Abstract
The current investigation explored the main and interactive effects of panic attacks in response to laboratory-induced bodily sensations and anxiety sensitivity in predicting acute nicotine withdrawal symptoms among daily smokers making a self-guided quit attempt. Participants were 99 daily smokers (58% women; Mage = 28.4 years, SD = 11.7) who completed a battery of questionnaires, a voluntary hyperventilation challenge, and a measure of nicotine withdrawal symptoms 12 hr after making a self-guided quit attempt. Results indicated that the interaction of anxiety sensitivity and panic responsivity to the challenge predicted quit-day nicotine withdrawal symptom severity above and beyond the main effects (p < .05). The form of the interaction indicated that the relationship between postchallenge panic attack status and acute nicotine withdrawal was more robust among individuals who were low in anxiety sensitivity. Individuals who did not experience a panic attack posthyperventilation who were also low in anxiety sensitivity reported the lowest levels of nicotine withdrawal. Results suggest that anxiety sensitivity may be less relevant with regard to acute nicotine withdrawal severity among individuals with panic-related problems.
Keywords: panic, anxiety sensitivity, nicotine withdrawal, smoking
Tobacco use and dependence are elevated among those with psychopathology compared with those without psychiatric conditions (Kalman, Morissette, & George, 2005). For example, persons with, relative to those without, psychiatric disorders are approximately twice as likely to be current smokers (Lasser et al., 2000). Some recent studies have shown clear linkages between smoking and anxiety disorders (Feldner, Babson, & Zvolensky, 2007; Morissette, Tull, Gulliver, Kamholz, & Zimering, 2007; Patton et al., 1998; Zvolensky, Feldner, Leen-Feldner, & McLeish, 2005). Some of the most robust, clinically significant relations documented in this domain have been between smoking and panic psychopathology (panic attacks, panic disorder, and agoraphobia; Goodwin, Zvolensky, & Keyes, 2008). Here, studies suggest that smoking co-occurs at significantly higher rates among those with panic attacks and panic disorder than found in the general population (Himle, Thyer, & Fischer, 1988; Lasser et al., 2000; McCabe et al., 2004; Pohl, Yeragani, Balon, Lycaki, & McBride, 1992). In addition, cigarette smoking, particularly at higher rates, has been shown to be concurrently and prospectively associated with an increased risk of more severe panic attack symptoms and life impairment related to such symptoms (Abrams, Zvolensky, et al., 2008; Breslau & Klein, 1999; Breslau, Novak, & Kessler, 2004; Goodwin, Lewinsohn, & Seeley, 2005; Isensee, Wittchen, Stein, Höfler, & Lieb, 2003; Johnson et al., 2000; McLeish, Zvolensky, & Bucossi, 2007; Zvolensky, Schmidt, & McCreary, 2003).
Other work has shown that panic psychopathology is related to greater difficulties quitting smoking (Lasser et al., 2000; Zvolensky & Bernstein, 2005; Zvolensky, Lejuez, Kahler, & Brown, 2004). Models of smoking and panic psychopathology co-occurrence suggest that individuals with panic psychopathology may be more reactive to interoceptive experiences during periods of smoking deprivation, and therefore more likely to relapse more quickly, than individuals without such problems because they desire to smoke to reduce negative affect (Zvolensky & Bernstein, 2005; Zvolensky, Schmidt, & Stewart, 2003). Some indirect empirical evidence is consistent with this perspective (Breslau, Kilbey, & Andreski, 1992; Pomerleau, Marks, & Pomerlau, 2000). Studies focused expressly on panic psychopathology have found that individuals with current panic disorder report greater withdrawal symptom severity during past quit attempts compared with individuals without such a history (Marshall et al., 2008). In addition, individuals with a recent history of nonclinical panic attacks (past 3 months) have been found to report significantly shorter latency to relapse during past quit attempts, as well as more intense retrospectively reported negative emotional reactions during past quits (e.g., intensity of anxiety symptoms; Zvolensky, Lejuez, et al., 2004). These data collectively suggest that having panic psychopathology may be related to a tendency to report symptoms during smoking deprivation as more intense and aversive.
A related body of work has focused on the role of anxiety sensitivity in anxiety–smoking relations. Anxiety sensitivity is a cognitive factor reflecting individual differences in the fear of anxiety and arousal-related sensations (McNally, 2002; Taylor, 1999). When anxious, individuals high in anxiety sensitivity become acutely fearful because of beliefs that these anxiety sensations have harmful physical, psychological, or social consequences (Bernstein & Zvolensky, 2007). Studies have found that cigarette smokers high, but not low, in anxiety sensitivity are more apt to report smoking because they believe that smoking can serve a coping function to down-regulate negative affective states (e.g., anxiety, depression; R. A. Brown, Kahler, Zvolensky, Lejuez, & Ramsey, 2001; Comeau, Stewart, & Loba, 2001; Novak, Burgess, Clark, Zvolensky, & Brown, 2003; Stewart, Karp, Pihl, & Peterson, 1997; Zvolensky, Bonn-Miller, Feldner, et al., 2006). In addition, smokers high compared with low in anxiety sensitivity report perceiving the prospect of quitting as both a more difficult and personally threatening experience (Zvolensky, Vujanovic, et al., 2007), possibly due to a hypersensitivity to aversive internal sensations, such as nicotine withdrawal symptoms (Zvolensky, Baker, et al., 2005) or elevated state anxiety (Mullane et al., 2008), both of which routinely occur on abstinence from smoking (Hughes, Higgins, & Hatsukami, 1990). In terms of smoking cessation, anxiety sensitivity is associated with an increased rate of smoking lapse (any smoking behavior) during the early phases of quitting (R. A. Brown et al., 2001; Mullane et al., 2008; Zvolensky, Bernstein, et al., 2007; Zvolensky, Bonn-Miller, Bernstein, & Marshall, 2006; Zvolensky, Stewart, Vujanovic, Gavric, & Steeves, 2009).
Although available work on the association between panic psychopathology, anxiety sensitivity, and various aspects of smoking behavior is promising, it is limited in a number of key ways. First, the vast majority of research addressing panic psychopathology and nicotine withdrawal, as well as anxiety sensitivity and nicotine withdrawal symptom intensity, has relied on retrospective reports of past withdrawal experiences. This limitation introduces the possibility of recall biases, possibly due to memory distortions. To address this issue, it would be advisable to elicit nicotine withdrawal in “real time” and directly monitor the relation between panic variables and anxiety sensitivity in relation to the experience of withdrawal. Second, panic responsivity has been characterized by current or past panic psychopathology as measured by self-report or interview methods rather than focusing on in-the-moment panic attack reactivity. Current theory suggests that individuals with panic psychopathology might experience nicotine withdrawal as more severe because of an aversion to negative interoceptive cues (e.g., abrupt bodily sensations; e.g., Zvolensky & Bernstein, 2005). Thus, an important extension of existing work would be to examine the role of panic responding to laboratory-induced bodily sensations as a predictor of nicotine withdrawal severity. Finally, past empirical work has focused on the main effects of panic psychopathology and anxiety sensitivity in terms of nicotine withdrawal symptoms. Although such work is a natural starting point for scientific inquiry in this domain, it would be advisable to explore the main and interactive effects of these variables (panic and anxiety sensitivity) in one overarching model. This limitation is important, as panic attacks and anxiety sensitivity appear to be related yet distinct constructs (e.g., Schmidt, Lerew, & Jackson, 1999). Theories of smoking–panic co-occurrence suggest that panic responsivity in combination with high anxiety sensitivity conveys the greatest risk for a more severe response to nicotine withdrawal symptoms.
Together, the overarching aim of the current investigation was to explore the main and interactive effects of panic attacks in response to laboratory-induced bodily perturbation and anxiety sensitivity in prospectively predicting acute nicotine withdrawal symptoms. Specifically, daily smokers attempting to quit smoking without professional or pharmacological assistance were examined. The primary predictor variables were anxiety sensitivity and panic attacks (yes/no) in response to a voluntary hyperventilation; both were assessed prior to quit day. The outcome variable was self-reported nicotine withdrawal symptoms that occurred during the first 12 hr of quitting smoking. It was expected that whereas both laboratory panic responding and higher levels of anxiety sensitivity would be significantly related to acute nicotine withdrawal symptom intensity, the combination of these factors would be uniquely associated with more intense withdrawal symptoms. Here, we expected that smokers with higher levels of anxiety sensitivity and a positive panic attack response to the challenge would endorse the most severe nicotine withdrawal symptoms relative to other variable combinations.
Method
Participants
Participants were 101 daily smokers (58% women) with a mean age of 28.4 years (SD = 11.7). The racial distribution generally reflected that of the State of Vermont (State of Vermont Department of Health, 2007), with 93% of the sample identifying themselves as Caucasian, 5% as Hispanic, 1% as African American, and 1% as “other.” Participants, on average, smoked 15.7 cigarettes per day (SD = 7.1) and reported having been regular (daily) smokers for approximately 11.3 years (SD = 10.3). On average, participants reported having made 2.7 (SD = 2.2) serious quit attempts in the past. The mean score on the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) was 3.0 (SD = 1.8), indicating relatively low levels of nicotine dependence.
Approximately 54.5% of the sample met criteria for one or more current Axis I disorders, as measured using the Anxiety Disorders Interview Schedule for DSM–IV: Client Interview Schedule (ADIS-IV; T. A. Brown, Di Nardo, & Barlow, 1994). Specifically, 15.2% of the sample met criteria for one psychological disorder, 13.1% met criteria for two disorders, 20.2% met criteria for three disorders, 4.0% met criteria for four disorders, and 2.0% met criteria for five disorders. Approximately 31.3% of the current sample met criteria for posttraumatic stress disorder, 23.2% for major depressive disorder, 20.2% for panic disorder with or without agoraphobia, 19.2% for generalized anxiety disorder, 18.2% for social phobia, 6.1% for specific phobia, and 1.0% for obsessive– compulsive disorder.
For inclusion in the study, participants were required to meet the following criteria: (a) be between 18 and 65 years of age, (b) have been a daily smoker for at least 1 year, (c) be currently smoking an average of at least 10 cigarettes per day, (d) report motivation to quit of at least 5 on a 10-point Likert-style scale (0 = no motivation to quit; 10 = extreme motivation to quit), (e) express interest in making a serious quit attempt in the next month, and (f) not have decreased the number of cigarettes smoked by more than half in the past 6 months. Exclusionary criteria for the investigation included (a) limited mental competency or the inability to provide informed, written consent; (b) current suicidal or homicidal ideation; (c) current or past history of psychotic-spectrum symptoms or disorders; (d) current major medical problems (e.g., heart disease, cancer); (e) current use of nicotine replacement therapy (e.g., patches or nicotine gum); (f) current, regular use of tobacco products other than cigarettes (e.g., cigars, chewing tobacco); (g) current substance dependence (other than nicotine); and (h) self-reported pregnancy (women only).
Measures
Anxiety Disorders Interview Schedule for DSM–IV: Client Interview Schedule (ADIS-IV; T. A. Brown et al., 1994)
The ADIS-IV is a semistructured diagnostic tool used to assess Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV) anxiety, mood, somatoform, and substance use disorders as well as screen for the presence of psychotic disorders. Reliability of this measure has shown good to excellent interrater agreement for the majority of anxiety and mood disorders among patients who were given two independent administrations of the ADIS-IV (T. A. Brown, Di Nardo, Lehman, & Campbell, 2001). The presence of current Axis I psychopathology was assessed using the ADIS-IV in the present study. Diagnostic reliability ratings by an independent rater (MJZ) were completed on a random selection of 20% of the protocols, with no cases of disagreement being noted.
Smoking History Questionnaire (SHQ; R. A. Brown, Lejuez, Kahler, & Strong, 2002)
The SHQ is a self-report questionnaire used to assess smoking history and pattern. The SHQ includes items pertaining to smoking rate, age of onset of smoking initiation, and years of being a daily smoker. The SHQ has been successfully used in previous studies as a measure of smoking history, pattern, symptoms, and related problems during quitting (Zvolensky, Leen-Feldner, et al., 2004; Zvolensky, Lejuez, et al., 2004).
Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991)
The FTND is a six-item scale designed to assess gradations in tobacco dependence (Heatherton et al., 1991). The FTND has shown good internal consistency, positive relations with key smoking variables (e.g., saliva cotinine; Heatherton et al., 1991; Payne, Smith, McCracken, McSherry, & Antony, 1994), and high degrees of test–retest reliability (Pomerleau, Carton, Lutzke, Flessland, & Pomerleau, 1994).
Carbon monoxide (CO) analysis
Biochemical verification of smoking history was completed by CO analysis of breath samples (10 ppm cutoff; Cocores, 1993). Expired air CO levels were assessed using a CMD/CO Carbon Monoxide Monitor (Model 3110; Spirometrics, Inc., Gray, ME).
Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986)
To assess sensitivity to, and discomfort with, anxious arousal, we used the 16-item ASI (Reiss et al., 1986). The ASI is a self-report measure on which respondents indicate, on a 5-point Likert-style scale (0 = very little to 4 = very much), the degree to which they fear the potential negative consequences of anxiety-related symptoms and sensations. The ASI has high internal consistency ranging from .84 for a sample of college students to .88 –.90 for a clinical sample of anxiety-disordered patients (Reiss et al., 1986). The ASI is unique from, and demonstrates incremental predictive validity relative to, trait anxiety (McNally, 2002) and negative affectivity (Zvolensky, Kotov, Antipova, & Schmidt, 2003).
Diagnostic Sensations Questionnaire (DSQ; Sanderson, Rapee, & Barlow, 1988, 1989)
The DSQ was administered to assess DSM–IV physical (e.g., “pounding or racing heart,” “breathlessness or smothering sensation”) and cognitive (e.g., “fear of going crazy,” “fear of losing control”) panic attack symptoms immediately postchallenge. Specifically, the DSQ consists of 12 physical symptom items, 3 cognitive symptom items, and 1 item targeting whether participants experienced a “sense of panic.” Participants were instructed to make ratings on the basis of how they felt during the challenge. This measure is frequently employed in challenge work (Zvolensky, Lejuez, & Eifert, 1998). Ratings for the DSQ are made on a 9-point Likert-type scale (0 = not at all to 8 = very strongly felt). Past work has successfully used the DSQ to index the intensity of panic symptoms and DSM–IV presence or absence of panic attacks in challenge studies (Forsyth, Eifert, & Canna, 2000; Schmidt et al., 2002). Consistent with past recommendation and biological challenge work and DSM–IV classification of panic (Barlow, Brown, & Craske, 1994), individuals who reported four or more postchallenge panic attack symptoms (at least one of which was cognitive) at a severity rating of 4 or greater, as well as a self-reported sensation of panic at a severity rating of 4 or greater, were coded as having had a panic attack during the hyperventilation challenge in the present study (Sanderson et al., 1989). On the basis of these criteria, a categorical variable (panic attack) was created wherein participants were dummy coded as either 0 (no panic attack during challenge) or 1 (panic attack during challenge).
Minnesota Nicotine Withdrawal Scale (MWS; Hughes & Hatsukami, 1986)
The MWS is a reliable and sensitive nine-item self-report scale used to measure current nicotine withdrawal symptoms. Participants are asked to rate their symptoms on a 4-point Likert-type scale. In the current study, participants completed the MWS on arrival to the laboratory on their scheduled quit day after their quit status was verified biochemically. As recommended by Hughes and Hatsukami (1998), only the eight DSM–IV withdrawal symptom items were included in the total score.
Physiological variables
A J&J Engineering (Poulsbo, WA) I-330-C2 system was used to digitally record physiological data online at a sample rate of 1,024 samples per second across all channels using J&J Engineering Physiolab software during both challenge procedures. In total, two physiological variables were measured: heart rate and respiration rate; a ground electrode was used for heart rate sampling. Raw electrocardiogram data were collected with disposable Ag/AgCl electrodes placed in a standard bilateral configuration on the palmar side of each wrist. The data were processed through a 1–100Hz bandpass filter designed to maximize R-wave frequency. Respiration rate was obtained using a pneumograph sensor cable with PS-2 sensors. The sensors were placed across the chest and secured with a Velcro strap, allowing a measure of chest excursion during respiration; a breaths-per-minute value was derived through software calculation. For the present investigation, prechallenge heart rate and prechallenge respiration rate values were obtained by averaging the values during the last minute of the baseline period prior to the initial hyperventilation challenge. Heart rate and respiration rate were obtained by averaging the values during the last minute of the initial hyperventilation challenge.
Procedure
The current study is a facet of a larger investigation (Zvolensky et al., 2008). Participation in the larger study consisted of eight total appointments: baseline assessment session, voluntary hyperventilation session, self-selected smoking cessation date (approximately within 28 days of the baseline session), and 3-day, 7-day, 14-day, 28-day, and 90-day postcessation follow-up appointments (see Zvolensky et al., 2008). Data from the baseline, hyperventilation, and quit-day appointments were used for the current investigation; these findings have not been reported previously and therefore represent a novel contribution.
During the baseline appointment, participants (a) provided verbal and written informed consent, (b) completed a medical screen, (c) underwent a diagnostic evaluation (ADIS-IV) to determine whether any exclusion criteria were met, and (d) completed an initial battery of self-report assessments. Eligible participants were then scheduled for the hyperventilation procedure within 4 weeks from the baseline appointment. Eligible participants were instructed not to smoke for 12 hr prior to their scheduled hyperventilation appointment. At the hyperventilation appointment, smoking abstinence was verified verbally and by CO analysis of breath samples (10 ppm cutoff; Society for Research on Nicotine and Tobacco Research Subcommittee on Biochemical Verification, 2002). Four participants who attended the hyperventilation session were excluded for failing to meet the CO analysis requirement. Participants were instructed not to use nicotine replacement therapy during the 12-hr quit period. Participants were not scheduled systematically over night or any other part of the day. Rather, they came to their appointment at a time that was convenient for them and the research team. Participants received $25 for completion of the baseline assessment session and $25 for completion of the hyperventilation procedure (and a total of $225 for completing the entire protocol for the larger smoking cessation study).
The hyperventilation procedure was administered in an 8-ft × 12-ft room. Participants sat alone in the experiment room throughout the procedure listening to an audiotape that guided them through the procedure. They were monitored by the experimenter (in the adjacent room) using audiovisual equipment. The hyperventilation procedure appointment consisted of the following five components: (a) 10-min baseline adaptation period; (b) 3-min voluntary hyperventilation period; (c) 10-min recovery period; (d) 5-min voluntary hyperventilation period, which participants were instructed to continue for as long as possible and to discontinue (i.e., stop the tape and breathe normally) when they could no longer continue (5-min maximum, if participant did not stop tape); and (d) 5-min final recovery period. All participants completed the full 3 min of the first hyperventilation procedure. All data for the current study come from the first hyperventilation challenge only.
At the outset of the procedure, the experimenter attached physiological electrodes and exited the room. A standardized audiotape provided directions at the outset of the procedure and then guided participants through the procedure. This procedure was used to standardize participants' breathing rates (at 30 breaths per minute). The audiotape described the overall procedure, reminded participants that they would be asked to participate in two hyperventilation procedures, and instructed the participant about the completion of questionnaires. Participants were informed initially (and reminded later) that they were to continue the second hyperventilation for as long as possible and to stop the tape when they could no longer continue.
This acute hyperventilation procedure reduces the partial pressure of arterial carbon dioxide, increases pH in the blood and cerebrospinal fluid (alkalosis; Nunn, 1987), and elicits a variety of panic-related symptoms such as dizziness, parethesias, palpitations, and dyspnea (Fried & Grimaldi, 1993). Physiological data were gathered continuously throughout the laboratory session. DSQ ratings were made immediately following both hyperventilation procedures.
For the quit-day appointment, participants self-selected a quit date within approximately 28 days of their baseline appointment. Participants were instructed to quit smoking for at least 12 hr before their quit-day appointment. Smoking abstinence was verified verbally and by CO analysis of breath samples (10 ppm cutoff; Society for Research on Nicotine and Tobacco Research Subcommittee on Biochemical Verification, 2002). Seven participants did not meet the CO cutoff at the quit-day appointment. At this appointment, participants completed a battery of questionnaires, including the MWS. Participants who did not meet the 10-ppm CO cutoff score were not included in the current investigation.
Results
A manipulation check was first conducted to ensure that the hyperventilation challenge sufficiently elicited physiological arousal. Specifically, paired samples t tests were conducted between prechallenge (i.e., baseline) and post-challenge heart rate and respiration rates for the first hyperventilation. A paired samples t test revealed that postchallenge heart rate levels (M = 88.71 beats/min, SD = 16.18) were significantly greater than prechallenge heart rate levels (M = 75.24 beats/min, SD = 13.76), t(90) = 9.35, p < .001. A paired samples t test revealed that postchallenge respiration levels (M = 25.32 breaths/min, SD = 3.80) were significantly greater than prechallenge respiration levels (M = 17.57 breaths/min, SD = 4.79), t(75) = 9.85, p < .001.
Second, individuals who did meet criteria for a panic attack postchallenge were compared with those who did not regarding physiological responding during the hyperventilation. Specifically, difference scores were created, such that prechallenge heart rate and respiration rate were subtracted from postchallenge heart rate and respiration rate. A one-way analysis of variance (ANOVA) revealed no significant differences between individuals who did and did not meet criteria for a panic attack on change in heart rate, F(1, 96) = .73, ns, or change in respiration rate, F(1, 82) = 1.21, ns.
Third, the interrelations between predictor and criterion variables were examined. See Table 1 for descriptive statistics and zero-order correlations. Note that the percentage of individuals who met criteria for a panic attack in this study is similar to that found in past work (Zvolensky, Leen-Feldner, et al., 2004). The mean ASI for the current sample (M = 21) may have been slightly lower than has been found in similar samples (M = 25; Mullane et al., 2008). The covariate of daily smoking rate was not significantly related to quit-day withdrawal (r = .10, ns). Both anxiety sensitivity and laboratory panic attack status were significantly positively correlated with quit-day nicotine withdrawal (r = .53, p < .001; r = .25, p < .05, respectively).
Table 1.
Descriptive and Zero-Order Relations Among Theoretically Relevant Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | Mean or % (SD) | Observed range |
|---|---|---|---|---|---|---|---|---|
| 1. Cigs/day | 1 | .08 | −.12 | .10 | .05 | .09 | 15.64 (7.07) | 10.0–50.0 |
| 2. ASI | 1 | .32** | .53*** | .00 | .09 | 20.88 (12.59) | 0.0–54.0 | |
| 3. PA | 1 | .25* | .09 | −.12 | 42.2% yes | |||
| 4. MWS | 1 | .01 | .14 | 8.88 (4.93) | 0.0–20.5 | |||
| 5. HR diff | 1 | .25* | 13.48 (14.20) | −47.75–47.89 | ||||
| 6. Resp diff | 1 | 7.74 (7.16) | −21.23–18.89 |
Note. Cigs/day = smoking rate from Smoking History Questionnaire; ASI = Anxiety Sensitivity Index total; PA = panic attack status during hyperventilation challenge (yes/no); MWS = Minnesota Nicotine Withdrawal Scale; HR diff = heart rate difference between prechallenge and postchallenge (beats/minute); Resp diff = respiration rate difference between prechallenge and postchallenge (breaths/ minute).
p < .05.
p < .01.
p < .001.
Fourth, a hierarchical multiple regression was conducted with smoking rate entered at Level 1, the main effects of prechallenge anxiety sensitivity and panic attack status (postchallenge) entered at Level 2, and their interaction entered at Level 3. The criterion variable was quit-day withdrawal symptoms (MWS scores). See Table 2. Results from the regression analysis suggested that the model was statistically significant, F(4, 101) = 12.15, p < .001. Level 1 of the model was not statistically significant (p > .05). Level 2 of the model accounted for a significant 28.5% of variance (p < .001), with ASI being the only significant predictor at that level, t(100) = 5.46, β = .49, sr2 = .21, p < .001. Level 3 of the model accounted for a significant 3.8% of variance (p < .05), with the interaction being a significant predictor, t(100) = −2.36, β = −.29, sr2 = .04, p < .05.
Table 2.
Hierarchical Regression Analysis: Interaction of Panic Attack Status and Anxiety Sensitivity Predicting Quit-Day Nicotine Withdrawal Symptoms
| Step/variable | Δ R2 | t(100) | β | sr2 | p |
|---|---|---|---|---|---|
| Step 1 | .01 | ns | |||
| Cigs/day | 1.02 | .10 | .01 | ns | |
| Step 2 | .29 | <.001 | |||
| ASI | 5.46 | .49 | .21 | <.001 | |
| PA | 1.12 | .10 | .01 | ns | |
| Step 3 | .04 | <.05 | |||
| AS × PA | −2.36 | −.29 | .04 | <.05 |
Note. Cigs/day = smoking rate from Smoking History Questionnaire; ASI = Anxiety Sensitivity Index total; PA = panic attack status during hyperventilation challenge (yes/no).
The form of the interaction was subsequently examined both graphically, as per recommendations from Cohen and Cohen (1983), and statistically (Holmbeck, 2002). First, on the basis of recommendations of Cohen and Cohen (pp. 323, 419), we examined the form of these interactions by inserting specific values for each predictor variable (0.5 SD above and below the mean) into the regression equation associated with the described analysis. As can be seen in Figure 1, individuals high in anxiety sensitivity reported the greatest levels of quit-day withdrawal symptoms, regardless of whether they met the postchallenge panic attack criteria. Individuals low in anxiety sensitivity who met the panic attack criteria reported greater withdrawal symptoms than individuals low in anxiety sensitivity who did not meet the panic attack criteria. Furthermore, on the basis of recommendations of Holmbeck (2002), we conducted post hoc probing analyses on the data to examine the significance of the simple slopes and interactions. The relation between postchallenge panic attack status and quit-day nicotine withdrawal symptoms was statistically significant when anxiety sensitivity was low, t(72) = 2.34, β = .33, p < .05, such that nicotine withdrawal was higher among those who had a panic attack (as compared with those who did not have a panic attack) when anxiety sensitivity was low. The relation between postchallenge panic attack status and quit-day nicotine withdrawal symptoms was not statistically significant when anxiety sensitivity was high, t = −0.46, β = −.07, p = .65.
Figure 1.
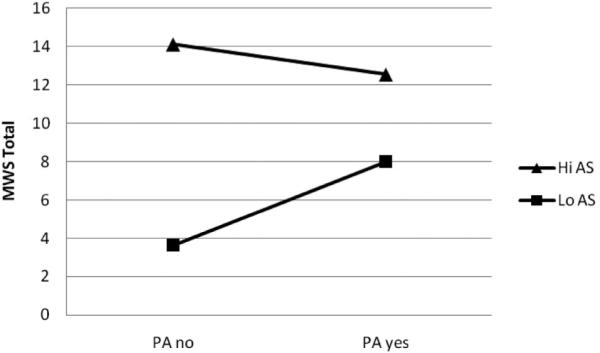
Panic attack status by anxiety sensitivity predicting quit-day nicotine withdrawal symptoms. Note: Values for each predictor variable were chosen on the basis of 0.5 SD above and below the means.
Discussion
Panic psychopathology (Marshall et al., 2008) and anxiety sensitivity (Zvolensky, Baker, et al., 2005) are related to greater retrospectively reported nicotine withdrawal symptom intensity during quit attempts. The current study aimed to address a number of limitations in past work on this topic by investigating the main and interactive effects of anxiety sensitivity and laboratory panic responding prospectively in relation to acute (quit-day) nicotine withdrawal symptoms among regular (daily) smokers making a self-guided quit attempt.
In terms of main effects, as hypothesized, higher levels of anxiety sensitivity were related to greater severity of acute nicotine withdrawal symptoms; the size of this effect was large in magnitude (Cohen, 1988). This finding is consistent with previous empirical work demonstrating that smokers high in anxiety sensitivity appear to be hypersensitive to interoceptive sensations, specifically those related to nicotine withdrawal or related aversive emotional states during the early phases of quitting (e.g., Mullane et al., 2008; Zvolensky, Baker et al., 2005). It is possible that individuals high in anxiety sensitivity lapse to smoking more quickly following a quit attempt (e.g., R. A. Brown et al., 2001), in part, because of their perceptions of nicotine withdrawal symptoms as being more aversive and harmful. Contrary to hypothesis, the main effect of panic attacks was not significantly related to quit-day nicotine withdrawal symptom severity in the current study. Although past work has demonstrated linkages between panic psychopathology (i.e., panic attacks, panic disorder, agoraphobia) and greater retrospective reports of nicotine withdrawal symptom severity using lifetime-reporting indices (Marshall et al., 2008), the current study did not find such relations using a prospective design. In addition, it may be the case that panic attacks experienced in response to bodily sensations induced by a laboratory provocation (voluntary hyperventilation), as opposed to those experienced apart from laboratory manipulation, are not independently related to “real-world” acute nicotine withdrawal symptom severity. However, it is also important to note that past work has not examined panic attacks and anxiety sensitivity concurrently in prediction of acute nicotine withdrawal symptom severity. Given that anxiety sensitivity and panic attacks in response to induced bodily sensations were simultaneously entered into the regression equation and were moderately correlated with one another (r = .32), it is possible that the robust effect for anxiety sensitivity may have mitigated the statistically significant panic attack-nicotine withdrawal symptom intensity effect (r = .25). Thus, although panic attack to the challenge was related to acute quit-day nicotine withdrawal symptom intensity, it was not as robust of an effect as anxiety sensitivity in the current investigation.
As expected, the interactive effect of panic responsivity to bodily sensations by anxiety sensitivity was significantly associated with levels of nicotine withdrawal symptoms on quit day. The size of the observed interactive effect was 3.8% of unique variance above and beyond the variance accounted for by smoking rate and the significant main effect of anxiety sensitivity (see Table 2). Inspection of the form of the interaction indicated that it was partially, but not uniformly, in accord with the a priori theoretical formulation (see Figure 1). Specifically, the relationship between panic attack status postchallenge and quit-day withdrawal symptoms was more robust among individuals low (compared with high) in anxiety sensitivity. In other words, it may be the case that having a panic attack in response to a hyperventilation challenge is less relevant in predicting acute nicotine withdrawal symptoms among individuals high in anxiety sensitivity. As hypothesized, nonpanic responding to the laboratory challenge and lower levels of anxiety sensitivity were associated with the lowest levels of acute nicotine withdrawal symptoms (see Figure 1). Overall, this novel pattern of findings highlights the possible clinically relevant interplay between panic responsivity to bodily sensations and anxiety sensitivity in regard to the experience of acute nicotine withdrawal symptoms among daily adult cigarette smokers attempting to make a self-guided quit attempt.
The current investigation provides a novel empirical perspective on the role of anxiety sensitivity and panic responsivity to bodily sensations in terms of acute nicotine withdrawal among daily smokers making a self-guided quit attempt. Findings from this study help elucidate the role of anxiety vulnerability in terms of nicotine withdrawal severity during the early phase of a quit experience. This work helps clarify putative mechanisms underlying the experience of more intense or personally problematic nicotine withdrawal symptoms among daily smokers.
The present investigation has a number of limitations that should be targeted in future research. First, the current findings were based on a community sample of relatively homogeneous participants in terms of race/ethnicity and age, thus limiting the generalizability of the findings. Future work might extend this line of inquiry to more diverse samples. Second, smokers in the current study reported relatively low levels of nicotine dependence, and given documented associations between higher levels of nicotine dependence and anxiety-related symptoms (e.g., Goodwin et al., 2008), future work might wish to sample more highly nicotine-dependent smokers. Third, the current findings were based on self-reported acute nicotine withdrawal symptoms experienced approximately 12 hr into a self-guided quit attempt. An important next step in this line of inquiry would be to obtain nicotine withdrawal symptom reports at multiple time points to evaluate the consistency of the present findings throughout the course of individuals' quit attempts. This test was not conducted in the current report because of high rates of lapse and relapse in the current sample within the early phases of the quit attempt. In other words, there was not enough statistical power to examine interactive effects of panic responding and anxiety sensitivity with regard to longer term withdrawal experiences in the current study. Future studies might benefit from recruiting larger samples with the understanding that many participants, particularly those with certain risk factors (e.g., high anxiety sensitivity), will lapse and relapse rapidly (e.g., Zvolensky, Bonn-Miller, Bernstein, & Marshall, 2006). Fourth, although the hyperventilation challenge was successful in eliciting significant physiological perturbation, future work might benefit from employing other even more potent biological challenge methodologies (e.g., carbon dioxide–enriched air challenge). Carbon dioxide–enriched air challenges may be a superior means of inducing panic sensations given that they tend to induce more intense bodily sensations, allow for greater manipulation related to the predictability of the administration, and may better mimic real-world panic sensations as compared with those induced via hyperventilation (Abrams, Schruers, Cosci, & Sawtell, 2008). Fifth, the exact duration of participants' nicotine deprivation prior to the hyperventilation session was not recorded, but rather, the 12-hr nicotine deprivation was verified biochemically. Future work would benefit from examining potential relations between duration of nicotine deprivation and panic responding to a biological challenge procedure. Sixth, data regarding differences in duration of time between the study appointments were not recorded. Future work might benefit from assessing this information. Finally, the self-quit methodological design employed in the current investigation involved “bonus” payment (extra $25) for verified abstinence on the quit day to help ensure a “serious” cessation attempt. This methodological tactic could have affected the nature of the quit experience; therefore, future studies might benefit from employing alternative strategies. For example, research efforts could evaluate the main and interactive effects of anxiety sensitivity and panic attacks among a treatment-seeking sample that receives an active smoking cessation intervention.
Together, the present findings uniquely extend previous work documenting a retrospective association between anxiety sensitivity and panic psychopathology and nicotine withdrawal symptoms among adult daily smokers. Results suggest that panic reactivity to bodily sensations may be less relevant with regard to acute nicotine withdrawal severity among high anxiety sensitivity individuals, who are already reporting elevated withdrawal symptoms, in comparison to low anxiety sensitivity individuals. These findings provide novel evidence that anxiety sensitivity and panic responsivity are important factors to consider in terms of the acute experience of nicotine withdrawal symptoms during self-guided quit attempts.
Acknowledgments
This research was supported by National Institute on Drug Abuse Research Grant 1 RO3 DA00101-01 awarded to Laura E. Gibson, as well as National Institute on Drug Abuse Research Grants 1 R01 DA018734-01A1, 1 R03 DA016566-01A2, and 1 R21 DA016227-01 awarded to Michael J. Zvolensky. This research also was supported by National Research Service Award 1 F31 MH080453-01A1 granted to Erin C. Marshall.
References
- Abrams K, Schruers K, Cosci F, Sawtell S. Biological challenge procedures used to study co-occurring nicotine dependence and panic disorder. Addictive Behaviors. 2008;33:1463–1469. doi: 10.1016/j.addbeh.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Abrams K, Zvolensky MJ, Dorflinger L, Galatis A, Blank M, Eissenberg T. Fear reactivity to bodily sensations among heavy smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2008;16:230–239. doi: 10.1037/1064-1297.16.3.230. [DOI] [PubMed] [Google Scholar]
- Barlow DH, Brown TA, Craske MG. Definitions of panic attacks and panic disorder in the DSM–IV: Implications for research. Journal of Abnormal Psychology. 1994;103:553–564. doi: 10.1037//0021-843x.103.3.553. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvolensky MJ. Anxiety sensitivity: Selective review of promising research and future directions. Expert Review of Neurotherapeutics. 2005;7(2):97–101. doi: 10.1586/14737175.7.2.97. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: Findings from an epidemiologic study of young adults. American Journal of Psychiatry. 1992;149:464–469. doi: 10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- Breslau N, Klein DF. Smoking and panic attacks: An epidemiologic investigation. Archives of General Psychiatry. 1999;56:1141–1147. doi: 10.1001/archpsyc.56.12.1141. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychological Medicine. 2004;34:323–333. doi: 10.1017/s0033291703008869. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Zvolensky MJ, Lejuez CW, Ramsey SE. Anxiety sensitivity: Relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addictive Behaviors. 2001;26:887–899. doi: 10.1016/s0306-4603(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111:180–185. [PubMed] [Google Scholar]
- Brown TA, DiNardo P, Barlow DH. Anxiety Disorders Interview Schedule for DSM–IV. Graywind Publications; Albany, NY: 1994. [Google Scholar]
- Brown TA, DiNardo P, Lehman CL, Campbell LA. Reliability of DSM–IV anxiety and mood disorders: Implications for the classification of emotional disorders. Journal of Abnormal Psychology. 2001;110:49–58. doi: 10.1037//0021-843x.110.1.49. [DOI] [PubMed] [Google Scholar]
- Cocores J. Nicotine dependence: Diagnosis and treatment. The Psychiatric Clinics of North America. 1993;16:49–60. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1983. [Google Scholar]
- Comeau N, Stewart SH, Loba P. The relations of trait anxiety, anxiety sensitivity, and sensation seeking to adolescents' motivations for alcohol, cigarette, and marijuana use. Addictive Behaviors. 2001;26:803–825. doi: 10.1016/s0306-4603(01)00238-6. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ. Smoking, traumatic event exposure, and posttraumatic stress: A critical review of the empirical literature. Clinical Psychology Review. 2007;27:14–45. doi: 10.1016/j.cpr.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth JP, Eifert GH, Canna MA. Evoking analogue subtypes of panic attacks in a non-clinical population using carbon dioxide–enriched air. Behaviour Research and Therapy. 2000;38:559–572. doi: 10.1016/s0005-7967(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Fried R, Grimaldi J. The psychology and physiology of breathing in behavioral medicine, clinical psychology, and psychiatry. Plenum Press; New York: 1993. [Google Scholar]
- Goodwin RD, Lewinsohn PM, Seeley JR. Cigarette smoking and panic attacks among young adults in the community: The role of parental smoking and anxiety disorders. Biological Psychiatry. 2005;58:686–693. doi: 10.1016/j.biopsych.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Zvolensky MJ, Keyes K. Nicotine dependence and mental disorders among adults in the United States: Evaluating the role of mode of administration. Psychological Medicine. 2008;38:1277–1286. doi: 10.1017/S0033291708003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Himle J, Thyer BA, Fischer DJ. Prevalence of smoking among anxious outpatients. Phobia Practice and Research Journal. 1988;1:25–31. [Google Scholar]
- Holmbeck GN. Post-hoc probing of significant moderational and meditational effects in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D. Effects of abstinence from tobacco: A critical review. Research Advances in Alcohol and Drug Problems. 1990;10:317–398. [Google Scholar]
- Isensee B, Wittchen HU, Stein MB, Höfler M, Lieb R. Smoking increases the risk of panic: Findings from a prospective community study. Archives of General Psychiatry. 2003;60:692–700. doi: 10.1001/archpsyc.60.7.692. [DOI] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. Journal of the American Medical Association. 2000;284:2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Comorbidity of smoking in patients with psychiatric and substance use disorders. American Journal on Addictions. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Marshall EC, Zvolensky MJ, Vujanovic AA, Gibson LE, Gregor K, Bernstein A. Evaluation of smoking characteristics among community-recruited daily smokers with and without posttraumatic stress disorder and panic psychopathology. Journal of Anxiety Disorders. 2008;22:1214–1226. doi: 10.1016/j.janxdis.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe RE, Chudzik SM, Antony MM, Young L, Swinson RP, Zvolensky MJ. Smoking behaviors across anxiety disorders. Journal of Anxiety Disorders. 2004;18:7–18. doi: 10.1016/j.janxdis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- McLeish AC, Zvolensky MJ, Bucossi MM. Interaction between smoking rate and anxiety sensitivity: Relation to anticipatory anxiety and panic-relevant avoidance among daily smokers. Journal of Anxiety Disorders. 2007;21:849–859. doi: 10.1016/j.janxdis.2006.11.003. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Anxiety sensitivity and panic disorder. Biological Psychiatry. 2002;52:938–946. doi: 10.1016/s0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychological Bulletin. 2007;133:245–272. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- Mullane JC, Stewart SH, Rhyno E, Steeves D, Watt M, Eisner A. Anxiety sensitivity and difficulties with smoking cessation. In: Columbus AM, editor. Advances in psychology research. Vol. 54A. NOVA Science; Hauppauge, NY: 2008. pp. 141–155. [Google Scholar]
- Novak A, Burgess ES, Clark M, Zvolensky MJ, Brown RA. Anxiety sensitivity, self-reported motives for alcohol and nicotine use and level of consumption. Journal of Anxiety Disorders. 2003;17:165–180. doi: 10.1016/s0887-6185(02)00175-5. [DOI] [PubMed] [Google Scholar]
- Nunn JF. Applied respiratory physiology. Butterworth; London: 1987. [Google Scholar]
- Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: A prospective study over 3 years. American Journal of Public Health. 1998;88:1518–1522. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, McCracken LM, McSherry WC, Antony MM. Assessing nicotine dependence: A comparison of the Fagerstrom Tolerance Questionnaire (FTQ) with the Fagerstrom Test for Nicotine Dependence (FTND) in a clinical sample. Addictive Behaviors. 1994;19(3):307–317. doi: 10.1016/0306-4603(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Pohl R, Yeragani VK, Balon R, Lycaki H, McBride R. Smoking in patients with panic disorder. Psychiatry Research. 1992;43:253–262. doi: 10.1016/0165-1781(92)90058-b. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerström Tolerance Questionnaire and the Fagerström Test for Nicotine Dependence. Addictive Behaviors. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Marks JL, Pomerlau OF. Who gets what symptom? Effects of psychiatric cofactors and nicotine dependence on patterns of smoking withdrawal symptom-atology. Nicotine & Tobacco Research. 2000;2:275–280. doi: 10.1080/14622200050147547. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency, and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Rapee RM, Barlow DH. Panic induction via inhalation of 5.5% CO2 enriched air: A single subject analysis of psychological and physiological effects. Behaviour Research and Therapy. 1988;26:333–335. doi: 10.1016/0005-7967(88)90086-1. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Rapee RM, Barlow DH. The influence of an illusion of control on panic attacks induced via inhalation of 5.5% carbon dioxide–enriched air. Archives of General Psychiatry. 1989;46:157–162. doi: 10.1001/archpsyc.1989.01810020059010. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Lerew DR, Jackson RJ. The role of anxiety sensitivity in the pathogenesis of panic: Prospective evaluation of spontaneous panic attacks during acute stress. Journal of Abnormal Psychology. 1997;106:355–364. doi: 10.1037//0021-843x.106.3.355. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco Research Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- State of Vermont Department of Health 2007 Retrieved June 30, 2007, from http://www.healthyvermonters.info.
- Stewart SH, Karp J, Pihl RO, Peterson RA. Anxiety sensitivity and self-reported reasons for drug use. Journal of Substance Abuse. 1997;9:223–240. doi: 10.1016/s0899-3289(97)90018-3. [DOI] [PubMed] [Google Scholar]
- Taylor S. Anxiety sensitivity. Erlbaum; Mahwah, NJ: 1999. [Google Scholar]
- Zvolensky MJ, Baker K, Yartz AR, Gregor K, Leen-Feldner E, Feldner MT. Mental health professionals with a specialty in anxiety disorders: Knowledge, training, and perceived competence in smoking cessation practices. Cognitive and Behavioral Practice. 2005;12:312–318. [Google Scholar]
- Zvolensky MJ, Bernstein A. Cigarette smoking and panic psychopathology. Current Directions in Psychological Science. 2005;14:301–305. [Google Scholar]
- Zvolensky MJ, Bernstein A, Cardenas SJ, Colotla VA, Marshall EC, Feldner MT. Anxiety sensitivity and early relapse to smoking: A test among Mexican daily, low-level smokers. Nicotine & Tobacco Research. 2007;9:483–491. doi: 10.1080/14622200701239621. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bonn-Miller MO, Bernstein A, Marshall EC. Anxiety sensitivity and abstinence duration to smoking. Journal of Mental Health. 2006;15:659–670. [Google Scholar]
- Zvolensky MJ, Bonn-Miller MO, Feldner MT, Leen-Feldner E, McLeish AC, Gregor K. Anxiety sensitivity: Concurrent associations with negative affect smoking motives and abstinence self-confidence among young adult smokers. Addictive Behaviors. 2006;31:429–439. doi: 10.1016/j.addbeh.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Leen-Feldner EW, McLeish AC. Smoking and panic attacks, panic disorder, and agoraphobia: A review of the empirical literature. Clinical Psychology Review. 2005;25:761–789. doi: 10.1016/j.cpr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, et al. Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research. 2008;10:1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Kotov R, Antipova AV, Schmidt NB. Cross-cultural evaluation of smokers' risk for panic and anxiety pathology: A test in a Russian epidemiological sample. Behaviour Research and Therapy. 2003;41:1199–1215. doi: 10.1016/s0005-7967(03)00031-7. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Leen-Feldner EW, Feldner MT, Bonn-Miller WO, Lejuez CW, Kahler C, et al. Emotional responding to biological challenge as a function of panic disorder and smoking. Journal of Anxiety Disorders. 2004;18:19–32. doi: 10.1016/j.janxdis.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez CW, Eifert GH. The role of offset control in anxious responding: An experimental test using repeated administrations of 20% carbon dioxide–enriched air. Behavior Therapy. 1998;29:193–209. [Google Scholar]
- Zvolensky MJ, Lejuez CW, Kahler CW, Brown RA. Nonclinical panic attack history and smoking cessation: An initial examination. Addictive Behaviors. 2004;29:825–830. doi: 10.1016/j.addbeh.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Schmidt NB, McCreary BT. The impact of smoking on panic disorder: An initial investigation of a pathoplastic relationship. Journal of Anxiety Disorders. 2003;17:447–460. doi: 10.1016/s0887-6185(02)00222-0. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Schmidt NB, Stewart SH. Panic disorder and smoking. Clinical Psychology: Science and Practice. 2003;10:29–51. [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic A, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine & Tobacco Research. 2009;11:323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Vujanovic AA, Bonn-Miller MO, Bernstein A, Yartz AR, Gregor KL, et al. Incremental validity of anxiety sensitivity in terms of motivation to quit, reasons for quitting, and barriers to quitting among community-recruited daily smokers. Nicotine & Tobacco Research. 2007;9:965–975. doi: 10.1080/14622200701540812. [DOI] [PubMed] [Google Scholar]


