Abstract
Obesity is associated with vascular endothelial dysfunction, as indicated by impaired endothelium-dependent dilation (EDD). Presently there is no direct evidence that energy intake restricted weight loss alone improves conduit or resistance artery EDD, the mechanisms involved, or if improvements differ with patient age. A total of 40 overweight or obese (body mass index ≥ 25<40 kg/m2) non-diabetic men and women aged 21–69 years completed 12 weeks of reduced energy intake (n=26, 15M) or attention control (n=14, 9M) and 4 weeks of weight maintenance (randomized trial). Energy intake restriction reduced estimated total energy intake (33%), body weight (10.5%), total and abdominal body fat, plasma leptin, oxidized LDL, and improved some metabolic risk factors. Brachial artery flow mediated dilation (FMD) was increased by 30% (6.0 ± 0.7 vs. 7.9 ± 0.7 % Δ, P=0.01, n=17). Peak forearm blood flow during intra-brachial artery infusion of acetylcholine was increased by 26% (16.8 ± 1.4 vs. 21.1± 1.9 ml/100ml/min, P<0.05, n=15); this was inversely related to the reduction in abdominal visceral:subcutaneous fat ratio (r=−0.46, P<0.05) and was abolished by inhibition of nitric oxide synthesis with Ng-monomethyl L-arginine. Improvements in EDD were not related to age: mean increases in subjects >50 years were similar to or greater than those <50. Energy intake restricted weight loss alone is an effective intervention for improving peripheral conduit and resistance artery endothelial function in young and older overweight/obese adults. The improvements in resistance artery function are mediated by an increase in nitric oxide bioavailability and are related to reductions in abdominal visceral fat.
Keywords: endothelium, obesity, intra-abdominal fat, nitric oxide, energy intake, adipokines, aging
Obesity is associated with increased risk of cardiovascular diseases (CVD), believed attributable in part to vascular endothelial dysfunction, as indicated by impaired endothelium-dependent dilation (EDD).1, 2 Thus, interventions that improve EDD in overweight and obese adults may have important clinical implications for the prevention of CVD.
As reviewed recently (2006),3 surgical treatment, short-term very-low calorie diets, and multi-component lifestyle interventions that include aerobic exercise and weight loss generally lead to improvements in EDD, particularly in overweight/obese adults with co-morbidities. However, presently there is no evidence that moderate energy intake restriction-based weight loss alone improves EDD in otherwise healthy overweight/obese adults,3 and no randomized trial with a non-weight loss control has been conducted on this question. This is important in that many overweight or obese adults who have not yet developed other clinical disorders are not candidates for gastric-bypass surgery, cannot sustain diets involving severe caloric restriction, and/or will not perform habitual exercise.
If energy intake restriction-induced weight loss does increase EDD in this group, one key question is whether improvements are observed in both brachial artery flow-mediated dilation (FMD), a measure of peripheral conduit artery EDD, and the increase in forearm blood flow in response to intra-brachial artery infusion of acetylcholine, a measure of peripheral resistance vessel EDD. Both of these expressions of EDD are predictors of future CV events,4–7 and it has been suggested that use of one or the other measure has contributed to inconsistent findings in previous studies of weight loss on EDD.3, 8
Another important question concerns the mechanisms involved. Generally, improvements in EDD with weight loss have not been related to reductions in body mass or total body fat.3, 8–11 However, endothelial dysfunction in human obesity may be more strongly related to body fat distribution, particularly abdominal visceral fat, than to total fat mass.12, 13 Circulating proinflammatory adipokines/acute phase reactants, oxidative stress, and certain neurohumoral factors have been linked with increased abdominal visceral fat as possible intermediary mechanisms contributing directly or indirectly to impaired EDD in overweight/obese adults.1 Moreover, impaired EDD in overweight/obese humans is associated with reduced nitric oxide (NO) bioavailability.1, 14 Presently, it is unknown if improvements in EDD with energy intake restriction-based weight loss are related to reductions in abdominal visceral fat or mediated by increased NO bioavailability.
Finally, risk of CVD increases progressively with age.15 As a result, it is important to establish the efficacy of interventions in adults varying in age. However, currently there is no information regarding the effects of energy intake restricted weight loss alone on EDD in adults over 50 years of age.
We hypothesized that energy intake restriction-based weight loss alone would improve EDD in both peripheral conduit and resistance arteries in otherwise healthy overweight and obese adults, and that these improvements would be related to reductions in abdominal visceral fat and changes in circulating adipokines and selective neurohormonal factors. We further hypothesized that middle-aged and older adults would demonstrate improvements in EDD as great or greater than those observed in young adults.
To address these issues we conducted a randomized, controlled intervention trial in which brachial artery FMD was measured before and at the end of energy intake restriction-induced weight loss alone or attention control (parallel group design) in overweight and obese adults varying in age who were free of clinical disease. Forearm blood flow responses to intra-brachial artery infusion of acetylcholine, with and without inhibition of NO production, were determined in a subgroup of the subjects who underwent weight loss. Body composition was assessed by dual x-ray absorptiometry and abdominal fat was determined by computed tomography in all subjects.
Methods
Subjects
A total of 56 overweight and obese (body mass index, BMI, ≥25<40 kg/m2) men (n=29) and women (n=27) aged 21–69 years were enrolled. Subjects were non-smokers, non-diabetic and were free of clinical diseases as assessed by medical history, physical examination, blood chemistry, and resting and exercise ECG (men >40 and women >50 years old only). All subjects self-reported that they were weight stable (± 2 kg) for the previous 6 months. Sixteen subjects, primarily from the attention control group, dropped out after randomization because of lack of time or interest in serving as a control, issues related to arterial catheterization, or non-compliance with treatment. A total of 40 subjects completed the weight loss (n=26) or attention control (n=14) treatments. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Measurements
All measurements were performed at the University of Colorado at Boulder General Clinical Research Center (GCRC) after an overnight fast and 24-hour abstention from alcohol and physical activity. For details on measurements of subject characteristics, body composition, humoral factors, and diet composition and physical activity please see the on-line Data Supplement (http://hyper.ahajournals.org).
EDD and Endothelium-Independent Dilation
EDD and endothelium-independent dilation of the brachial conduit artery was performed as previously described by our laboratory.16–20 For details please see the on-line Data Supplement (http://hyper.ahajournals.org).
Complete pre-post data were obtained on 17 (11M/6F) and 11 (7M/4F) subjects in the weight loss and attention control conditions, respectively: data from 12 subjects were lost because of electronic file corruption (n=6) or poor quality ultrasound images in the pre and/or post treatment measurements (n=6). All FMD analysis was performed and analyzed by the same investigator (GLP) who was blinded to treatment condition.
Forearm resistance vessel EDD and endothelium-independent dilation were determined as previously described by our laboratory.21 For details please see the on-line Data Supplement (http://hyper.ahajournals.org). Complete pre-post data were obtained on 15 (9M/6F) subjects in the weight loss conditions because of issues related to arterial catheterization (n=5) or poor quality forearm blood flows during infusion (n=6). All analyses were performed by the same investigator (AJD) who was blinded to treatment condition.
Energy Intake Restriction and Attention Control Treatments
After completion of baseline measurements subjects were randomly assigned to either energy intake restriction weight loss or attention control treatments. Subjects assigned to the weight loss intervention received counseling and consumed an individualized research diet prepared by a GCRC bionutritionist designed to reduce their caloric intake to meet a goal of 10% weight loss within 12 weeks.22 The diet ensured that total caloric intake was not <1200 kcal/day in order to maintain vitamin and mineral adequacy.23 Subjects gradually substituted the GCRC food with self-prepared meals. To isolate the effects of weight loss from energy intake restriction, subjects remained weight stable (±2 kg) for 4 weeks before the end-treatment measurements were performed. During the entire treatment period, weight loss subjects reported to the GCRC every week to have their body weight assessed and meet with a GCRC bionutritionist to ensure compliance to the diet. Subjects assigned to the attention control condition underwent the same testing every 2 weeks and other interactions with the study staff, but maintained their baseline diet. These subjects were offered the resources for a weight loss program after completion of the study.
Data Analysis
All data are presented as mean ± standard error. For details on statistical analysis please see the on-line Data Supplement (http://hyper.ahajournals.org).
Results
Subject Characteristics, Body Composition, Humoral Factors, Energy Intake and Physical Activity
Baseline and end-treatment values for the weight loss and attention control groups are shown in Tables 1–3 and Table S1 of the Data Supplement (http://hyper.ahajournals.org). At baseline the weight loss group was slightly older (P<0.05) and had a higher body weight (P<0.05), but there were no other group differences.
Table 1.
Subject characteristics
| Variable | Weight Loss (n=26) | Control (n=14) | ||
|---|---|---|---|---|
| Baseline | 16 Weeks | Baseline | 16 Weeks | |
| Age (years) | 49.5 ± 2.5 | ------ | 40.8 ± 3.3‡ | ------ |
| Male/Female (no.) | 15/11 | ------ | 9/5 | ------ |
| Systolic BP (mmHg) | 122 ± 2 | 116 ± 3 | 119 ± 4 | 121 ± 3 |
| Diastolic BP (mmHg) | 72 ± 2 | 69 ± 2 | 70 ± 2 | 67 ± 2 |
| Rest HR (beats/min) | 67 ± 2 | 62 ± 2*† | 64 ± 2 | 68 ± 3 |
| Total cholesterol (mg/dl) | 207 ± 8 | 184 ± 6*† | 197 ± 6 | 189 ± 6 |
| LDL-cholesterol (mg/dl) | 128 ± 8 | 113 ± 6 | 119 ± 6 | 116 ± 6 |
| HDL-cholesterol (mg/dl) | 48 ± 3 | 47 ± 2 | 42 ± 3 | 41 ± 3 |
| Triglycerides (mg/dl) | 157 ± 21 | 113 ± 10 | 181 ± 30 | 155 ± 23 |
| VLDL-cholesterol (mg/dl) | 31 ± 4 | 23 ± 2 | 36 ± 6 | 31 ± 5 |
| Total/HDL-cholesterol ratio | 4.6 ± 0.3 | 4.1 ± 0.2 | 5.1 ± 0.5 | 4.7 ± 0.4 |
| Glucose (mg/dl) | 93 ± 2 | 91 ± 1 | 92 ± 1 | 90 ± 1 |
| Insulin (μU/L) | 9.0 ± 1.1 | 6.7 ± 0.9*† | 9.8 ± 1.2 | 10.4 ± 1.2 |
| HOMA-IR | 2.1 ± 0.3 | 1.5 ± 0.2*† | 2.2 ± 0.3 | 2.4 ± 0.3 |
| Si ([mU/L]−1/min) | 3.7 ± 0.5 | 5.6 ± 0.6 | 3.0 ± 0.6 | 4.3 ± 1.2 |
| Sg ([mU/L−1/min) | 0.023 ± 0.004 | 0.020 ± 0.004 | 0.036 ± 0.012 | 0.019 ± 0.002 |
Values are mean ± standard error;
P<0.05 vs. Pre;
P<0.05 group (Weight loss, Control) × time (Pre, Post) interaction;
P<0.05 vs. Weight Loss; BP, blood pressure;
LDL, low-density lipoprotein; HDL, high density lipoprotein; VLDL, very-low density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; Si, insulin sensitivity; Sg, glucose sensitivity.
Table 3.
Humoral factors
| Variable | Weight Loss (n=26) | Control (n=14) | ||
|---|---|---|---|---|
| Baseline | 16 Weeks | Baseline | 16 Weeks | |
| C-reactive protein (mg/L) | 2.3 ± 0.5 | 1.7 ± 0.4 | 2.0 ± 0.6 | 1.4 ± 0.3 |
| Interleukin-6 (pg/ml) | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.17 | 1.4 ± 0.2 |
| Tumor-necrosis factor-α (pg/ml) | 1.6 ± 0.2 | 1.4 ± 0.1 | 2.9 ± 1.1 | 2.5 ± 0.7 |
| Oxidized LDL (U/L) | 69 ± 4 | 57 ± 4*† | 65 ± 5 | 67 ± 4 |
| Total antioxidant status (mg/dl) | 1.34 ± 0.04 | 1.27 ± 0.04 | 1.33 ± 0.05 | 1.34 ± 0.06 |
| Adiponectin (μg/ml) | 11.7 ± 1.7 | 11.4 ± 1.4 | 8.0 ± 1.6 | 8.0 ± 1.5 |
| Leptin (ng/ml) | 17.6 ± 2.2 | 10.5 ± 1.9*† | 13.5 ± 1.5 | 14.5 ± 2.1 |
| Norepinephrine (pg/ml) | 209 ± 15 | 211 ± 25 | 167 ± 19 | 174 ± 18 |
| Aldosterone (ng/dl) | 5.6 ± 0.6 | 5.2 ± 0.8 | 7.0 ± 1.2 | 7.2 ± 1.3 |
| Endothelin-1 (pg/ml) | 6.6 ± 0.3 | 6.6 ± 0.3 | 5.9 ± 0.4 | 6.6 ± 0.4 |
| Cortisol (μg/ml) | 12.0 ± 1.0 | 9.8 ± 0.7 | 12.8 ± 1.3 | 10.0 ± 1.3 |
| Free fatty acids (μmol/L) | 715 ± 56 | 655 ± 47 | 577 ± 62 | 604 ± 66 |
Values are mean ± standard error;
P<0.05 vs. Pre;
P<0.05 group (Weight loss, Control) × time (Pre, Post) interaction.
Weight loss resulted in reductions in fasting plasma total cholesterol, insulin, and the homeostasis model assessment of insulin resistance (HOMA-IR, P<0.05). There were trends for improvements in plasma low-density lipoprotein cholesterol (LDL-C, P=0.08) and systolic blood pressure (P=0.05), but diastolic blood pressure, plasma high-density lipoprotein cholesterol (HDL-C), total:HDL-C ratio, triglycerides, very-low density lipoprotein cholesterol (VLDL-C), fasting glucose, insulin sensitivity (Si) and glucose sensitivity (Sg) were not significantly changed (Table 1). Body weight decreased by 10.4% and total body fat (% and kg), fat-free mass, and total abdominal fat, visceral fat, and subcutaneous fat were reduced (P<0.05) (Table 2). The abdominal visceral:subcutaneous fat ratio and waist:hip ratio were unchanged. Plasma oxidized LDL-C and leptin were reduced (P<0.05), whereas plasma C-reactive protein, interleukin-6 (IL-6), tumor-necrosis factor-α (TNFα), total antioxidant status, adiponectin, norepinephrine, cortisol, endothelin-1, aldosterone, and free fatty acids were not significantly changed (Table 3). Estimated total energy, fat, carbohydrate, and protein intake were reduced (P<0.05), but there was no change in estimated physical activity (Table S1 Data Supplement, http://hyper.ahajournals.org).
Table 2.
Body composition
| Variable | Weight Loss (n=26) | Control (n=14) | ||
|---|---|---|---|---|
| Baseline | 16 Weeks | Baseline | 16 Weeks | |
| Body weight (kg) | 85 ± 3 | 76 ± 2*† | 94 ± 3‡ | 95 ± 3 |
| Body mass index (kg/m2) | 29 ± 1 | 26 ± 1*† | 31 ± 1 | 31 ± 1 |
| Waist circumference (cm) | 95 ± 2 | 88 ± 2*† | 98 ± 3 | 100 ± 3 |
| Hip circumference (cm) | 109 ± 2 | 102 ± 1*† | 112 ± 1 | 112 ± 2 |
| Waist:hip ratio | 0.88 ± 0.01 | 0.87 ± 0.02 | 0.88 ± 0.02 | 0.89 ± 0.02 |
| Fat-free mass (kg) | 53 ± 2 | 51 ± 2*† | 59 ± 3 | 61 ± 3 |
| Total body fat (kg) | 32 ± 2 | 26 ± 2*† | 35 ± 2 | 35 ± 1 |
| Total body fat (%) | 39 ± 2 | 33 ± 2*† | 38 ± 2 | 39 ± 2 |
| Total abdominal fat (cm2) | 513 ± 25 | 374 ± 24*† | 509 ± 33 | 533 ± 29 |
| Abdominal visceral fat (cm2) | 128 ± 10 | 84 ± 7*† | 150 ± 19 | 154 ± 19 |
| Abdominal subcutaneous fat (cm2) | 385 ± 24 | 290 ± 22*† | 359 ± 26 | 379 ± 26 |
| Abdominal visceral:subcutaneous fat ratio | 0.38 ± 0.04 | 0.33 ± 0.04 | 0.44 ± 0.06 | 0.44 ± 0.07 |
Values are mean ± standard error;
P<0.05 vs. Pre;
P<0.05 group (Weight loss, Control) × time (Pre, Post) interaction;
P<0.05 vs. Weight Loss.
In the attention control group, all variables were unchanged at end-treatment compared with baseline (Table 1–3; Table S1 Data Supplement, http://hyper.ahajournals.org).).
Peripheral Conduit Artery FMD and Endothelium-Dependent Dilation
At baseline, brachial artery diameter (Table S2 Data Supplement, http://hyper.ahajournals.org) and brachial artery FMD and endothelium-independent dilation (Figure 1) were not different in the weight loss and attention control groups. In the overall group, baseline FMD was inversely related to serum triglycerides (r=−0.34, P<0.05) and VLDL (r=−0.34, P<0.05).
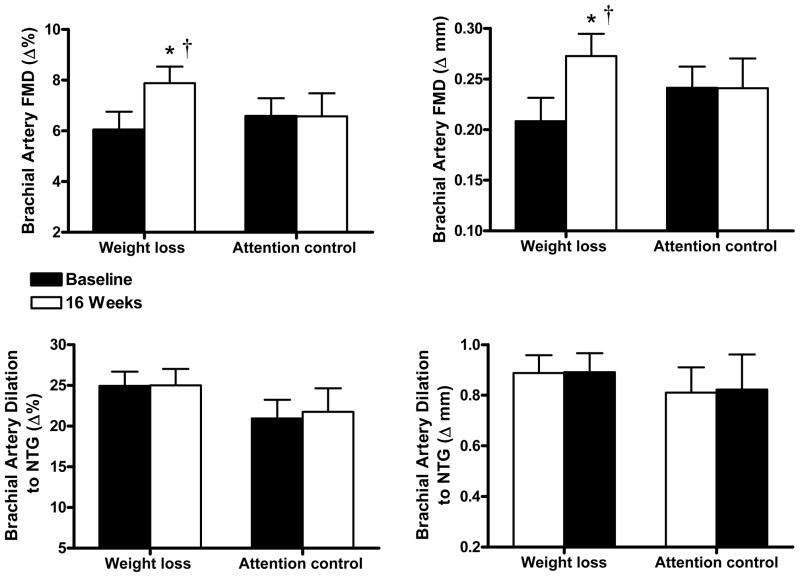
Figure 1.
Endothelium-dependent dilation (brachial artery flow mediated dilation, FMD; percentage change [Δ%], absolute change [Δ mm]) (top) at baseline and after treatment (16 weeks) in the weight loss (n=17) and attention control (n=11) groups and endothelium-independent dilation (brachial artery dilation in response to sublingual nitroglycerin, NTG) (bottom) at baseline and after 16 weeks of treatment in the weight loss (n=11) or attention control (n=7) groups. Values are mean ± SE. *P<0.05 vs. baseline; †P<0.05 group (weight loss, attention control) × time (baseline, 16 weeks) interaction.
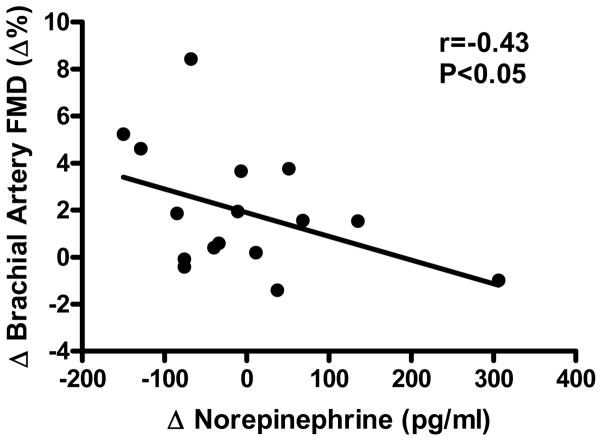
Baseline brachial artery diameter was not different before and after weight loss (Table S2 Data Supplement, http://hyper.ahajournals.org). Weight loss resulted in an ~30% mean increase in FMD, but no change in endothelium-independent dilation (Figure 1). Among individuals, the % change in FMD from baseline to end-treatment in the weight loss group was inversely related to changes in plasma norepinephrine concentrations (r=−0.43, P<0.05, Figure 2). Changes in FMD were not related to changes in any other variable (all P>0.05), including subject age (r=0.21, P>0.05). Brachial artery FMD increased with weight loss in subjects <50 (+28%: 6.0 ± 1.4 vs. 7.7 ± 0.9%, P<0.05, n=8) as well as ≥50 (+31%: 6.1 ± 0.6 vs. 8.0 ± 1.0%, P<0.05, n=9) years of age.
Figure 2.
Relation between changes (Δ) in brachial artery flow-mediated dilation (FMD) and plasma norepinephrine concentrations in response to weight loss (n=16).
In the attention control group, brachial artery diameter (Table S2 Data Supplement, http://hyper.ahajournals.org), FMD and endothelium-independent dilation were unchanged at end-treatment compared with baseline (Figure 1).
Peripheral Resistance Vessel EDD and Endothelium-Dependent Dilation
At baseline, peak forearm blood flow in response to acetylcholine was inversely related to fasting plasma insulin (r=−0.53) and HOMA-IR (r=−0.51) (both P<0.05).
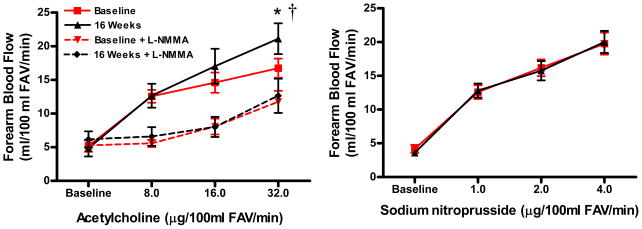
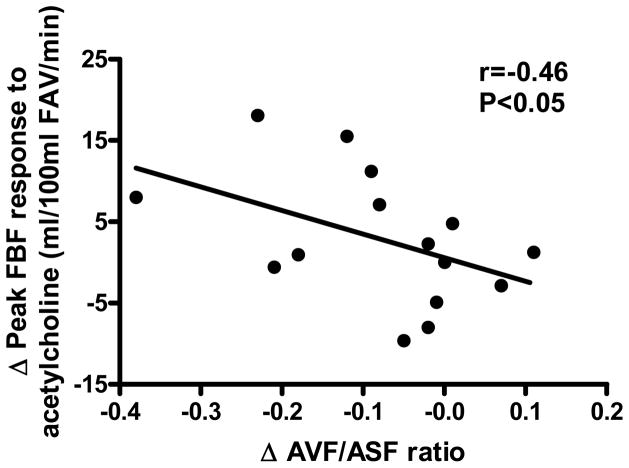
Weight loss resulted in an increase in the forearm blood flow response to acetylcholine (peak +26%), but not to sodium nitroprusside (Figure 3). The change in the peak forearm blood flow response to acetylcholine was inversely related to changes in abdominal visceral fat (r=−0.43, P=0.05) and the abdominal visceral:subcutaneous fat ratio (r=−0.46, P<0.05, Figure 4), and was positively related to changes in plasma leptin (r=0.48) and aldosterone (r=0.61) concentrations (both P<0.05), but was not related to subject age (r=0.20, P>0.05). The increases in the forearm blood flow response to acetylcholine with weight loss were significant in subjects ≥50 years of age (+38%: peak FBF 16.8 ± 2.0 vs. 23.1 ± 2.9 ml/100 ml FAV/min, P<0.05, n=9), but not in the smaller subgroup of subjects <50 (+9%: peak FBF 16.6 ± 2.0 vs. 18.1 ± 3.7 ml/100 ml FAV/min, P=0.34, n=6).
Figure 3.
Endothelium-dependent dilation (during control acetylcholine and inhibition of nitric oxide synthesis with Ng-monomethyl L-arginine [L-NMMA]) and endothelium-independent dilation (forearm blood flow responses to intra-brachial artery infusion of acetylcholine and sodium nitroprusside, respectively) at baseline and after weight loss (16 weeks; n=15). Values are mean ± SE. *P<0.05 vs. baseline; †P<0.05 dose × time interaction; FAV=forearm volume.
Figure 4.
Relation between changes (Δ) in peak forearm blood flow (FBF) to acetylcholine and the abdominal visceral fat:abdominal subcutaneous fat (AVF/ASF) ratio in response to weight loss (n=15). FAV=forearm volume.
In contrast to the greater forearm blood flow response to acetylcholine administration alone after compared with before weight loss, the increase in forearm blood flow to acetylcholine was similar before and after weight loss when NO synthesis was inhibited by co-administration of L-NMMA (Figure 3). This was the case because inhibition of NO production had a greater suppressive effect on the forearm blood flow response to acetylcholine after compared with before weight loss, suggesting that the greater response to acetylcholine after weight loss was mediated by enhanced NO bioavailability.
Relations Between Peripheral Conduit Artery and Resistance Vessel EDD
Measurements of brachial artery FMD and increases in forearm blood flow to acetylcholine were available in 15 overall subjects at baseline and in 9 subjects before and after weight loss. The 2 measures of EDD were not related at baseline (r=0.14, P=0.30), nor were the changes with weight loss related (r=0.30, P=0.22).
Discussion
The primary new finding of this study was that moderate energy intake restriction-based weight loss alone (i.e., in the absence of other lifestyle or pharmacological interventions) can improve EDD in overweight and obese men and women without CVD or other major risk factors for CVD. Endothelium-independent dilation was unaffected by weight loss, demonstrating that the effects of the intervention were specific for the vascular endothelium. Previous studies have shown that weight loss produced by a combination of moderate caloric restriction and aerobic exercise can increase EDD.24, 25 However, regular exercise improves EDD independent of weight loss.21 Thus, the present randomized controlled trial is the first to demonstrate that a conventional weight loss program alone can improve EDD in otherwise healthy overweight and obese adults.
Our findings of improved EDD differ from previous studies that found no changes with weight loss in non-diabetic premenopausal women with a history of gestational diabetes 11 or in overweight adults with hypertriglyceridemia.26 Addition of the drug orlistat to facilitate energy intake restricted weight loss has produced mixed results: one study found improvements in EDD related to reductions in plasma LDL-C,11 whereas another found no effect on EDD.8 All of the studies produced significant weight loss, but there are many methodological differences that could contribute to the inconsistent results.
A novel finding from our study was that improvements in EDD with weight loss were observed in both peripheral conduit arteries and resistance vessels. Previous investigations have measured only one of these expressions of EDD. This is important because both measures are independent predictors for future CV events.4, 6, 7, 27 We found that the mean improvements in brachial artery FMD and the forearm blood flow responses to acetylcholine were similar (~25–30%) with weight loss. However, among the small group of subjects who had both measures, the improvements in conduit artery and resistance vessel EDD did not correlate, as was the case at baseline. These observations are consistent with previous findings that the 2 measures are not related 28, 29 and likely reflect distinct expressions of endothelium-mediated vasodilatory function.
In the present study, the absence of significant relations between changes in EDD and body mass or total body fat with weight loss is in agreement with most previous reports.3 A unique feature of the present investigation, however, was the measurement of abdominal fat and its distribution with computed tomography. We found that changes in brachial artery FMD with weight loss were not related to corresponding reductions in any measure of abdominal fat. In contrast, changes in the peak forearm blood flow response to acetylcholine were inversely related to changes in abdominal visceral fat and the abdominal visceral:subcutaneous fat ratio. The latter findings provide evidence that improvements in vascular endothelial function with weight loss may be linked to reductions in abdominal visceral fat. It is unclear why improvements in brachial FMD did not show such a relation. The facts that these are different functions and that the 2 samples did not include the same subjects may explain the differences. Alternatively, it is possible that improvements in peripheral resistance vessel EDD are selectively related to abdominal visceral fat.
Impaired forearm blood flow responses to acetylcholine in overweight/obese compared with normal weight adults is mediated at least in part by reduced NO bioavailability.1, 14 In the present study, we found that the blood flow response to acetylcholine was improved after weight loss, but not during co-infusion with L-NMMA, which inhibits NO production by endothelial NO synthase. Thus, another novel finding of our investigation is that energy intake restriction-based weight loss alone improves peripheral resistance vessel EDD in otherwise healthy overweight and obese adults by increasing NO bioavailability. The mechanisms by which weight loss per se improved NO bioavailability are uncertain. Oxidative stress is thought to develop in human obesity,13, 14, 30 and the resulting excess of reactive oxygen species can react with NO, reducing its bioavailability and impairing EDD. The fact that plasma oxidized LDL was lower after weight loss is consistent with the possibility of reduced oxidative stress, and this may have contributed to the increase in NO bioavailability and improved EDD.
We observed several expected changes in body composition and metabolic profile in response to our weight loss intervention. The reductions in body mass and total body fat were as great or greater than in previous studies on weight loss and EDD that did not involve gastric bypass surgery,3 and the intervention also produced substantial reductions in regional adiposity, including total, subcutaneous and visceral abdominal fat. Heart rate, plasma fasting total cholesterol and insulin were reduced. Although Sg was not improved in response to weight loss, fasting glucose was normal in our subjects at baseline and was maintained with less circulating insulin after weight loss, as indicated by ~25% and 30% reductions in fasting insulin and HOMA-IR score, respectively. Together, these changes along with the ~50% increase in Si observed, are consistent with an improvement in insulin sensitivity after weight loss. Thus, the intervention produced a marked improvement several CVD risk factors in these overweight and obese adults. However, other than abdominal visceral fat, none of these favorable changes in risk factors correlated with improvements in EDD, as generally observed in previous weight loss studies.3, 8, 10, 26
Several changes in humoral factors with potential vascular effects also were noted with weight loss. Plasma leptin concentrations were decreased, as expected with the reduction in fat mass, and were positively related to changes in peak forearm blood flow with acetylcholine. A similar relation between changes in plasma leptin and brachial FMD with weight loss has been reported,8 indicating that subjects with the greatest decrease in leptin had no change or a decrease in EDD.8 Although mean plasma concentrations of aldosterone did not decrease with weight loss, a positive relation was found with the change in peak forearm blood flow among individual subjects. Given that these hormones exert NO-mediated vasodilatory effects,31, 32 reductions could modulate other beneficial influences of weight loss on EDD. We found no significant changes in circulating levels of several neural, hormonal, inflammatory and local vasoconstrictor factors, or in adiponectin. In contrast to some previous observations,9, 26, 33, 34 but in agreement with others,8, 35 plasma concentrations of CRP, IL-6 and TNFα were not reduced after weight loss compared with attention control. However, among individuals, changes in plasma norepinephrine concentration with weight loss were inversely related to changes in brachial artery FMD, suggesting a possible link between reductions in sympathetic activity and peripheral conduit artery EDD.
Aging is associated with impaired vascular endothelial function,17–19, 21, 36, 37 and increased risk of CVD, independent of obesity.15 As such, interventions that can improve endothelial function in overweight and obese middle-aged and older adults have important clinical implications for preventing age-associated CVD. In the present study, the improvements in EDD with weight loss were not related to subject age, and subjects ≥50 years of age showed improvements in both measures of EDD. To our knowledge, these are the first data concerning the effects of weight loss alone on EDD in overweight and obese adults differing in age, and show that middle-aged and older adults respond as well or better to this intervention than young adults.
At least 2 important limitations should be emphasized. First, there was a high rate of dropout in the attention control group and incomplete data for our measures of EDD. Although the weight loss and attention control groups generally were similar at baseline and we were able to demonstrate improvements in EDD with weight loss, these factors could have biased our results. For example, it is possible that the weight loss subjects on whom we did not obtain interpretable measures of EDD would not have shown improvements, thus reducing the size or significance of the overall group increases in EDD. Second, the rate of weight loss in the present study (10% of baseline body weight over 3 months) is more aggressive than recommended by NIH guidelines (decrease of 10% over a 6-month period). Therefore, individuals undergoing the present weight loss regimen should do so only under medical supervision.
Perspectives
The current study provides the first experimental support for the efficacy of energy intake restriction-induced weight loss alone for restoring vascular endothelial function in otherwise healthy overweight and obese adults. Our results indicate that weight loss improves both peripheral conduit artery and resistance vessel EDD, with the latter mediated by increases in NO bioavailability. The improvements in conduit artery FMD may be related to reductions in sympathetic activity, whereas increases in resistance vessel EDD are related to reductions in abdominal visceral fat. Importantly, middle-aged and older overweight and obese adults demonstrate similar or greater improvements in EDD with weight loss as young adults. These findings have important clinical implications for the therapeutic role of conventional, moderate energy intake restriction-based weight loss programs to improve vascular endothelial function and perhaps contribute to the prevention of age-associated CVD in overweight and obese men and women.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health awards AG006537, AG013038, AG022241, AG000279 and RR00051.
Footnotes
Disclosures
None
References
- 1.Williams I, Wheatcroft S, Shah A, Kearny M. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes. 2002;26:754–764. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook RD. Obesity, weight loss, and vascular function. Endocrine. 2006;29(1):21–25. doi: 10.1385/endo:29:1:21. [DOI] [PubMed] [Google Scholar]
- 4.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 5.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 6.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 7.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 8.Brook R, Bard R, Glazewski L, Kehrer C, Bodary P, Eitzman D, Rajagopalan S. Effect of short term weight loss on the metabolic syndrome and conduit vascular endothelial function in overweight adults. Am J Cardiol. 2004;93:1012–1016. doi: 10.1016/j.amjcard.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Raitakari M, Ilvonen T, Ahotupa M, Lehtimaki T, Harmoinen A, Suominen P, Elo J, Hartiala J, Raitakari OT. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24:124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Sasaki S, Hara K, Matsuura H, Goto C, Oshima T, Chayama K. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15:302–309. doi: 10.1016/s0895-7061(01)02322-6. [DOI] [PubMed] [Google Scholar]
- 11.Bergholm R, Tiikkainen M, Vehkavaara S, Tamminen M, Teramo K, Rissanen A, Yki-Jarvinen H. Lowering of LDL cholesterol rather than moderate weight loss improves endothelium-dependent vasodilatation in obese women with previous gestational diabetes. Diabetes Care. 2003;26:1667–1672. doi: 10.2337/diacare.26.6.1667. [DOI] [PubMed] [Google Scholar]
- 12.Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 13.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli P. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress. Protective effect of vitamin c. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 14.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Sasaki S, Hara K, Matsuura H, Goto C, Oshima T, Chayama K, Yoshizumi M. Low body mass index is a risk factor for impaired endothelium-dependent vasodilation in humans: role of nitric oxide and oxidative stress. J Am Coll Cardiol. 2003;42:256–263. doi: 10.1016/s0735-1097(03)00630-2. [DOI] [PubMed] [Google Scholar]
- 15.Stevens J, Cai J, Pamuk E, Williamson D, Thun M, Wood J. Effect of age on the association between body mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 16.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol. 2007;102:63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- 17.Eskurza I, Kahn Z, Seals D. Xanthine oxidase does not contribute to impaired peripheral artery conduit artery endothelium-dependent dilatation with aging. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskurza I, Monahan K, Robinson J, Seals D. Effect of acute and chronic ascorbic acid augmentation on flow-mediated dilation with physically active and sedentary aging. J Physiol. 2004;556:215–224. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskurza I, Myerburgh L, Khan Z, Seals D. Tetrahydrobiopterin augments endothelial-dependent dilation in sedentary but not habitually exercising older adults. J Physiol. 2005;568.3:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 21.DeSouza C, Shapiro L, Clevenger C, Dinenno F, Monahan K, Tanaka H, Seals D. Regular aerobic exercise prevents and restores age-related declines in endothelium- dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 22.Nonas CA. A model for chronic care of obesity through dietary treatment. J Am Diet Assoc. 1998;98:S16–22. doi: 10.1016/s0002-8223(98)00705-6. [DOI] [PubMed] [Google Scholar]
- 23.Meisler JG, St Jeor S. Summary and recommendations from the American Health Foundation’s Expert Panel on Healthy Weight. Am J Clin Nutr. 1996;63:474S–477S. doi: 10.1093/ajcn/63.3.474. [DOI] [PubMed] [Google Scholar]
- 24.Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Caselli A, Caballero AE, Economides PA, Veves A, Horton ES. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26:2119–2125. doi: 10.2337/diacare.26.7.2119. [DOI] [PubMed] [Google Scholar]
- 25.Sciacqua A, Candigliota M, Ceravolo R, Scozzafava A, Sinopoli F, Corsonello A, Sesti G, Perticone F. Weight loss in combination with physical activity improves endothelial dysfuntion in human obesity. Diabetes Care. 2003;26:1673–1678. doi: 10.2337/diacare.26.6.1673. [DOI] [PubMed] [Google Scholar]
- 26.Clifton PM, Keogh JB, Foster PR, Noakes M. Effect of weight loss on inflammatory and endothelial markers and FMD using two low-fat diets. Int J Obes (Lond) 2005;29:1445–1451. doi: 10.1038/sj.ijo.0803039. [DOI] [PubMed] [Google Scholar]
- 27.Gokce N, Keaney J, Hunter L, Watkins M, Menzoian J, Vita J. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelila function. A prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 28.Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol. 2001;88:1067–1069. doi: 10.1016/s0002-9149(01)01997-x. [DOI] [PubMed] [Google Scholar]
- 29.Lind L, Fors N, Hall J, Marttala K, Stenborg A. A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Arterioscler Thromb Vasc Biol. 2005;25:2368–2375. doi: 10.1161/01.ATV.0000184769.22061.da. [DOI] [PubMed] [Google Scholar]
- 30.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 31.Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–173. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]
- 32.Nietlispach F, Julius B, Schindler R, Bernheim A, Binkert C, Kiowski W, Brunner-La Rocca HP. Influence of acute and chronic mineralocorticoid excess on endothelial function in healthy men. Hypertension. 2007;50:82–88. doi: 10.1161/HYPERTENSIONAHA.107.088955. [DOI] [PubMed] [Google Scholar]
- 33.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, D’Andrea F, Molinari AM, Giugliano D. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 34.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces C-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–569. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 35.Vazquez LA, Pazos F, Berrazueta JR, Fernandez-Escalante C, Garcia-Unzueta MT, Freijanes J, Amado JA. Effects of changes in body weight and insulin resistance on inflammation and endothelial function in morbid obesity after bariatric surgery. J Clin Endocrinol Metab. 2005;90:316–322. doi: 10.1210/jc.2003-032059. [DOI] [PubMed] [Google Scholar]
- 36.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 37.Gerhard M, Roddy M, Creager S, Creager M. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.