Abstract
The majority of cells in early/colostrum milk are breast milk macrophages (BrMMø) expressing dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM3) grabbing nonintegrin (DC-SIGN), and the expression level of DC-SIGN on BrMMø will determine cell-to-cell human immunodeficiency virus type 1 (HIV-1) transmissibility. Thus, one of the strategies to prevent vertical transmission of HIV-1 through breast-feeding is to find a way to suppress DC-SIGN expression on BrMMø. As for the expression of Toll-like receptors (TLRs) in BrMMø, TLR3 was always seen in BrMMø but not in peripheral blood monocytes (PBMo). Also, the expression of TLR3 was slightly enhanced in BrMMø when the cells were treated with interleukin (IL)-4. Moreover, when TLR3 was stimulated with its specific ligand, the double-stranded RNA (dsRNA) poly(I:C), DC-SIGN expression on BrMMø was reduced even in the IL-4-mediated enhanced state. Some reduction may be caused by type I interferons (IFNs), such as IFN-α/β, secreted from BrMMø. Indeed, both IFNs, particularly IFN-β, showed a strong capacity to suppress the enhancement of DC-SIGN expression on IL-4-treated BrMMø and such TLR3-mediated DC-SIGN suppression was partially abrogated by the addition of anti-IFN-α/β-receptor-specific antibodies. As expected, DC-SIGN-mediated HIV-1 transmission to CD4-positive cells by BrMMø was inhibited by either poly(I:C) stimulation or by treatment with type I IFNs. These findings suggest a possible strategy for preventing mother-to-child transmission (MTCT) of HIV-1 via breast-feeding through TLR3 signalling.
Keywords: breast milk macrophages, colostrum/early breast milk, dendritic cell-specific intercellular adhesion molecule 3 (ICAM3) grabbing nonintegrin (DC-SIGN), human immunodeficiency virus type 1 mother-to-child transmission, Toll-like receptor 3, type I interferons
Introduction
Although mother-to-child transmission (MTCT) of human immunodeficiency virus type 1 (HIV-1) has been markedly reduced by antiretroviral treatment and avoidance of breast-feeding,1 around 400 000 newly infected children have been born, particularly in resource-limited countries (AIDS epidemic update. UNAIDS, http://www.UNAIDS.org accessed 29 July 2008), via vertical transmission during pregnancy, delivery and breast-feeding. Among these three distinct routes, breast-feeding is still a major public health concern in developing countries. The risk of HIV-1 infection of infants via breast-feeding has been found to be influenced by breast milk virus load, which is significantly higher in early/colostrum milk than in mature breast milk.2
The majority of cells in colostrum milk have been identified as unique large cells, termed breast milk macrophages (BrMMø), expressing both CD4 and CD14.3 Importantly, BrMMø also express chemokine receptors such as chemokine (C-X-C motif) receptor 4 (CXCR4) and chemokine (C-C motif) receptor 5 (CCR5), which permit HIV-1 entrance, as well as CD83, a maturation marker of dendritic cells (DCs).4 Thus, BrMMø have been identified as DC-lineage HIV-1-vulnerable cells and also express C-type lectin DC-specific intercellular adhesion molecule 3 (ICAM3) grabbing nonintegrin (DC-SIGN),5 which will tightly capture free HIV-1 virions and transmit them to HIV-1-susceptible infant CD4-positive cells.3 Moreover, after co-culture with interleukin (IL)-4, BrMMø were found to have enhanced DC-SIGN expression,4 and became resistant to HIV-1 infection. Therefore, IL-4-treated BrMMø will not be infected by HIV-1 but will rather capture free virus particles via DC-SIGN, and such cell-associated virions would more readily be transmitted to HIV-1-susceptible cells via breast-feeding.
Local production of IL-4 in mastitis may up-regulate the expression of DC-SIGN in BrMMø, which may explain why mastitis is linked to higher HIV load in breast milk and a higher risk of mother-to-infant vertical transmission of the virus.6 Indeed, it has recently been reported that increased cell-associated HIV-1 but not cell-free virion shedding in breast milk could mediate the association between mastitis and MTCT.7 In addition, we reported previously that high transmissibility was mediated through HIV-1 virions captured by DC-SIGN but not through cell-free virus particles released from HIV-1-infected cells,3 although some reports indicate that cell-free HIV-1 in breast milk may contribute to vertical transmission.8 Therefore, in order to prevent vertical transmission of HIV-1 through breast-feeding, it is necessary to find a way to inhibit the acquisition of free HIV-1 virions via DC-SIGN by suppressing its expression on BrMMø.
In the present study, careful examination of BrMMø revealed the apparent expression of Toll-like receptor 3 (TLR3) in freshly isolated BrMMø, although we could not detect TLR3 in peripheral blood monocytes (PBMo). However, TLR3 was detected in PBMo when they were stimulated with granulocyte–macrophage colony-stimulating factor (GM-CSF), which is spontaneously produced in BrMMø.4 Moreover, freshly isolated TLR3-positive BrMMø also expressed DC-SIGN and the expression of TLR3 was slightly enhanced in IL-4-treated BrMMø, in which DC-SIGN expression is significantly enhanced. Thus, we attempted to stimulate TLR3 with one of its ligands, poly(I:C), which is a double-stranded RNA (dsRNA),9,10 to investigate its effect on DC-SIGN expression, and found a reduction in DC-SIGN expression in both freshly isolated BrMMø and IL-4-treated BrMMø. Also, poly(I:C)-stimulated BrMMø secreted considerable amounts of type I interferons (IFNs), such as IFN-α and IFN-β. As expected, DC-SIGN-mediated HIV-1 transmission to susceptible CD4-positive cells by BrMMø was inhibited by TLR3 signalling with poly(I:C) or treatment of BrMMø with their products, type I IFNs. We discuss our findings in terms of this unique feature of BrMMø and propose a possible strategy for preventing MTCT of HIV-1 via breast-feeding through TLR3 signalling.
Materials and methods
Isolation and culture of BrMMø and PBMo
Breast milk was collected from healthy women within 2–6 days of delivery after informed consent had been obtained under a protocol approved by the Institutional Review Board of Nippon Medical School. Breast milk cells were isolated from freshly obtained breast milk by Ficoll-Hypaque (Amersham-Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation methods as described previously.4 BrMMø were isolated from freshly collected breast milk cells, and allowed to adhere to polystyrene tissue culture dishes (Corning, New York, NY) for 1–2 hr at 37°. After non-adhering cells had been gently removed, adherent cells were washed with warm RPMI-1640 medium containing 2% fetal calf serum (FCS) (HyClone Laboratories, Logan, UT). The remaining adherent cells were then removed by incubation with 5 mm ethylenediaminetetraacetic acid (EDTA) for 30 min at 4° and confirmed to express homogeneous CD14+ cells at approximately 95% using a FACScan (BD Biosciences, Mountain View, CA). To obtain PBMo, CD14+ monocytes were isolated from peripheral blood of healthy volunteers by magnetic depletion using a monocyte isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) containing hapten-conjugated antibodies to CD3, CD7, CD16, CD19, CD56, CD123 and Glycopholin A and a magnetic antibody cell sorter (MACS; Miltenyi Biotec) according to the manufacturer’s instructions, routinely resulting in > 90% purity of CD14+ cells. The isolated monocytes were cultured in 24-well culture plates (Corning) for 6 days in RPMI-1640-based complete culture medium (CCM)11 supplemented with 10% FCS (HyClone Laboratories), 20 mm HEPES (Invitrogen, Carlsbad, CA), 50 mm 2-mercapto-ethanol (2-ME) (Sigma-Aldrich, St Louis, MO), 2 mm l-glutamine (Sigma-Aldrich) and 100 units of penicillin-streptomycin (Sigma-Aldrich), together with 100 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ) and 20 ng/ml IL-4 (Biosource Intl., Camarillo, CA) to obtain immature DCs (iDCs).
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted from 3 × 105 cells of each cell preparation using the commercial RNeasy Kit (Qiagen, Hilden, Germany), and first-strand DNA was synthesized as described previously.12 Transcripts of TLRs as well as the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were amplified by PCR reaction. The primer sets were: GAPDH sense, 5′-GCC TCA AGA TCA TCA GCA ATG C-3′; GAPDH anti-sense, 5′-ATG CCA GTG AGC TTC CCG TTC-3′; TLR1 sense, 5′-CGC ATG GTC CAC ATG CTT T-3′; TLR1 anti-sense, 5′-GCC ACA TCC AGG AAG GTC AGT-3′; TLR2 sense, 5′-CCC TGG GCA GTC TTG AAC ATT-3′; TLR2 anti-sense, 5′-GCC TCC GGA TTG TTA ACG TTT-3′; TLR3 sense, 5′-GGG TCC CAG CCT TAC AGA GAA-3′; TLR3 anti-sense, 5′-CTA GGT GGC CCA ACC AAG AG-3′; TLR4 sense, 5′-TGG TGT CCC AGC ACT TCA TC-3′; TLR4 anti-sense, 5′-CTG CAT ATC TAG TGC ACC ATG G-3′; TLR5 sense, 5′-TCC ACG GAA GGT TGT GAT GA-3′; TLR5 anti-sense, 5′-GAC CCA ACC ACC ACC ATG A-3′; TLR6 sense, 5′-CCT CAT GCA CCA AGC ACA TT-3′; TLR6 anti-sense, 5′-TCT GGC AGC TCT GGA AGA AA-3′; TLR7 sense, 5′-CGA ACC TCA CCC TCA CCA TTA-3′; TLR7 anti-sense, 5′-GGG ACG GCT GTG ACA TTG TTA-3′; TLR8 sense, 5′-GCC AGC GAG TCT CAC TGA ACT-3′; TLR8 anti-sense, 5′-GCC AGG GCA GCC AAC ATA-3′; TLR9 sense, 5′-TGG ACA CTC CCA GCT CTG AAG-3′; TLR9 anti-sense, 5′-TGG GAC ACT TGG CTG TGG ATG-3′. After 35 cycles of PCR reaction, the PCR products were resolved by electrophoresis in agarose gels and visualized by ethidium bromide staining using an ultraviolet (UV) light source.
BrMMø treatment with various reagents
To treat BrMMø with IL-4, cells were plated at 1 × 106 cells/ml in 10% FCS containing CCM, and cultured at 37° for 5 days in the presence of IL-4 (20 ng/ml) (Biosource Intl.). To treat BrMMø with poly(I:C) (Amersham-Pharmacia Biotech), cells were placed at 1 × 106 cells/ml on a 24-well flat-bottom tissue culture plate in CCM with or without 50 μg/ml poly(I:C). After incubating for 24 hr at 37°, poly(I:C) was washed out three times with RPMI-1640 medium. Cells were further cultured for 4 days with or without IL-4. In some experiments, after culture of BrMMø with CCM for 24 hr at 37°, IL-4 was added and the cells were further cultured for 3 days, and then poly(I:C) was added and the cells were incubated for an additional 24 hr. To treat BrMMø with IFNs, freshly isolated BrMMø (1 × 106) placed in a 24-well plate were stimulated with 100 IU/ml of either IFN-α (INTRON® A; Schering-Plough, Kenilworth, NJ) or IFN-β (Toray Medical Co. Ltd, Tokyo, Japan) together with IL-4 for 5 days.
Measurement of IFN by enzyme-linked immunosorbent assay (ELISA)
Freshly isolated BrMMø (1 × 106 cells/ml) were stimulated in a 24-well flat-bottomed tissue culture plate with either 50 μg/ml poly(I:C) or 50 μg/ml poly(I:C) plus 20 ng/ml IL-4 in 1 ml of CCM for 24 hr at 37°, and the levels of IFN-α and IFN-β in culture supernatants were measured by ELISA using human an IFN-α and IFN-β ELISA kit (PBL Interferon Source, Piscataway, NJ).
Antibodies and flow cytometry
Phycoerythrin (PE)-conjugated and unlabelled anti-human monoclonal antibodies (mAbs) to DC-SIGN (120507) for blocking experiments were purchased from R & D Systems (Minneapolis, MN). PE-conjugated isotype-matched control antibody (MOPC-21), PE-conjugated anti-human monoclonal antibody (mAb) to CD4 (RPA-T4) and fluorescein isothiocyanate (FITC)-conjugated anti-human mAb to CD14 (M5E2) were purchased from BD Biosciences.
Cells were stained with the relevant antibody on ice for 30 min in phosphate-buffered saline (PBS) with 2% FCS and 0·01 m sodium azide (PBS-based medium), washed twice, and re-suspended in PBS-based medium. Labelled cells were then analysed with a FACScan (BD Biosciences) using CellQuest software (BD Biosciences). Live cells were gated based on propidium iodide gating.
For intracellular staining of TLR3, cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences) for 20 min on ice. After washing twice with Perm/Wash solution (BD Biosciences), cells were incubated with AB serum to prevent non-specific binding for 30 min and further incubated with anti-human mAb to TLR3 (TLR3.7) (eBioscience, San Diego, CA) for 30 min on ice in the dark. After washing twice, cells were incubated with PE-conjugated secondary anti-mouse immunoglobulin G (IgG) (Beckman Coulter, Fullerton, CA) for 30 min on ice in the dark and re-suspended in PBS-based medium for analysis by FACScan.
Infection of cultured BrMMø with NL(AD8) isolate
After treatment with either poly(I:C) or IFNs, BrMMø were harvested and added to a 1·5-ml micro-tube (Greiner Bio-one, Frickenhausen, Germany). Spanned cells were then incubated with the macrophage-tropic NL(AD8)13 HIV-1 isolate, which has a multiplicity of infection (MOI) of 0·2, at 1–2 × 105 cells for 2 hr at 37°. Cells were washed three times with RPMI-1640 containing 2% FCS and used as HIV-1-loaded cells.
HIV-1 transmission assay
An an indicator cell line, Ghost X4/R5 cells,14 kindly provided by the National Institutes of Health (NIH) AIDS Reagent Repository, were used to examine the capacity of NL(AD8)-sensitized cells to transmit that isolate. Ghost X4/R5 cells (1 × 104 cells/well) were plated in a flat-bottomed 96-well micro-plate (Corning) with Dulbecco’s modified Eagle’s minimal essential medium (DMEM) (Sigma-Aldrich) supplemented with 10% FCS (D-10) the day before co-culturing with target cells. After removal of the medium from each well, 5 × 103 thoroughly washed NL(AD8)-infected BrMMø were added. After incubation for an additional 16 hr, the loaded BrMMø were removed by gentle washing with warmed D-10. After 24 hr, NL(AD8)-infected Ghost X4/R5 cells expressing green fluorescent protein (GFP) were analysed by flow cytometry to estimate transmissibility.
Results
TLR3 expression in freshly isolated BrMMø
It has previously been shown that freshly isolated BrMMø are DC-lineage cells expressing DC-SIGN, which will capture free HIV virions and transmit them to HIV-susceptible CD4-positive cells.3 Such DC-SIGN-mediated spread of HIV appears to be the principal route of MTCT via breast milk. The magnitude of DC-SIGN expression on BrMMø is greatly enhanced when BrMMø are treated with IL-4.4 In order to find a way to prevent MTCT of HIV via BrMMø through down-modulation of DC-SIGN, we first examined the TLR expression of BrMMø, through which they might be regulated. As demonstrated in Fig. 1a, although TLR3 expression was not detected in freshly isolated PBMo by RT-PCR analysis, TLR3 was clearly observed in both DCs and BrMMø. Also, as shown in Fig. 1b, the expression of TLR3 was slightly enhanced in BrMMø when they were treated with IL-4 at both the transcriptional level, as determined by RT-PCR [Fig. 1b, left panel; the mean fold increase (MFI) ± standard deviation (SD) was 1·5 ± 0·3], and the protein level, as determined by flow cytometry (Fig. 1b, middle panel; MFI ± SD was 1·4 ± 0·1). As in a previous study,4 we found marked enhancement of DC-SIGN expression on IL-4-treated BrMMø (Fig. 1b, right panel; MFI ± SD was 21·9 ± 7·6). TLR3 expression among a series of TLRs seems to be a critical difference between PBMo and BrMMø.
Figure 1.
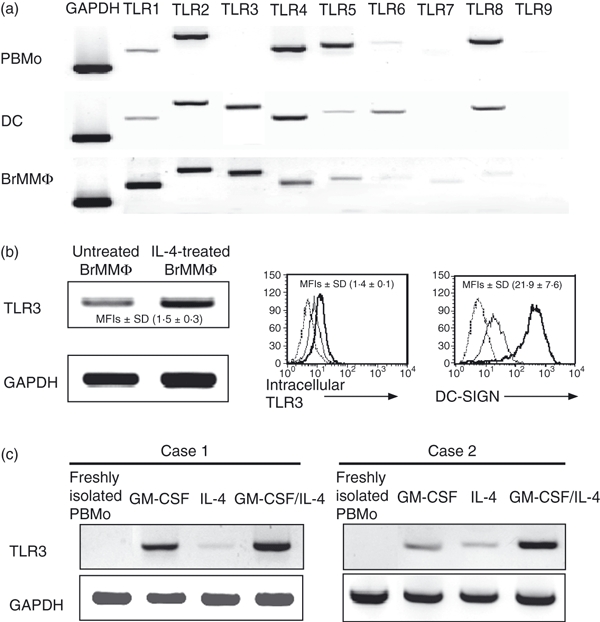
Analysis of Toll-like receptors (TLRs) in breast milk macrophages (BrMMø). (a) Expression of all TLRs in freshly isolated peripheral blood monocytes (PBMo), dendritic cells (DCs) generated from isolated PBMo with 20 ng/ml interleukin (IL)-4 plus 100 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF), and freshly isolated BrMMø was analysed by reverse transcription–polymerase chain reaction (RT-PCR). (b) The expression level of TLR3 in freshly isolated BrMMø was slightly increased in IL-4-treated BrMMø both at the transcriptional level, as determined by RT-PCR [the mean fold increase (MFI) ± standard deviation (SD), determined using a densitometer and the bio-imaging software ImageJ (widely provided by NIH, USA) in four separate experiments, was 1·5 ± 0·3; left panel), and at the protein level, as determined by flow cytometry (the MFI ± SD for four separate experiments was 1·4 ± 0·1; middle panel). The expression level of DC-SIGN was markedly increased in the IL-4-treated BrMMø (the MFI ± SD for four separate experiments was 21·9 ± 7·6; right panel). The dotted lines indicate isotype control, the thin line freshly isolated BrMMø, and the thick line IL-4-treated BrMMø in these panels. (c) Effect of externally added GM-CSF on TLR3 detection in PBMo. TLR3 was detected in PBMo when cells were co-cultured with 100 ng/ml GM-CSF. We used samples from four donors, and two representative cases are shown (cases 1 and 2). In case 2, it should be noted that weak expression of TLR3 was found when cells were incubated with IL-4 alone. Each experiment shown was performed for at least four donors and representative results are shown. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
In the light of our previous finding4 that freshly isolated BrMMø produced GM-CSF spontaneously, but PBMo did not, and that TLR3-expressing DCs could be obtained from PBMo by GM-CSF plus IL-4 stimulation, we stimulated freshly isolated PBMo with GM-CSF to see whether they would express TLR3. As expected, TLR3 was detected in PBMo by RT-PCR analysis when cells were co-cultured with GM-CSF (Fig. 1c; cases 1 and 2). It should be noted that low levels of expression of TLR3 was sometimes found in PBMo from individual donors (e.g. case 2) when they were incubated with IL-4 alone. This could be because PBMo may produce a small amount of GM-CSF during co-culture with IL-4.15 These results suggest that TLR3 expression in freshly isolated BrMMø may be regulated by their internal production of GM-CSF, which was not blocked by the external addition of anti-GM-CSF-specific antibodies (data not shown).
Effect of stimulation with the TLR3 ligand poly(I:C) on DC-SIGN expression on BrMMø
As shown in Fig. 1b (middle panel), the slight enhancement of TLR3 expression in IL-4-treated BrMMø with augmented DC-SIGN (right panel) was confirmed by flow cytometry. This finding may suggest a relationship between DC-SIGN and TLR3. Thus, we investigated the effect of TLR3 stimulation with a known dsRNA ligand, poly(I:C), on DC-SIGN expression on BrMMø. Because a number of freshly isolated BrMMø died after co-culture with 200 μg/ml poly(I:C) for more than 24 hr, we incubated cells with 50 μg/ml poly(I:C) for 24 hr after confirming that there was no toxic effect associated with this procedure, and used them in the following experiments after extensive washing to remove free poly(I:C).
Using two-colour staining, dot-plot analyses were performed by FACScan (Fig. 2a). In the upper left panel of Fig. 2a, the expression of both DC-SIGN and CD14 on freshly isolated BrMMø was confirmed. Dot-plot data to evaluate the pattern of CD14 and DC-SIGN on IL-4- and/or poly(I:C)-treated-BrMMø are shown in the other panels of Fig. 2a. The total incubation period for BrMMø was 5 days. The CD14 expression (indicating a macrophage lineage) observed on the freshly isolated BrMMø completely disappeared after treatment with 20 ng/ml IL-4 (upper right, lower middle and lower right panels) and decreased on cultured BrMMø. The up-regulation of DC-SIGN expression on BrMMø produced by IL-4 treatment (upper right) was significantly inhibited when freshly isolated BrMMø pretreated with 50 μg/ml poly(I:C) for 24 hr were further stimulated for an additional 4 days with IL-4 (lower middle), and was slightly inhibited when BrMMø treated with IL-4 for 4 days were incubated with 50 μg/ml poly(I:C) for the final 24 hr (lower right). Moreover, DC-SIGN expression on untreated BrMMø (upper middle) was decreased by pretreatment with 50 μg/ml poly(I:C) for 24 hr (lower left).
Figure 2.
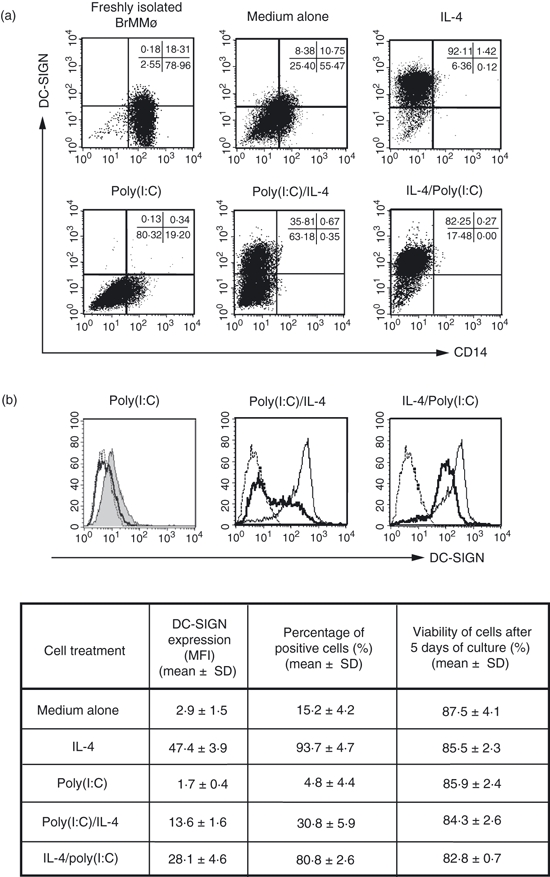
Effect of stimulation with a known Toll-like receptor 3 (TLR3) ligand, poly(I:C), on dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM3) grabbing nonintegrin (DC-SIGN) expression of breast milk macrophages (BrMMø). (a) Dot-plot data from two-colour FACScan analysis are shown for evaluation of the patterns of DC-SIGN and CD14 expression on freshly isolated BrMMø (upper left panel), as well as on interleukin (IL)-4- and/or poly(I:C)-treated BrMMø (other panels). The total incubation period for BrMMø was 5 days. CD14 expression, indicating a macrophage lineage, observed on the freshly isolated BrMMø completely disappeared after treatment with 20 ng/ml IL-4 (upper right, lower middle and lower right panels) and that on cultured BrMMø was reduced. Up-regulation of DC-SIGN expression on BrMMø by 4 days of IL-4 treatment (upper right panel) was strongly inhibited when freshly isolated BrMMø pretreated with 50 μg/ml poly(I:C) for 24 hr were stimulated for an additional 4 days with IL-4 (lower middle panel) and slightly inhibited when BrMMø treated with IL-4 for 4 days were incubated with 50 μg/ml poly(I:C) for the final 24 hr (lower right panel). Moreover, DC-SIGN expression on untreated BrMMø was decreased by pretreatment with 50 μg/ml poly(I:C) for 24 hr (lower left panel). (b) The results presented in (a) were confirmed by flow cytometry analysis. The effect of poly(I:C) on DC-SIGN expression in BrMMø is shown in the table, which presents the mean fluorescence intensity (MFI), the percentage of positive cells, and their viability (mean ± standard deviation). Dotted lines show isotype controls and thick lines indicate poly(I:C)-treated BrMMø. The shaded line in the left panel indicates DC-SIGN expression of BrMMø cultured for 5 days with medium alone, and thin lines in the middle and right panels indicate DC-SIGN expression of BrMMø stimulated for 4 days with IL-4. Each experiment shown was carried out on samples from at least four different donors, and representative results for the same donor are shown in (a) and (b) for comparison.
The above results were confirmed by flow cytometry analysis (Fig. 2b). It should be noted that DC-SIGN expression on BrMMø in culture medium alone [Fig. 2a, upper middle panel and Fig. 2b, left panel (shaded line)] was decreased by treatment with 50 μg/ml poly(I:C) for 24 hr [Fig. 2a, lower left panel and Fig. 2b, left panel (thick line)]. Moreover, up-regulation of DC-SIGN by IL-4 treatement was also strongly inhibited when freshly isolated BrMMø were pretreated with 50 μg/ml poly(I:C) for 24 hr and stimulated for an additional 4 days with 20 ng/ml IL-4 [Fig. 2a, lower middle panel and Fig. 2b, middle panel (thick line)]. In addition, the already enhanced DC-SIGN expression induced by IL-4 co-culture for 4 days was partially suppressed when IL-4-treated BrMMø were incubated with 50 μg/ml poly(I:C) for the final 24 hr [Fig. 2a, lower right panel and Fig. 2b, right panel (thick line)]. Thus, DC-SIGN expression on BrMMø can be down-regulated by brief TLR3 stimulation with the dsRNA poly(I:C).
Effect of poly(I:C) on the production of type I IFNs (IFN-α and IFN-β) by BrMMø
Upon dsRNA stimulation via TLR3, cells will produce and secrete type I IFNs, such as IFN-α and IFN-β. We thus investigated whether BrMMø were able to produce and secrete type I IFNs after brief exposure to the dsRNA poly(I:C). As expected, not only freshly isolated but also IL-4-treated BrMMø produced and secreted both IFN-α and IFN-β after stimulation with poly(I:C) (Fig. 3a,b). These results suggest that freshly isolated BrMMø as well as IL-4-stimulated BrMMø with high HIV-1 transmissibility will gain the capacity to produce and secrete antiviral cytokines, such as type I IFNs, upon stimulation of TLR3 with viral substances such as poly(I:C).
Figure 3.

Measurement of type I interferon (IFN) secretion in the culture supernatant of breast milk macrophages (BrMMø) stimulated with poly(I:C). Both freshly isolated and interleukin (IL)-4-treated BrMMø produced and secreted both IFN-α and IFN-β after incubation with 50 μg/ml poly(I:C) for 24 hr, as measured by enzyme-linked immunosorbent assay (ELISA). The data shown are representative of at least four different experiments and results are expressed as the mean ± standard deviation.
Effect of type I IFNs on DC-SIGN expression on BrMMø
We further examined whether type I IFNs can affect the DC-SIGN expression of IL-4-treated BrMMø with high HIV-1 transmissibility. As shown in Fig. 4a, both IFNs at non-toxic concentrations, particularly IFN-β, showed a strong capacity to suppress the enhancement of DC-SIGN expression on IL-4-treated BrMMø in a dose-dependent manner when IFNs were added together with IL-4. However, once the enhanced DC-SIGN expression on BrMMø had been established by co-culture with IL-4 for 4–5 days, IFN treatment did not affect the expression of DC-SIGN (data not shown). The finding that treatment of BrMMø with type I IFN, particularly IFN-β, seemed to reduce the enhancement of DC-SIGN expression mediated by external factors, such as IL-4, suggests that such treatment may inhibit DC-SIGN-mediated MTCT of HIV-1. As also shown in the dot-plot analyses in the two right-hand panels of Fig. 4b, co-culture of BrMMø with IFNs and IL-4 usually generated two subpopulations, DC-SIGN positive and negative. We found that the percentage of CD4 expression in the DC-SIGN-negative population was always higher than that in the DC-SIGN-positive population, indicating that the HIV-1-susceptible population may persist in the presence of IFNs, in particular IFN-β, when BrMMø are co-cultured with IL-4 (data not shown).
Figure 4.
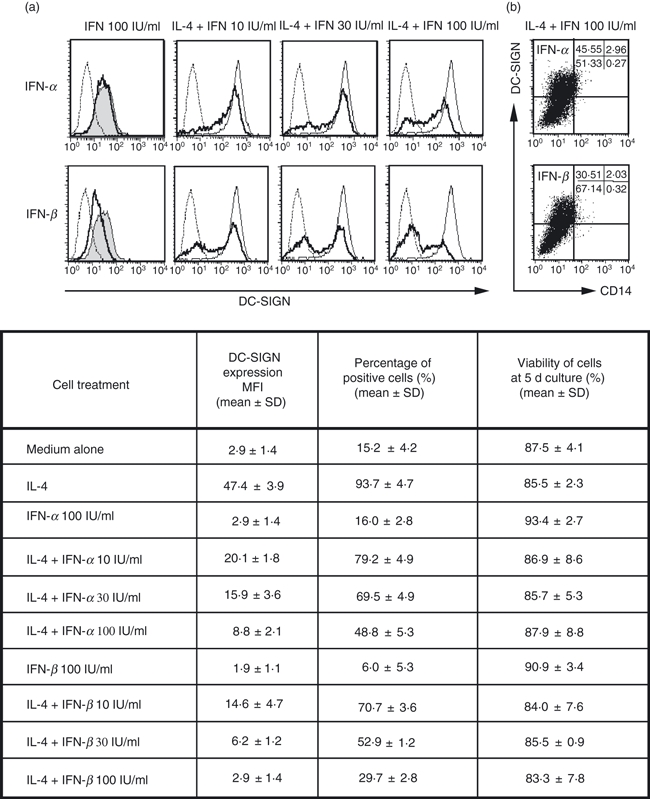
Effect of type I interferons (IFNs) on dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM3) grabbing nonintegrin (DC-SIGN) expression on breast milk macrophages (BrMMø). BrMMø co-cultured with IFN for 5 days are shown as thick lines and isotype controls as dotted lines (a). Both type I IFNs, particularly IFN-β (lower panels), showed a strong capacity to suppress the enhancement of DC-SIGN expression in BrMMø cultured with IFN for 5 days in the presence of 20 ng/ml IL-4 (indicated as thin lines), and this suppression was dose-dependent. As controls (the two left-hand panels), DC-SIGN expression in BrMMø cultured for 5 days in medium alone is shown as shaded lines, that for isotype controls as dotted lines, and that for IFN-treated BrMMø as thick lines. (b) Dot-plot analyses for BrMMø co-cultured with IFNs and IL-4. The effect of IFNs on DC-SIGN expression on BrMMø co-cultured with IL-4 is shown in the table, which gives the percentage of positive cells and their mean fluorescence intensity (MFI) and viability (mean ± standard deviation). Data shown are representative of at least three separate experiments.
Moreover, we previously demonstrated that a dramatic reduction in chemokine receptor expression for both CXCR4 and CCR5 on BrMMø led to weak susceptibility to HIV-1 when cells were co-cultured with IL-4.3 The reduced expression of CXCR4 and CCR5 as well as CD4 on IL-4-treated BrMMø was not augmented by treatment with IFNs (data not shown).
Taken together, the results suggest that, although co-culture of BrMMø with IFNs and IL-4 may reduce their capacity to transmit HIV-1 via DC-SIGN, IFN treatment of IL-4-stimulated BrMMø will not affect their HIV-1 transmissibility and susceptibility. It is also important to note that pretreatment of BrMMø with anti-IFN-α/β-receptor-specific antibodies produced a remarkable abrogation of poly(I:C)-mediated inhibition of DC-SIGN expression on BrMMø (data not shown). The findings indicate that IFNs secreted by stimulated BrMMø may down-modulate DC-SIGN expression via their IFN-α/β receptors.
DC-SIGN-mediated transmission of HIV-1 via BrMMø was significantly inhibited by treatment not only with poly(I:C) but also with IFNs
Collectively, the above findings indicate that not only IFN inducers, such as poly(I:C), but also IFNs themselves have the capacity to inhibit mother-to-child HIV-1 transmission via breast milk through down-modulation of DC-SIGN. Thus, we first co-cultured BrMMø for 24 hr with 50 μg/ml poly(I:C) and further incubated them with IL-4 for an additional 4 days after extensive washing to remove free poly(I:C). Poly(I:C)-treated, IL-4-co-cultured BrMMø were infected with a 0·2 MOI dose of the R5-type macrophage-tropic HIV-1 isolate NL(AD8) for 2 hr at 37°. After washing three times to remove free NL(AD8) virions, 1 × 104 Ghost X4/R5 cells expressing GFP were added to NL(AD8)-pulsed BrMMø and incubated for an additional 16 hr. After incubation, the loaded BrMMø were removed by gentle washing with warmed medium (D-10) and NL(AD8)-infected Ghost X4/R5 cells expressing GFP were analysed by flow cytometry to estimate transmissibility. As expected, in comparison with IL-4-treated BrMMø, poly(I:C)-pretreated BrMMø had a markedly reduced capacity to transmit HIV-1 virions to susceptible cells (Fig. 5a). Also, IL-4-mediated enhancement of HIV-1 transmission was slightly inhibited by post-treatment with poly(I:C) (Fig. 5a). It should be noted that blocking with anti-DC-SIGN-specific antibody produced a 60–70% reduction of IL-4-mediated HIV-1 transmission of BrMMø (Fig. 5a). Similarly high transmissibility of IL-4-treated BrMMø was strongly inhibited by pretreatment with type I IFNs (Fig. 5b). As also shown in Fig. 5b, it should be noted that the magnitude of inhibition was always much greater with IFN-β than with IFN-α treatment. These results indicate that, although there may be DC-SIGN-independent pathways for HIV-1 transmission of BrMMø, down-modulation of DC-SIGN expression on BrMMø through TLR3 signalling mediated by poly(I:C) or through their product IFNs may reduce HIV-1 transmission via breast-feeding.
Figure 5.
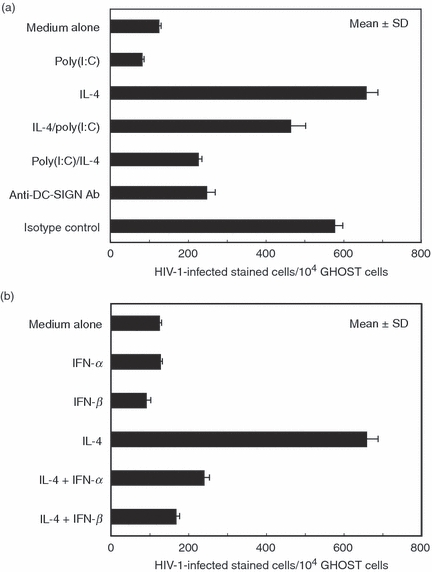
Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM3) grabbing nonintegrin (DC-SIGN)-mediated transmission of human immunodeficiency virus type 1 (HIV-1) via breast milk macrophages (BrMMø) was inhibited by treatment not only with poly(I:C) (a) but also with interferons (IFNs) (b). (a) To examine the effect of poly(I:C) treatment on DC-SIGN-mediated HIV-1 transmission, BrMMø incubated for 24 hr with 50 μg/ml poly(I:C) were further incubated with either culture medium or interleukin (IL)-4 for an additional 4 days after extensive washing to remove free poly(I:C). Also, BrMMø co-cultured with IL-4 for 4 days were further incubated with 50 μg/ml poly(I:C) for the final 24 hr. These poly(I:C)-treated BrMMø were infected with a 0·2 multiplicity of infection (MOI) dose of the R5-type macrophage-tropic HIV-1 isolate NL(AD8) for 2 hr at 37°. After extensive washing to remove free NL(AD8) virions, 1 × 104Ghost X4/R5 cells were added to NL(AD8)-pulsed BrMMø and incubated for an additional 16 hr. After incubation, the HIV-1-loaded BrMMø were gently removed with warmed D-10, and NL(AD8)-infected Ghost X4/R5 cells expressing green fluorescent protein (GFP) were analysed by flow cytometry to estimate transmissibility. Also, to examine the possibility of a DC-SIGN-independent pathway for HIV-1 transmission by BrMMø, IL-4-stimulated BrMMø pretreated with 20 μg of anti-DC-SIGN monoclonal antibody (mAb) for 30 min on ice were infected with NL(AD8) for 2 hr at 37° and added to Ghost X4/R5 cells. (b) Similarly, to investigate the effect of IFNs on DC-SIGN-mediated HIV-1 transmission, BrMMø co-cultured with IFNs and IL-4 for 5 days were infected with a 0·2 MOI dose of NL(AD8) for 2 hr at 37°, washed to remove free virions, and incubated with 1 × 104Ghost X4/R5 cells for an additional 16 hr before analysis by flow cytometry. Data are representative of at least four experiments and the results are expressed as the mean ± standard deviation.
Discussion
In the present study, although TLR3 was not detected in PBMo, TLR3 expression was clearly observed in freshly isolated BrMMø producing GM-CSF intracellularly. As expected, TLR3 was detected in PBMo when the cells were incubated with GM-CSF. In contrast, freshly isolated neutrophils that expressed all TLRs except TLR3 did not express TLR3 even when co-cultured with GM-CSF.16 It has also been reported that GM-CSF enhances the expression of TLR2 or TLR4 in neutrophils and TLR2 in monocytes.17 These findings indicate that there may be at least two distinct subpopulations of PBMo, one that provides protection against extracellular bacteria, such as neutrophils expressing TLR2 and TLR4, and one that provides protection against intracellular viruses, such as macrophages expressing TLR3, and that GM-CSF seems to have the capacity to reinforce the innate alert system in PBMo providing protection against various invasive pathogens through enhancement of the TLR network.
As we reported previously, GM-CSF production was detected in freshly isolated BrMMø, and PBMo gained the ability to produce GM-CSF upon exposure to breast milk.4 The findings strongly suggest that breast milk has the potential to stimulate PBMo to become TLR3-positive cells. Moreover, freshly isolated TLR3-positive BrMMø also expressed DC-SIGN, which captures various virions, such as HIV-1 and West Nile virus (WNV), and transmits them to susceptible cells. It has recently been reported that binding of the glycosylated WNV envelope protein to DC-SIGN leads to a reduction in the expression of TLR3 in macrophages from young donors via the signal transducer and activator of transcription 1 (STAT1)-mediated pathway.18 Thus, binding signals of DC-SIGN mediated by target virions may down-modulate the internal expression of TLR3, which would act as an alert signalling the invasion of foreign viruses. Indeed, we have observed a similar reduction of TLR3 expression in IL-4-treated BrMMø, which have up-regulated expression of DC-SIGN and down-regulated expression of chemokine receptors,3 when incubated with HIV-1 (Y.Y., E.W., and H.T., unpublished observations). The findings suggest that the direct attachment of HIV-1 to DC-SIGN on BrMMø may cause a negative signal to down-regulate TLR3 expression.
Conversely, TLR3-mediated alert signalling seems to inhibit both intracellular virus replication and intercellular virus transmission via DC-SIGN. Here we found a reduction in DC-SIGN expression on both freshly isolated BrMMø and IL-4-treated BrMMø when they were stimulated with a synthetic TLR3 ligand, poly(I:C). These findings indicate that TLR3 in BrMMø appears to act as a ‘button’ that can be pressed to cancel DC-SIGN-mediated virus transmission, and this cancelling signal is sent via the products of internally replicated virions. Collectively, DC-lineage BrMMø seem to be unique HIV-1 carriers equipped with receptors allowing entrance of the virus (CD4 and chemokine receptors), a mechanism for virus attachment (DC-SIGN), and a ‘cancel button’ (TLR3, the cellular expression of which seems be regulated by GM-CSF). The reason why BrMMø have such equipment for use against viruses remains to be determined.
Breast milk from an HIV-infected women may contain both cell-free HIV-1 and cell-associated virus.19 However, as we reported previously, high transmissibility is mediated through HIV-1 virions captured by DC-SIGN but not through cell-free virus particles released from HIV-1-infected cells,3 although the actual impact of human breast milk on HIV infection in CD4 cells remains poorly understood. Nevertheless, it has recently been reported that breast milk itself may have a protective function against cell-free HIV-1 but may be less effective at blocking infection by cell-associated virus.20 Thus, to prevent vertical transmission of HIV-1 through breast-feeding, it may be important to inhibit the acquisition of free HIV-1 virions via specific receptors such as DC-SIGN on the BrMMø of HIV-infected women.
When TLR3 in BrMMø was stimulated by the dsRNA poly(I:C), a considerable amount of type I IFNs, such as IFN-α and IFN-β, was detected in the culture supernatant. In addition, significant down-modulation of DC-SIGN on BrMMø was observed when they were stimulated with IL-4 together with IFN-α or IFN-β. As expected, in comparison with IL-4-treated BrMMø, poly(I:C)-pretreated and IL-4-expanded BrMMø had markedly reduced capacities to transmit HIV-1 virions to susceptible cells. Similarly, the high transmissibility of IL-4-treated BrMMø was also markedly inhibited by pretreatment with either IFN-α or IFN-β. Also, DC-SIGN expression on IL-4-treated BrMMø was slightly down-modulated by 24 hr of poly(I:C) exposure, which also inhibited HIV-1 transmission to some extent (Fig. 5a). Moreover, as shown in Fig. 5a, anti-DC-SIGN antibody treatment produced a 60–70% reduction of HIV-1 transmission by IL-4-treated mature BrMMø. Therefore, blocking of DC-SIGN-mediated transmission of HIV-1 may have great potential to prevent MTCT HIV transmission, although the possible existence of a DC-SIGN-independent transmission pathway should also be considered.21
These results indicate that decreased DC-SIGN expression may be the cause of decreased transmission of HIV-1 virus, and that BrMMø have the ability to reduce their external DC-SIGN expression by stimulating their internal TLR3, and thus the TLR3 in BrMMø seems to be a critical molecule to determine the MCTC of HIV-1. The findings presented here may be of use in developing strategies for preventing MTCT of HIV-1 via breast-feeding.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Science, Sport, and Culture, from the Ministry of Health and Labor and Welfare, Japan, and from the Japanese Health Sciences Foundation, and by the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Disclosures
The authors have no competing financial interests or conflicts of interests to disclose.
References
- 1.McIntyre J. Strategies to prevent mother-to-child transmission of HIV. Curr Opin Infect Dis. 2006;19:33–8. doi: 10.1097/01.qco.0000200290.99790.72. [DOI] [PubMed] [Google Scholar]
- 2.Rousseau CM, Nduati RW, Richardson BA, Steele MS, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187:741–7. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satomi M, Shimizu M, Shinya E, et al. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J Infect Dis. 2005;191:174–81. doi: 10.1086/426829. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa M, Sugita M, Takahashi M, Satomi M, Takeshita T, Araki T, Takahashi H. Breast milk macrophages spontaneously produce granulocyte-macrophage colony-stimulating factor and differentiate into dendritic cells in the presence of exogenous interleukin-4 alone. Immunology. 2003;108:189–95. doi: 10.1046/j.1365-2567.2003.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 6.Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci. 2000;918:156–62. doi: 10.1111/j.1749-6632.2000.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 7.Kantarci S, Koulinska IN, Aboud S, Fawzi WW, Villamor E. Subclinical mastitis, cell-associated HIV-1 shedding in breast milk, and breast-feeding transmission of HIV-1. J Acquir Immune Defic Syndr. 2007;46:651–4. doi: 10.1097/QAI.0b013e31815b2db2. [DOI] [PubMed] [Google Scholar]
- 8.Lewis P, Nduati R, Kreiss JK, John GC, Richardson BA, Mbori-Ngacha D, Ndinya-Achola J, Overbaugh J. Cell-free human immunodeficiency virus type 1 in breast milk. J Infect Dis. 1998;177:34–9. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto C, Nakagawa Y, Ohara K, Takahashi H. Polyriboinosinic polyribocytidylic acid [poly(I:C)]/TLR3 signaling allows class I processing of exogenous protein and induction of HIV-specific CD8+ cytotoxic T lymphocytes. Int Immunol. 2004;16:55–63. doi: 10.1093/intimm/dxh025. [DOI] [PubMed] [Google Scholar]
- 11.Shinya E, Owaki A, Shimizu M, et al. Endogenously expressed HIV-1 nef down-regulates antigen-presenting molecules, not only class I MHC but also CD1a, in immature dendritic cells. Virology. 2004;326:79–89. doi: 10.1016/j.virol.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi J, Watari E, Shinya E, et al. Down-regulation of Toll-like receptor expression in monocyte-derived Langerhans cell-like cells: implications of low-responsiveness to bacterial components in the epidermal Langerhans cells. Biochem Biophys Res Commun. 2003;306:674–9. doi: 10.1016/s0006-291x(03)01022-2. [DOI] [PubMed] [Google Scholar]
- 13.Englund G, Theodore TS, Freed EO, Engelman A, Martin MA. Integration is required for productive infection of monocyte-derived macrophages by human immunodeficiency virus type 1. J Virol. 1995;69:3216–9. doi: 10.1128/jvi.69.5.3216-3219.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freed EO, Englund G, Martin MA. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J Virol. 1995;69:3949–54. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy KC, Bandyopadhyay G, Rakshit S, Ray M, Bandyopadhyay S. IL-4 alone without the involvement of GM-CSF transforms human peripheral blood monocytes to a CD1a(dim), CD83(+) myeloid dendritic cell subset. J Cell Sci. 2004;117:3435–45. doi: 10.1242/jcs.01162. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 17.O’Mahony DS, Pham U, Iyer R, Hawn TR, Liles WC. Differential constitutive and cytokine-modulated expression of human Toll-like receptors in primary neutrophils, monocytes, and macrophages. Int J Med Sci. 2008;5:1–8. doi: 10.7150/ijms.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong KF, Delroux K, Wang X, Qian F, Arjona A, Malawista SE, Fikrig E, Montgomery RR. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–23. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore JS, Rahemtulla F, Kent LW, Hall SD, Ikizler MR, Wright PF, Nguyen HH, Jackson S. Oral epithelial cells are susceptible to cell-free and cell-associated HIV-1 infection in vitro. Virology. 2003;313:343–53. doi: 10.1016/s0042-6822(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 20.Lyimo MA, Howell AL, Balandya E, Eszterhas SK, Connor RI. Innate factors in human breast milk inhibit cell-free HIV-1 but not cell-associated HIV-1 infection of CD4+ cells. J Acquir Immune Defic Syndr. 2009;51:117–24. doi: 10.1097/QAI.0b013e3181a3908d. [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo-Useros N, Blanco J, Erkizia I, et al. Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J Virol. 2007;81:7559–70. doi: 10.1128/JVI.02572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


