Abstract
Osteocalcin was recently identified as an osteoblast-secreted hormone regulating insulin secretion and sensitivity. In mice and humans, osteocalcin can be present in the serum in carboxylated or undercarboxylated forms and it has been shown that it is the undercarboxylated form of osteocalcin which acts as a hormone. The study of osteocalcin different circulating forms in mouse serum, however, has been hampered by the absence of quantitative methodology. Here we described a triple enzyme-linked immunosorbent assay (ELISA) system for quantification of mouse total, carboxylated and uncarboxylated osteocalcin. That carboxylation of osteocalcin was decreased in mouse osteoblasts cultures treated with warfarin, an inhibitor of carboxylation validated this assay. This ELISA could also detect elevated levels of undercarboxylated osteocalcin in the serum of mice treated with warfarin and in the serum of Esp−/− mice, a mouse model known to have more undercarboxylated i.e. active osteocalcin. These results show that this new ELISA system is a reliable method to assess of carboxylation status of osteocalcin in cell culture supernatants as well as in mouse serum. Its use should facilitate the analysis of culture system or mouse model in which the hormonal activity of osteocalcin needs to be evaluated.
Keywords: Osteocalcin, Carboxylation, ELISA, Serum
INTRODUCTION
In mammals, glucose homeostasis depends on the correct integration of multiple signals emanating from endocrine organs such as pancreatic islets, liver, fat, muscle and brain. Recently, we demonstrated that bone also exerts an endocrine regulation of energy metabolism [1–3]. Indeed, the osteoblast, the cell responsible for bone formation, secretes a hormone, osteocalcin, which stimulates insulin secretion by pancreatic β-cells and favours insulin sensitivity in muscle, liver and fat [4–6]. Supporting this model, mice lacking osteocalcin have a decrease in insulin secretion and sensitivity; causing glucose intolerance. In contrast, Esp-deficient mice, which are a model of osteocalcin gain of activity, have improved insulin secretion and sensitivity [4; 7].
Osteocalcin is a small 46 amino acids-long protein containing three glutamic acids (GLU13, GLU17 and GLU20) in mice that are γ-carboxylated during it synthesis in the osteoblast [8]. However, osteocalcin exists in serum as fully carboxylated or partially carboxylated forms and completely uncarboxylated [9–13]. Cell-based assays and in vivo infusions have shown that recombinant uncarboxylated osteocalcin promotes insulin secretion, insulin gene expression and insulin sensitivity in cells and mice [4; 7]. Remarkably, Esp−/− mice present an increase in undercarboxylated osteocalcin, further supporting that this is the metabolically active form of this hormone. More recently, we have shown that the low pH generated during bone resorption can preferably decarboxylate osteocalcin on glutamic acid 13 and therefore that osteoclasts determine the carboxylation status and function of osteocalcin in vivo [14].
In mice and human carboxylation levels of osteocalcin in serum and in bone samples have been previously assessed indirectly by hydroxyapatite (HA) pull down followed by measurement of the unbound fraction of osteocalcin using commercially available ELISA or RIA [15–17]. This assay is based on the fact that undercarboxylated osteocalcin has a lower binding affinity for HA compared to carboxylated osteocalcin [15; 16]. Such HA-based measurement of undercarboxylated osteocalcin are, however, a semi-quantitative method that does not precisely quantify the serum concentration of undercarboxylated or carboxylated osteocalcin. Hence, there is a need for a method for the direct quantitative measurement of the different forms of osteocalcin in mouse serum samples. Here, we described the development of a triple enzyme-linked immunosorbend assay (ELISA) system for the precise quantification of mouse carboxylated, uncarboxylated and total osteocalcin. We also report that this assay could successfully measure the carboxylation status of osteocalcin in osteoblasts culture mediums as well as in mice serums corresponding to well-know models where osteocalcin carboxylation is altered.
MATERIALS AND METHODS
Plasmid constructs
The GST-OCN11-26 and the GST-OCN25-46 constructs were generated by cloning double strand oligonucleotides encoding mouse OCN11-26 (PLEPTREQCELNPAC) and OCN25-46 (CDELSDQYGLKTAYKRIYGITI) into the BamHI and EcoRI sites of pGEX 4T3. The GST-OCN expression vector has been described previously [7].
Antibodies production and purification
For the generation of anti-uncarboxylated osteocalcin, anti-carboxylated osteocalcin and anti-C-terminal osteocalcin antibodies, 5 mg of full-length recombinant uncarboxylated osteocalcin was purified as described [7] or 5 mg of full-length carboxylated osteocalcin was chemically synthesized (Biosynthesis, TX). Half of each preparation was conjugated to KLH by the glutaraldehyde conjugation method and a 1:1 mixture of native and conjugated protein was used for the immunization of 2 goats (Genscript, NJ). For the primary immunization, 0.5 mg of mixture per goat were injected subcutaneously in combination with complete Freund’s adjuvant. Animals were then given boosts of 0.5 mg with incomplete Freund’s adjuvant every 3 weeks for a total of 3 boosts. Final bleeds resulted in 500 ml of anti-serum per goat.
One to 10 mg of peptides or recombinant proteins (GST-OCN11-26, GST-OCN25-46, GLA13-OCN11-26 and GLA-OCN) were coupled to HiTrap NHS-activated columns (GE Healthcare) according to the manufacturer instruction. For affinity purifications (see Figure 1 for the complete strategy of purification of all antibodies), 25 ml of goat antiserum was heat inactivated at 56°C for 30 minutes, filtered through a 0.45 μm filter and diluted 5 times in start buffer (75 mM Tris-HCl, pH 8.0). The diluted anti-serum was then applied onto the affinity column using a peristaltic pump at a flow rate of 0.5 ml/min. The column was next washed with 10 column volumes of start buffer. Purified antibodies were eluted with 3 column volumes of elution buffer (100 mM glycine-HCl, 0.5 M NaCl pH 2.7). Eluted antibodies were immediately neutralized with 1/10 volume of 1 M Tris-HCl pH 8, glycerol was added (50% final) and aliquots were stored at −80°C. To deplete antibodies, purified antibodies were dialyzed in PBS 1X, circulated on the column and the unbound flow through fraction was stored at −80°C.
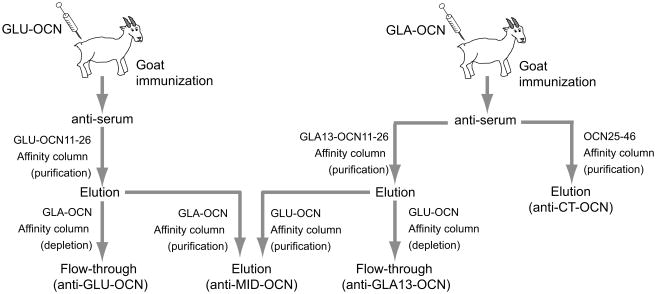
FIGURE 1. Production and purification of anti-OCN goat antibodies.
Schematic representation of the methodology used to obtain the GLU-OCN, the GLA13-OCN, the MID-OCN and the CT-OCN goat polyclonal antibodies. See also the Materials and Methods section.
Dot blot
Synthetic uncarboxylated and carboxylated osteocalcin were diluted in PBS 1X containing 0.01% BSA and spotted on nitrocellulose membranes. Membranes were allowed to dry and subjected to western blotting according to standard procedures.
HRP conjugation to the CT-OCN antibodies
One mg of affinity purified anti-CT-OCN antibodies was conjugated to horseradish peroxidase (HRP) using EZ-Link® Plus Activated Peroxidase reagent (Pierce) according to the manufacturer instruction. A Conjugate Purification Kit (Pierce) was then used to remove free HRP and further purify the conjugated IgG. The HRP conjugate was then transferred to 1X PBS using a desalting column, and 1 volume of Peroxidase Conjugate Stabilizer (Pierce) was added before storage in aliquots at −20°C.
GLU, GLA13 and total mouse osteocalcin ELISA
Antibody Coating Buffer (CB1), ELISA Wash Buffer (WB1), General Blocker Buffer (BB1), General Assay Diluent (AD1) and Stop Solution for TMB (STOP1) were all obtained from ImmunoChemistry Technologies. 1-Step™ Ultra TMB ELISA substrate was purchased from Pierce. ELISA plates (R&D system) were coated overnight at room temperature with 100 μl of a 12 μg/ml solution of affinity purified anti-GLU-OCN, anti-GLA13-OCN or anti-MID-OCN diluted in 1X antibody coating buffer. Following 2 washes with 1X wash buffer, the plate was blocked with 300 μl of 1X General Blocker Buffer for 3–6h at room temperature. Blocking solution was removed, the plate firmly tapped on absorbent paper to remove excess liquid and used immediately. Ninety five μl of General assay diluent was added to each well, followed by 5 μl of blank, standards (purified GLU-OCN or synthetic GLA-OCN) or serum samples. Plate was then sealed and incubated overnight at 4°C. Following 5 washes with 1X washing buffer, 100 μl of HRP-conjugated anti-CT-OCN (1 μg/ml in 1X General assay diluent) was added to each well and the plate incubated for 1h at room temperature on a shaker (~200–300 rpm). The plate was washed again 5 times and after complete removal of excess solution by taping on absorbent paper, 100 μl of TMB substrate was added to each well. After 15 minutes incubation at room temperature, 100 μl of stop solution was added and the absorbance at 450 nm was read using a plate reader (Biorad). Concentrations of GLU-OCN, GLA13-OCN and total OCN in the samples were calculated from polynomial second order or exponential standard curve obtained from the standard included in each assay.
Primary osteoblasts cultures
Mouse osteoblasts were isolated from calvaria as described [18]. Cells were cultured in α-MEM supplemented with 10% Fetal Bovine Serum (FBS) for 4 days, after what osteoblast differentiation was induced by changing the medium to α-MEM/10% FBS supplemented with 5 mM β-glycerophosphate and 100 μg/ml L-ascorbic acid. Ten days later, cells were washed twice with PBS 1X before being incubated in the same medium in the presence or absence of Warfarin (5 μM) for 48h. Supernatant were then collected and osteocalcin levels measured by ELISA.
Warfarin treatment
Eight week-old C57BL/6 male mice were injected subcutaneously once a day with saline or with warfarin (1mg/kg). Following a 2 weeks treatment, serum was collected by tail vein bleeding and osteocalcin levels measured by ELISA.
Statistics
Data are presented as means ± SE. Statistical significance was analyzed by two-tailed Student’s t test. The value of P < 0.05 was considered as significant.
RESULTS
Generation and purification of specific osteocalcin antibodies
To generate antibodies recognizing specifically carboxylated, uncarboxylated and total osteocalcin, goats were immunized with full-length bacterially produced recombinant uncarboxylated mouse osteocalcin or synthetic fully carboxylated mouse osteocalcin (Fig. 1 and Materials and Methods). Polyclonal antibodies recognizing either the C-terminal portion of osteocalcin (CT), the middle part of the protein (MID), the carboxylated glythamic acid 13 (GLA13) or the central uncarboxylated region (GLU) of the protein were then affinity purified. To obtain highly specific antibodies recognizing only GLU-OCN or GLA13-OCN we developed a double purification method (Fig. 1). Antibodies were first enriched against the desired epitope, then were next applied on a second column to deplete the antibodies recognizing non-specifically GLA- or GLU-OCN, leaving only antibodies recognizing specifically GLA13-OCN or GLU-OCN. Non-specific antibodies were pooled to obtain the anti-MID-OCN antibodies.
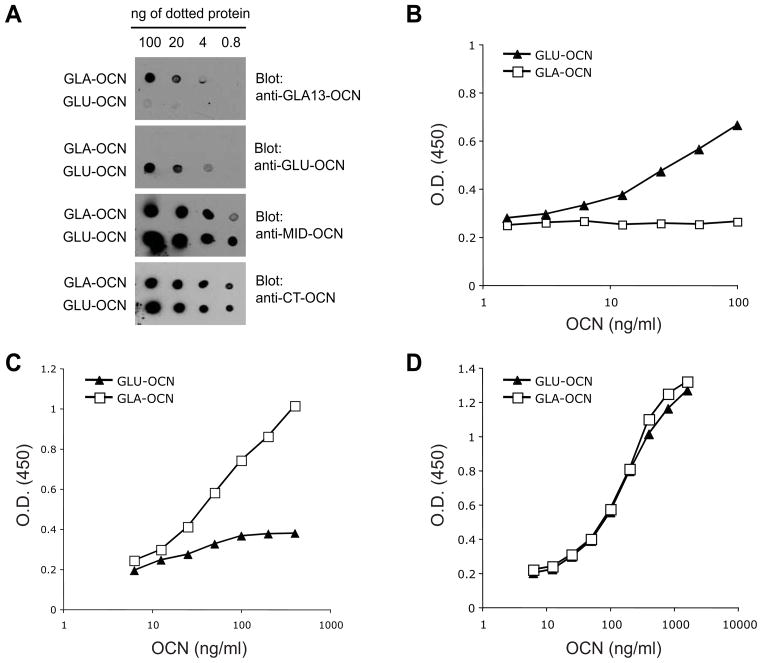
The respective specificity of these antibodies was tested in dot blot through serial dilution of carboxylated osteocalcin (GLA-OCN) or uncarboxylated osteocalcin (GLU-OCN). As shown in Fig. 2A, the anti-GLU-OCN specifically recognizes GLU-OCN protein with little cross-reactivity toward GLA-OCN protein. Conversely, the anti-GLA13-OCN recognized very specifically GLA-OCN (Fig. 2A). As expected both the anti-CT-OCN directed against the C-terminal region of osteocalcin and the non-specific anti-MID-OCN recognized with equal affinity the GLU- and GLA-OCN proteins (Fig. 2A).
FIGURE 2. Characterization of the anti-OCN antibodies and establishment of a triple ELISA method.
(A) The specificity of the different goat antibodies were tested by western blotting on dot blot membranes. GLA-OCN: fully carboxylated osteocalcin. GLU-OCN: uncarboxylated osteocalcin.
(B–D) Dose-responses and specificity analysis. Sensitivity and specificity of the GLU-OCN (B), the GLA13-OCN (C) and the total OCN ELISAs was evaluated by applying the indicated amounts (ng/ml) of GLA-OCN or GLU-OCN and plotting the OD results on a semilogarithmic scale.
Establishment of quantitative ELISAs for GLU-OCN, GLA13-OCN and total OCN
We next tested if the antibodies specific for the various forms of osteocalcin we generated could be used in combination to establish sandwich ELISAs. To generate GLU-OCN, GLA13-OCN and total osteocalcin ELISAs, we respectively coated 96-wells plates with anti-GLU-OCN, anti-GLA13-OCN or anti-MID-OCN antibodies and detected concentrations of captured osteocalcin using the anti-CT-OCN antibodies coupled to horseradish peroxidase (HRP) (see Materials and Methods). As shown in Fig. 2B, the GLU-OCN ELISA could detect concentration of GLU-OCN ranging from 1.5 to 100 ng/ml, without any cross reactivity toward GLA-OCN. Conversely, the GLA13-OCN ELISA could detect GLA-OCN concentrations ranging from 6.25 to 400 ng/ml, with little cross detection of GLU-OCN (Fig. 2C). Finally, the total osteocalcin ELISA could allow linear quantification of osteocalcin concentrations between 6.25 ng/ml to 800 ng/ml (Fig. 2D). Importantly, this latter assay could detect equally GLU-OCN and GLA-OCN.
Quantification of carboxylated osteocalcin in culture medium
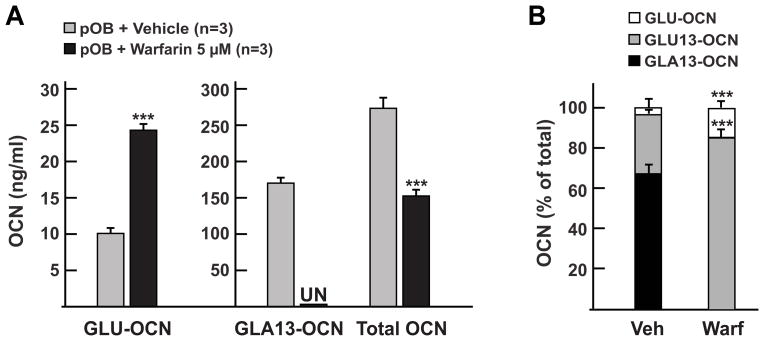
γ-carboxylation of osteocalcin occurs inside the endoplasmic reticulum of the osteoblast i.e. before osteocalcin is secreted [8]. Since the γ-glutamyl carboxylase requires reduced vitamin K as a co-factor [19] it can be inhibited in vivo or in vitro by warfarin, a drug that blocks the vitamin K epoxide reductase enzyme (Vkorc1) [20; 21]. Thus, to test our ELISA method we first quantified the levels of carboxylated, uncarboxylated and total osteocalcin in the supernatant obtained from primary differentiated osteoblast cultures treated or not with warfarin (5 μM) for 48h. As expected, levels of uncarboxylated osteocalcin (GLU-OCN) were significantly higher in the supernatant of osteoblasts treated with warfarin compared to vehicle-treated cells (Fig. 3A). In contrast, levels of carboxylated osteocalcin, as measured with the GLA13-OCN ELISA, were decreased from 167 ng/ml to undetectable levels by the warfarin treatment (Fig. 3A). As previously reported, total osteocalcin secreted by osteoblasts was decreased by about 40 percent following the same treatment [22]. When expressed as a percentage of total osteocalcin, we calculated that 63 percent of osteocalcin was carboxylated on GLA13 in control osteoblasts supernatant, while this level was decreased to 0 percent in the supernatant from warfarin-treated cells (Fig. 3B). The same warfarin treatment increased the level of uncarboxylated osteocalcin from 4 to 16 percent. Based on the measurement from the GLA13-OCN and total OCN ELISAs, we also estimated that decarboxylation of osteocalcin on glutamic acid 13 (GLU13-OCN) increased from ~30% in vehicle-treated cells to more than 80% in warfarin-treated cells (Fig. 3B). This indicates that, as expected, the drug is efficiently blocking γ-carboxylation of osteocalcin on it 3 glutamic acid residues, but that carboxylation on GLA13 is more sensitive than the other GLA residues to the warfarin treatment. Together, these results indicate that the triple ELISA system we developed can define osteocalcin carboxylation status in osteoblast cultures.
FIGURE 3. Quantification of osteocalcin carboxylation status in osteoblasts culture supernatants.
(A) Quantitative measurement of GLU-OCN, GLA13-OCN and total osteocalcin in the supernatant of primary osteoblasts cultures (pOB) treated with a vehicle or with warfarin. *** p < 0.001 compared to vehicle treated osteoblasts.
(B) Representation of the different fractions of osteocalcin quantified by the ELISAs in (A) as percentage of total osteocalcin. GLU13-OCN is calculated by subtracting GLA13- and GLU-OCN from total osteocalcin. *** p < 0.001 compared to vehicle treated osteoblasts.
Quantification of osteocalcin carboxylation in mouse serum
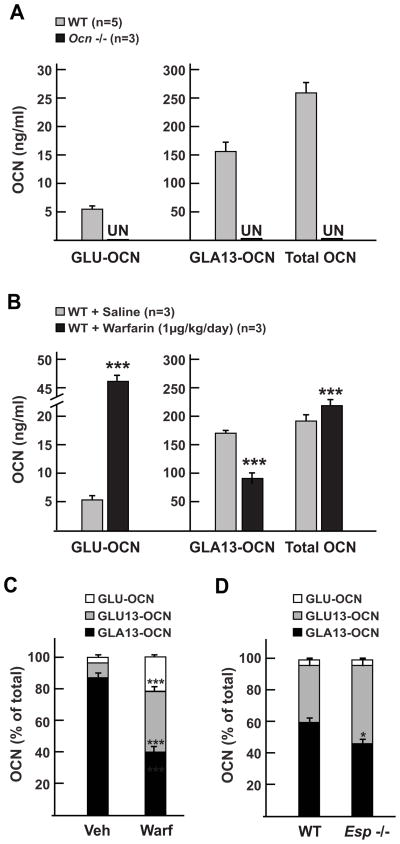
Next, we asked whether our triple ELISA could be used to assess osteocalcin carboxylation in vivo in mice. To address the specificity of the three assays, we compared the concentration of osteocalcin detected in wild type mice sera and in sera obtained from Osteocalcin-deficient mice (Ocn −/−) [23]. As shown in Fig. 4A we could detect and measure the concentration of GLU-OCN, GLA13-OCN and total OCN in wild type serum, but all three forms were undetectable in Ocn −/− serum. This result excluded any cross reactivity of the assays with other serum proteins and thus confirmed the high level of specificity of our ELISA system.
FIGURE 4. Quantification of osteocalcin carboxylation status in mice serums.
(A) Quantitative measurement of GLU-OCN, GLA13-OCN and total osteocalcin in serums from 2 months old wild type (WT) and Ocn −/− mice. All three forms were undetectable (UN) in Ocn −/− serums.
(B) Quantitative measurement of GLU-OCN, GLA13-OCN and total osteocalcin in serums from 3 months old wild type (WT) mice treated with saline solution or warfarin for 2 weeks. *** p < 0.001 compared to saline treated mice.
(C) Representation of the different fractions of osteocalcin quantified by the ELISAs in (B) as percentage of total osteocalcin. GLU13-OCN is calculated by subtracting GLA13- and GLU-OCN from total osteocalcin. *** p < 0.001 compared to saline treated mice.
(D) Osteocalcin carboxylation fraction in 2 months old wild type (WT) and Esp −/− mice. * p < 0.05 compared to WT mice.
We then injected wild type mice with either warfarin (1 mg/kg/day), which decreases osteocalcin carboxylation [24–26] or a saline solution for 2 weeks and measured osteocalcin carboxylation in their sera. As expected, warfarin treatment resulted in a 9-fold increase in uncarboxylated osteocalcin (GLU-OCN) while carboxylated osteocalcin (GLA13-OCN) was decreased 2-fold (Fig. 4B). When these values were expressed as percentage of total osteocalcin, we observed that, as in the osteoblast culture experiment, warfarin treatment results in an increase in both uncarboxylated osteocalcin (GLU-OCN) and in osteocalcin decarboxylated on GLU13 (GLU13-OCN), while the proportion of osteocalcin carboxylated on GLA13 was decreased (Fig. 4C).
We have previously demonstrated that the metabolic phenotype of the Esp −/− mice is caused by an increase in the bioactive, i.e. undercarboxylated fraction of osteocalcin present in their serum [4; 5]. We thus used these mice to validate our assay. In agreement with our previous studies, our ELISA system could detect a significant decreased in the fraction of GLA13 osteocalcin in Esp −/− serum and an increase in the GLU13 fraction, while the fraction of uncarboxylated osteocalcin (GLU-OCN) was not changed in these mice (Fig. 4D). Thus, in contrast to the warfarin-treated mice in which carboxylation of all residues was affected, it is only GLA13 carboxylation that is specifically decreased in Esp −/− mice.
DISCUSSION
Since our initial identification of osteocalcin as a bone-derived hormone regulating energy metabolism [4; 7], a growing number of studies have confirmed and expanded our finding in mice and humans [5; 6; 14; 27–35]. Although these studies have presented compelling evidence that undercarboxylated osteocalcin is the physiologically active form of this hormone, measuring its levels in mice has been hampered by the absence of a convenient and a reliable method. The new triple ELISA method described here provides such assay for both serum and culture medium.
Until now, researchers have relied on an hydroxyapatite pull-down assay to estimate the percentage of undercarboxylated osteocalcin present in serum [15–17]. When compared to this assay, the triple ELISA method presents several advantages. First, this method allows the quantification of the exact serum concentration of GLU-, GLA13- and total osteocalcin. Second, this assay can distinguish between completely decarboxylated osteocalcin (GLU-OCN) and the osteocalcin decarboxylated on GLU13, a residue that appears to be critical to osteocalcin activation in vivo [14]. Third, the ELISA approach allows simultaneous processing of a large number of samples.
Using this assay, we have shown that carboxylation of osteocalcin is dramatically decreased by warfarin both in vitro and in vivo. Interestingly, we found that although all residues could be affected carboxylation of the glutamic acid 13 is more sensitive to warfarin than carboxylation of the two other residues. This result is in line with a study in human which showed by direct sequencing that the first glutamic acid of human osteocalcin is the most often decarboxylated [11].
We have recently demonstrated that during bone resorption, a process occurring at a low pH, osteocalcin bound to the bone extracellular matrix is decarboxylated on glutamic acid 13 and thereby activated [14]. Accordingly, the Esp−/− mice present an increase in both bone resorption and osteocalcin activity. In agreement with these observations, our new method could show that in Esp−/− mice the fraction of osteocalcin decarboxylated on GLU13 is significantly increased. This result provides support to the conclusion that undercarboxylated or GLU13 osteocalcin is the physiologically active form of this hormone. In conclusion, this new triple ELISA method is a convenient and reliable tool to address the carboxylation status of osteocalcin in mouse, and its use should facilitate the analysis of this process in culture assays as well as in genetically engineered mouse models. As such it should contribute to expand our understanding of the regulation of osteocalcin bioactivity and function.
Acknowledgments
This work was supported by a fellowship from the Fond de la recherche en santé du Québec (M.F.) and grants from the NIH (G.K.) and the Juvenile Diabetes Research Foundation (P.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab. 2009;20:230–6. doi: 10.1016/j.tem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. J Musculoskelet Neuronal Interact. 2008;8:351. [PubMed] [Google Scholar]
- 3.Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008;19:161–6. doi: 10.1016/j.tem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizawa T, Hinoi E, Jung DY, Kajimura D, Ferron M, Seo J, Graff JM, Kim JK, Karsenty G. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. J Clin Invest. 2009;119:2807–17. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr, Chua SC, Jr, Kim JK, Kaestner KH, Karsenty G. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183:1235–42. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–70. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 9.Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest. 1993;91:1769–74. doi: 10.1172/JCI116387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plantalech L, Guillaumont M, Vergnaud P, Leclercq M, Delmas PD. Impairment of gamma carboxylation of circulating osteocalcin (bone gla protein) in elderly women. J Bone Miner Res. 1991;6:1211–6. doi: 10.1002/jbmr.5650061111. [DOI] [PubMed] [Google Scholar]
- 11.Cairns JR, Price PA. Direct demonstration that the vitamin K-dependent bone Gla protein is incompletely gamma-carboxylated in humans. J Bone Miner Res. 1994;9:1989–97. doi: 10.1002/jbmr.5650091220. [DOI] [PubMed] [Google Scholar]
- 12.Schilling AF, Schinke T, Munch C, Gebauer M, Niemeier A, Priemel M, Streichert T, Rueger JM, Amling M. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res. 2005;20:274–82. doi: 10.1359/JBMR.041101. [DOI] [PubMed] [Google Scholar]
- 13.Vergnaud P, Garnero P, Meunier PJ, Breart G, Kamihagi K, Delmas PD. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab. 1997;82:719–24. doi: 10.1210/jcem.82.3.3805. [DOI] [PubMed] [Google Scholar]
- 14.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. Insulin signaling in osteoblast intergrates bone remodeling and energy metabolism. Cell. 2010 doi: 10.1016/j.cell.2010.06.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poser JW, Price PA. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979;254:431–6. [PubMed] [Google Scholar]
- 16.Merle B, Delmas PD. Normal carboxylation of circulating osteocalcin (bone Gla-protein) in Paget’s disease of bone. Bone Miner. 1990;11:237–45. doi: 10.1016/0169-6009(90)90062-k. [DOI] [PubMed] [Google Scholar]
- 17.Bunyaratavej N, Soontrapa S, Rojanasthin S, Kitimanon N, Lektrakul S. Level of undercarboxylated osteocalcin in reproductive Thai females. J Med Assoc Thai. 2005;88(Suppl 5):S37–9. [PubMed] [Google Scholar]
- 18.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 19.Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999;93:1798–808. [PubMed] [Google Scholar]
- 20.Wallin R, Hutson SM. Warfarin and the vitamin K-dependent gamma-carboxylation system. Trends Mol Med. 2004;10:299–302. doi: 10.1016/j.molmed.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427:541–4. doi: 10.1038/nature02254. [DOI] [PubMed] [Google Scholar]
- 22.Nishimoto SK, Price PA. The vitamin K-dependent bone protein is accumulated within cultured osteosarcoma cells in the presence of the vitamin K antagonist warfarin. J Biol Chem. 1985;260:2832–6. [PubMed] [Google Scholar]
- 23.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 24.Price PA, Williamson MK, Lothringer JW. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981;256:12760–6. [PubMed] [Google Scholar]
- 25.Price PA, Williamson MK. Effects of warfarin on bone. Studies on the vitamin K-dependent protein of rat bone. J Biol Chem. 1981;256:12754–9. [PubMed] [Google Scholar]
- 26.Obrant KJ, Kakonen SM, Astermark J, Lilja H, Lovgren T, Akesson K, Pettersson K. The proportion of carboxylated to total or intact osteocalcin in serum discriminates warfarin-treated patients from control subjects. J Bone Miner Res. 1999;14:555–60. doi: 10.1359/jbmr.1999.14.4.555. [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:45–9. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- 28.Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, Mellstrom D. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24:785–91. doi: 10.1359/jbmr.081234. [DOI] [PubMed] [Google Scholar]
- 29.Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94:827–32. doi: 10.1210/jc.2008-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aonuma H, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low serum levels of undercarboxylated osteocalcin in postmenopausal osteoporotic women receiving an inhibitor of bone resorption. Tohoku J Exp Med. 2009;218:201–5. doi: 10.1620/tjem.218.201. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Real JM, Izquierdo M, Ortega F, Gorostiaga E, Gomez-Ambrosi J, Moreno-Navarrete JM, Fruhbeck G, Martinez C, Idoate F, Salvador J, Forga L, Ricart W, Ibanez J. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J Clin Endocrinol Metab. 2009;94:237–45. doi: 10.1210/jc.2008-0270. [DOI] [PubMed] [Google Scholar]
- 32.Hwang YC, Jeong IK, Ahn KJ, Chung HY. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev. 2009;25:768–772. doi: 10.1002/dmrr.1045. [DOI] [PubMed] [Google Scholar]
- 33.Ueland T, Fougner SL, Godang K, Lekva T, Schurgers LJ, Scholz H, Halvorsen B, Schreiner T, Aukrust P, Bollerslev J. Associations between body composition, circulating interleukin-1 receptor antagonist, osteocalcin, and insulin metabolism in active acromegaly. J Clin Endocrinol Metab. 95:361–8. doi: 10.1210/jc.2009-0422. [DOI] [PubMed] [Google Scholar]
- 34.Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. doi: 10.1007/s00198-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 35.Rached MT, Kode A, Silva BC, Jung DY, Gray S, Ong H, Paik JH, DePinho RA, Kim JK, Karsenty G, Kousteni S. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest. 2010;120:357–68. doi: 10.1172/JCI39901. [DOI] [PMC free article] [PubMed] [Google Scholar]