Abstract
Background
Fatigue is a common symptom in Western high-income countries but is often medically unexplained and little is known about its presentation in other populations.
Aims
To explore the epidemiology and aetiology of fatigue in Sri Lanka, and of its overlap with depression.
Method
A total of 4024 randomly selected twins from a population-based register in Sri Lanka (Colombo district) completed home interviews including the Chalder Fatigue Questionnaire.
Results
The prevalence of fatigue was similar to that in other countries, although prolonged fatigue may be less common. There was substantial comorbidity with a screen for lifetime depression. Non-shared environmental factors made the largest contributions, although genetic/family factors also contributed. The aetiology appeared consistent across the spectrum of severity.
Conclusions
The aetiology of fatigue is broadly similar in Sri Lanka and Western high-income countries. Abnormal experiences of fatigue appear to be the extreme form of more common fatigue, rather than representing independent entities with different genetic or environmental risk factors.
Most fatigue research has been conducted in high-income Western countries despite a greater rate of fatigue presentations to health services in low- and middle-income countries.1 Most studies of symptomatic fatigue and chronic fatigue (i.e. present for at least 6 months) find that, although a small proportion of cases can be accounted for by defined biomedical disease (e.g. cancer, rheumatoid arthritis) or treatments, the majority of cases are ‘medically unexplained’.2,3 In Europe and the USA, about a third of people experience troublesome fatigue,2,4 5–12% chronic fatigue2,5,6 and 0.5–2% chronic fatigue syndrome.7 Twin studies are one method by which the overall aetiological contributions to fatigue can be investigated; in European-derived populations twin studies have suggested a substantial role for genes but a larger contribution from environmental factors.8–10 Fatigue falls on a continuum without notable cut-points,11 which suggests that studying broader definitions could be informative about more severe and prolonged cases. Most clinical and epidemiological studies4,12,13 indicate that risk factors are similar across the spectrum of severity. This suggests that more severe forms are quantitatively, but not qualitatively, different from milder fatigue; however, this has not been tested using a genetically sensitive method. Despite the potential heterogeneity within fatigue, and although fatigue symptoms can be separated from mood symptoms, there are striking associations between fatigue or chronic fatigue syndrome and psychiatric symptoms or disorders in the general population.8,14,15 Although the aetiology of this overlap is poorly understood, evidence from birth cohorts indicates that psychiatric disorder may often predispose individuals to fatigue or chronic fatigue syndrome15 and twin studies implicate genetic pleiotropy across symptoms.8,9 Thus fatigue is an important, prevalent, disabling but poorly understood phenomenon. The aim of the current study was to investigate whether these patterns of findings are the same in a non-Western low-income country, Sri Lanka, where the important environmental impacts and cultural contexts might be expected to be considerably different to those previously studied.
Method
The study received approval from the Institute of Psychiatry, King’s College London Research Ethics Committee; the Ethical Review Committee, University of Sri Jayewardanepura; and the World Health Organization’s (WHO’s) Research Ethics Committee.
Study design and participants
The study aimed to examine the prevalence and aetiology of fatigue in Sri Lanka, and of its overlap with depressive episodes. In addition, it aimed to clarify the relationship between the range of normal variation and more pronounced fatigue.
This was a population-based twin study, the twin component of the Colombo Twin And Singleton Study (CoTASS). Full details of the design and implementation of the study are described elsewhere.16 Briefly, the study took place in the Colombo District of Sri Lanka, an area with a population of 2.2 million that includes the island’s capital and urban to semi-urban areas. We added a question to the update of the annual census asking whether the householder knew of any twins and identified 19 302 individual twins by this method. Of these, we randomly selected 4387 individual twins who were at least 15 years old to take part in the project on common mental disorders. A total of 4024 (91.7%) participated, including 1954 complete twin pairs. We included all consenting individuals aged 15 years or older who spoke sufficient Sinhala to understand the interview, excluding those who failed a mini-mental state examination and those whose data was provided by a proxy. The mean age of the twins was 34.0 years, 46.2% were male and 90.1% were of Sinhalese ethnicity. High-school-educated research workers visited the participants’ homes to interview them each separately. Interviews took place between 2006 and 2007 when Sri Lanka had been experiencing violent civil war for over 20 years. However, much of the conflict has centred in areas to the North and East of the island, far from the location of the current study. Nonetheless, a small minority (2.6%) of the participants reported directly participating in the conflict as combatants.
Measures
All interviews and questionnaires were translated at least twice independently into Sinhala, and were then reviewed by a group of professionals and a scholar in Sinhala, and finally trialled to confirm lay people could understand it.16 All questionnaires were administered as interviews.
Participants were assessed with the Chalder Fatigue Questionnaire.17 This includes 11 core fatigue items, and 2 assessing muscle pain, experienced over the past month (scored as: less than usual, no more than usual, more than usual or much more than usual, which were coded as 0, 0, 1, 1 for categorical analysis and 0, 1, 2, 3 for continuous analysis). Those who had had fatigue for a long time were asked to rate their past month’s experiences compared with when they last felt well. It also assesses what percentage of the time fatigue is present, and among those who are currently fatigued, it assesses how long this has lasted. We created three variables to analyse fatigue both as a continuum and as dichotomies indicating potentially clinically significant fatigue. ‘Fatigue severity’ is a continuous scale using all 13 symptom items each scored 0–3. For genetic analyses, this scale was log-transformed to remove skew, and the mean effects of age and gender were regressed out (in order to avoid artificially inflated estimates of shared environmental factors). ‘Abnormal fatigue’ indicates that 3 of the 11 fatigue items were present at least ‘more than usual’; ‘prolonged fatigue’ indicates that at least 4 of the 11 fatigue items were present at least ‘more than usual’ and for at least 50% of the time and for at least 6 months.
A dimensional approach to measurement can be useful especially where there appears to be no ‘point of rarity’ marking a discrete disorder and where there may be a complex combination of risk factors involved in aetiology, which is unlikely to be adequately indexed by a single diagnostic category.18 It is also appropriate where there is no clear Mendelian pattern of inheritance, suggesting that any genetic effect may come from many separate loci, each of small effect size, and each of which may contribute to the range of normal variation as well as differentiating cases from controls (i.e. the quantitative trait loci hypothesis).19 The current study used a mix of dimensional and categorical definitions of fatigue in order to test the consistency of the findings across different severity levels. Prior population-based twin studies have only examined approximations of chronic fatigue syndrome rather than the official definition, partly because of the large sample sizes required for twin analysis of uncommon conditions in representative populations. The current study also takes this approach, but explicitly tests the validity of making comparisons between broader and narrower measures of fatigue. It is also the first genetically informative exploration of fatigue symptoms outside of the Western high-income world.
All participants were interviewed with the Composite International Diagnostic Interview.20 This includes two ‘probe’ questions relating to lifetime ever depression (which are the two core symptoms of depression according to DSM–IV/ICD–10).21,22 These are low mood and loss of interest in things normally enjoyed for at least a 2-week period (thus neither item tapped fatigue as a symptom within the depressive episode). We used a positive score on either of these as a screen for lifetime ever depression, ‘D-probe’, because this relatively broad definition is suitable for twin analysis especially in the context of broad definitions of fatigue and because it avoids potential cultural biases in the assessment of impairment.
An item from the Short Form–36 was used to indicate social/functional impairment: ‘During the past 4 weeks, how much of the time has your physical health or emotional problems interfered with social activities (like visiting friends, relatives etc)?’23 This item was scored on a Likert scale but was collapsed into a binary (yes/no) score. Zygosity was assessed using a validated questionnaire24,25 administered to both twins.
Analyses
A database was constructed, and correlational and extremes analyses were performed in Stata version 10.1 for Windows. Analyses were corrected for non-independence within pairs where appropriate. Genetic model fitting was performed in Mx for Windows (www.vcu.edu/mx/index.html).
The twin analyses involve calculating the proportion of individual differences in a given trait that can be detected as a result of additive genetics (A), environments shared between twins (C) and environments unique to each twin in a pair (E). The size of these parameters is estimated based on the knowledge that monozygotic (MZ) pairs of twins share 100% of their genetic material, whereas dizygotic (DZ) pairs share on average 50% of their genetic material, relative to the rest of the population. By definition, both MZ and DZ twin pairs share 100% of their shared environments and 0% of their non-shared environments.
Extremes analyses (comparing extreme groups with normal variation)
In order to test the hypothesis that the aetiology of fatigue is the same at the extreme as in the range of normal variation, we ran DeFries–Fulker extremes regression models.26 This method typically requires a continuously measured trait, onto which a cut-point is made in order to create a dichotomy to identify probands. The co-twin mean score is compared with the proband mean and the population mean. In this study we used fatigue severity as the continuous measure and abnormal fatigue as the categorical variable to define probands. We are in essence conducting a bivariate analysis to describe the relationship between an extreme dichotomy and the range of fatigue reported in the population measured as a continuous trait.27 To increase power, we compared both male and female MZ pairs to male, female and opposite gender DZ pairs (this approach is supported by a lack of gender differences in the univariate models below). The regression models allow calculation of group heritability (gA) and group environmentality (gC and gE). These indicate the extent to which A, C and E factors are responsible for the probands having a higher mean than the rest of the population. Evidence of gA and/or gC indicates that some of the A or C factors that influence the extreme also influence the normal distribution.
In more detail, the fatigue severity mean scores of the co-twins of the probands are standardised on the proband mean scores. This is referred to as the group co-twin correlation (or transformed co-twin mean) because it focuses on the mean score of the co-twins rather than individual differences.27 The degree of regression of this transformed co-twin mean to the population mean is examined. If there are genetic factors that influence the extreme as well as normal variation of fatigue, we would expect the MZ co-twins to regress less towards the mean than DZ co-twins.
Twin variance component models (individual differences)
Univariate genetic models decompose the variance in each trait into that resulting from A, C and E (i.e. the variation between individuals is divided into that accountable by genetic, shared environmental and non-shared environmental factors). The estimated model is compared with the observed data in order to produce the maximum likelihood fit of the model. This model fit is compared with that of a fully saturated model. Separate thresholds or means were estimated according to gender. Univariate genetic models were performed for the continuously measured fatigue severity and categorical abnormal fatigue. Twin variance components models using categorical data assume a liability threshold model (for more details, see footnotes to Tables 4 and 5).
Table 4.
Univariate genetic models: fatigue severity and abnormal fatiguea
| Parameter estimates, % (95% CI)
|
Fit of model
|
||||||
|---|---|---|---|---|---|---|---|
| A | C | E | Δχ2 | Δd.f. | P | ΔAIC | |
| Fatigue severityb | |||||||
| ACE | 21 (4–34) | 7 (0–20) | 72 (66–79) | 2.93c | 2 | 0.231 | –1.07 |
| AE | 30 (24–35) | 0 | 70 (65–76) | 1.08d | 1 | 0.300 | –0.92 |
| CE | 0 | 22 (17–26) | 78 (74–83) | 5.72d | 1 | 0.017 | 3.72 |
| E |
0
|
0
|
100
|
93.47d |
2
|
<0.001
|
89.47
|
| Abnormal fatigue | |||||||
| ACE | 29 (0–48) | 8 (0–32) | 62 (52–74) | 5.70c | 2 | 0.058 | 1.70 |
| AE | 39 (29–49) | 0 | 60 (51–71) | 0.47d | 1 | 0.492 | –1.53 |
| CE | 0 | 29 (22–37) | 71 (63–78) | 3.40d | 1 | 0.065 | 1.40 |
| E | 0 | 0 | 100 | 54.75d | 2 | <0.001 | 50.75 |
A, additive genetics; C, environmental influences shared across twins; E, non-shared environments; AIC, Akaike’s information criterion.
a. Results for the best fit models (based on χ2 and AIC) shown in bold. The results for categorical abnormal fatigue (and those in Table 5) assume a liability threshold model. This is the assumption that everyone in the population is to a greater or lesser degree liable to the disorder (on a continuous, normally distributed scale). Those people above a certain threshold of liability display the disorder (tetrachoric correlations also operate on this basis). Rather than estimating a mean score, the threshold is estimated. If the disorder’s prevalence varies with age, this can be corrected for by including an age regression term in the model of the thresholds.31 For abnormal fatigue, we tested the significance of both linear and quadratic effects of age, and whether they could be equated across genders. We then fixed them to the estimated values prior to estimating the variance components.
b. A scalar model was fitted as a result of a significantly higher variance in women than men.
c. Compared with model estimating parameters separately for each gender.
d. Compared with ACE model.
Table 5.
Bivariate genetic model of the overlap between abnormal fatigue and a screen for lifetime depressive episodes (D-probe) (same-gender twin pairs only)a
| Bivariate phenotypic correlation (within-person) | Aetiology of the bivariate correlation, % (95% Cl)b |
Fit compared with saturated model
|
||||||
|---|---|---|---|---|---|---|---|---|
| ACE model | A | C | E | Δχ2 | Δd.f. | P | ΔAIC | |
| Gender | 9.88 | 10 | 0.451 | –10.12 | ||||
| Men | 0.40 | –11 (–19 to 21) | 23 (–3 to 34) | 27 (14 to 41) | ||||
| Women | 0.36 | 17 (–9 to 43) | 10 (–10 to 33) | 8 (–2 to 20) | ||||
A, additive genetics; C, environmental influences shared across twins; E, non-shared environments; AIC, Akaike’s information criterion.
Men: A: Δχ2 = 0.82, d.f. = 1, P = 0.36, AIC = –1.18; C: Δχ2 = 3.21, d.f. = 1, P = 0.07, AIC = 1.21; E: Δχ2 = 14.15, d.f. = 1, P<0.001, AIC = 12.15.
Women: A: Δχ2 = 1.33, d.f. = 1, P = 0.25, AIC = –0.67; C: Δχ2 = 0.60, d.f. = 1, P = 0.44, AIC = –1.40; E: Δχ2 = 2.27, d.f. = 1, P = 0.13, AIC = 0.27.
Note: these results were calculated by dropping covariance paths in the Cholesky model, but for ease of presentation the results are presented as the mathematically equivalent correlated factors solution, in which these tests correspond to dropping rA, rC and rE (Fig. 1).
a. In the bivariate model the regression parameters correcting for the effect of age on the thresholds (for abnormal fatigue and D-probe) were fixed as those estimated in the respective univariate models.
b. Tests of A, C and E covariance paths are as follows:
A bivariate categorical genetic model was used to decompose the covariance between abnormal fatigue and D-probe into A, C and E influences. Due to the gender differences in the heritability of D-probe reported in a previous study,28 the bivariate model was run to examine males and females separately (opposite-gender pairs were not included). A Cholesky model was fitted, in which the latent A, C and E factors for the first variable (abnormal fatigue) are also allowed to load onto the second variable (D-probe). There is also a separate set of A, C and E factors that load only on the second variable (D-probe). However, for ease of interpretation, we will present the mathematically equivalent correlated factors solution. Here, separate A, C and E parameters are estimated for each variable, and the extent that the phenotypic overlap is a result of A, C or E is calculated (Fig. 1).
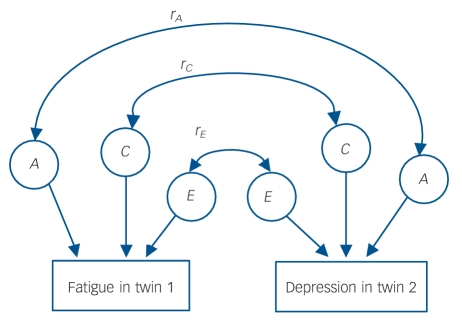
Fig. 1.
Diagram representing the bivariate twin model.
Only one twin from each pair is shown. The double-headed arrows represent the correlations between latent factors (e.g. r, the correlation between the genetic influence (A) on fatigue and the genetic influence on depression). The genetic contribution to the overall phenotypic overlap is found by examining the size of three paths: the genetic contribution to fatigue, r, and the genetic contribution to depression. This is also calculated for C (shared environment) and E (non-shared environment). Since the total phenotypic correlation consists of contributions from A, C and E, percentage contributions can then be calculated.
Results
Table 1 shows that men had less fatigue than women, both when measured as a continuous variable (t = 5.48, P<0.001) and as a categorical variable, presence/absence of abnormal fatigue (z = 5.11, P<0.001). The prevalence of prolonged fatigue was low, giving low power to detect gender differences.
Table 1.
Severity and prevalence of fatigue
| Men | Women | All | Men, n | Women, n | All, n | |
|---|---|---|---|---|---|---|
| Fatigue severity (range 0–39),a mean (95% CI)
|
14.5 (14.3–14.6)
|
15.1 (14.9–15.2)
|
14.8 (14.7–14.9)
|
|||
| Categorical: % prevalence (95% CI) | ||||||
| Abnormal fatigue | 21.4 (19.5–23.3) | 28.6 (26.7–30.5) | 25.3 (23.9–26.7) | 381 | 594 | 975 |
| Prolonged fatigue | 1.1 (0.6–1.6) | 1.0 (0.6–1.4) | 1.1 (0.7–1.4) | 20 | 21 | 41 |
a. Prior to transformation.
Low prevalence of prolonged fatigue
To assess the duration of fatigue, participants were asked ‘If you are tired at the moment, please indicate approximately how long this has lasted’. In total, 72% of those who reported sufficient specific fatigue symptoms over the past month for the most extreme definition denied experiencing fatigue at the moment of the interview when asked about its duration (for comparison, in a random sample of UK armed forces personnel using the same questionnaire29 this figure was roughly a quarter). This subset, who did admit to substantial generalised fatigue over the past month (only 20% claimed to be not tired in response to the question: ‘Overall, what percentage of the time do you feel tired’), were thus ineligible for a duration score, and this apparent disparity partially accounts for the low prevalence of prolonged fatigue.
The data were double checked, and the translation of the relevant items was confirmed to be accurate and to reflect the same meaning as the English version, so there is a high level of confidence in the validity of these responses. The disparity may instead relate to the context of the study population (e.g. what is viewed as an abnormal level of tiredness in Sri Lanka), in which case the individuals meeting criteria for prolonged fatigue may be representing a more severe (and thus possibly more reliable and informative) subset of the phenotype than those picked up under similar criteria in other countries. The disparity may also have been triggered by the administration of the questionnaire as a face-to-face interview, whereas in other settings it has usually been completed by the participant alone, using pen and paper. Alternatively, this result may suggest that although fatigue is a common condition in Sri Lanka, prolonged fatigue is considerably rarer. Thus the responses are valid in respect of the items asked, but precise wording of the questions (i.e. reference to the specific moment of the interview) may have influenced the prevalence. Because of this uncertainty, the prolonged fatigue category was used as a supplementary category for comparison rather than a focus of the analyses in this paper.
Impairment
Reporting of social impairment over the past month was higher among those who either reported fatigue over the past month or 2 weeks of depressive symptoms over their lifetime (i.e. D-probe) (Table 2). Impairment was significantly more common among those reporting both abnormal fatigue and lifetime depressive symptoms compared with abnormal fatigue only (Wald test of equality of odds ratios: χ2 = 14.2, P <0.001), but this fell short of significance when repeated using prolonged fatigue (χ2 = 2.1, P = 0.14). So, having both abnormal fatigue and D-probe is a marker of heightened impairment, which makes it particularly interesting to explore the aetiological factors explaining why some people report both, as we have done in the bivariate model reported at the end of the results section.
Table 2.
Social impairment among participants with fatigue and/or a screen for lifetime depressive episodes (D-probe)
| % impaired | Odds ratioa | |
|---|---|---|
| Whole sample
|
15.8
|
–
|
| Model 1: abnormal fatigue | ||
| Fatigue only | 28.5 | 4.0 (3.3–5.0) |
| D-probe only | 21.7 | 2.8 (2.1–3.8) |
| Fatigue + D-probe
|
41.2
|
7.1 (5.4–9.3)
|
| Model 2: prolonged fatigue | ||
| Fatigue only | 56.7 | 7.7 (3.9–15.3) |
| D-probe only | 29.2 | 2.8 (2.2–3.4) |
| Fatigue + D-probe | 76.2 | 17.7 (6.8–46.0) |
a. Adjusted for age, gender and ethnicity.
Extremes analysis
The Defries–Fulker extremes analysis using abnormal fatigue as the cut-off (and fatigue severity to index normal variation) suggested an important influence of group heritability but not group-shared environmentality (gA = 24%, P = 0.026; gC = 4%, P = 0.309; n = 774 pairs of twins selected for analysis, i.e. with at least one twin with abnormal fatigue). This indicates that there is aetiological overlap between the extreme and normal variation.
Correlations
In order to examine the heritability of the different definitions of fatigue, we first examined the cross-twin correlations for fatigue severity and abnormal fatigue (Table 3). We did not examine cross-twin correlations or variance decomposition models for the narrower definition of prolonged fatigue because of low prevalence. The MZ correlations were slightly greater than the DZ correlations among men, whereas the correlations were more similar across zygosity for women, perhaps indicating greater genetic influence in men and greater shared environmental influences in women. However, the confidence intervals around these estimates are wide. None of the correlations were greater than 0.48 indicating a considerable role for non-shared environmental factors.
Table 3.
Cross-twin and within-person correlations (95% CIs) for fatigue and a screen for lifetime depressive episodes (D-probe)a
| Monozygotic, male | Dizygotic, male | Monozygotic, female | Dizygotic, female | Dizygotic, opposite gender | Men (n = 1764) | Women (n = 2056) | All (n = 3820) | |
|---|---|---|---|---|---|---|---|---|
| Univariate (cross-twin) correlation, n pairs | 356 | 254 | 453 | 301 | 513 | |||
| Fatigue severity, correlation (95% CI) | 0.29 (0.20 to 0.39) | 0.25 (0.14 to 0.37) | 0.26 (0.18 to 0.35) | 0.20 (0.10 to 0.31) | 0.15 (0.06 to 0.23) | |||
| Abnormal fatigue, correlation (95% CI)
|
0.48 (0.32 to 0.64)
|
0.36 (0.15 to 0.58)
|
0.35 (0.20 to 0.49)
|
0.35 (0.17 to 0.52)
|
0.16 (0.01 to 0.31)
|
|||
| Bivariate (within-person) correlation with D-probe (95% CI) | ||||||||
| Fatigue severity | 0.33 (0.26 to 0.39) | 0.26 (0.20 to 0.31) | 0.28 (0.24 to 0.33) | |||||
| Abnormal fatigue | 0.38 (0.29 to 0.47) | 0.39 (0.32 to 0.47) | 0.39 (0.34 to 0.45) | |||||
| Prolonged fatigue
|
0.50 (0.32 to 0.68)
|
0.33 (0.13 to 0.53)
|
0.41 (0.28 to 0.55)
|
|||||
| Bivariate (cross-twin) correlation with D-probe, n pairs | 359 | 256 | 457 | 306 | 518 | |||
| Abnormal fatigue correlation (95% CI) | 0.03 (–0.14 to 0.20) | 0.29 (0.11 to 0.47) | 0.31 (0.18 to 0.43) | 0.16 (0.00 to 0.32) | 0.13 (0.01 to 0.25) |
a. Correlations are tetrachorlc for binary data; intraclass for continuous data; point-biserial where there is a combination of categorical and continuous data.
A history of D-probe (the screen for lifetime depressive episodes) was associated with abnormal fatigue (odds ratio (OR) = 3.19, 95% CI 2.64–3.84) and prolonged fatigue (OR = 6.47, 95% CI 3.36–12.47), when looking within individuals but controlling for familial relatedness. All three of the fatigue indices correlated significantly with D-probe; the magnitude of this correlation was slightly less when assessing the full range of fatigue variation (fatigue severity) than the two categorical indices (Table 3). This is consistent with research from a WHO study in 14 countries30 that showed a stronger relationship between depression and (unexplained) fatigue syndromes with narrower definitions of fatigue. The bivariate cross-twin correlations suggest genetic factors are involved in this association for women only (because the MZ cross-trait correlation is roughly double that for DZs, Table 3). However, the confidence intervals are wide, and the relatively low magnitude of the MZ male bivariate correlation suggests a large influence from non-shared environmental factors.
Univariate twin models
Fatigue severity had a larger standard deviation in women than in men (0.17 and 0.14 respectively, F = 0.704, P<0.001), so we ran a scalar variance components model to the saturated model, which fit well (Δχ2 = 10.06, d.f. = 9, P = 0.346). The variance components models for abnormal fatigue also fit the respective saturated model well (Δχ2 = 0.34, d.f. = 1, P = 0.560). For both definitions, there was no evidence of qualitative gender differences (P-values >0.56). There were no quantitative gender differences (there was a significant familial influence in both genders, although men tended to have higher A and lower C estimates, and this was marginally significant for abnormal fatigue (P = 0.058)). We proceeded using models with ACE parameters equated for men and women (Table 4).
The ACE parameter estimates were similar across the two definitions. The largest contribution to the variance came from the non-shared environment (72% in fatigue severity and 62% in abnormal fatigue), followed by additive genetics (21% and 29%) and with minimal estimated contributions from the shared environment (7% and 8%). These values are very similar to the group heritability and group-shared environmentality obtained in the extremes analyses. The effect of additive genetics was significant in the fatigue severity model (P = 0.017) and was marginally significant for abnormal fatigue (P = 0.065). Neither of the shared environmental parameters were significantly greater than zero (P-values >0.30). The data are best explained by the AE models (i.e. additive genetic plus non-shared environmental effects).
It should be noted that statistical power to detect gender differences was low, particularly for the category of abnormal fatigue. This is partly because the large magnitude of the E parameter makes it hard to distinguish the relative contributions of (or possibly the mix of) A and C. Therefore these results only provide tentative evidence of lack of gender differences, although they are useful in telling us that an AE model best explains the data for men and women combined. Confidence in the abnormal fatigue univariate results is improved as a result of the similar magnitude of aetiological effects identified both with the continuously measured fatigue severity, and the extremes analyses examining the overlap between fatigue severity and abnormal fatigue.
Bivariate twin model
Finally, we ran a bivariate model to decompose the covariation between abnormal fatigue and D-probe (see Table 5 for parameter estimates). As a result of previously identified gender differences in D-probe,28 we did not attempt to equate the model across genders. The only significant individual contribution to the overlap in men was from E. In women, none of the three parameters were individually significant using a χ2-test, although E was the most likely contributor based on Akaike’s information criterion (AIC). However, although we could not distinguish between A and C, there was significant familial influence (combination of A and C) in both men (Δχ2 = 7.03, d.f. = 2, P = 0.03, AIC = 3.03) and women (Δχ2 = 24.29, d.f. = 2, P<0.001, AIC = 20.29). So the overlap between fatigue and history of depressive symptoms is associated with high levels of impairment, and this overlap is explained by a combination of person-specific environments and familial factors.
Discussion
The aims of this study were to examine the characteristics and determinants of fatigue in a population-representative sample from outside Western high-income countries; and to describe the relationship between the normal range of fatigue and more extreme presentations. Key findings were that troublesome fatigue was common in Sri Lanka with a prevalence of 25%, but prolonged fatigue was considerably less common (roughly 1%). The prevalence of troublesome fatigue is in line with findings in other countries,2,4 but prolonged fatigue in Sri Lanka was lower than in many Western high-income countries;2,5,6 however, this may have been influenced by different interpretations of the precise wording of the item assessing duration.
We found the largest aetiological influence came from non-shared environmental factors and familial similarity in fatigue appears to be a result of shared genes rather than shared environment. An aetiological overlap was identified between the determinants of variation in the normal range of fatigue and in more extreme cases. This means that the risk and protective factors contributing to the normal range of fatigue also contribute to more severe and prolonged fatigue.
Fatigue was associated with a positive screen for past depressive episodes, and both contributed to impairment in functioning. Impairment was especially marked among people affected by both, making it particularly pertinent to understand the reasons behind this overlap. In other countries, genetic or familial factors have been found to be responsible for the overlap between fatigue and mental distress.8,9,32 Although familial factors did contribute in Sri Lanka, we also found influences from non-shared environmental factors.
Low prevalence of prolonged fatigue
The low prevalence of fatigue in Sri Lanka compared with estimates from high-income Western countries mirror findings from a WHO multinational study, which found that fatigue assessed via direct questioning was less prevalent in low-compared with high-income countries.1 The prevalence in Sri Lanka was particularly low for prolonged fatigue (which required the participant to respond positively to further questioning, including being fatigued at the time of the interview). This could be a true difference in underlying prevalence, suggesting a difference in the identity or prevalence of some of the risk factors, or an effect of the wider social or physical environment buffering the effect of specific risk factors. Alternatively, the low prevalence may reflect a tendency for participants to play down symptoms upon further questioning (Sri Lankan participants might be more likely to view fatigue as a fact of life rather than something unusual to be commented on or investigated). The WHO study conversely found more spontaneous fatigue presentations in low-compared with high-income countries1 and a study in Goa, India,33 found rates comparable with high-income countries using questions from the Revised Clinical Interview Schedule (CIS–R). These patterns suggest that the precise wording of questions is important and interpretation of the questions may differ across countries. However, the initial items on the Chalder Fatigue Questionnaire used in this study (to generate the fatigue severity and abnormal fatigue classifications) may be particularly useful, because they specifically enquire about the past month’s symptoms in relation to what is usual for the participant (i.e. compared with the previous month, except where the participant has been fatigued for a long time, in which case the comparison is with when they last felt well).
Aetiology
The overall aetiology of fatigue was similar to that in high-income Western countries,8–10 although power constraints make it hard to be precise. This is noteworthy given the different profile of communicable versus non-communicable disease in Sri Lanka, which might be expected to lead to a different aetiological profile than that seen in high-income Western countries, if fatigue is typically a consequence of medical conditions each with differing risk factors. Nonetheless, the results did suggest a small contribution from genetic factors, in keeping with genetically informative research on chronic fatigue syndrome that has suggested larger genetic influences on objective domains (e.g. sleep latency, cold pain threshold and tolerance) than subjective domains (e.g. reports of fatigue or pain).34 There was evidence that suggested that environmental influences on the overlap between fatigue and an indicator of depression were higher in Sri Lanka than in high-income Western countries. This is a tentative finding because of the uncertainty in the estimates of the bivariate analyses, and may be partly as a result of low power (we could not rule out an overall familial effect). Alternatively, it might reflect differences in measurement, or possibly more varied experiences of actual environmental factors influencing both fatigue and mental health in Sri Lanka. A previous study on the same sample35 has shown how factors including poverty, housing and working conditions are related to depression in Sri Lanka, especially among men (a corresponding gender difference was not found in relation to fatigue in the current report, except when examining the overlap with depression).
Although non-shared environmental influences (E) strongly influence fatigue both in Sri Lanka and in other countries, the precise nature of the exposure may differ. E could include each twin’s separate occupational and social activities and thus their differential exposure to stressors and infections over the past month or beyond, where these exposures would lead to cross-twin differences in tiredness. Some research has linked chronic fatigue syndrome to infections such as the Epstein–Barr virus.36,37 However, other research has downplayed a link to infections,38 or else suggests that individual responses to infection such as immune activation or prolonged convalescence are important,39,40 particularly when considering (in relation to Epstein–Barr virus) the high proportion of cases that do not go on to develop chronic fatigue syndrome. More longstanding influences could also be important, e.g. lifestyle factors such as childhood over-exercise (which is a prospective risk factor)41 or childhood trauma which is linked to psychopathology as well as chronic fatigue syndrome42 – such experiences often differ for each twin in a pair, or are perceived differently by each twin. The E influences in the univariate models also incorporate measurement error.
The finding that the aetiology of fatigue is similar for the narrow and broad definitions (i.e. abnormal fatigue and fatigue severity) concurs with data from over 30 000 Swedish twins. The Swedish study compared the 95% confidence intervals for varying definitions of fatigue (including chronic fatigue syndrome-like illness assessed using medical exclusions), and showed that the A/C/E proportions did not change according to the stringency of the definition.10 The current study adds that the genetic and environmental risk factors that contribute to excessive and prolonged experiences of fatigue (abnormal fatigue and prolonged fatigue) also appear to influence the normal range of fatigue (fatigue severity), and that the different definitions were all associated with a screen for history of depressive episodes. This suggests that, although fatigue may have non-psychiatric medical causes, the links with psychiatric morbidity are substantial and may be more important than environmental exposures or disease burden on a population level.
We found no evidence of gender differences in the aetiology of fatigue, which is interesting in the light of the relative status of men and women in Sri Lanka. The gender discrepancy experienced in everyday life in Sri Lanka may be more pronounced than in many Western and/or higher-income countries, but in certain aspects it is less pronounced than other countries of the Indian subcontinent, for example gender equality has already been reached in Sri Lanka in primary and secondary education.43
Limitations
The current study assessed fatigue over the past month (and for prolonged fatigue, whether this had been present for the past 6 months). This might have led to underestimates of true heritability because of the possibility of twins being discordant over the month but concordant over their lifetimes. However, these analyses are useful for the purposes of making comparisons with data from other countries that used similar methods.
The specificity of our findings are limited because we did not use any medical exclusions. However, previous results from Sweden found that the aetiology of fatigue was similar regardless of the stringency of the definition used, or the use of medical exclusions.10 Moreover, this is a relatively young population-based sample,16 few of whom are likely to be affected by medically diagnosable physical disorders. If there were a range of undetected medical diagnoses that contributed to reporting of fatigue, this is likely to have increased measurement error and so the calculated heritability could be an underestimate.
The precise wording of the fatigue duration item may have influenced the prevalence of the ‘prolonged fatigue’ category, so it is not clear whether this reflects a true difference in the rate of extended experiences of fatigue in Sri Lanka. However, the other two definitions are much more likely to have been interpreted in a consistent manner across countries because they focus on recent experiences in comparison with what is usual for the participant.
The assumptions of the twin method apply to these findings. For example, it is assumed that MZ pairs are not treated any more similarly than DZ pairs purely on the basis of zygosity or that such differential treatment is not relevant for the phenotypes under investigation. Such assumptions have been supported through tests in relation to psychiatric disorders.44
Conclusions
Fatigue in Sri Lanka is, as in Western countries, common and strongly associated with depressive symptoms. There is evidence from our work that both fatigue as a spectrum and abnormal fatigue are mainly determined by non-shared environmental influences, although there is a modest genetic component, and that the aetiology is consistent across the spectrum of severity.
Funding
The Wellcome Trust provided funding for the CoTASS study, and the Institute for Research and Development, Sri Lanka, provided infrastructural support. H.A.B. is supported by an ESRC research studentship. M.H. is funded by the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King’s College London, National Institute of Health Research, Biomedical Research Centre.
Declaration of interest
P.M. has received honoraria from Eli Lilly and GlaxoSmithKline and has acted as a consultant in the recent past for GlaxoSmithKline and AstraZeneca. N.G. has received honoraria from Sanofi-Aventis and Servier.
See editorial pp. 86–87, this issue.
References
- 1.Skapinakis P, Lewis G, Mavreas V. Cross-cultural differences in the epidemiology of unexplained fatigue syndromes in primary care. Br J Psychiatry 2003; 182: 205–9. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer H, Gill B, Petticrew M. The Prevalence of Psychiatric Morbidity Among Adults Aged 16–64, Living in Private Households, in Great Britain. Office of Population Censuses and Surveys Social Surveys Division, 1994.
- 3.Wessely S. The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health 1997; 87: 1449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJM, Wessely SC. Population based study of fatigue and psychological distress. BMJ 1994; 308: 763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res 1998; 45: 53–65. [DOI] [PubMed] [Google Scholar]
- 6.Jason LA, Jordan KM, Richman JA, Rademaker AW, Huang CF, McCready W, et al. A Community-based study of prolonged fatigue and chronic fatigue. J Health Psychol 1999; 4: 9. [DOI] [PubMed] [Google Scholar]
- 7.Ranjith G. Epidemiology of chronic fatigue syndrome. Occup Med 2005; 55: 13–9. [DOI] [PubMed] [Google Scholar]
- 8.Williamson R, Purcell S, Sterne A, Wessely S, Hotopf M, Farmer A, et al. The relationship of fatigue to mental and physical health in a community sample. Soc Psychiatry Psychiatr Epidemiol 2005; 40: 126–32. [DOI] [PubMed] [Google Scholar]
- 9.Hickie I, Kirk K, Martin N. Unique genetic and environmental determinants of prolonged fatigue: a twin study. Psychol Med 1999; 29: 259. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan PF, Evengard B, Jacks A, Pedersen NL. Twin analyses of chronic fatigue in a Swedish national sample. Psychol Med 2005; 35: 1327. [DOI] [PubMed] [Google Scholar]
- 11.David A, Pelosi A, McDonald E, Stephens D, Ledger D, Rathbone R, et al. Tired, weak, or in need of rest: fatigue among general practice attenders. BMJ 1990; 301: 1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane TJ, Manu P, Matthews DA. Depression and somatization in the chronic fatigue syndrome. Am J Med 1991; 91: 335–44. [DOI] [PubMed] [Google Scholar]
- 13.Wessely S, Chalder T, Hirsch S, Wallace P, Wright D. Psychological symptoms, somatic symptoms, and psychiatric disorder in chronic fatigue and chronic fatigue syndrome: a prospective study in the primary care setting. Am J Psychiatry 1996; 153: 1050–9. [DOI] [PubMed] [Google Scholar]
- 14.Skapinakis P, Lewis G, Meltzer H. Clarifying the relationship between unexplained chronic fatigue and psychiatric morbidity: results from a community survey in Great Britain. Am J Psychiatry 2000; 157: 1492–8. [DOI] [PubMed] [Google Scholar]
- 15.Harvey SB, Wadsworth M, Wessely S, Hotopf M. The relationship between prior psychiatric disorder and chronic fatigue: evidence from a national birth cohort study. Psychol Med 2008; 38: 933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siribaddana S, Ball H, Hewage S, Glozier N, Kovas Y, Dayaratne DARK, et al. Colombo Twin and Singleton Study (CoTASS): a description of a population based twin study of mental disorders in Sri Lanka. BMC Psychiatry 2008; 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalder T, Berelowitz C, Pawlikowska T. Development of a fatigue scale. J Psychosom Res 1993; 37: 147–54. [DOI] [PubMed] [Google Scholar]
- 18.Widiger TA, Samuel DB. Diagnostic categories or dimensions? A question for the Diagnostic and Statistical Manual of Mental Disorders – fifth edition. J Abnorm Psychol 2005; 114: 494–504. [DOI] [PubMed] [Google Scholar]
- 19.Plomin R, Owen MJ, McGuffin P. The genetic basis of complex human behaviors. Science 1994; 264: 1733–9. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Composite International Diagnostic Interview (CIDI). World Health Organization Division of Mental Health, 1990.
- 21.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th edn) (DSM–IV). APA, 1994.
- 22.World Health Organization. The ICD–10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. WHO, 1992.
- 23.Ware Jr JE, Sherbourne CD. The MOS 36-item short-form health survey (SF–36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–83. [PubMed] [Google Scholar]
- 24.Ooki S, Yamuda K, Asaka A, Hayakawa K. Zygosity diagnosis of twins by questionnaire. Acta Genet Med Gemellol (Roma) 1990; 39: 109–15. [DOI] [PubMed] [Google Scholar]
- 25.Sumathipala A, De SN, Siribaddana SH, Abeysingha MR, Fernando DJ. Cross-cultural adaptation and preliminary validation of a zygosity determination questionnaire for twins in Sri Lanka. Twin Res 2000; 3: 205–12. [PubMed] [Google Scholar]
- 26.Defries JC, Fulker DW. Multiple regression analysis of twin data: etiology of deviant scores versus individual differences. Acta Genet Med Gemellol (Roma) 1988; 37: 205–16. [DOI] [PubMed] [Google Scholar]
- 27.Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychol Bull 2005; 131: 592–617. [DOI] [PubMed] [Google Scholar]
- 28.Ball HA, Sumathipala A, Siribaddana SH, Kovas Y, Glozier N, McGuffin P, et al. Genetic and environmental contributions to depression in Sri Lanka. Br J Psychiatry 2009; 195: 504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotopf M, Hull L, Fear NT, Browne T, Horn O, Iversen A, et al. The health of UK military personnel who deployed to the 2003 Iraq war: a cohort study. Lancet 2006; 367: 1731. [DOI] [PubMed] [Google Scholar]
- 30.Skapinakis P, Lewis G, Mavreas V. Unexplained fatigue syndromes in a multinational primary care sample: specificity of definition and prevalence and distinctiveness from depression and generalized anxiety. Am J Psychiatry 2003; 160: 785–7. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds CA, Fiske A, Fratiglioni L, Pedersen NL, Gatz M. Heritability of an age-dependent categorical phenotype: cognitive dysfunction. Twin Res Hum Genet 2006; 9: 17–23. [DOI] [PubMed] [Google Scholar]
- 32.Fowler TA, Rice F, Thapar A, Farmer A. Relationship between disabling fatigue and depression in children. Genetic study. Br J Psychiatry 2006; 189: 247–53. [DOI] [PubMed] [Google Scholar]
- 33.Patel V, Kirkwood BR, Weiss H, Pednekar S, Fernandes J, Pereira B, et al. Chronic fatigue in developing countries: population based survey of women in India. BMJ 2005; 330: 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson NF, Jacobsen C, Goldberg J, Kapur V, Buchwald D. Subjective and objective sleepiness in monozygotic twins discordant for chronic fatigue syndrome. Sleep 2004; 27: 973–7. [DOI] [PubMed] [Google Scholar]
- 35.Ball HA, Siribaddana SH, Kovas Y, Glozier N, McGuffin P, Sumathipala A, et al. Epidemiology and symptomatology of depression in Sri Lanka: a cross-sectional population-based survey in Colombo District. J Affect Disord 2009; 123: 188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White PD, Thomas JM, Amess J, Crawford DH, Grover SA, Kangro HO, et al. Incidence, risk and prognosis of acute and chronic fatigue syndromes and psychiatric disorders after glandular fever. Br J Psychiatry 1998; 173: 475–81. [DOI] [PubMed] [Google Scholar]
- 37.Petersen I, Thomas JM, Hamilton WT, White PD. Risk and predictors of fatigue after infectious mononucleosis in a large primary-care cohort. QJM 2006; 99: 49–55. [DOI] [PubMed] [Google Scholar]
- 38.Wessely S, Chalder T, Hirsch S, Pawlikowska T, Wallace P, Wright DJ. Postinfectious fatigue: prospective cohort study in primary care. Lancet 1995; 345: 1333–8. [DOI] [PubMed] [Google Scholar]
- 39.Candy B, Chalder T, Cleare AJ, Peakman A, Skowera A, Wessely S, et al. Predictors of fatigue following the onset of infectious mononucleosis. Psychol Med 2003; 33: 847–55. [DOI] [PubMed] [Google Scholar]
- 40.Hotopf M, Noah N, Wessely S. Chronic fatigue and minor psychiatric morbidity after viral meningitis: a controlled study. J Neurol Neurosurg Psychiatry 1996; 60: 504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey SB, Wadsworth M, Wessely S, Hotopf M. Etiology of chronic fatigue syndrome: testing popular hypotheses using a national birth cohort study. Psychol Med 2008; 70: 488. [DOI] [PubMed] [Google Scholar]
- 42.Heim C, Wagner D, Maloney E, Papanicolaou DA, Solomon L, Jones JF, et al. Early adverse experience and risk for chronic fatigue syndrome: results from a population-based study. Arch Gen Psychiatry 2006; 63: 1258. [DOI] [PubMed] [Google Scholar]
- 43.United Nations Economic and Social Commission for Asia and the Pacific, United Nations Development Programme, Asian Development Bank. The Millennium Development Goals: Progress in Asia and the Pacific 2006. United Nations, 2007 (http://www.mdgasiapacific.org/files/shared_folder/documents/MDG-Progress2006.pdf).
- 44.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the equal-environment assumption in twin studies of psychiatric illness. Behav Genet 1993; 23: 21–7. [DOI] [PubMed] [Google Scholar]