control DCs. Our results support the notion that histamine, by acting on DCs, increases the severity of allergic processes.
Keywords: allergy, dendritic cells, histamine, interleukin-5, CD8+ T lymphocytes
Introduction
Dendritic cells (DCs) have the unique ability to activate resting T lymphocytes and play a critical role not only in the priming of adaptive immune responses, but also in the induction of self-tolerance.1,2 Upon stimulation by inflammatory stimuli or pathogens in the periphery, DCs undergo a number of changes, leading to their maturation.3 Mature DCs activate naïve T cells and direct the differentiation of CD4+ T cells into cells with distinct profiles.1–4
Histamine (HIS) plays an important role in the development of lung inflammation during the course of allergic processes by inducing airway constriction, mucus secretion and recruitment of immune cells.5,6 Histamine is involved in the regulation of DC function. It stimulates the chemotaxis of immature DCs,7,8 increases the ability of DCs to induce the differentiation of CD4+ T cells into cells with a T helper type 2 (Th2) profile,9 and induces the cross-presentation of antigens by DCs through major histocompatibility complex (MHC) class I,10 supporting the theory that histamine plays a role in the activation of CD8+ T cells in response to allergens.
Adoptive transfer of allergen-pulsed DCs is a useful tool with which to examine the role of DCs in the course of allergic lung inflammation.11,12 It has been shown that injection of antigen-pulsed DCs into the airways leads to sensitization to inhaled antigen and to the development of antigen-induced airway eosinophilia.12–14 Moreover, modulation of the functional profile of DCs has been shown to be able to regulate the course of allergic inflammation. Administration of ovalbumin (OVA)-pulsed DCs treated with bacillus Calmette–Guérin (BCG) in allergic mice reduced eosinophilic airway inflammation, airway hyperreactivity (AHR) and mucus production while enhancing the accumulation of regulatory Foxp3(+) T cells.15 Administration of interleukin (IL)-10-treated DCs markedly suppressed the development of AHR, inflammation, and Th2 cytokine production.16 Similarly, activation of DCs with antibodies directed to a member of the family of B7 costimulatory molecules PD-1 costimulatory molecule ligand ex vivo before adoptive transfer into pre-sensitized mice was shown to be sufficient to protect animals from inflammatory lung disease induced by subsequent repeated airway exposure to the offending antigen.17 In this study, we investigated whether transfer of histamine-treated allergen-pulsed DCs changed the course of the allergic response, in a well-defined model of OVA-induced allergic airway inflammation.18
Materials and methods
Mice
All experiments were carried out using 2-month-old virgin female BALB/c mice raised at the National Academy of Medicine, Buenos Aires, Argentina. Mice were housed six per cage and kept at 20 ± 2° under an automatic 12 hr light/dark schedule. Animal care was in accordance with institutional guidelines.
Sensitization and challenge of mice with OVA
Mice were sensitized using a standard protocol, as described previously.18 Briefly, mice were injected intraperitoneally (i.p.) with 20 μg of OVA (grade V; Sigma-Aldrich, Sigma, San Louis, MO) in 2 mg of aluminium hydroxide (alum) at days 0 and 7. Control mice received a saline injection instead of OVA/alum solution. On day 14, sensitized mice were challenged intranasally with 50 μl of phosphate-buffered saline (PBS) containing 3% OVA for 5 days. Control mice were instillated with PBS.
DC generation from bone marrow cultures
The procedure used to obtain DCs was as described by Inaba et al.,19 with minor modifications.20 Briefly, bone marrow was flushed from the long bones of the limbs using 2 ml of RPMI-1640 (Invitrogen, Carlsbad, CA) with a syringe and 25-gauge needle. Red cells were lysed with ammonium chloride. After washing, cells were suspended at a concentration of 1·5 × 106 cells/ml in 70% RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 5·5 × 10−5 mercaptoethanol (Sigma, San Louis, MO) (complete medium) and 30% J588-GM cell line supernatant. The cultures were fed every 2 days by gently swirling the plates, aspirating 50% of the medium, and adding fresh medium with J588-GM cell line supernatant. At day 9 of the culture, > 90% of the harvested cells expressed MHC class II, CD40 and CD11c, but not Gr-1 (not shown).
Intratracheal injection of DCs
DCs obtained from bone marrow precursors were incubated in the absence or presence of histamine (1 μm) (DCs and DCHISs, respectively) for 30 min at 37°. Cells were then incubated for 3 hr at 37° in the presence or absence of OVA (100 μg/ml). Finally, DCs were washed and injected intratracheally (i.t.) into BALB/C mice after intranasal challenge of sensitized mice with OVA. For this purpose, mice were anaesthetized with embuthal (2% v/v in PBS), and 100 μl of PBS, DCs or DCHISs (5 × 105 cells) was injected.
Treatment of lung tissues to obtain a cell suspension
Lungs were cut into small pieces and treated with Type I collagenase (250 U/ml) (Roche; Bs.As., Argentina) for 30 min at 37°. At the end of the incubation time, the reaction was stopped by the addition of PBS supplemented with 5% FCS. Subsequently, the fragments were incubated with DNase I (50 U/ml) (Invitrogen) for 40 min at 37°. Finally, the cell suspensions were collected through a gauze mesh and washed with cold PBS.
Migration of DCs
DCs were labelled with carboxyfluorescein succinimidyl ester (CFSE; 5 μm) for 40 min at 37°. Cells were extensively washed and re-suspended in PBS. DCs (1 × 106) were injected i.t. into BALB/c mice. Six hours later, lung tissues were collected and processed as described above. The presence of CFSE-labelled DCs in the lung suspensions was analysed by flow cytometry.
Bronchoalveolar lavage
A week after the treatment of allergic mice with PBS, DCs or DCHISs, lungs were washed via a tracheal tube with PBS. Cells were washed and leucocyte counts were determined by optical microscopy. Cytospin slides were stained with toluidine to determine the percentages of eosinophils.
Flow cytometry
Cell staining was performed using the following monoclonal antibodies (mAbs): anti-CD11c, anti-CD8α, anti-CD4, anti-CD8, anti-CD11b and anti-GR1 [conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or peridinin chrorophyl protein complex] (BD Pharmingen, San Diego, CA). The data were collected using a FACSCalibur (Bs.As., Argentina) flow cytometer and analysed using the CellQuest program (BD Biosciences; Bs.As., Argentina).
Analysis of serum levels of IgE antibodies directed to OVA
Serum samples were obtained from mice at the end of experiments by cardiac puncture. OVA-specific IgE antibodies were detected using plates coated overnight with 1 μg/ml OVA in sodium carbonate buffer (pH 9·5; Sigma-Aldrich). Plates were treated with Tween 0·5% in PBS (TPBS) supplemented with 1% bovine serum albumin (BSA) for 2 hr at room temperature. Serial dilutions of sera were added and, after 2 hr, the plates were washed three times with TPBS and an appropriate dilution of biotinylated detection antibody (rat anti-mouse IgE; BD Pharmingen) was added for 1 hr. After the plates had been washed, the enzyme avidine peroxidase (eBiosciences; San Diego, CA) was added for 20 min. 3,3′,5,5′-tetramethylbenzidine (TMB) was used as a substrate. Absorbance was measured at 450 nm.
Cell purification
T cells and DCs were purified from lung cell suspensions using an autoMACS separator in accordance with the manufacturer’s protocols (Miltenyi Biotec; Bergisch Gladbach, Germany). DCs and T cells were purified by positive selection using magnetic beads coupled to anti-CD11c and anti-CD3 antibodies, respectively.
Intracellular cytokine staining
Purified T cells from lungs were stimulated for 18 hr with OVA (10 ng/ml) in the presence of brefeldin A (10 μg/ml). Cells were stained for cell surface markers with FITC-conjugated anti-CD4 or CD8 antibodies (BD Pharmingen). After washing, cells were fixed in 4% paraformaldehyde and permeabilized with saponin (0·1% in PBS). Permeabilized cells were incubated with PE-conjugated antibodies directed to IL-4, IL-13, interferon (IFN)-γ, IL-5 (BD Pharmingen) or isotype-mached control antibodies for 30 min. The stained cells were washed with saponin buffer twice, suspended in isoflow, and analysed by flow cytometry.
Leukotriene B4 (LTB4) production
Production of LTB4 was analysed in the supernatants of CD11c+ cells purified from the lungs (1·5 × 105 cells/200 μl cultured for 18 hr) by enzyme-linked immunosorbent assay (ELISA) (IBL Internat.; IBL-America Minniapolis, MN).
Statistical analysis
Differences between means were analysed using Student’s t-test, and values of P< 0·05 were considered to indicate statistical significance. All calculations were performed with GraphPad Prism 4 for Windows (GraphPad Software; La Jolla, CA).
Results
Airway inflammation was induced in BALB/c mice by i.p. administration of OVA followed by challenge with aerosolized OVA, as described in the Materials and Methods. Control mice were challenged with saline instead of OVA. Five days after the challenge with aerosolized OVA, we collected the BAL to confirm the development of the allergic process. This was confirmed by the high number of eosinophils found in the BAL of allergic mice (4·6 ± 2·3 × 105 cells/ml; eosinophil percentage 47 ± 9%) but not in control mice (2·8 ± 1·2 × 104 cells/ml; eosinophil percentage 2·3 ± 1·9%) [mean ± standard error of the mean (SEM), n = 6, P < 0·001, for allergic versus control mice]. Also revealing the development of the allergic status, we found high levels of serum IgE antibodies directed to OVA (Fig. 1a).
Figure 1.
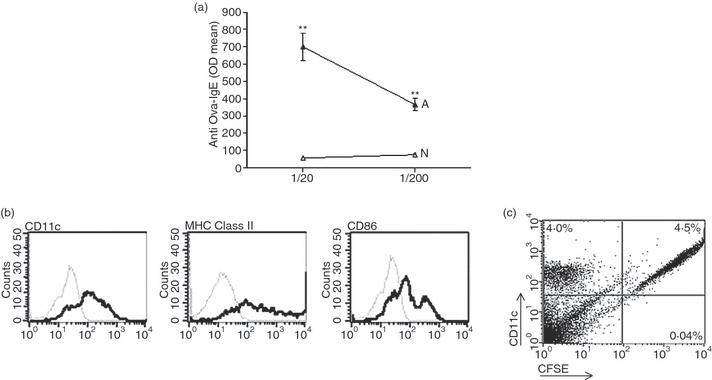
High levels of serum immunoglobulin E (IgE) antibodies directed to ovalbumin (OVA) in allergic mice. (a) BALB/c mice were inoculated intraperitoneally (i.p.) with OVA on days 0 and 7. On day 14, sensitized mice were challenged intranasally with OVA for 5 days. After 7 days, serum samples were obtained from allergized (A) or control (N) mice and the levels of IgE antibodies directed to OVA were determined by enzyme-linked immunosorbent assay (ELISA). Values are expressed as the arithmetic mean of the optical density (OD) ± standard error of the mean (SEM) (n = 6–8). Asterisks indicate statistical significance (**P ≤ 0·01) versus controls. (b) Representative histograms of the phenotypes of immature DCs obtained from bone marrow precursors. The thin line represents the isotype control. (c) Carboxyfluorescein succinimidyl ester (CFSE)-labelled DCs (1 × 106) were injected intratracheally (i.t.). After 6 hr, lungs were processed as described in the ‘Materials and methods’. Cells were labelled with phycoerythrin (PE)-conjugated antibodies directed to CD11c and analysed by flow cytometry. A representative experiment (n = 3) is shown.
DCs were differentiated from bone marrow precursors, as described in the Materials and Methods. Figure 1(b) shows the phenotype of these DCs, while Fig. 1c shows that i.t. inoculated DCs effectively arrived to lung tissues 6 hr after inoculation.
We then investigated whether i.t. inoculation of histamine-treated DCs pulsed with OVA was able to modulate lung infiltration by T cells in allergic mice. Airway inflammation was induced in BALB/c mice as described in the Materials and Methods. Histamine-treated DCs (DCHISs) were prepared by incubating DCs and histamine (1 μm) for 30 min at 37°. Then, either control DCs (DCs) or DCHISs were pulsed with OVA (100 μg/ml) for 3 hr at 37° and, after washing, they were injected i.t. into BALB/c mice 3 days after OVA challenge. Control mice were inoculated i.t. with PBS instead of DCs. Lung tissues were collected in all cases 2 weeks later. Cell suspensions were obtained from the lungs after collagenase treatment, and T cells were purified by magnetic isolation, using a monoclonal antibody directed to CD3 coupled to magnetic beads (> 80% purity). The total number of T cells purified from the lungs was similar for mice inoculated with PBS, DCs or DCHISs (not shown). Interestingly, a significant increase in the percentage of CD8+ T cells was observed in T cells purified from the lungs of DCHIS-treated mice (Fig. 2a,b) compared with T cells from mice treated with either PBS or control DCs. No changes in the percentage of CD4+ T cells were detected (Fig. 2c,d). We then analysed the pattern of cytokine production by lung CD8+ T cells. Figure 3(a,b) shows that CD8+ T cells obtained from the lungs of mice inoculated with OVA-pulsed DCHISs had a higher percentage of IL-5(+) cells compared with CD8+ T cells obtained from the lungs of mice treated with either PBS or OVA-pulsed control DCs. By contrast, no differences in the percentage of CD8+ T cells stained with antibodies directed to IFN-γ, IL-4 and IL-13 were observed.
Figure 2.
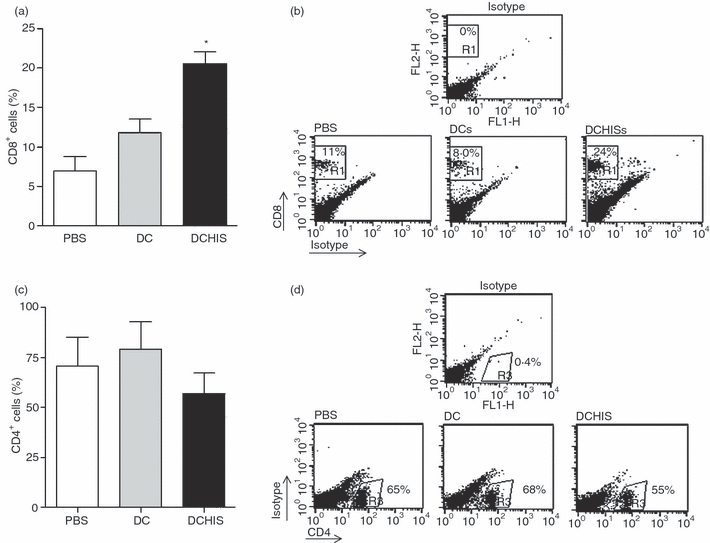
Histamine-pretreated dendritic cells (DCs) stimulate the recruitment of CD8+ T lymphocytes in the lungs of allergic mice. BALB/c mice were inoculated intraperitoneally (i.p.) with ovalbumin (OVA) on days 0 and 7. On day 14, sensitized mice were challenged intranasally with OVA for 5 days. Then, DCs obtained from bone marrow precursors, pretreated or not with 1 μm histamine (30 min at 37°) and pulsed with 100 μg/ml OVA (3 hr at 37°), were injected intratracheally (i.t.) (5 × 105 cells/mouse). Control mice were injected with phosphate-buffered saline (PBS). After 7 days, mice were killed and the lungs were processed. Lung T cells were purified using anti-CD3 antibodies coupled to magnetic beads. Purified T cells were stained with phycoerythrin (PE)-labelled anti-CD8 antibodies and fluorescein isothiocyanate (FITC)-labelled anti-CD4 antibodies. Cells were analysed by flow cytometry. (a, c) Results are expressed as the percentage of CD8+ and CD4+ T cells, respectively, and represent the mean ± standard error of the mean (SEM) for eight experiments. Asterisks indicate statistical significance (*P ≤ 0·01) versus controls (PBS). (b, d) Representative dot-plots (n = 8) are shown.
Figure 3.
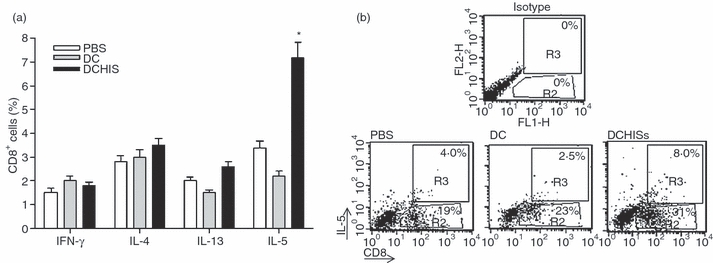
Histamine-pretreated dendritic cells (DCs) stimulate the production of interleukin (IL)-5 by a subset of CD8+ T lymphocytes recruited in the lungs of allergic mice. DCs pretreated or not with histamine (1 μm; 30 min at 37°) and pulsed with ovalbumin (OVA) (100 μg/ml; 3 hr at 37°) were intratracheally (i.t.) injected (5 × 105 cells/allergized mouse), as described in the Materials and Methods. After 7 days, mice were killed, the lungs were processed, and T cells were purified using anti-CD3 antibodies coupled to magnetic beads. T cells (2 × 105/200 μl) were then stimulated in vitro with OVA (10 ng/ml) in the presence of brefeldin (10 μg/ml) for 18 hr. The percentages of interferon (IFN)-γ-, IL-4-, IL-13-, and IL-5-positive CD8 T cells were determined by flow cytometry. In (a), data are expressed as the percentage of CD8+ T cells positive for the production of each of the cytokines analysed. Data represent the mean ± standard error of the mean (SEM) for five experiments. Asterisks indicate statistical significance (*P ≤ 0·01) versus controls (phosphate-buffered saline). In (b), a representative experiment (n = 5) is shown.
Because CD8α+ DCs have been implicated as the main DC subset for cross-presentation and cross-priming of CD8+ T cells,21–23 we investigated whether treatment of allergic mice with OVA-pulsed DCHISs also resulted in the accumulation of CD8α+ DCs in the lungs. Figure 4(a,b) shows that i.t. injection of both OVA-pulsed control DCs and OVA-pulsed DCHISs resulted in a higher proportion of CD8α+ cells in the population of CD11c+ cells. However, the proportion of lung CD8α+ cells was significantly higher (P < 0·05) for mice treated with OVA-pulsed DCHISs versus OVA-pulsed control DCs. Moreover, we found that CD11c+ cells isolated from the lungs of mice treated with DCHISs released higher levels of LTB4 compared with CD11c+ cells isolated from the lungs of mice treated with control DCs (Fig. 4c). Because LTB4 displays a potent chemotactic effect on CD8 T cells,24 this result could explain the infiltration of the lungs by CD8+ T cells found in mice treated with DCHISs.
Figure 4.
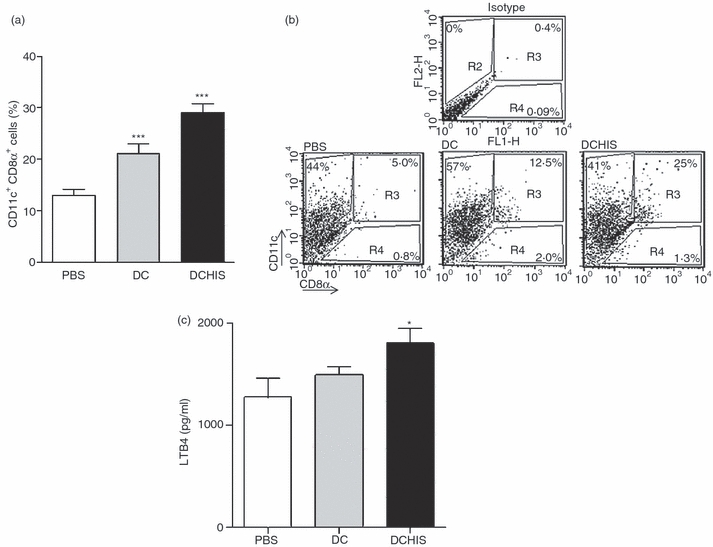
Histamine-pretreated dendritic cells (DCs) stimulate the recruitment of CD11c+ CD8 alpha+ DCs in the lungs of allergic mice and the production of Leukotriene B4 (LTB4). DCs pretreated or not with histamine and pulsed with ovalbumin (OVA) were intratracheally (i.t.) injected (5 × 105 cells/allergized mouse), as described in the Materials and Methods. After 7 days, mice were killed and the lungs were processed. Lung cell suspensions were enriched in CD11c using antibodies directed to CD11c coupled to magnetic beads. Isolated cells were analysed for the expression of CD11c and CD8α, using specific antibodies labelled with phycoerythrin (PE) and fluorescein isothiocyanate (FITC), respectively. (a) Results are expressed as the percentage of cells double-positive for CD11c and CD8α [mean ± standard error of the mean (SEM); n = 6]. (b) A dot-plot of a representative experiment is shown. (c) CD11c+ cells purified from the lungs (1·5 × 105 cells/200 μl) were cultured for 18 hr at 37° and the production of LTB4 was analysed in the supernatants by enzyme-linked immunosorbent assay (ELISA). Results are expressed in pg/ml, and represent the mean ± SEM of eight experiments. Asterisks indicate statistical significance (*P ≤ 0·01) versus controls (phosphate-buffered saline).
We finally investigated whether administration of OVA-pulsed DCs to allergic mice resulted in changes in serum levels of specific IgE antibodies or the percentages of eosinophils found in the BAL. In these experiments, OVA-pulsed DCs were injected 3 days after challenge of mice with aerosolized OVA, and BAL and serum samples were obtained 2 weeks later. Figure 5(a,b) shows that administration of OVA-pulsed DCHISs resulted in: (i) a significant increase in serum levels of specific IgE antibodies directed to OVA, and (ii) an increase of eosinophils percentages of eosinophils in BAL compared with mice treated with OVA-pulsed control DCs.
Figure 5.
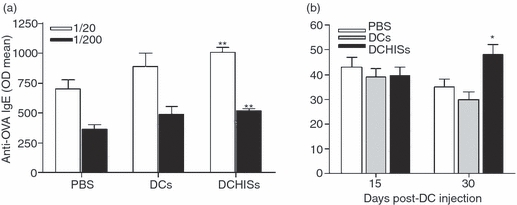
Transfer of histamine-pretreated dendritic cells (DCs) to allergic mice increases serum levels of specific immunoglobulin E (IgE) antibodies directed to ovalbumin (OVA) and leads to a more persistent infiltration of the lungs by eosinophils. DCs pretreated or not with histamine and pulsed with OVA were intratracheally (i.t.) injected (5 × 105 cells/allergized mouse), as described in the Materials and Methods. (a) After 15 days, serum samples were obtained and the levels of serum IgE antibodies directed to OVA were determined by enzyme-linked immunosorbent assay (ELISA). Data are expressed as the arithmetic mean of the optical density (OD) ± standard error of the mean (SEM) (n = 6). Asterisks indicate statistical significance (**P ≤ 0·01) versus controls [phosphate-buffered saline (PBS)]. (b) After 15 or 30 days, the percentages of eosinophils in bronchoalveolar lavage (BAL) were determined. Data are expressed as the percentage of eosinophils in BAL (arithmetic mean ± SEM; n = 6). Asterisks indicate statistical significance (*P ≤ 0·01) versus controls (PBS).
Discussion
Asthma is a complex respiratory disease characterized by persistent airway inflammation and AHR.25 Eosinophils, Th2 cells and mast cells play a critical role in asthma.26,27 These cells are recruited in the lung and upon activation they release a number of cytokines and chemokines inducing airway inflammation. In contrast to the well-defined role of Th2 cells in the induction of IgE production, eosinophilia and AHR, the role of CD8+ T cells is less well established.28,29 A number of reports, however, have shown that CD8+ T cells are essential for the development of AHR and allergic inflammation.30 An increased number of CD8+ T cells were observed in the blood and in the BAL of asthmatic patients, while animal models of airway inflammation have revealed substantial CD8+ T-cell infiltration of the bronchial mucosa after allergic sensitization.31,32 Like CD4+ T cells, CD8+ T cells can differentiate into cells with a Th2-like profile characterized by the production of IL-4, IL-5 and/or IL-10, but not IFN-γ or IL-2.33 Interestingly, in patients with asthma, it has been shown that airway-infiltrating CD8+ T cells have the ability to produce Th2 cytokines.34–36 Moreover, using the model of OVA-induced allergic airway inflammation, it was shown that CD8+ T cell-depleted mice did not develop AHR, and that this failure was associated with the inability to recruit eosinophils into the lung as a result of diminished production of IL-5.37,38
In the present study, we found that adoptive transfer of OVA-pulsed DCs previously treated with histamine to allergic mice resulted in the selective stimulation of lung infiltration by CD8+ T cells but not by CD4+ T cells. These cells did not produce IFN-γ but a subpopulation of them produced IL-5, suggesting that they had differentiated into cells with a CD8+ T-cell type 2 profile. Interestingly, these changes were associated with a significant increase in the serum levels of specific IgE antibodies directed to OVA and more persistent lung infiltration by eosinophils. This last effect could be attributable to the higher levels of IL-5 in the lungs of mice treated with DCHISs.
Histamine plays a critical role in immediate-type allergic reactions, and also modulates the function of DCs.31,39,40 Histamine inhibits IL-12 and increases IL-10 production by activated DCs, promoting the differentiation of CD4+ T cells into cells with a Th2 profile,5,8 and thus increasing the severity of atopic diseases. Histamine also induces the chemotaxis of immature DCs.7 Moreover, it has been shown that histamine is produced during the differentiation of DCs and that inhibition of histamine biosynthesis results in the impairment of DC development.41 We previously reported a new mechanism through which histamine might modulate the function of DCs.10 We found that histamine stimulates cross-presentation of soluble antigens by DCs. Thus, histamine may enhance the ability of DCs to activate CD8+ T cells. This mechanism, however, does not explain the greater ability of DCHISs to induce the recruitment of CD8+ T cells in the lung. This response could be related to the higher production of LTB4, a master chemotactic stimulus for CD8+ T cells,24,42 by DCs isolated from the lungs of allergic mice treated with DCHISs.
Our results reveal a new pathway through which histamine, via its effects on DCs, may increase the severity of allergic airway inflammation. Further experiments are required to elucidate the underlying mechanisms by which DCHISs stimulate lung infiltration by CD8+ T cells and their differentiation into cells with a type 2 profile.
Acknowledgments
We thank Beatriz Loria and Edith Mabel Horvat for their technical assistance. This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), School of Medicine, Buenos Aires University, and Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
Glossary
Abbreviations:
- DC
dendritic cell
- HIS
histamine
- MFI
mean fluorescence intensity
- Tc2
type 2 CD8+ T cell
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;32:2325–30. doi: 10.1002/eji.200939548. [DOI] [PubMed] [Google Scholar]
- 2.Worsley AG, LeibundGut-Landmann S, Slack E, Phng LK, Gerhardt H, Reis e Sousa C, MacDonald AS. Dendritic cell expression of the Notch ligand jagged2 is not essential for Th2 response induction in vivo. Eur J Immunol. 2008;38:1043–9. doi: 10.1002/eji.200737335. [DOI] [PubMed] [Google Scholar]
- 3.Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–47. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald AS, Straw AD, Dalton NM, Pearce EJ. Cutting edge: Th2 response induction by dendritic cells: a role for CD40. J Immunol. 2002;168:537–40. doi: 10.4049/jimmunol.168.2.537. [DOI] [PubMed] [Google Scholar]
- 5.Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA. Immune regulation by histamine. Curr Opin Immunol. 2002;14:735–40. doi: 10.1016/s0952-7915(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 6.Simmons FE. Allergic rhinobronchitis: the asthma-allergic rhinitis link. J Allergy Clin Immunol. 1999;104:534–40. doi: 10.1016/s0091-6749(99)70320-9. [DOI] [PubMed] [Google Scholar]
- 7.Caron G, Delneste Y, Roelandts E, et al. Histamine induces CD86 expression and chemokine production by human immature dendritic cells. J Immunol. 2001;166:6000–6. doi: 10.4049/jimmunol.166.10.6000. [DOI] [PubMed] [Google Scholar]
- 8.Schneider E, Rolli-Derkinderen M, Arock M, Dy M. Trends in histamine research: new functions during immune responses and hematopoiesis. Trends Immunol. 2002;23:255–63. doi: 10.1016/s1471-4906(02)02215-9. [DOI] [PubMed] [Google Scholar]
- 9.Sabatté J, Maggini J, Nahmod K, et al. Interplay of pathogens, cytokines and other stress signals in the regulation of dendritic cell function. Cytokine Growth Factor Rev. 2007;18:5–17. doi: 10.1016/j.cytogfr.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Amaral MM, Davio C, Ceballos A, Salamone G, Cañones C, Geffner J, Vermeulen M. Histamine improves antigen uptake and cross-presentation by dendritic cells. J Immunol. 2007;179:3425–33. doi: 10.4049/jimmunol.179.6.3425. [DOI] [PubMed] [Google Scholar]
- 11.Blank F, von Garnier C, Obregon C, Rothen-Rutishauser B, Gehr P, Nicod L. Role of dendritic cells in the lung: in vitro models, animal models and human studies. Expert Rev Respir Med. 2008;2:215–8. doi: 10.1586/17476348.2.2.215. [DOI] [PubMed] [Google Scholar]
- 12.van Rijt LS, Lambrecht BN. Dendritic cells in asthma: a function beyond sensitization. Clin Exp Allergy. 2005;35:1125–34. doi: 10.1111/j.1365-2222.2005.02321.x. [DOI] [PubMed] [Google Scholar]
- 13.Hammad H, Lambecht BN. Recent progress in the biology of airway dendritic cells and implications for understanting the regulation of asthmatic inflammation. J Allergy Clin Immunol. 2006;118:331–6. doi: 10.1016/j.jaci.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- 15.Ahrens B, Grüber C, Rha RD, et al. BCG priming of dendritic cells enhances T regulatory and Th1 function and suppresses allergen-induced Th2 function in vitro and in vivo. Int Arch Allergy Immunol. 2009;150:210–20. doi: 10.1159/000222673. [DOI] [PubMed] [Google Scholar]
- 16.Bianco NR, Kim SH, Morelli AE, Robbins PD. Modulation of the immune response using dendritic cell-derived exosomes. Methods Mol Biol. 2007;380:443–55. doi: 10.1007/978-1-59745-395-0_28. [DOI] [PubMed] [Google Scholar]
- 17.Ruffner MA, Kim SH, Bianco NR, Francisco LM, Sharpe AH, Robbins PD. B7-1/2, but not PD-L1/2 molecules, are required on IL-10-treated tolerogenic DC and DC-derived exosomes for in vivo function. Eur J Immunol. 2009;39:3084–90. doi: 10.1002/eji.200939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd CM, Gonzalo JA, Coyle AJ, Gutierrez-Ramos JC. Mouse models of allergic airway disease. Adv Immunol. 2001;77:263–95. doi: 10.1016/s0065-2776(01)77019-8. [DOI] [PubMed] [Google Scholar]
- 19.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Maramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermeulen M, Giordano M, Trevani AS, et al. Acidosis improves uptake of antigens and MHC I-restricted presentation by dendritic cells. J Immunol. 2004;172:3196–204. doi: 10.4049/jimmunol.172.5.3196. [DOI] [PubMed] [Google Scholar]
- 21.Hochrein H, Shortmen K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–55. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 22.den Hann JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CM, Belz GT, Wilson NS, Villadangos JA, Shortman K, Carbone FR, Heath WR. Cutting edge: conventional CD8(+) alpha dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2003;172:5327–40. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 24.Miyahara N, Miyahara S, Takeda K, Gelfand EW. Role of the LTB4/BLT1 pathway in allergen-induced airway hyperresponsiveness and inflammation. Allergol Int. 2006;55:91–7. doi: 10.2332/allergolint.55.91. [DOI] [PubMed] [Google Scholar]
- 25.Cohn L, Elías JA, Chupp GL. Asthma: mechanism of disease, persistent and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 26.Rogmanani S. Regulation of the development of type-2 T-helper cells in allergy. Curr Opin Immunol. 1994;6:838–46. doi: 10.1016/0952-7915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 27.Pisi G, Olivieri D, Chetta A. The airway neurogenic inflammation: clinical and pharmacological implications. Inflamm Allergy Drug Targets. 2009;8:176–81. doi: 10.2174/187152809788681047. [DOI] [PubMed] [Google Scholar]
- 28.Coyle AJ, Bertrand C, Tsuyuki S, Pircher H, Walti S, Le Gros G, Erard F. IL-4 differentiates naive CD8 T cells to a ‘‘Th2-like’’ phenotype: a link between viral infections and bronchial asthma. Ann N Y Acad Sci. 1996;796:97–103. doi: 10.1111/j.1749-6632.1996.tb32571.x. [DOI] [PubMed] [Google Scholar]
- 29.Kemp RA, Bäckström BT, Ronchese F. The phenotype of type 1 and type 2 CD8(+) T cells activated in vitro is affected by culture conditions and correlates with effector activity. Immunology. 2005;115:315–24. doi: 10.1111/j.1365-2567.2005.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells JW, Cowled CJ, Giorgini A, Kemeny DM, Noble A. Regulation of allergic airway inflammation by class I-restricted allergen presentation and CD8 T-cell infiltration. J Allergy Clin Immunol. 2007;119:226–34. doi: 10.1016/j.jaci.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Jarjour NN, Calhoun WJ, Scwartz LB, Busse WW. Elevated bronchoalveolar lavage fluid histamine levels in allergic asthmatics are associated with increased airway obstruction. Am Rev Respir Dis. 1991;144:83–7. doi: 10.1164/ajrccm/144.1.83. [DOI] [PubMed] [Google Scholar]
- 32.Baena-Cagnani CE, Berger WE, DuBuske LM, Gurne SE, Stryszak P, Lorber R, Danzig M. Comparative effects of desloratine versus montelukast on asthma symptoms and use of beta 2-agonist in patients with seasonal allergic rhinitis and asthma. Int Arch Allergy Immunol. 2003;130:307–13. doi: 10.1159/000070218. [DOI] [PubMed] [Google Scholar]
- 33.Ying S, Humbert M, Corrigan J, Pfister CJ, Mentz R. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1997;158:3539–43. [PubMed] [Google Scholar]
- 34.Betts RJ, Kemeny DM. CD8(+) T cells in asthma: friend or foe? Pharmacol Ther. 2009;121:123–31. doi: 10.1016/j.pharmthera.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Cho SH, Stanciu LA, Holgate ST, Johnston S. Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD4(+) and CD8(+) T cells in atopic asthma. Am J Respir Crit Care Med. 2005;171:224–30. doi: 10.1164/rccm.200310-1416OC. [DOI] [PubMed] [Google Scholar]
- 36.Krug N, Madden J, Redington AE, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–26. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 37.Miyahara S, Miyahara N, Matsubara S, Takeda K, Koya T, Gelfand EW. IL-13 is essential to the late-phase response in allergic rhinitis. J Allergy Clin Immunol. 2006;118:1110–6. doi: 10.1016/j.jaci.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Miyahara N, Takeda K, Kodama T, et al. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J Immunol. 2004;172:2549–58. doi: 10.4049/jimmunol.172.4.2549. [DOI] [PubMed] [Google Scholar]
- 39.Dunford PJ, O'Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062–70. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 40.Pearce FL. Biological effects of histamine: an overwiew. Agents Actions. 1991;33:4–7. doi: 10.1007/BF01993112. [DOI] [PubMed] [Google Scholar]
- 41.Koarai A, Ichinose M, Ishigaki-Suzuki S, et al. Disruption of L-histidine decarboxylase reduces airway eosinophilia but not hyperresponsiveness. Am J Respir Crit Care Med. 2003;167:758–63. doi: 10.1164/rccm.200206-619OC. [DOI] [PubMed] [Google Scholar]
- 42.Gelfand EW, Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. J Allergy Clin Immunol. 2006;117:577–82. doi: 10.1016/j.jaci.2005.12.1340. [DOI] [PubMed] [Google Scholar]


