Abstract
We have recently demonstrated that dramatic alteration in mucosal microvascular blood content termed early increase in blood supply (EIBS) is a hallmark of early colon carcinogenesis. In the current study, we elucidate the mechanism of EIBS by assessing iNOS/nitric oxide axis in the histologically normal colonic mucosa of rats treated with the colon-specific carcinogen, azoxymethane. We demonstrate that there was a strong temporal correlation between EIBS and iNOS expression/activity. Importantly, we also observed that short-term treatment with nitric oxide inhibitor abrogated EIBS. This data indicates that iNOS induction may have a critical role in augmenting the predysplastic mucosal blood supply and thereby fostering colon carcinogenesis.
1. Introduction
The recent discovery of the role of the vascular alterations in the colorectal cancer (CRC) has major biological and clinical implications. Biologically, angiogenesis is essential to meet the burgeoning metabolic needs of the growing colon cancer [1]. From a clinical perspective, suppressing angiogenesis with agents such as bevacizumab (a monoclonal antibody to vascular endothelial growth factor (VEGF)) causes profound regression in CRC thus becoming a stalwart in the therapeutic armamentarium [2]. While the importance of blood supply is unequivocal in established CRCs, the carcinogenic stage at which it begins to augment remains unclear. Previous studies indicate that microvessel density is increased in small adenomas or even aberrant crypt foci (ACF), the earliest morphological stages in colon carcinogenesis [3-5]. However, there is limited information on whether vascular abnormalities occur in the predysplastic mucosa (i.e. carcinogenesis initiation). From a teleological perspective, one would expect increased mucosal blood supply at the earliest stages given that the premalignant epithelium is hyperproliferative (resulting from field carcinogenesis) [6] and therefore hypermetabolic. However, the conventional methodologies have lacked the requisite ability to accurately quantify the micro-circulation in the premalignant mucosa.
Our group has developed a novel optical technique, four dimensional elastic light-scattering fingerprinting (4D-ELF), that allows heretofore unattainable quantitative assessment of the epithelial microcirculation [7,8]. We have previously reported that in the azoxymethane (AOM)-treated rat model of colon carcinogenesis, there was an increase in pericryptal capillary blood content that preceded the earliest morphological manifestations of colon carcinogenesis [8]. We termed this phenomenon as the early increase in blood supply (EIBS). While immunoblot analysis of mucosal hemoglobin also confirmed EIBS, it was considerably less sensitive than the optical approach [8].
Using 4D-ELF we have been able to also document EIBS in humans ex vivo (endoscopic biopsies) and more recently in situ (using colonoscopically-compatible 4D-ELF probe) studies [9]. Thus, while the concept of EIBS is firmly established in colon carcinogenesis, one major unresolved question is the mechanisms through which EIBS occurs. It has been shown that a number of vascular modulating genes (cyclooxygenase 2, osteopontin etc), are overexpressed in the histologically normal appearing mucosa of patients who harbor neoplasia elsewhere in the colon [10]. However, the rapidity of the EIBS occurrence would argue against neo-angiogenesis (insufficient time for new vessel formation). A more plausible hypothesis for EIBS would be augmentation of blood flow through dilation of existing vessels (e.g. vasodilation).
Nitric oxide (NO) would represent an attractive potential molecular candidate to mediate the putative vasodilation. In CRC, NO can be produced by inducible nitric oxide synthase (iNOS), which is known to be overexpressed in majority of invasive colon cancers [11,12]. However, since little is known about the timing of iNOS upregulation (and hence NO production) in the early stages of colon carcinogenesis, it is unclear whether the timing of iNOS upregulation would be consonant for its possible role as an EIBS mediator. In the present study we utilized AOM-treated rat model of colon carcinogenesis to demonstrate that iNOS induction is temporally correlates with EIBS. Furthermore, we prove the causality of the two phenomena by observing that pharmacological inhibition of iNOS abrogated EIBS.
2. Materials and Methods
2.1 Animal Studies
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee for Evanston-Northwestern Healthcare. Male Fisher 344 rats (150-200g; Harlan, Indianapolis, IN) were treated either with two injections (i.p.) of 15 mg/kg AOM (Midwest research Institute, Kansas City, MO) or saline. Based on the experimental protocols, rats were euthanized either after two or 8 weeks of second AOM/saline injections. For the iNOS inhibition experiment, animals seven weeks post second AOM/saline injections were gavaged with a pharmacological NOS inhibitor, N6-nitro-L-arginine-methyl ester (L-NAME) or vehicle once a day for 7 days. Colons were removed and aliquots (matched between control and treatment group) were subjected to 4D-ELF analysis within 2 hours of harvest. Comparable sections were formalin fixed and paraffin embedded for future immunohistochemical analysis.
2.2 4D-ELF Analysis
4D-ELF was used to measure blood content in the subepithelial compartment [7,8]. Briefly, a collimated beam of light (from a 75 W xenon arc lamp and passed through a polarizer and a beam-splitter) was allowed to illuminate approximately ten sites on the distal mucosa surface. The light scattered by the specimen was collected through the beam-splitter, a lens, and a second polarizer into the detection system, which consisted of a spectrograph coupled with a CCD (Charge-Coupled Device) camera. The instrument measured two polarization components of scattered light: polarized parallel to the incident polarization direction (co-polarized component) as well as polarized perpendicular to the incident polarization direction (cross-polarized component). The unique absorption spectrum of hemoglobin in the visible range and the depth-selectivity of the differential polarization signal enabled us to extract blood concentration in the superficial mucosa (∼50-100 micrometers below the mucosal surface) that corresponded to the capillary plexus immediately below the epithelium. Detailed descriptions and validations of the measurements of tissue blood content are published elsewhere [7,8,13].
2.3 Immunohistochemical Staining
Formalin fixed and paraffin embedded colonic segments were subjected to immunohistochemical analysis as described previously [14]. Briefly, 5μM paraffin-embedded sections were mounted on Superfrost+ slides (Vector Laboratories, Burlingame, CA), heated at 60°C for 1h and then deparaffinized with two 5 min washes of xylene followed by series of graded ethanol washes. Antigen retrieval for iNOS, VEGF and nitrotyrosine were accomplished by pressure microwaving (NordicWare, Minneapolis, MN) in antigen unmasking reagent (Vector Laboratories) at high power setting for 9 minutes. Endogenous peroxidase activity was quenched by 5 minute wash in 3% H2O2 while the nonspecific binding was blocked by 2 h incubation with 5% horse serum at room temperature. Sections were then incubated overnight with primary antibodies [anti-VEGF (1:100) and anti-iNOS (1:200) from Santa Cruz Biotechnology, CA; anti-nitrotyrosine (1:200) from Cayman Laboratories, An Arbor, MI] followed by incubation with appropriate biotinylated secondary antibodies. After three 5 minute washings in PBS, the specimen were further developed using Vectastatin Elite ABC kit (Vector Laboratories). Staining intensity was measured on a five-point scale (0, none; 1, equivocal; 2, low; 3, moderate; 4 and above intense) by a gastrointestinal pathologist (J.H.) blinded to the treatment group.
2.4 Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
We evaluated iNOS mRNA from colonic scrapings of rats two weeks after carcinogen initiation. Briefly, after euthanizing rats (both AOM/Saline treated), the colons were isolated, washed and opened longitudinally. The distal colonic mucosa was isolated through gentle scraping with a microscope slide as previously described [15]. Total RNA was extracted using TRI Reagent (Sigma). After DNase treatment, cDNA synthesis and PCR amplification for iNOS was carried out using the RT-PCR kit (Invitrogen, CA) following the manufacturer’s protocol. RT-PCR expression was normalized to cyclophilin expression.
3. Results
3.1 iNOS but not VEGF increase at pre-ACF stage
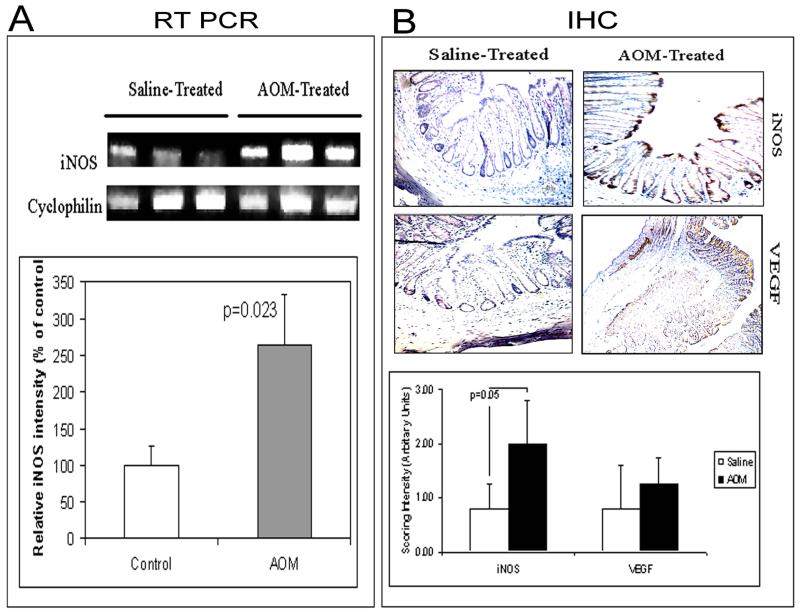
Previous studies from our laboratory indicated that microvascular blood content is augmented early in colon carcinogenesis (i.e. 2 weeks after AOM-injection) [8]. To keep this in perspective, in this well-validated model of colon carcinogenesis, ACF typically develops at 4-6 weeks post carcinogen treatment while adenomas typically require ∼20 weeks and carcinomas ∼35-40. [8]. Thus, we assessed iNOS mRNA in the colonic mucosal aliquots and found that it was markedly induced at this early time point when compared to age-matched control (saline-injected) rats (Figure 1A).
Figure 1.
iNOS induction occurs in a temporally consonant with EIBS (previously demonstrated to be 2 weeks post-AOM, prior to ACF development [8]). Fisher rats were euthanized 2 weeks after 2nd weekly injections of AOM (see methods section)in Panel A, iNOS expression (by RT-PCR) was upregulated in AOM treated colons (by ∼2.5 fold; p=0.02) compared to controls. Similarly as shown in Panel B, the protein expression (by IHC scoring intensity) was significantly upregulated in AOM rats (p< 0.05) compared to controls. However, at this stage no significant change was observed in the immunoreactivity of VEGF in AOM sample compared to controls.
We confirmed this upregulation of iNOS protein through immunohistochemcial analysis. Specifically, we noted a dramatic augmentation of iNOS protein in the colonic epithelium of AOM-treated rats compared to age-matched control (Figure 1B). In order to support that this resulted in increased nitric oxide production we assayed the tissue sections for nitrotyrosine immunoreactivity. Nitrotyrosine residues are produced by covalent interactions (peroxinitration) of nitric oxide to protein and thus serve as a well validated surrogate marker of iNOS activity [16]. We noted that nitrotyrosine increase mirrored iNOS in the mucosa (data not shown).
We then assessed VEGF levels in the mucosa as a surrogate marker for angiogenic status of the mucosa given that this is the most powerful mediator of blood vessel formation in colon carcinogenesis [17]. While there was colonic mucosal staining noted, there was no evidence of alterations between AOM-treated and control animals (Figure 1B).
3.2 iNOS Expression/Marker of Activity temporally correlates with EIBS
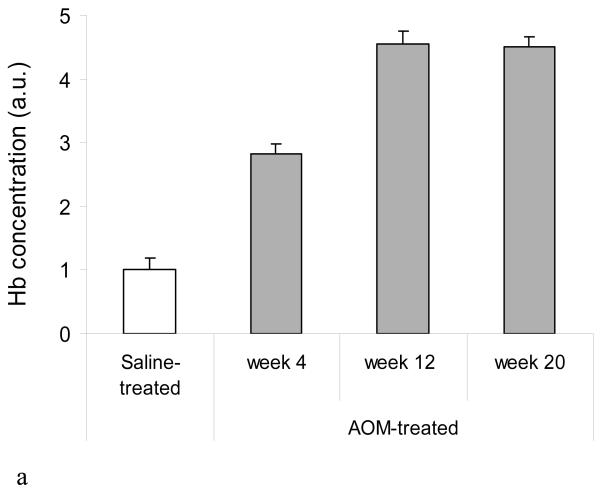
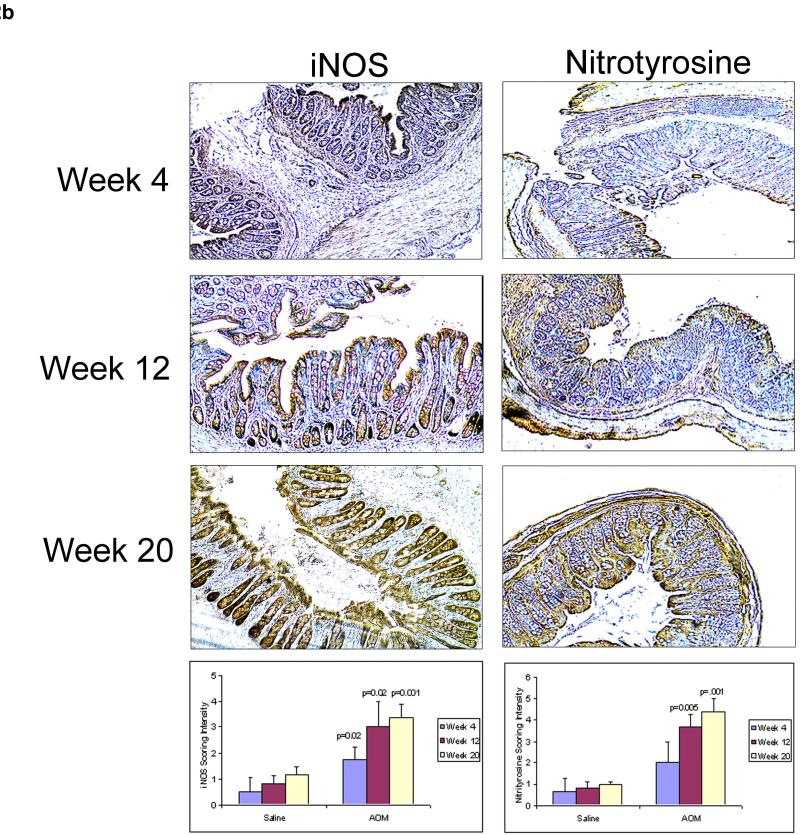
To further explore the role of iNOS in EIBS, we examined the temporal correlation of these parameters. We first assessed EIBS and noted that this was also elevated at week 4 and increased in magnitude by week 12 where it plateaued. There was no significant alteration in microvascular blood content in the saline treated animals over time and therefore the saline controls are represented by a single composite measure (Figure 2A). This was paralleled by colonic mucosal iNOS protein levels in AOM-treated rats when compared to age-matched controls (Figure 2b). In order to demonstrate that the induction of iNOS actually lead to nitric oxide production, we probed these specimens further for nitrotyrosine immunoreactivity. We demonstrate that nitrotyrosine was markedly increased at these early time points (3.0 fold over age-matched controls at week 4) and continued relatively unabated at the later time points (Figure 2b).
Figure 2.
iNOS induction/activity temporally correlates with the EIBS. To determine the associations of EIBS and iNOS induction during the carcinogenic progression animals were sacrificed serially at 4, 12 and 20 weeks post initiation (see text) * p<0.01 versus age-matched saline control. EIBS was measured by 4D-ELF, while iNOS and nitrotyrosine was quantified using immunohistochemistry.
3.3 Pharmacological Inhibition of iNOS Abrogates EIBS
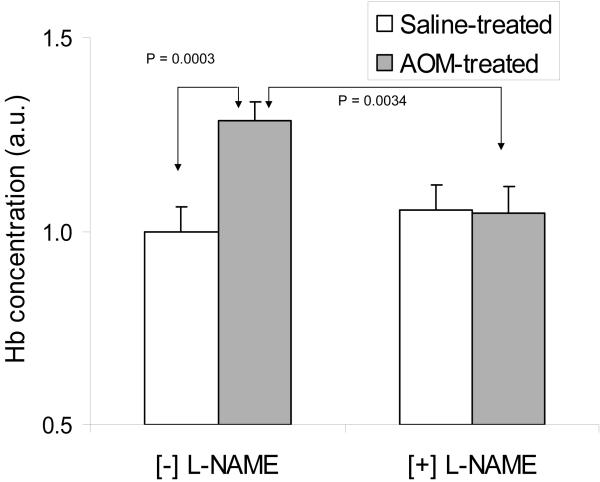
In order to establish that iNOS induction is in fact causal and not simply correlated with EIBS, we studied the effect of iNOS inhibitor, L-NAME [18], on the microvascular blood content as determined by 4D-ELF analysis in both the saline- and AOM-treated rats. We observed that inhibition of iNOS by L-NAME normalized AOM induced blood content in the superficial mucosa to the control levels (P-value = 0.0034, compared to superficial blood content of AOM-treated rats gavaged with vehicle-saline). Importantly, in the saline-treated rats, L-NAME had no significant effect on microvascular blood content, suggesting that the effect of L-NAME is specific to carcinogenesis associated microvascular blood content changes and not simply a non-specific vasoconstriction (Figure 3).
Figure 3.
Short term treatment with L-NAME mitigated EIBS in AOM-treated animals but had no effect on the colonic microvascular blood content in saline-treated rats (see text).
4. Discussion
We herein provide the first mechanistic insights into the augmentation of microvascular blood supply during early colonic carcinogenesis. We demonstrate that increased expression of iNOS is an early event in colon carcinogenesis that temporally mirrors EIBS. Moreover, short-term use of the iNOS inhibitor, L-NAME abrogated EIBS providing compelling evidence for causality of the two phenomena. These studies were made possible through the technological breakthrough of 4D-ELF to reliably identify and quantitate EIBS [7]. Specifically, 4D-ELF allows probing the epithelial capillaries without contamination from the deeper mucosal/submucosal arterioles/venules [8]. Since EIBS is predominantly a mucosal capillary phenomenon (serving the metabolic needs of the hyperproliferative mucosa), whereas most of the total blood supply resides deeper, gating of the superficial mucosal capillaries is critical for the identification of EIBS. Indeed, while EIBS was also noted by immunoblot analysis of hemoglobin, its magnitude was considerably lower than that noted by 4D-ELF, because it is essentially impossible to limit tissue scarping required for immunoblot to the mucosal capillaries only [8].
Our current work provides the biological underpinnings for the recently described phenomenon of EIBS. There is emerging evidence to support the clinical and biological significance of EIBS. Specifically, EIBS has promise for a powerful marker of field carcinogenesis. In the initial studies conducted on experimental models (AOM-treated rat and MIN mouse), EIBS not only occurred prior to morphological markers (adenoma or ACF) but, importantly, it was confined to the colonic regions where future neoplasia would occur. EIBS is also a hallmark of early colon carcinogenesis in humans as indicated by our demonstration that microvascular blood content was markedly increased in the endoscopically normal mucosa from patients harboring neoplasia when compared to those who were neoplasia-free [19]. These findings have been bolstered by our recent work utilizing an endoscopically compatible 4D-ELF probe which enables in situ EIBS measurements during colonoscopy [9]. Using this approach, we have demonstrated that the augmentation of microvascular blood content extends within the third of the colon that harbors the neoplasia (adenomatous polyps) and its magnitude mirrors the proximity to the lesion.
The phenomenon of EIBS lends itself to several potential applications. For instance the spatial correlation of EIBS to tumors serves as a marker for the presence of neoplasia. We are currently exploring its utility in both risk stratification and polyp identification. Another application of EIBS may be a biomarker for chemoprevention. It is well-established that the vasculature is a very effective therapeutic target in established colon cancers (e.g. bevacizumab) [2]. Recently, there has been emerging interest in “angioprevention”, inhibiting vasculature-modulating proteins (e.g. cyclooxygenase 2, AKT, osteopontin, nuclear factor kappa B) for chemoprevention of colonic neoplasia [20,21].
Our data on iNOS expression and its pharmacological inhibition, taken together, strongly supports a role for iNOS induction in EIBS. A number of previous studies have mainly focused on the role of iNOS in established colon cancer. For example, iNOS has long been known to be upregulated in most (∼80%) of all human and AOM-induced colon cancers [22,23]. It is noteworthy that iNOS expression has been shown to be an independent prognostic marker of colon cancer [24]. However, to date, there has been limited data on the levels of iNOS in earlier stages of colon carcinogenesis. Rao and colleagues had reported that iNOS activity was augmented at 8 weeks post AOM injection (when ACF are well-established) [25]. Our data clearly indicates that iNOS is upregulated prior to ACF development. While we did not explore the mechanisms involved in iNOS regulation, there is evidence that protein kinase C (PKC) may be involved in iNOS induction by deoxycholate, a colon carcinogen [26]. Many groups, including our own, have shown that induction of PKC in the histologically normal mucosa is an early event in colon carcinogenesis [27]. Indeed, upstream regulators of PKC such as epidermal growth factor receptor and phospholipase C have been shown to be induced within 5 days of carcinogen treatment [28].
While there are numerous molecular/cellular consequences of iNOS overexpression (e.g. production of mutagenic reactive nitric oxide species), we believe that microvascular blood flow induction is of major importance in early colon carcinogenesis in order to meet the metabolic needs of the hyperproliferative epithelium. In support of the role of metabolic demand, our in vivo data in humans demonstrated that the elevation in deoxygenated hemoglobin concentration was more significant than that of the total (deoxygenated and oxygenated) hemoglobin in the proximity of neoplasia [9]. Unfortunately, the ex vivo nature of our present rat studies precluded the ability to extract information about the oxygen status of hemoglobin. Further supporting the role of blood supply in the initiation of colon carcinogenesis is the demonstration that germline mutation of the vascular modulator, endoglin, resulted in an increased risk of colon carcinogenesis in humans [29].
The mechanism through which iNOS induces EIBS was not directly investigated in this study. We believe that this most likely represents increased flow in existing blood vessels as the vasodilatory ability of NO is well established. In addition, the rapid reversal of EIBS with iNOS inhibitors would argue against neo-angiogenesis and favor vasodilation. However, several studies suggest that iNOS is also an important mediator of angiogenesis in colon cancer [30]. For instance, iNOS expression closely correlates with the degree of angiogenesis in colorectal cancers. The mechanisms remain unclear but the ability of iNOS to induce cyclooxygenase 2 has been implicated [31]. While our data strongly suggests that vasodilation is responsible for pre-adenoma stage EIBS, this does not preclude the possibility that angiogenesis may play a role at later time points. However, it is interesting to note that studies from murine cancers showed that iNOS inhibitors decreased flow in tumor blood vessels while there was no alterations in flow through non-neoplastic vessels [32]. This indicates that the most plausible explanation is that EIBS is mediated through NO-induced vasodilation.
There are several limitations to our study that need to be acknowledged. The use of pharmacological inhibitors raises potential concerns about specificity. However, there is a wealth of evidence that L-NAME is a very specific suppressor of NO production through competitive inhibition of NOS [33]. Another potential concern is that we did not assay the degree of inhibition in colonic iNOS. However, the dosage selected (∼100ppm) is well established for colonic chemopreventive studies with marked reduction in neoplasia [18]. While it is possible that other nitric oxide synthase species may be involved, we believe that given its role in colon carcinogenesis, iNOS is clearly the most compelling molecular candidate.
In conclusion, we present herein the first mechanistic insights into the augmentation of microvascular blood content in colon carcinogenesis. Specifically, we provide both correlational and pharmacological inhibitor data to support the central role of iNOS in governing EIBS. This important finding has ramifications for understanding biology of colon carcinogenesis and also potential clinical applications with regards to early detection and chemoprevention.
5. Acknowledgements
Supported by grants from the National Cancer Institute R01CA109861, R03CA119261, U01CA111257 (Early Detection Research Network)
Footnotes
Presented in part at Digestive Disease Week meeting May 14-19 2005 Chicago IL
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].De Vita F, et al. Elevated perioperative serum vascular endothelial growth factor levels in patients with colon carcinoma. Cancer. 2004;100:270–8. doi: 10.1002/cncr.11911. [DOI] [PubMed] [Google Scholar]
- [2].Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shpitz B, Gochberg S, Neufeld D, Grankin M, Buklan G, Klein E, Bernheim J. Angiogenic switch in earliest stages of human colonic tumorigenesis. Anticancer Res. 2003;23:5153–7. [PubMed] [Google Scholar]
- [4].Xu MH, Deng CS, Zhu YQ, Lin J. Role of inducible nitric oxide synthase expression in aberrant crypt foci-adenoma-carcinoma sequence. World J Gastroenterol. 2003;9:1246–50. doi: 10.3748/wjg.v9.i6.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aotake T, Lu CD, Chiba Y, Muraoka R, Tanigawa N. Changes of angiogenesis and tumor cell apoptosis during colorectal carcinogenesis. Clin Cancer Res. 1999;5:135–42. [PubMed] [Google Scholar]
- [6].Anti M, et al. Rectal epithelial cell proliferation patterns as predictors of adenomatous colorectal polyp recurrence. Gut. 1993;34:525–30. doi: 10.1136/gut.34.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roy HK, Liu Y, Wali RK, Kim YL, Kromine AK, Goldberg MJ, Backman V. Four-dimensional elastic light-scattering fingerprints as preneoplastic markers in the rat model of colon carcinogenesis. Gastroenterology. 2004;126:1071–81. doi: 10.1053/j.gastro.2004.01.009. discussion 948. [DOI] [PubMed] [Google Scholar]
- [8].Wali RK, et al. Increased microvascular blood content is an early event in colon carcinogenesis. Gut. 2005;54:654–60. doi: 10.1136/gut.2004.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roy HKV, et al. Improving Colonoscopic Polyp Detection Through Microvascular Blood Content Analysis with Four Dimensional Elastic Light Scattering Fingerprinting (4D-ELF) Gastroenterology. 2007;132:95. [Google Scholar]
- [10].Chen LC, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64:3694–700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- [11].Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–70. [PubMed] [Google Scholar]
- [12].Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319–27. [PubMed] [Google Scholar]
- [13].Siegel MP, Kim YL, Roy HK, Wali RK, Backman V. Assessment of blood supply in superficial tissue by polarization-gated elastic light-scattering spectroscopy. Appl Opt. 2006;45:335–42. doi: 10.1364/ao.45.000335. [DOI] [PubMed] [Google Scholar]
- [14].Roy HK, et al. Chemoprevention of colon carcinogenesis by polyethylene glycol: suppression of epithelial proliferation via modulation of SNAIL/beta-catenin signaling. Mol Cancer Ther. 2006;5:2060–9. doi: 10.1158/1535-7163.MCT-06-0054. [DOI] [PubMed] [Google Scholar]
- [15].Roy HK, Karolski WJ, Ratashak A. Distal bowel selectivity in the chemoprevention of experimental colon carcinogenesis by the non-steroidal anti-inflammatory drug nabumetone. Int J Cancer. 2001;92:609–15. doi: 10.1002/ijc.1226. [DOI] [PubMed] [Google Scholar]
- [16].Barreiro E, Comtois AS, Gea J, Laubach VE, Hussain SN. Protein tyrosine nitration in the ventilatory muscles: role of nitric oxide synthases. Am J Respir Cell Mol Biol. 2002;26:438–46. doi: 10.1165/ajrcmb.26.4.4634. [DOI] [PubMed] [Google Scholar]
- [17].Zheng S, Han MY, Xiao ZX, Peng JP, Dong Q. Clinical significance of vascular endothelial growth factor expression and neovascularization in colorectal carcinoma. World J Gastroenterol. 2003;9:1227–30. doi: 10.3748/wjg.v9.i6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Watanabe K, Kawamori T, Nakatsugi S, Wakabayashi K. COX-2 and iNOS, good targets for chemoprevention of colon cancer. Biofactors. 2000;12:129–33. doi: 10.1002/biof.5520120120. [DOI] [PubMed] [Google Scholar]
- [19].Roy HK, et al. Spectroscopic Microvascular Assessment From Endoscopically Normal Mucosa for Colon Adenoma Identification. Gastroenterology. 2006;130:A102. [Google Scholar]
- [20].Albini A, Dell’Eva R, Vene R, Ferrari N, Buhler DR, Noonan DM, Fassina G. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-kappaB and Akt as targets. Faseb J. 2006;20:527–9. doi: 10.1096/fj.05-5128fje. [DOI] [PubMed] [Google Scholar]
- [21].Dell’Eva R, Ambrosini C, Minghelli S, Noonan DM, Albini A, Ferrari N. The Akt inhibitor deguelin, is an angiopreventive agent also acting on the NF-kappaB pathway. Carcinogenesis. 2007;28:404–13. doi: 10.1093/carcin/bgl162. [DOI] [PubMed] [Google Scholar]
- [22].Ambs S, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–41. [PubMed] [Google Scholar]
- [23].Takahashi M, Fukuda K, Ohata T, Sugimura T, Wakabayashi K. Increased expression of inducible and endothelial constitutive nitric oxide synthases in rat colon tumors induced by azoxymethane. Cancer Res. 1997;57:1233–7. [PubMed] [Google Scholar]
- [24].Lagares-Garcia JA, Moore RA, Collier B, Heggere M, Diaz F, Qian F. Nitric oxide synthase as a marker in colorectal carcinoma. Am Surg. 2001;67:709–13. [PubMed] [Google Scholar]
- [25].Rao CV, Kawamori T, Hamid R, Reddy BS. Chemoprevention of colonic aberrant crypt foci by an inducible nitric oxide synthase-selective inhibitor. Carcinogenesis. 1999;20:641–4. doi: 10.1093/carcin/20.4.641. [DOI] [PubMed] [Google Scholar]
- [26].Hirose Y, Rao CV, Reddy BS. Modulation of inducible nitric oxide synthase expression in rat intestinal cells by colon tumor promoters. Int J Oncol. 2001;18:141–6. [PubMed] [Google Scholar]
- [27].Gokmen-Polar Y, Murray NR, Velasco MA, Gatalica Z, Fields AP. Elevated protein kinase C betaII is an early promotive event in colon carcinogenesis. Cancer Res. 2001;61:1375–81. [PubMed] [Google Scholar]
- [28].Malecka-Panas E, Tureaud J, Majumdar AP. Enhanced ligand-induced activation of EGF-receptor and overall tyrosine kinase and phospholipase C in colonocytes isolated from azoxymethane-treated rats. Hepatogastroenterology. 1998;45:733–7. [PubMed] [Google Scholar]
- [29].Sweet K, et al. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. Jama. 2005;294:2465–73. doi: 10.1001/jama.294.19.2465. [DOI] [PubMed] [Google Scholar]
- [30].Cullis ER, Kalber TL, Ashton SE, Cartwright JE, Griffiths JR, Ryan AJ, Robinson SP. Tumour overexpression of inducible nitric oxide synthase (iNOS) increases angiogenesis and may modulate the anti-tumour effects of the vascular disrupting agent ZD6126. Microvasc Res. 2006;71:76–84. doi: 10.1016/j.mvr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- [31].Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 2004;555:107–19. doi: 10.1016/j.mrfmmm.2004.05.022. [DOI] [PubMed] [Google Scholar]
- [32].Andrade SP, Hart IR, Piper PJ. Inhibitors of nitric oxide synthase selectively reduce flow in tumor-associated neovasculature. Br J Pharmacol. 1992;107:1092–5. doi: 10.1111/j.1476-5381.1992.tb13412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun P, Wang J, Mehta P, Beckman DL, Liu L. Effect of nitric oxide on lung surfactant secretion. Exp Lung Res. 2003;29:303–14. doi: 10.1080/01902140303787. [DOI] [PubMed] [Google Scholar]