Abstract
Objective
This pilot study explores the reported symptoms in African Americans and Caucasians with asthma.
Methods
Asthma patients in an inner-city pulmonary clinic were given a brief questionnaire of asthma symptoms and the BORG scale, followed by spirometry.
Results
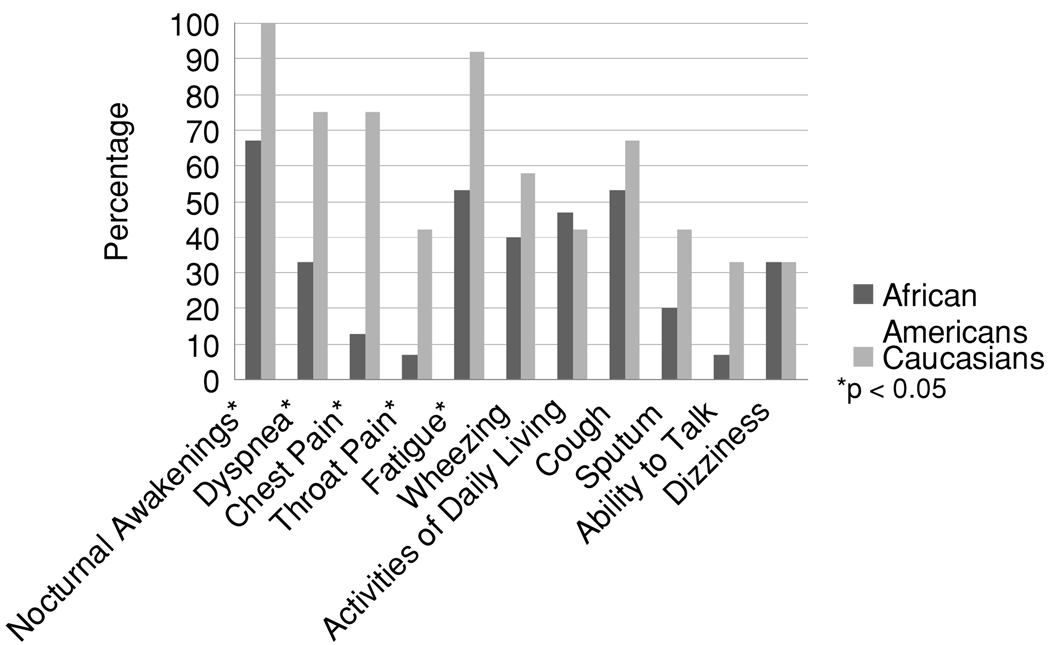
African Americans were less likely to report nocturnal awakenings (67% vs. 100%; p = 0.037), complain of dyspnea (33% vs. 75%; p = 0.038), or experience chest pain (13% vs. 75%; p=0.002) than Caucasians.
Conclusions
This is the first study to demonstrate that there are clinically significant differences in the reporting of asthma symptoms between African Americans and Caucasians.
Keywords: symptoms, African Americans, asthma, perception, disparities
INTRODUCTION
African Americans with asthma suffer greater morbidity and mortality than do Caucasians (1, 2) and have an estimated lifetime prevalence of disease of 12% (3). Perception or usage of different reports of asthma symptoms may account, in part, for the disparity in African Americans. A focus group study of African Americans in Nashville, Tennessee, revealed that African-American asthmatics may perceive and describe their symptoms differently from Caucasians (in press). Understanding the differences in symptom perception will not only allow physicians to improve their ability to diagnose African-American patients with asthma, but also better judge their asthma severity and control.
Although small studies have investigated symptom perception of induced bronchospasm (5), none have directly compared in vivo (naturally occurring) symptoms of asthma in African Americans and Caucasians. Most of the studies performed to date have been in Caucasian children in Europe and Australia (6). The Asthma Control Test (ACT) (7) is one of several scales currently in wide use for the chronic management of patients with asthma; however, it has never been validated for use in minority populations. These scales have been incorporated into the most recently published version of the National Asthma Education and Prevention Program guidelines (8), despite the lack of validity and cultural sensitivity in African-American populations with asthma. Updated asthma guidelines (8) stress the importance of asthma control as the primary means to assess asthmatics. Physicians and patients with asthma must be able to communicate in a language that both parties understand in order for optimal care to be delivered (9, 10). This pilot study compares the description and perception of self-reported asthma symptoms by African-Americans and Caucasians in the setting of in vivo asthma.
METHODS
We performed an observational study using a culturally sensitive questionnaire designed from a combination of NAEPP guidelines, the ACT, and information gained from our previous African-American focus groups (performed on inner-city asthmatics) discussing the reporting of asthma symptoms (Table 1). We limited the duration of nocturnal symptoms that participants were asked to the prior two nights, whereas the ACT asks about this symptom over the preceding month. We did this to better reflect acute symptoms at the time of spirometry rather than to assess their overall control. Patients presenting to the pulmonary clinic at an academic inner-city hospital were recruited for the study. We used a convenience sample to recruit participants; all asthmatic patients undergoing spirometry were approached to enter the study. Asthma severity at the time of spirometry was not a factor used in the recruitment of patients.
TABLE 1.
The questionnaire.
| Race (circle one) | Caucasian | African American |
|---|---|---|
| Today, do you have any of the following: | ||
| • Chest pain | No | Yes |
| • Cough | No | Yes |
| • Phlegm | No | Yes |
| • Throat hurts | No | Yes |
| • Can’t breathe | No | Yes |
| • Feel tired | No | Yes |
| • Feel dizzy | No | Yes |
| • Wheezing | No | Yes |
| • Woke up within last 2 nights | No | Yes |
| • Can’t do what I want last 2 days | No | Yes |
| • Trouble talking | No | Yes |
| Please rate your breathing from 0 (absolutely normal) to 10 (as bad as it gets): |
African Americans and Caucasians age 18 and older were recruited in Nashville, Tennessee, from April through August 2007. All participants signed an Internal Review Board approved consent form that was verbally explained. Surrogate consent was not permitted. We strictly adhered to the Helsinki and Belmont protocols.
Inclusion criteria were patients with a diagnosis of asthma of at least one year’s duration, requiring, as needed, use of a bronchodilator within the previous year. Asthma was diagnosed by spirometry (before and after the administration of a bronchodilator) showing a minimum of a 12% and 200-mL increase in forced expiratory volume in one second (FEV1) at some time during the patient’s illness using Crapo standards on a Puritan Bennett Spirometer (NPB 500,Tyco Healthcare, Pleasanton, CA, USA). All subjects self-identified their race. Subjects had to speak fluent English; literacy was only required to sign the consent form. The questionnaire was administered verbally to all participants.
Exclusion criteria were patients with a history of chronic bronchitis or chronic obstructive pulmonary disease (COPD). Patients with a daily productive cough were excluded from the study even if they were undergoing treatment for asthma. Patients working night shifts were excluded. Subjects with other comorbid illnesses, such as diabetes, hypertension or smoking (less than 10 pack-years), were not excluded.
Participants were asked for their answers to the questionnaire by certified respiratory therapists trained in the administration of the questionnaire (SP, NW). These same therapists then performed spirometry. It is important to note that patients were asked to describe their symptoms before spirometry was performed. Bronchoconstriction was not artificially induced by the administration of methacholine or histamine. A copy of the spirometry was attached to the consent and questionnaire and then given to the investigator for statistical analysis.
The Fisher’s exact test was performed for comparison between nominal variables. The Mann-Whitney U test was used for unmatched nonparametric interval variables. Spearman’s rs was used to correlate perceived dyspnea with measured percent FEV1 (%FEV1 calculated as FEV1/forced vital capacity [FVC]). Linear regression was used to model predictors of %FEV1 and best-fit curves with p-values based on the regression of the model. One-sided p-values were reported for the Fisher’s exact test and the Mann-Whitney U test. Two-sided p-values were calculated for Spearman’s rs. For all tests, a p-value less than 0.05 was considered statistically significant. All tests were performed using SPSS statistical software (SPSS Inc., release 15.0, 2006, SPSS, Inc., Chicago, IL, USA).
RESULTS
Demographics of the sample population are in Table 2. Twenty-seven patients participated in the study. African Americans comprised 15 of the participants, Caucasians 12. We matched the participants in terms of race, age, sex, smoking status, and baseline percent predicted FEV1 (race adjusted). There were no statistic differences between ethnic groups.
TABLE 2.
Demographics.
| African Americans | Caucasians | Total | p-value | |
|---|---|---|---|---|
| Agea | 53 ± 27 | 44 ± 26 | n/ab | 0.107 |
| Race | 15 | 12 | 27 | 0.252 |
| Females | 8 | 6 | 14 | 0.707 |
| Smoker | 9 | 4 | 13 | 0.252 |
| % Predicted FEV1a | 50.73 ± 16.9 | 59.08 ± 30.5 | n/ab | 0.407 |
Mean ± standard deviation.
Totals not applicable as continuous variables.
Statistical significance was calculated by the Mann-Whitney U test.
African Americans often did not awaken at night complaining of asthma symptoms nor using their rescue medication, although all Caucasians in the study woke at night (67% vs. 100%; p=0.037) (Figure 1). African Americans complained less of dyspnea (33% vs. 75%; p = 0.038) and experienced less chest pain than Caucasians (13% vs. 75%; p = 0.002). The former group were also less likely to complain of their throat hurting (7% vs. 42%; p = 0.043) or feeling tired (53% vs. 92%; p = 0.038).
FIGURE 1. Percentage of symptoms reported by race.
All values calculated as Fisher's exact test between the races by symptom. A p < 0.05 is considered to be a statistically significant difference between the races and is indicated by *.
There were no statistically significant differences between the races in terms of wheezing (40% vs. 58%; p = 0.288), ability to perform activities of daily living (47% vs. 42%; p= 0.552), cough (53% vs. 67%; p = 0.381), sputum production (20% vs. 42%; p = 0.212), ability to talk (7% vs. 33%; p = 0.102), or dizziness (33% vs. 33%; p = 0.660).
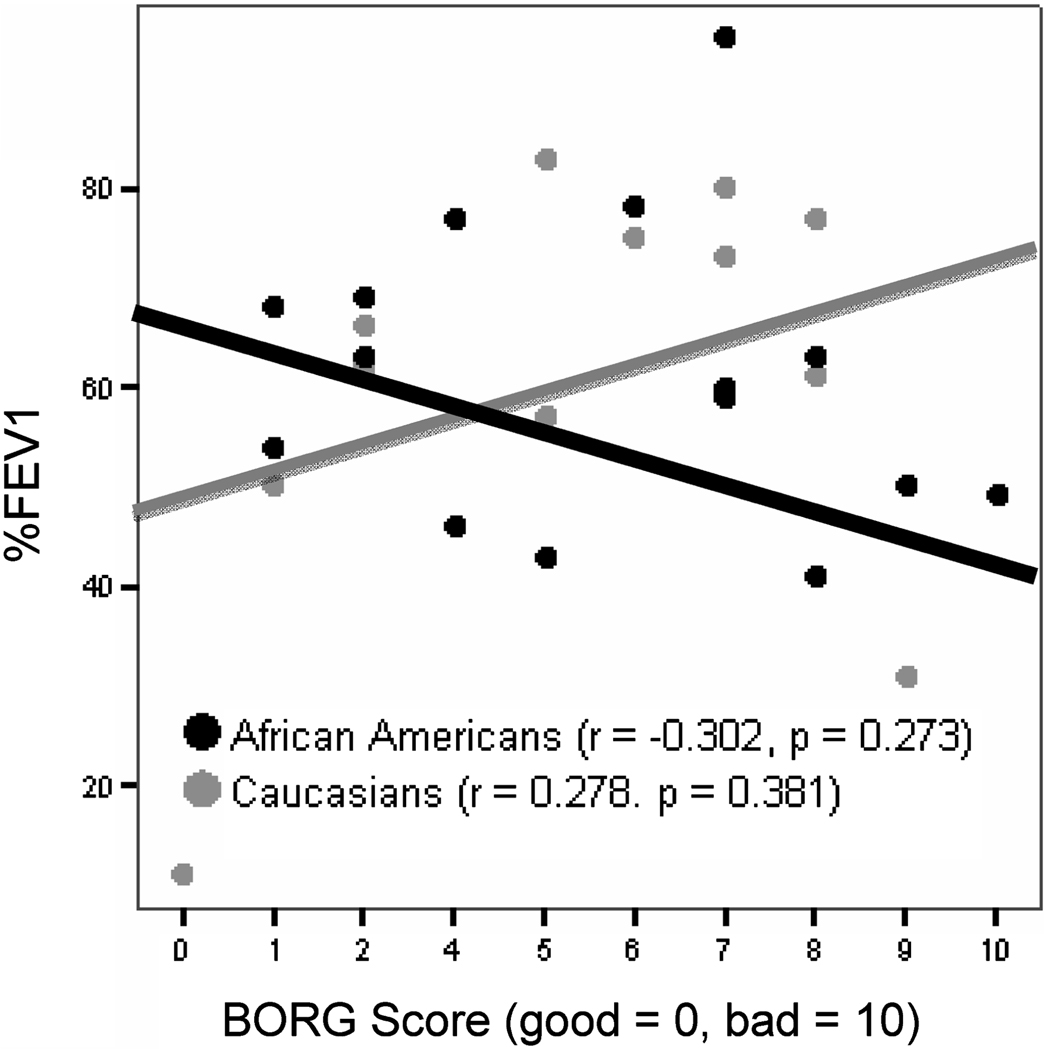
Neither race was able to predict the severity of their bronchoconstriction using the BORG scale (Figure 2). The overall correlation for both races was 0.024 (p = 0.906). African Americans were no better than Caucasians in predicting their %FEV1. The correlation for African Americans was −0.302 (p = 0.273); for Caucasians it was 0.278 (p = 0.381).
FIGURE 2. Perception of bronchoconstriction.
Neither ethnic group was able to estimate their degree of bronchoconstriction in vivo using the BORG scale immediately before spirometry was performed. Spearman's rs test used. Curves fit by linear regression.
In subgroup analysis within African Americans, there were no differences between the sexes in terms of nocturnal awakenings (p = 0.713), dyspnea (p = 0.294), chest pain (p = 0.343), cough (p = 0.231), sputum production (p = 0.341), throat hurting (p = 0.600), fatigue (p = 0.378), dizziness (p = 0.287), wheezing (p = 0.455), activities of daily living (p = 0.622), and ability to talk (p = 0.600).
Similarly, there were no differences in Caucasians reporting of symptoms in terms of sex: nocturnal awakenings (p=n/a), dyspnea (p=0.091), chest pain (p=0.091), cough (p=0.273), sputum production (p = 0.500), throat hurting (p= 0.500), fatigue (p = 0.500), dizziness (p = 0.727), wheezing (p = 0.121), activities of daily living (0.121), and ability to talk (p = 0.727).
We found differences between smokers and nonsmokers. Smokers were more likely to complain of cough (p = 0.041), sputum production (p = 0.021), and fatigue (p = 0.011). There were no statistically significant differences in other reported symptoms: nocturnal awakenings (p = 0.462), dyspnea (p=0.1710), chest pain (p=0.079), throat hurting (p= 0.362), dizziness (p = 0.249), wheezing (p = 0.087), activities of daily living (p=0.288), and ability to talk (p=0.186).
In linear regression models, the predictors of %FEV1 were different for each race. In African Americans, the best-fit symptoms were the combination of chest pain, cough, throat pain, difficulty talking, and wheeze (p = 0.029). In Caucasians, chest pain and fatigue were statistically significant predictors (p = 0.016); however, the combination of chest pain, fatigue, and dyspnea neared statistical significance (p = 0.051); this may represent the small sample size. Nocturnal symptoms do not add to the Caucasian model as all of these subjects had this symptom. When the combinations of best-fit in vivo symptoms were reversed and applied to the opposite race, they no longer predict %FEV1 (African Americans p = 0.117, Caucasians p = 0.160).
DISCUSSION
In this pilot study, we found that African Americans report their asthma symptoms differently from Caucasians. Many African Americans do not report any asthma-related nocturnal symptoms despite this being the sine qua non of asthma in the ACT (6) and the new NAEPP guidelines (7). African Americans are similarly less likely than Caucasians to complain of dyspnea, another hallmark of asthma control. However, the new guidelines stress the assessing of control of asthma as the way to evaluate and treat asthma. Having a reliable, culturally sensitive method of assessment of asthma control is critical in the treatment of all asthmatics. Therefore, classic symptom-based severity assessment may not be depended upon in African Americans with asthma.
In vivo reported symptom descriptors of dyspnea are poorly understood in patients with asthma. Methacholine or histamine challenge has been the method to evoke bronchoconstriction in the majority of studies in the literature evaluating these self-reported symptom descriptors. Diminished perception of dyspnea has been noted with methacholine challenge (11, 12) and histamine (13). Pharmacologically evoked (ex vivo) symptoms include dose-dependent sensations of inspiratory difficulty, chest tightness, and unsatisfactory inspiration (14). Chest tightness and work of breathing was not consistently reported by subjects in one study using methacholine (15). The reporting of symptoms even differs based on the ex vivo method of inducing bronchospasm (16), raising the question if reported symptoms depend on whether in vivo or ex vivo methods are used.
Emotional state (affect) at the time of the inhalation challenge has been shown to effect the perception and descriptors of dyspnea in asthmatics (17–19). Many asthmatics perceive dyspnea differently when reported in vivo rather than ex vivo evoked by histamine challenge, especially during an exacerbation of their illness (20). Fritz et al. found that reported in vivo symptoms better predicted asthma morbidity than resistive loading (21). In another study, almost half of the subjects given methacholine did not report a worsening of their symptoms at a PC30 (the dosage of methacholine at which the FEV1 falls by 30%) (22). BORG scores in adolescents either in remission or symptomatic did not correlate with induced bronchoconstriction (23). In fact, methacholine challenge may not even be able to differentiate asthmatic from nonasthmatic children (24). This provides further evidence that, as in our pilot study, in vivo symptoms may more accurately reflect the patient’s perception of dyspnea and airway narrowing than that evoked by bronchoconstrictors. Thus, bronchoprovocation may not reliably reproduce the symptoms of in vivo asthma.
Patients with more severe asthma often underestimate the severity of their disease because of a blunted perception of dyspnea (25–27). We found that neither African Americans nor Caucasians were able to predict their %FEV1 when using the 10-point BORG scale. This may be due to failure to correctly perceive dyspnea; it is unlikely that patients failed to understand the BORG scale as it was completely explained to the participants by the respiratory therapist who administered the questionnaire. This contradicts a previous study that showed a correlation between BORG scores and %FEV1 (28). In that retrospective study, BORG scores were often reduced without a concomitant improvement in %FEV1. A study by Laveneziana (29) evoking bronchospasm ex vivo with methacholine found that BORG scores correlated with feelings of chest tightness and effort. Our study, however, showed that African Americans may be less likely to experience dyspnea and chest discomfort than Caucasians when reporting in vivo asthma symptoms.
The perception of some of the typical symptoms of asthma (cough, phlegm, and wheezing) (8, 30) did not statistically differ between the races. Although wheezing is one of the best discriminators of asthma (31), many of our participants did not perceive this symptom. A large pharmacy study also confirmed suboptimal symptom perception by tracking the usage of symptom-based rescue medications; however, there was no comparison of ethnic groups in that study (32). Important differences in the descriptors of asthma have been noted by Vincent et al., with patients and physicians using different words, such as “exacerbations” and “attacks” (33). In vivo symptoms in African Americans may overestimate the control of their asthma due to under-reporting of the typical symptoms of uncontrolled asthma. This under-reporting may lead to suboptimal asthma therapy administered to African Americans and partially contribute to the observed disparity in outcomes between the races.
Of particular importance is the absence of nocturnal symptoms in nearly one-third of the African Americans tested in our study. In a focus group study performed by the authors (in press), we found only a single African American reported nocturnal symptoms, even when he presented to the Emergency Department during an exacerbation of his disease. Nocturnal symptoms are a key feature in the diagnosis (7) and treatment of asthma (8), although it is often under-diagnosed in general practice (33). Nocturnal symptoms in otherwise clinically mild disease have been shown to be a predictor of worsening asthma severity (34). Ironically, one study found that frequency of nocturnal symptoms correlated with urgent care visits in minority groups (35). However, another study by Chugh et al. found that clinically stable children with asthma often failed to report nocturnal symptoms despite objective daytime hypersomnia (36). Similarly, in a large cross-sectional survey, 40% of asthmatics did not report nocturnal symptoms, although the authors do not clearly state whether such symptoms were actually present (37). It has also been found that nocturnal symptoms are frequently missed by clinicians (38, 39). Last, there is debate whether nocturnal asthma is part of the spectrum of disease severity or represents a separate phenotype of asthma (40); nonetheless, the treatment and prognostic implications of nocturnal symptoms are unchanged by this debate.
The lack of self-reported nocturnal symptoms may not serve as a measure of asthma control within this minority population: in regression models of predictors of %FEV1, inclusion of nocturnal symptoms results in a lack of statistical significance. Thus, it may be necessary to modify asthma classification and therapy guidelines in African Americans to better reflect the level of their disease control.
Our pilot study did not support the findings of Hardie in terms of descriptors of asthma in African Americans (5). In the Hardie study, African Americans almost exclusively used upper airway descriptors of their asthma symptoms (such as a tight or itchy throat), whereas only throat pain was different between the races in my study (with African Americans less likely to report this symptom). Unlike her results, in our study there was no statistically significant difference between racial groups in lower airway symptoms, except that African Americans were less likely to report dyspnea. We have stressed that artificially provoked bronchoconstriction may not accurately reflect the naturally occurring in vivo symptoms of asthma. This most likely explains the different results between our studies. Our focus group study (in press), using open-ended probes, confirm these findings.
Regression models of symptom-based predictors of %FEV1 in this pilot study display a difference between the races. Although the presence of nocturnal awakenings, dyspnea, chest pain, and fatigue predicts %FEV1 in African Americans when present, it must be remembered that the majority of asthmatics in this ethnic group did not experience these symptoms. This observation suggests that the ACT (7), NAEPP (8), and Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) (41) guidelines may not be culturally sensitive to African-American asthmatics. Qualitative techniques will be required to develop a culturally sensitive, responsive scale to judge the severity of asthma within this minority population. Until a scale is developed, it is prudent to perform frequent spirometry on African Americans to gather objective measurement of their disease control.
Limitations to our study include the small catchment area from which participants were recruited, making it difficult to generalize our findings to the African-American community outside of Nashville, Tennessee. The study relied on a convenience sample of consecutive asthma patients from a single inner-city pulmonary clinic; private practice primary care and pulmonary practices were not included in the study. A strength of the study is that recall bias was minimized by the patients completing the questionnaire of their current in vivo symptoms at the same time spirometry was performed. The mean %FEV1 was less than 80% in this group, thus the participants should have been symptomatic. Another strength of the study is that in vivo symptoms were assessed, avoiding potential confounders from artificially evoked bronchospasm.
CONCLUSIONS
In conclusion, the description and perception of symptoms is instrumental in judging the control of asthma in any given patient. The usage of controller medications (such as inhaled steroids) is predominantly based on self-reported symptoms by using a scale such as ACT. Inadequate reporting of symptoms may lead to under-treatment and the potential for greater morbidity and mortality in minority patients with asthma. A number of African Americans, by their failure to perceive and report nocturnal symptoms and dyspnea, are at increased risk of being considered controlled when they are actuality unstable. This misclassification of asthma severity in minority populations may explain some of the disparities in treatment and outcomes observed in African Americans. The differing predictors of %FEV1 between the races needs to be further studied, and culturally sensitive scales need to be developed to help eliminate the ethnic disparity is asthma outcomes.
ACKNOWLEDGMENT
The authors would like to think Dr. John J. Murray for his mentorship of this project.
This work was supported by NIH grants U01 HL072431, P20RR011792, CRECD NCRR 1R25RR17577, and NIH P20RR011792 CRC and RCMI Clinical Research Infrastructure Initiative.
REFERENCES
- 1.Ford JG, Meyer IH, Sternfels P, Findley SE, McLean DE, Fagan JK, Richardson L. Patterns and predictors of asthma-related emergency department use in Harlem. Chest. 2001;120(4):1129–1135. doi: 10.1378/chest.120.4.1129. [DOI] [PubMed] [Google Scholar]
- 2.Grant EN, Lyttle CS, Weiss KB. The relation of socioeconomic factors and racial/ethnic differences in US asthma mortality. Am J Pub Health. 2000;90(12):1923–1925. doi: 10.2105/ajph.90.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinn K, Shalowitz MU, Berry CA, Mijanovich T, Wolf RL. Racial and ethnic disparities in diagnosed and possible undiagnosed asthma among public-school children in Chicago. Am J Pub Health. 2006;96(9):1599–1603. doi: 10.2105/AJPH.2005.071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trochtenberg DS, BeLue R. Descriptors and perception of dyspnea in African Americans with asthma. J Asthma. 2007;44(10):811–815. doi: 10.1080/02770900701645769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardie GE, Janson S, Gold WM, Carrieri-Kohlman V, Boushey HA. Ethnic differences: word descriptors used by African-American and white asthma patients during induced bronchoconstriction. Chest. 2000;117(4):935–943. doi: 10.1378/chest.117.4.935. [DOI] [PubMed] [Google Scholar]
- 6.Pearce N, Weiland S, Keil U, Langridge P, Anderson HR, Strachan D, Bauman A, Young L, Gluyas P, Ruffin D, et al. Self-reported prevalence of asthma symptoms in children in Australia, England, Germany, and New Zealand: an international comparison using the ISAACprotocol. Eur Respir J. 1993;6:1455–1461. [PubMed] [Google Scholar]
- 7.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 8.NIH. National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland, USA: NIH Draft Publication; 2007
- 9.Kendrick KR. Can a self-rating 0–10 scale for dyspnea yield a common language that is understood by ED nurses, patients, and their families? J Emerg Nurs. 2000;26(3):233–234. doi: 10.1016/s0099-1767(00)90095-3. [DOI] [PubMed] [Google Scholar]
- 10.Chetta A, Castagnaro A, Foresi A, Del Donno M, Pisi G, Malorgio R, Olivieri D. Assessment of breathlessness perception by BORG scale in asthmatic patients: reproducibility and applicability to different stimuli. J Asthma. 2003;40(3):323–329. doi: 10.1081/jas-120018632. [DOI] [PubMed] [Google Scholar]
- 11.Malakauskas K, Sitkauskiene B, Stravinskaite K, Salalauskas R. Dyspnea perception and reversibility methacholine-induced unlimited airway narrowing in asthmatics. J. Asthma. 2006;43:463–467. doi: 10.1080/02770900600758366. [DOI] [PubMed] [Google Scholar]
- 12.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Jr, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am. J. Respir. Crit. Care Med. 2004;170(4):426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 13.Bijl-Hofland ID, Folgering HT, van den Hoogen H, Cloosterman SG, Van Weel C, Donkers JM, van Schayck CP. Relation of the perception of airway obstruction to the severity of asthma. Thorax. 1999;54(1):15–19. doi: 10.1136/thx.54.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lougheed MD, Fisher T, O’Donnell DE. Dynamic hyperinflation during bronchoconstriction in asthma: implications for symptom perception. Chest. 2006;130:1072–1081. doi: 10.1378/chest.130.4.1072. [DOI] [PubMed] [Google Scholar]
- 15.Coli C, Picaricello M, Stendardi L, et al. Is there a link between the qualitative descriptors and the quantitative perception of dyspnea in asthma? Chest. 2006;130:436–441. doi: 10.1378/chest.130.2.436. [DOI] [PubMed] [Google Scholar]
- 16.Bijl-Hofland ID, Cloosterman SG, van Schayck CP, v d Elshout FJ, Akkermans RP, Folgering HT. Perception of respiratory sensation assessed by means of histamine challenge and threshold loading tests. Chest. 2000;117(4):954–959. doi: 10.1378/chest.117.4.954. [DOI] [PubMed] [Google Scholar]
- 17.De Peuter S, Lemaigre V, Van Diest I, Verleden G, Demedts M, Van den Bergh O. Differentiation between sensory and affective aspects of histamine-induced bronchoconstriction in asthma. Resp Med. 2007;101:925–932. doi: 10.1016/j.rmed.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 18.von Leupoldt A, Dahme B. Psychological aspects in the perception of dyspnea in obstructive lung diseases. Resp Med. 2007;101(3):411–422. doi: 10.1016/j.rmed.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Chetta A, Gerra G, Foresi A, Zaimovic A, Del Donno M, Chittolini B, Malorgio R, Castagnaro A, Olivieri D. Personality profiles and breathlessness perception in outpatients with different gradings of asthma. Am J Respir Crit Care Med. 1998;157(1):116–122. doi: 10.1164/ajrccm.157.1.9702093. [DOI] [PubMed] [Google Scholar]
- 20.Ekici M, Ekici A, Kara T, Keles H, Karlidag A, Altunkaya V, Bulcun E. Perception of dyspnea during exacerbation and histamine-related bronchospasm in patients with asthma. Ann Asthma Allergy Immunol. 2006;96:707–712. doi: 10.1016/S1081-1206(10)61069-1. [DOI] [PubMed] [Google Scholar]
- 21.Fritz GK, Adams SK, McQuaid EL, Klein R, Kopel S, Nassau J, Mansell A. Symptom perception in pediatric asthma : resistive loading and in vivo assessment compared. Chest. 2007;132(3):884–889. doi: 10.1378/chest.06-2140. [DOI] [PubMed] [Google Scholar]
- 22.Hardie GE, Gold WM, Janson S, Carrieri-Kohlman V, Boushey HA. Understanding how asthmatics perceive symptom distress during a methacholine challenge. J Asthma. 2002;39(7):611–618. doi: 10.1081/jas-120014925. [DOI] [PubMed] [Google Scholar]
- 23.van den Toorn LM, Overbeek SE, Prins JB, Hoogsteden HC, De Jongste JC. Dyspnoea perception during clinical remission of atopic asthma. Eur Respir J. 2002;19:1047–1050. doi: 10.1183/09031936.02.01712001. [DOI] [PubMed] [Google Scholar]
- 24.Pattemore PK, Asher MI, Harrison AC, Mitchell EA, Rea HH, Stewart AW. The interrelationship among bronchial hyperresponsiveness, the diagnosis of asthma, and asthma symptoms. Am Rev Respir Dis. 1990;142(3):549–554. doi: 10.1164/ajrccm/142.3.549. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi Y, Okabe S, Tamura G, Hida W, Homma M, Shirato K, Takishima T. Chemosensitivity and perception of dyspnea in patients with a history of near-fatal asthma. N. Engl J Med. 1994;330:1329–1334. doi: 10.1056/NEJM199405123301901. [DOI] [PubMed] [Google Scholar]
- 26.Veen JC, Smits HH, Ravensberg AJ, Hiemstra PS, Sterk PJ, Bel EH. Impaired perception of dyspnea in patients with severe asthma: relation to sputum eosinophils. Am J Respir Crit Care Med. 1998;158(4):1134–1141. doi: 10.1164/ajrccm.158.4.9710087. [DOI] [PubMed] [Google Scholar]
- 27.Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121(2):329–333. doi: 10.1378/chest.121.2.329. [DOI] [PubMed] [Google Scholar]
- 28.Choi IS, Chung SW, Han ER, Lim JH, Cho JS, Lee YC, Cho S, Jang AS. Effects of anti-asthma therapy on dyspnea perception in acute asthma patients. Resp Med. 2006;100:855–861. doi: 10.1016/j.rmed.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Laveneziana P, Lotti P, Coli C, Binazzi B, Chiti L, Stendardi L, Duranti R, Scano G. Mechanisms of dyspnoea and its language in patients with asthma. Eur Respir J. 2006;27:742–747. doi: 10.1183/09031936.06.00080505. [DOI] [PubMed] [Google Scholar]
- 30.Yu IT, Wong TW, Li W. Using child reported respiratory symptoms to diagnose asthma in the community. Arch Dis Child. 2004;89(6):544–548. doi: 10.1136/adc.2003.033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.d’Andriran G, Schindler C, Leuenberger P. The absence of dyspnoea, cough, and wheezing: a reason for undiagnosed airflow obstruction? Swiss Med Wkly. 2006;136:425–433. doi: 10.4414/smw.2006.11128. [DOI] [PubMed] [Google Scholar]
- 32.Laforest L, Van Ganse E, Devouassoux G, Osman LM, Brice K, Massol J, Bauguil G, Chamba G. Asthmatic patients’ poor awareness of inadequate disease control: a pharmacy-based survey. Ann Allergy Asthma Immunol. 2007;98:146–152. doi: 10.1016/S1081-1206(10)60687-4. [DOI] [PubMed] [Google Scholar]
- 33.Raherison C, Abouelfath A, Le Gros V, et al. Underdiagnosis of nocturnal symptoms in asthma in general practice. J Asthma. 2006;43(3):199–202. doi: 10.1080/02770900600566744. [DOI] [PubMed] [Google Scholar]
- 34.Strunk RC, Sternberg AL, Bacharier LB, Szefler SJ. Nocturnal awakening caused by asthma in children with mild-to-moderate asthma in the childhood asthma management program. J Allergy Clin Immunol. 2002;110(3):395–403. doi: 10.1067/mai.2002.127433. [DOI] [PubMed] [Google Scholar]
- 35.Stingone JA, Claudio L. Disparities in the use of urgent health care services among asthmatic children. Ann Allergy Asthma Immunol. 2006;97(2):244–250. doi: 10.1016/S1081-1206(10)60021-X. [DOI] [PubMed] [Google Scholar]
- 36.Chugh IM, Khanna P, Shah A. Nocturnal symptoms and sleep disturbances in clinically stable asthmatic children. Asian Pac J Allergy Immunol. 2006;24(2–3):135–142. [PubMed] [Google Scholar]
- 37.Shigemitsu H, Afshar K. Nocturnal asthma. Curr Opin Pulm Med. 2007 Jan;13(1):49–55. doi: 10.1097/MCP.0b013e328010a890. [DOI] [PubMed] [Google Scholar]
- 38.Meijer GG, Postma DS, Wempe JB, Gerritsen J, Knol K, van Aalderen WM. Frequency of nocturnal symptoms in asthmatic children attending a hospital out-patient clinic. Eur Respir J. 1995;8(12):2076–2080. doi: 10.1183/09031936.95.08122076. [DOI] [PubMed] [Google Scholar]
- 39.Turner-Warwick M. Nocturnal asthma: a study in general practice. J R Coll Gen Pract. 1989;39(323):239–243. [PMC free article] [PubMed] [Google Scholar]
- 40.Calhoun WJ. Nocturnal asthma. Chest. 2003;123:399–405. doi: 10.1378/chest.123.3_suppl.399s. [DOI] [PubMed] [Google Scholar]
- 41.Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) [accessed 1 October 2007];2006 Available at: http://www.ginasthma.org. [Google Scholar]