Abstract
Objective
The purpose of this study was to compare the effects of intraocular injections of ranibizumab (RBZ) and bevacizumab (BVZ) in transgenic mouse models in which human vascular endothelial growth factor (VEGF) causes subretinal neovascularization (NV) and/or exudative retinal detachment.
Design
Randomized trials in animal models
Participants
Transgenic mice in which the rhodopsin promoter drives expression of human VEGF in photoreceptors (rho/VEGF mice) and double transgenic mice with doxycycline-inducible expression of human VEGF in photoreceptors (Tet/opsin/VEGF mice).
Methods
Rho/VEGF mice) received intraocular injections of RBZ, BVZ, or vehicle and after various time periods the area of subretinal NV was measured. Tet/opsin/VEGF mice were given an intraocular injection of RBZ, BVZ, or vehicle and after 5 days of doxycycline treatment the presence or absence of retinal detachment was determined.
Main Outcome Measures
Area of subretinal neovascularization per retina in rho/VEGF mice and the occurrence of retinal detachment in Tet/opsin/VEGF mice
Results
In rho/VEGF mice, intraocular injections of RBZ or BVZ strongly suppressed subretinal NV, but the duration of effect was greater for BVZ. Three injections of 10 μg of BVZ over the course of 2 weeks not only suppressed subretinal NV in the injected eye, but also caused significant suppression in the fellow eye indicating a systemic effect. In doxycycline-treated Tet/opsin/VEGF mice, intraocular injection of 10 μg of BVZ significantly reduced the incidence of exudative retinal detachment compared to injection of 10 μg of RBZ. Injection of 25 μg of BVZ reduced the incidence of retinal detachment in both eyes.
Conclusions
Intraocular injections of RBZ and BVZ had similar efficacy in rho/VEGF mice, but the duration of effect was greater for BVZ. In Tet/opsin/VEGF mice which expression levels of human VEGF are very high and the phenotype is severe, BVZ showed greater efficacy than RBZ. In both models, higher doses or repeated injections of BVZ, but not RBZ, resulted in a systemic effect. These data suggest that BVZ is not inferior to RBZ for treatment of subretinal NV in mice and is superior in a severe model. The systemic effects of BVZ after intraocular injection deserve further study and consideration of their potential consequences.
Introduction
Choroidal neovascularization (NV) occurs in diseases of the retinal pigmented epithelium (RPE)-Bruch's membrane complex, the most common of which is age-related macular degeneration (AMD),1 but choroidal NV also occurs in other diseases in which Bruch's membrane is damaged such as pathologic myopia, ocular histoplasmosis, multifocal choroiditis, and angioid streaks. Rupturing Bruch's membrane with laser photocoagulation reliably causes choroidal NV in mice2 providing a useful animal model. In this model, vascular endothelial growth factor (VEGF) has been implicated as a critical stimulus, because expression of VEGF occurs in association with development of choroidal NV3 and VEGF antagonists strongly suppress the choroidal NV.4 Additional evidence implicating VEGF was provided by transgenic mice in which the rhodopsin promoter drives expression of VEGF in photoreceptors resulting in subretinal NV.5, 6
As evidence accumulated suggesting that VEGF played important roles in both tumor and ocular NV, Genentech Inc. developed bevacizumab (BVZ), a full-length humanized monoclonal antibody that binds all isoforms of VEGF-A for treatment of tumors.7 It was felt that the 150 kDa molecular weight of bevacizumab would limit its penetration through the retina after intraocular injection; therefore, ranibizumab (RBZ), a 48 kDa Fab that binds all isoforms of VEGF-A was developed for ocular NV. As a result of affinity maturation, RBZ is 5 to 20-fold more potent on a molar basis in binding VEGF-A than BVZ.8 The half-life after a single intraocular injection of RBZ in monkeys was 3 days and serum levels were very low, approximately 1000-fold lower than levels in the eye.9 The half-life after an intraocular injection of the full-length antibody, trastuzumab (148 kDa), which is comparable in size to BVZ, is 5.6 days10.
Addition of infusions of BVZ to the regimen of patients with metastatic colorectal cancer modestly prolonged survival11 leading to its approval by the FDA. A few years later, RBZ was approved after it was demonstrated that intraocular injections of 0.5 mg of RBZ caused an increase in visual acuity of 3 or more lines in 34-40% of patients with neovascular AMD.12, 13 However, in the interval between the approval of BVZ and RBZ, off-label testing of BVZ was done in patients with neovascular AMD and young patients with CNV due to causes other than AMD and in both patient populations strong efficacy was seen.14-17 A substantial number of older patients treated with BVZ developed hypertension and therefore intraocular injections of BVZ were tried. To adjust for the reduced potency of BVZ compared to RBZ, 1.25 mg of BVZ, a dose 2.5-fold higher than the 0.5 mg dose of RBZ was empirically selected. Intraocular injections of 1.25 mg of BVZ reduced subretinal and intraretinal fluid and improved vision in a substantial number of patients with neovascular AMD.18 Case series have provided supporting data suggesting a beneficial effect of BVZ in neovascular AMD.19, 20 Currently both RBZ and BVZ are widely used in clinical care. A clinical trial has been organized to compare the efficacy of intraocular injections of 0.5 mg of RBZ with 1.25 mg of BVZ, but it will be quite some time before the data are available. Comparative data in animal models would be helpful, but BVZ and RBZ do not cross-react with mouse or rat VEGF21 and therefore rodent models which have been useful for evaluating many agents cannot be used. However, there are two mouse transgenic models in which human VEGF is expressed in photoreceptors and as noted above, they helped to provide important evidence implicating VEGF in the pathogenesis of subretinal NV. Transgenic mice in which the rhodopsin promoter drives expression of human VEGF165 in photoreceptors (rho/VEGF mice) sprout new vessel from the deep capillary bed of the retina starting at post natal day (P) 10 that grow into the subretinal space.5 The production of VEGF is sustained and therefore the new vessels continue to grow and enlarge and form large nets in the subretinal space similar to those seen in humans with neovascular AMD.6 Since the new vessels originate from retinal capillaries and not choroidal vessels, it is technically a model of retinal angiomatous proliferation (RAP) which occurs in roughly 30% of patients with neovascular AMD,22 but in general it mimics critical features of neovascular AMD because the production of VEGF is sustained and results in progressive growth and spread of new vessels in the subretinal space. In a second model, the tetracycline-inducible promoter system (Tet/on system) was employed to generate double transgenic mice with doxycycline-inducible expression of human VEGF165 in photoreceptors (Tet/opsin/VEGF mice).23 These mice are completely normal until they are given doxycycline which turns on the expression of VEGF in photoreceptors. A dose of 2 mg/ml of doxycycline in drinking water results in high expression of VEGF in photoreceptors (at least 30-fold higher than that seen in rho/VEGF mice) and results in very severe NV and massive leakage causing total exudative retinal detachment in 80-90% of mice within 5 days.23-25 Retinal detachment provides a great outcome measure to test efficacy in this model and the severe phenotype has proven extremely useful to distinguish between treatments that are moderately effective and those that are very effective.24, 25 In this study, we compared effects of intraocular injections of RBZ and BVZ in these mouse models.
Materials and Methods
Testing of RBZ and BVZ in rho/VEGF transgenic mice
Mice were treated in accordance with the Association for Research in Vision and Ophthalmology guidelines for the use of animals in research. The protocol was approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee. At postnatal day (P) 14, hemizygous rho/VEGF mice5 were given an intraocular injection of 1 μl of phosphate buffered saline (PBS) or 1 μl of PBS containing 1 or 10 μg of RBZ (Genentech, Inc., South San Francisco, CA) or 1, 10 or 25 μg of BVZ (Genentech, Inc., South San Francisco, CA) in one eye. Intraocular injections were done under a dissecting microscope with a Harvard Pump Microinjection System and pulled glass micropipettes as previously described.26 At P21, P28, or P35 the total area of subretinal NV per eye was quantified as previously described.6 Briefly, mice were anesthetized and perfused with 1 ml of phosphate-buffered saline containing 25 mg/ml of fluorescein-labeled dextran (2×106 average mw, Sigma, St. Louis, MO). The eyes were removed and fixed for 1 hour in 10% phosphate-buffered formalin. The cornea and lens were removed and the entire retina was carefully dissected from the eyecup, radially cut from the edge of the retina to the equator in all 4 quadrants, and flat-mounted in mounting medium (Aquamount; Polysciences, Warrington, PA) with photoreceptors facing upward. The retinas were examined by fluorescence microscopy at 200× magnification, which provides a narrow depth of field so that when focusing on NV on the outer surface of the retina, the remainder of the retinal vessels are out-of-focus allowing easy delineation of the NV. The outer edge of the retina, which corresponds to the subretinal space in vivo, is easily identified and therefore there is standardization of focal plane from slide to slide. Images were digitized using a 3 CCD color video camera and a frame grabber. Using Image analysis software (Image-Pro Plus; Media Cybernetics, Silver Spring, MD), an investigator masked with respect to treatment group allowed the software to recognize and calculated the total area of subretinal NV per eye as previously described.6 To determine the maximum effect of equivalent doses of RBZ and BVZ in rho/VEGF mice, (P) 14 mice were given an intraocular injection of 1 μl of phosphate buffered saline (PBS) or 1 μl of PBS containing 10 μg of RBZ or BVZ in one eye. The injections were repeated on P19 and P24 and on P28 the total area of subretinal NV per eye was measured as described above.
Testing of RBZ and BVZ in Tet/opsin/VEGF double transgenic mice
Four to six week old double hemizygous Tet/opsin/VEGF double transgenic mice23 were divided into 4 groups. Group A was given an intraocular injection of 25 μg of BVZ in one eye and 10 μg of RBZ in the other eye. Group B was given 25 μg of BVZ in one eye and phosphate-buffered saline (PBS) in the other eye. Group C was given 10 μg of RBZ in one eye and PBS in the other eye. Group D was given 10 μg of BVZ in one eye and PBS in the other eye. The mice were given 2 mg/ml of doxycycline in their drinking water. After 5 days, with the investigator masked with respect to treatment group, the retinas were examined under an operating microscope and it was determined if there was a total retinal detachment (TRD), partial retinal detachment (PRD), or no retinal detachment (no RD).
Results
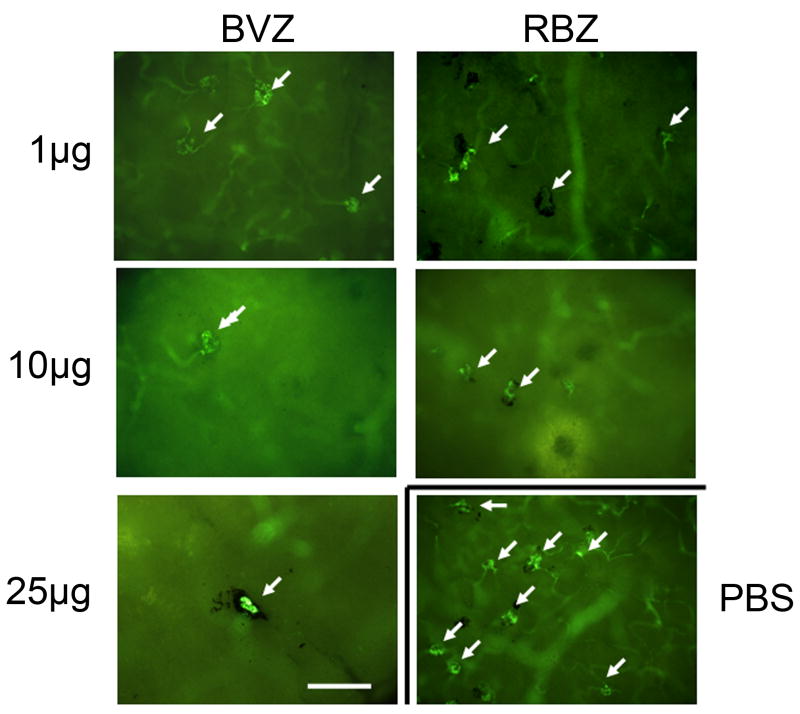
Intraocular injections of 50 μl of a 10 μg/μl solution of RBZ provide substantial benefit in patients with neovascular AMD.12, 13 There is also substantial evidence that intraocular injections of 50 μl of a 25 μg/μl solution of BVZ are beneficial in patients with neovascular AMD.19, 20 To provide the same relative dose, 1 μl of a 10 μg/μl solution of RBZ or 1 μl of a 25 μg/μl solution of BVZ was injected into the eyes of rho/VEGF transgenic mice. In addition, we tested lower doses of each, 1 μg of RBZ and 1 and 10 μg of BVZ. The injections appeared to be well-tolerated and there were no deaths. At various time points after injections, mice were perfused with fluorescein-labeled dextran and retinas were flat mounted with the photoreceptor side facing up. Figure 1 shows fluorescent microscopy of retinas one week after injection and at this magnification the depth of field is fairly narrow so that the buds of neovascularization on the outer surface of the retina which corresponds to the subretinal space are in focus (arrows) while the retinal vessels are out of focus. For many of the neovascular buds feeder vessels can be seen and many are partially or completely surrounded by retinal pigmented epithelial (RPE) cells. Compared to retinas from eyes injected with PBS (second column, third row), those injected with each of the doses of BVZ or RBZ appeared to have fewer buds of neovascularization. The total area of neovascularization on the outer surface of the retina was measured by image analysis with the investigator masked with respect to treatment group. One week after injection, eyes treated with each of these doses of RBZ or BVZ had significantly less subretinal NV than eyes injected with PBS (Figure 2A). Fellow eyes did not have significantly less subretinal NV than PBS-injected eyes indicating that the predominant anti-angiogenic effect of these doses of RBZ and BVZ in this model was local and not systemic. When the area of subretinal NV was evaluated 2 weeks after injection, compared to PBS-injected eyes, it was significantly less in eyes injected with 10 μg of RBZ or 1, 10, or 25 μg of BCZ, but not those injected with 1 μg of RBZ (Figure 2B). This indicates that despite its lower potency, equivalent doses of BVZ have a longer duration of effect compared to RBZ in this model. Three weeks after injection there was no significant reduction in mean area of subretinal NV for any of the doses of RBZ or BVZ, indicating that a single injection of 1 μl of these doses cannot suppress NV for 3 weeks in this model (Figure 2C).
Figure 1. Representative images of fluorescent microscopy of retinal whole mounts from transgenic mice with over-expression of vascular endothelial growth factor (VEGF) in photoreceptors (rho/VEGF mice) one week after injection of bevacizumab (BVZ), ranibizumab (RBZ), or phosphate-buffered saline (PBS).
One week after intraocular injection of PBS or one of the designated doses of BVZ or RVZ, mice were perfused with fluorescein-labeled dextran and retinal flat mounts with the photoreceptor side facing up were examined by fluorescence microscopy. Compared to eyes injected with PBS, there appeared to be fewer neovascular buds (arrows) on the outer surface of the retina (which corresponds to the subretinal space) in eyes injected with any of the doses of BVZ or RBZ. Many of the tufts of neovascularization are partially or completely surrounded by retinal pigmented epithelial cells and for some feeder vessels can be seen.
Bar = 100 μm
Figure 2. Effect of intraocular injection of ranibizumab (RBZ) or bevacizumab (BVZ) in transgenic mice with over-expression of vascular endothelial growth factor (VEGF) in photoreceptors (rho/VEGF mice).
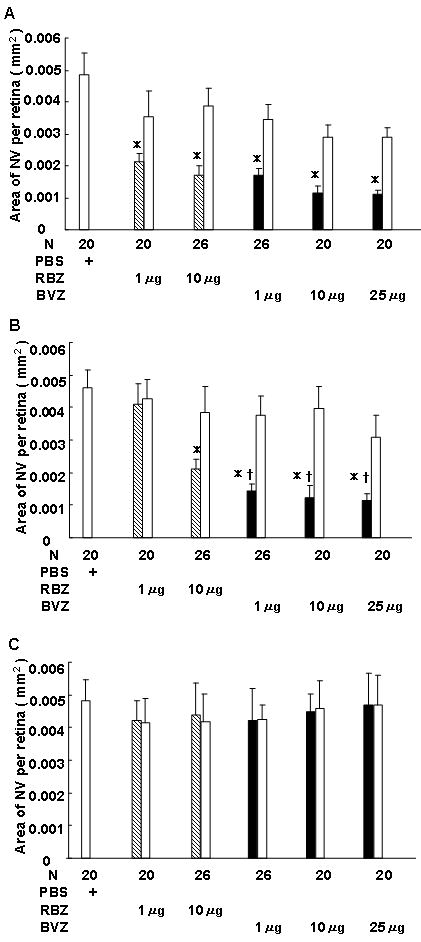
At postnatal day (P) 14, hemizygous rho/VEGF transgenic mice were given an intraocular injection of 1 μl of phosphate buffered saline (PBS) or 1 μl of PBS containing 1 or 10 μg of RBZ or 1, 10 or 25 μg of BVZ in one eye. At P21 (A), P28 (B), or P35 (C), mice were perfused with fluorescein-labeled dextran and the total area of subretinal neovascularization (NV) was measured in each eye on retinal flat mounts by image analysis with the investigator masked with respect to treatment group. The bars show the mean (±SEM) area of subretinal NV for injected eyes and fellow eyes.
(A) One week after injection, compared to eyes injected with PBS, the mean area of subretinal NV was significantly less in eyes injected with 1 or 10 μg of RBZ or 1, 10 or 25 μg of BVZ (*p<0.001 by ANOVA with Bonferroni/Dunn's correction for multiple comparisons). The area of subretinal NV in fellow eyes was not significantly different from eyes injected with PBS, indicating a local effect in eyes injected with RBZ or BVZ.
(B) Two weeks after injection, compared to eyes injected with PBS, the mean area of subretinal NV was not significantly less in eyes injected with 1 μg of RBZ. The mean area of subretinal NV was significantly less in eyes injected with 10 μg of RBZ or 1, 10, or 25 μg of BVZ compared to eyes injected with PBS (*p<0.001 by ANOVA with Bonferroni/Dunn's correction for multiple comparisons) or compared to eyes injected with 1 μg of RBZ (†p<0.005).
(C) Three weeks after injection, compared to eyes injected with PBS, the mean area of subretinal NV was not significantly less in any of the eyes injected with RBZ or BVZ.
In order to determine the maximum effect of equivalent doses of RBZ or BVZ in rho/VEGF mice, the mice were given injections of PBS (group A), 10 μg of RBZ (group B), or 10 μg of BVZ in one eye on P14, P19, and P24 and then the area of subretinal NV was measured in both eyes on P28 (n=20 in each group). Eyes injected with RBZ or BVZ had significantly less subretinal NV than eyes injected with PBS (Figure 3; *p<0.005, **p<0.001 by analysis of variance with Bonferroni/Dunn's correction for multiple comparisons), but no significant difference from each other. Eyes contralateral to those injected with BVZ, but not those contralateral to RBZ-injected eyes had significantly less subretinal NV than eyes injected with PBS (*p<0.005 by analysis of variance with Bonferroni/Dunn's correction), indicating that with this regimen of 3 intraocular injections of 10 μg over 2 weeks, BVZ, but not RBZ had a substantial systemic effect.
Figure 3. Effect of three intraocular injection of ranibizumab (RBZ) or bevacizumab (BVZ) over the course of 2 weeks in transgenic mice with over-expression of vascular endothelial growth factor (VEGF) in photoreceptors (rho/VEGF mice).
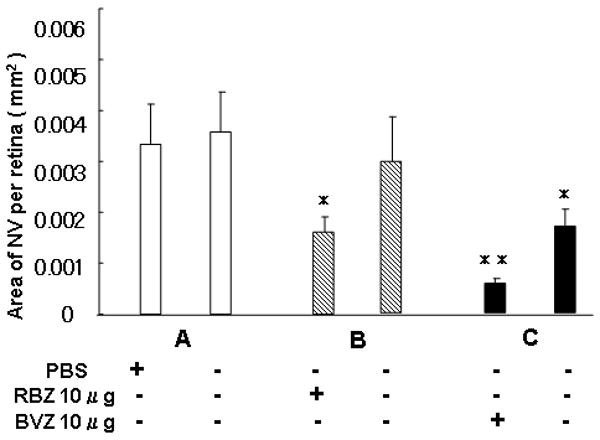
At postnatal day (P) 14, hemizygous rho/VEGF transgenic mice were given an intraocular injection of 1 μl of phosphate buffered saline (PBS) or 1 μl of PBS containing 10 μg of RBZ or BVZ in one eye. The injections were repeated on P19 and P24 and on P28 the mice were perfused with fluorescein-labeled dextran and the total area of subretinal neovascularization (NV) was measured in each eye on retinal flat mounts by image analysis with the investigator masked with respect to treatment group. The bars show the mean (±SEM) area of subretinal NV for injected eyes and fellow eyes. Compared to eyes injected with PBS, eyes injected with RBZ or BVZ, or eyes contralateral to those injected with BVZ has significantly less subretinal NV (*p<0.005; **p<0.001 by ANOVA with Bonferroni/Dunn's correction for multiple comparisons).
Double transgenic mice with doxycycline-inducible expression of VEGF (Tet/opsin/VEGF mice) express higher levels of VEGF in photoreceptors than rho/VEGF mice and have a more severe phenotype. When given 2 mg/ml of doxycycline in drinking water, 80-90% of Tet/opsin/VEGF mice develop a total retinal detachment (TRD) within 5 days.23-25 When Tet/opsin/VEGF mice were given an intraocular injection of 10 μg of RBZ in one eye and PBS in the fellow eye and treated with 2 mg/ml of doxycycline in their drinking water, 90% of the mice developed TRD in each eye (Figure 4, group C) indicating that 10 μg of RBZ is ineffective in this severe model. Compared to the 90% rate of TRD in eyes injected with 10 μg of RBZ when the fellow eye was injected with PBS, the rate of TRD was significantly less (34.8%; p<0.001 by chi-square) in eyes injected with 25 μg of BVZ when the fellow eye was injected with PBS (Figure 4, group B). The PBS-injected fellow eye had a 56.5% rate of TRD which was significantly less than the 90% rate in eyes injected with 10 μg of RBZ (p<0.05) indicating that the systemic effect of an intraocular injection of 25 μg of BVZ was greater than the local effect of 10 μg of RBZ in this model. In view of this systemic effect of BVZ, it is not surprising that Tet/opsin/VEGF mice injected with 25 μg of BVZ in one eye and 10 μg of RBZ in the fellow eye (Figure 4, group A), showed fewer TRDs in each eye (50% and 54.2%, respectively) compared to eyes injected with 10 μg of RBZ when PBS was injected in the fellow eye. When 10 μg of BVZ was injected in one eye and PBS was injected in the fellow eye (Figure 4, group D), the rate of TRD was 60% in the BVZ-injected eyes and 75% in PBS-injected fellow eyes. The former, but not the latter, was significantly less than the 90% seen in eyes injected with 10 μg of RBZ indicating that the local effect of BVZ is superior to that of RBZ at equivalent doses in this model. Figure 5 shows a representative ocular section from an eye injected with PBS showing a total retinal detachment and one from an eye injected with BVZ showing no retinal detachment.
Figure 4. Effect of intraocular injection of ranibizumab (RBZ) or bevacizumab (BVZ) in double transgenic mice with inducible over-expression of vascular endothelial growth factor (VEGF) in photoreceptors (Tet/opsin/VEGF mice).
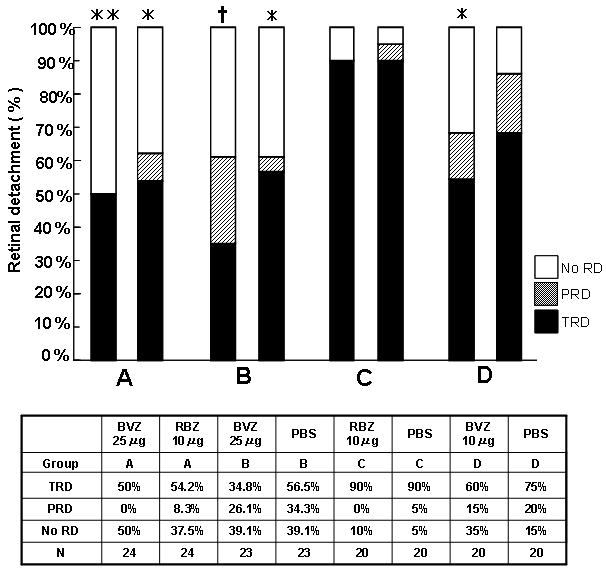
Adult Tet/opsin/VEGF mice were divided into 4 groups. Group A was given an intraocular injection of 25 μg of BVZ in one eye and 10 μg of RBZ in the other eye. Group B was given 25 μg of BVZ in one eye and phosphate-buffered saline (PBS) in the other eye. Group C was given 10 μg of RBZ in one eye and PBS in the other eye. Group D was given 10 μg of BVZ in one eye and PBS in the other eye. The mice were given 2 mg/ml of doxycycline in their drinking water. After 5 days, with the investigator masked with respect to treatment group, the retinas were examined under an operating microscope and it was determined if there was a total retinal detachment (TRD), partial retinal detachment (PRD), or no retinal detachment (no RD). In group C mice, 90% of eyes injected with 10 μg of RBZ and 90% of fellow eyes injected with PBS had TRD indicating that 10 μg of RBZ was ineffective in this model. In group B mice, 34.8% of eyes injected with 25 μg of BVZ and 56.5% of fellow eyes injected with PBS had TRD, both significantly less than RBZ-injected eyes in group C (*p<0.05;†p<0.001 by chi-square). This suggests that injection of 25 μg of BVZ in one eye significantly reduced TRD in both eyes. Similarly, in group A injection of 25 μg of BVZ in one eye resulted in significant reduction in TRD in both eyes compared to the RBZ-injected eye in group C (*p<0.05; **p<0.01 by chi-square). In group D, injection of 10 μg of BVZ in one eye resulted in a significant reduction in TRD compared to the RBZ-injected eye, but not in the fellow eye injected with PBS.
Figure 5. Ocular sections from eyes injected with phosphate-buffered saline (PBS) or 25 μg of bevacizumab (BVZ).
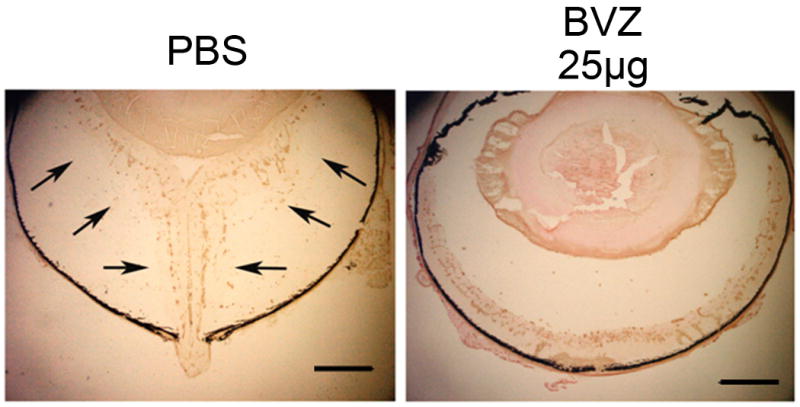
Five days after institution of doxycycline treatment and an intraocular injection of PBS or BVZ, an eye that was injected with PBS shows a total retinal detachment, while an eye injected with BVZ shows no retinal detachment.
Bar = 500 μm
Discussion
Monthly intraocular injections of 0.5 mg of RBZ over the course of 1 year cause improvement of 3 or more lines of visual acuity in 34-40% of patients with neovascular AMD12, 13 and that improvement is maintained for at least another year of continued treatment. This has revolutionized the treatment of neovascular AMD, but despite these impressive results many retina specialists have substituted intraocular injections of 1.25 mg of BVZ for 0.5 mg of RBZ. The 2.5-fold higher dose of BVZ was empirically selected to compensate for the higher binding affinity for VEGF of RBZ compared to BVZ. While there is some suggestion from case series that intraocular injections of 1.25 mg of BVZ provide benefit in patients with neovascular AMD, it is not known how the effects compare with those obtained with RBZ. There is an ongoing clinical trial in which the effects of intraocular injections of 0.5 mg of RBZ and 1.25 mg of BVZ will be compared.
This study provides the first comparison of the efficacy of intraocular RBZ and BVZ in animal models of subretinal NV. Rho/VEGF mice have sustained expression of human VEGF in photoreceptors and develop progressive subretinal NV and therefore have phenotype similar to that seen in patients with neovascular AMD. In this model, a single intraocular injection of 1 μg of RBZ or BVZ caused significant suppression of subretinal NV over the course of a week, but while the effect of BVZ was maintained 2 weeks after injection, the effect of RBZ was not. These data indicate that at an equivalent dose, BVZ has a longer duration of effect than RBZ.
In order to try to assess the maximum effect of equivalent doses of RBZ and BVZ in this model, 3 injections of 10 μg of each was given over the span of 2 weeks. Although the mean area of subretinal NV was somewhat smaller in BVZ-injected eyes compared to RBZ-injected eyes, the difference was not statistically different. We must conclude that if there is a difference in efficacy, this fairly large experiment was not sufficiently powered to detect it. Another finding from this experiment was that with this regimen of multiple injections, a systemic effect was identified for BVZ, but not RBZ.
When treated with 2 mg/ml of doxycycline, Tet/opsin/VEGF mice express at least 30-fold higher levels of VEGF in photoreceptors than rho/VEGF mice and within 5 days of the onset of doxycycline, 80-90% develop severe subretinal NV and exudative retinal detachment. 23 Compared to intraocular injection of 10 μg of RBZ, injection of 10 μg of BVZ significantly reduced the number of TRDs indicating that in this severe model, BVZ has greater efficacy than RBZ.
As was true in rho/VEGF mice with 3 injections of 10 μg, in Tet/opsin/VEGF mice a single injection of 25 μg or multiple injections of 10 μg of BVZ significantly suppressed subretinal NV in the fellow eye indicating a systemic effect, while there was no detectable systemic effect after multiple injections of 10 μg RBZ. The presence of an Fc domain in BVZ, but not RBZ, probably contributes because it reduces clearance from the circulation thereby allowing higher levels to be achieved as BVZ exits from the eye. Also, the presence of neonatal Fc receptors in the ciliary epithelium may increase egress of intact BVZ from the vitreous to the systemic circulation and therefore boost systemic levels. 27 While this could be beneficial in patients with bilateral subretinal NV, it may increase the risk of systemic complications for intraocular BVZ compared to intraocular RBZ.
The current study predicts that the Comparison of AMD Treatments Trial trial will show that treatment with intraocular injections of 1.25 mg of BVZ is not inferior to treatment with injections of 0.5 mg of RBZ in patients with neovascular AMD. Whether the superior efficacy of BVZ compared to RBZ in Tet/opsin/VEGF mice is sufficient to translate into superior efficacy in patients with neovascular AMD is uncertain. The greater systemic antiangiogenic effect after intraocular injection of BVZ compared to RBZ raises the question of whether RBZ should be preferred over BVZ in patients with a recent history of stroke or myocardial infarction. Such patients have a greater risk of thrombotic complications from systemic blockade of VEGF,28 but it is unknown whether the relatively small amount of systemic anti-VEGF activity associated with intraocular injections of BVZ would result in similar complications as the more profound systemic anti-VEGF activity achieved by systemically administered VEGF antagonists. However, concerns have been raised that intraocular injections RBZ may increase the risk of stroke, particularly in patients with diseased vascular endothelium, which can be indicated by a prior history of stroke.29 It is still not clear if there is any justification for concern, but if there is, concern regarding BVZ should be greater than that for RBZ. This issue deserves further consideration and study.
Acknowledgments
Supported by EY12609 and core grant P30EY1765 from the NEI. PAC is the George S. and Dolores Dore Eccles Professor of Ophthalmology and Neuroscience.
Footnotes
PAC has an institutional consulting agreement with Genentech, Inc. for which payment is made to Johns Hopkins University, has research funding for clinical trials sponsored by Genentech but not for this study, and is on the Data and Safety Monitoring Committee for the View 1 Trial sponsored by Regeneron, Inc. No other authors have any potential conflict of interest.
References
- 1.Klein R, Klein BEK, Linton KP. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137:190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- 2.Tobe T, Ortega S, Luna JD, et al. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–6. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi X, Ogata N, Komada M, et al. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Lab Invest. 1997;235:313–9. doi: 10.1007/BF01739641. [DOI] [PubMed] [Google Scholar]
- 4.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is an important stimulator in a model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41:3158–64. [PubMed] [Google Scholar]
- 5.Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151(1):281–91. [PMC free article] [PubMed] [Google Scholar]
- 6.Tobe T, Okamoto N, Vinores MA, et al. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest Ophthalmoll Vis Sci. 1998;39(1):180–8. [PubMed] [Google Scholar]
- 7.Presta LG, Chen H, O'Conner SJ, et al. Humanization of an anti-vascular endothelial growth facor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 8.Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–70. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 9.Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 10.Mordenti J, Thomsen K, Licko V, et al. Intraocular pharmacokinetics and safety of a humanized monoclonal antibody in rabbits after intravitreal administration of a solution or a PLGA microsphere formulation. Toxicol Sci. 1999;52:101–6. doi: 10.1093/toxsci/52.1.101. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Eng J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 14.Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Ophthalmology. 2005;112:1035–47. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen QD, Shah SM, Tatlipinar S, et al. Bevacizumab suppresses choroidal neovascularization due to pathologic myopia. Br J Ophthalmol. 2005;89:1368–70. [PMC free article] [PubMed] [Google Scholar]
- 16.Moshfeghi AA, Rosenfeld PJ, Puliafito CA, et al. Systemic Bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113:2002–11. doi: 10.1016/j.ophtha.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen QD, Shah SM, Hafiz G, et al. Intravenous bevacizumab causes regression of choroidal neovascularization secondary to diseases other than age-related macular degeneration. Am J Ophthalmol. 2007;145:257–66. doi: 10.1016/j.ajo.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intavitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophtalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- 19.Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–90. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Wu X, Cheng Z, et al. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008;49:522–7. doi: 10.1167/iovs.07-1175. [DOI] [PubMed] [Google Scholar]
- 22.Yannuzzi LA, Negrao S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–34. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Ohno-Matsui K, Hirose A, Yamamoto S, et al. Inducible expression of vascular endothelial growth factor in photoreceptors of adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. 2002;160:711–9. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, Saishin Y, Saishin Y, et al. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J. 2003;17:896–8. doi: 10.1096/fj.02-0824fje. [DOI] [PubMed] [Google Scholar]
- 25.Nambu H, Nambu R, Oshima Y, et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11:865–73. doi: 10.1038/sj.gt.3302230. [DOI] [PubMed] [Google Scholar]
- 26.Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–63. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 27.Kim H, Fariss RN, Zhang C, et al. Mapping of the neonatal Fc receptor in the rodent eye. Invest Ophthalmol Vis Sci. 2008;49:2025–9. doi: 10.1167/iovs.07-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elice F, Rodeghiero F, Falanga A, Rickels F. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol. 2009;22:115–28. doi: 10.1016/j.beha.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Dafer RM, Schneck M, Friberg TR, Jay WM. Intravitreal ranibizumab and bevacizumab; a review of risk. Semin Ophthalmol. 2007;22:201–4. doi: 10.1080/08820530701543024. [DOI] [PubMed] [Google Scholar]