INTRODUCTION
Since its development in 1994 by Colebatch et al., the sound-evoked cervical vestibular evoked myogenic potential (VEMP) test has been widely used as a clinical test of saccular function (1). The human test is based on animal studies that have shown that saccular afferents are responsive to sound, while utricular afferents are additionally responsive to vibration. McCue and Guinan found that acoustic stimuli activated irregular afferent neurons that innervated the saccule in the cat (2). Curthoys, et al. showed that bone-conducted vibration best activated utricular irregular otolith afferent neurons in the guinea pig (3). In humans, intense auditory clicks and tone bursts delivered to the ear stimulate saccular afferents, leading to inhibition of the sternocleidomastoid (SCM) muscle via the vestibulo-collic pathway (4–7). These inhibitory potentials are electromyographically detected with surface electrodes overlying the sternocleidomastoid muscle while the subject maintains tension of that muscle. The resultant waveform consists of a positivity, called the p13 potential, followed by a negativity, called the n23 potential.
Cervical VEMPs (cVEMPs) have been consistently and reliably recorded in healthy subjects across all age groups, but have been found to be absent in subjects after vestibular nerve sectioning and abnormal in patients with acoustic neuromas, Ménière's disease, vestibular neuritis, multiple sclerosis, and lower brainstem lesions (8–13). Cervical VEMPs are used as a test of otolith function in these disorders. In addition, cVEMPs have found an important role in the diagnosis of superior canal dehiscence syndrome (SCDS), where the third window creates a low-impedance pathway that diverts sound energy to the labyrinth, causing decreased VEMP thresholds and increased cVEMP amplitudes (14–16).
A decade after the development of the cervical VEMP test, Rosengren et al. (17) and Iwasaki et al. (18) reported that bone-conducted vibrations produced extra-ocular potentials of vestibular origin, leading to the development of the ocular VEMP (oVEMP). Subsequently, it was found that the oVEMP was also obtained in response to the same auditory clicks and tone bursts used for evoking the cVEMP, and that patients with vestibular loss had absent oVEMP responses, while those with normal vestibular function had typical responses (19). Whereas the cVEMP represents a relaxation response in a tonically-contracting muscle, the oVEMP represents excitation of the extra-ocular muscles via the vestibulo-ocular pathways. Additionally, the cVEMP response is mediated by a primarily uncrossed pathway such that stimulation of one ear mostly affects the ipsilateral sternocleidomastoid muscle, while the oVEMP response follows a primarily crossed pathway where stimulation of one ear affects mostly the contralateral extraocular muscles.
Studies have confirmed that the oVEMP is not an auditory response, a blink response, a facial nerve or trigeminal nerve response, or a corneo-retinal potential produced by eye movements (17;19;19;20). The oVEMP is optimally recorded on upgaze with surface electrodes placed inferior to the eyes on the cheeks, and the typical waveform consists of a negativity occurring at approximately 10 ms, which has been referred to as the n10 potential, followed by a positivity occurring at approximately 16 ms, which we shall refer to as the p16 potential (18). Of these waves, only the n10 response has been found to be both absent in patients with vestibular loss and present in patients with hearing loss but intact vestibular function, suggesting that later waves may involve non-vestibular components (18). The cVEMP and oVEMP techniques used together test the integrity of otolith projections to the neck and eyes, respectively.
Akin to the cVEMP, oVEMP responses have been consistently obtained in healthy individuals, and studies have suggested that they are useful in evaluating patients with vestibular disorders (19–23). OVEMPs have significantly increased amplitudes and decreased thresholds in SCDS (24;25). Compared with cVEMPs, oVEMPs are less arduous for the subject to perform, and symmetrical responses can be obtained without monitoring background activation. As the oVEMP test becomes a more widely-recognized test of otolith function, it is likely that it will be used in addition to the cVEMP test to assess end organ function and as a complementary technique when assessing central vestibular disorders.
Bone-conducted cVEMPs and oVEMPs create vibrational waves that conduct across the skull to directly stimulate the bilateral otolith organs (19;21;26;27). Recent investigation has shown that head taps at Fz oscillate the mastoid bone, causing deflection of the vestibular hair cells and thus activating the otolithic afferent neurons (3). Since they circumvent the middle ear apparatus, vibration-evoked VEMPs and can be especially useful for testing the vestibular responses in patients with conductive hearing loss. Vibration-evoked VEMPs have been studied in patients with various vestibular disorders, including vestibular schwannomas (21), superior canal dehiscence syndrome (17;25), Ménière's disease (28), and vestibular neuritis (23). Vibration-evoked oVEMPs have been found to be significantly reduced or abolished in patients with superior vestibular neuritis and vestibular schwannomas (29), and also reduced in patients with superior canal dehiscence syndrome (25).
While the normal cVEMP parameters and test-retest reliability in response to sound stimuli have been the subject of several investigations, little is known about the test-retest reliability of the sound- or vibration-evoked oVEMPs or of vibration-evoked cVEMPs (30–33). In this study, we examine the test-retest reliability of the oVEMP and cVEMP parameters in response to both sound and vibration stimuli. We also examine age-related trends in cVEMP and oVEMP response parameters.
MATERIALS AND METHODS
Subjects
Fifty-three healthy individuals (24 males and 29 females, with a mean age of 35 and a range of 20–70 years) with no hearing or vestibular deficits enrolled in this study, and their VEMP data were used for the analysis of age-related trends in cVEMP and oVEMP test parameters. Twelve of these subjects (4 males and 8 females, with a mean age of 30 years and a range of 22–59 years) underwent a second testing session, and their data from the first and second testing sessions were used for the test-retest reliability analysis. All subjects gave informed consent for the cVEMP and oVEMP testing through a protocol approved by the Institutional Review Board at the Johns Hopkins University School of Medicine, the institution where testing was performed.
Testing Sessions
A mean of 10 weeks (range 4 – 18 weeks) elapsed between the first testing session and the second testing session, both conducted by the same investigator (KDN). CVEMP and oVEMP testing were performed separately within each testing session.
Stimuli and recording techniques
A commercial electromyographic (EMG) system (Medelec Synergy, CardinalHealth Neurocare, software version 14.1, Dublin, OH) was used for VEMP testing. Sound stimuli were delivered monaurally via intra-auricular speakers from VIASYS Healthcare (Madison, WI) with foam eartips (Aearo Company Auditory Systems, Indianapolis, IN). Two types of air-conducted stimuli were delivered: (1) 0.1-ms, 105 dB nHL (140 dB peak SPL) clicks of positive polarity at a repetition rate of 5 per second; and (2) 500 Hz, 125 dB SPL tone bursts of positive polarity, with a linear envelope (1 ms rise/fall time, 2 ms plateau), at a repetition rate of 5 per second. Two types of midline vibration stimulation were delivered at Fz (in the midline at the hairline, 30% of the distance between the inion and nasion): (1) manual taps delivered with an Aesculap model ACO12C reflex hammer fitted with an inertial microswitch trigger; and (2) “mini taps,” as described by Iwasaki, et al. (18) were delivered with a Brüel and Kjær Mini-Shaker Type 4810 (1-ms clicks of positive polarity, with a repetition rate of 5 per second) (19). EMG signals were amplified (2500×) and band-pass filtered (20 Hz – 2000 Hz). One hundred sweeps were averaged for each test.
Protocol for cVEMP Testing
Subjects lay semi recumbent on an examination table with their upper bodies elevated at a 30-degree angle from horizontal. They were instructed to lift their heads up from the head rest by flexing their necks to provide tonic background muscle activity during cVEMP stimulation and recording. A break was given at every 10 second interval.
CVEMPs were recorded with disposable, self-adhesive, pre-gelled, Ag/AgCl electrodes with attached 40” safety leadwires from GN Otometrics (Schaumburg, IL). The electrode montage consisted of a non-inverting electrode placed at the midpoint of the sternocleidomastoid muscle belly, an inverting electrode placed on the sternoclavicular junction, and a ground electrode placed on the manubrium sterni. The skin overlying both sternocleidomastoid muscles and the manubrium sterni was cleansed with alcohol preps prior to electrode placement.
Protocol for oVEMP Testing
Subjects lay semi recumbent with their upper bodies elevated at a 30 degree angle from horizontal. They were instructed to maintain maximum upgaze during oVEMP stimulation and recording. A break was given at every 10–20 second interval.
The electrode montage consisted of a non-inverting electrode placed on the cheek approximately 3 mm below the eye and centered beneath the pupil, an inverting electrode centered 2 cm below the non-inverting electrode, and a ground electrode placed on the manubrium sterni. The skin overlying the cheeks and the manubrium sterni was cleansed with alcohol preps prior to electrode placement.
Before testing with sound and vibration stimulation, 20° vertical saccades were performed to ensure that symmetrical signals were recorded from both eyes. If the signal change showed > 25% asymmetry, the electrodes were removed and new ones applied.
cVEMP Response Parameters
The p13 potential was identified as the first distinctive trough in the waveform, occurring approximately 10–14 ms after stimulus onset, and the n23 potential was identified as the first distinctive peak in the waveform, occurring approximately 19–23 ms after stimulus onset. In some cases, the n23 waveforms were not clearly separable from later-occurring, non-vestibular waveforms. These cases were not included in the analysis.
The raw peak-to-peak amplitude was calculated as the sum of the p13 amplitude and the n23 amplitudes. The corrected peak-to-peak amplitude (referred to hereafter simply as the peak-to-peak amplitude) was calculated by dividing the raw peak-to-peak amplitude by the rectified background EMG activity recorded during the 10-ms interval prior to stimulus onset. The corrected p13 amplitude (referred to hereafter as the p13 amplitude) was calculated in a similar manner. This correction factor accounts for the varying tonic muscle tone that affects cVEMP amplitudes. The asymmetry ratio (AR) between a subject's ears was calculated according to the following formula:
oVEMP Response Parameters
The n10 potential was identified as the first distinctive peak in the waveform, occurring approximately 7–11 ms after stimulus onset, and the p16 potential was identified as the first distinctive trough in the waveform, occurring approximately 12–16 ms after stimulus onset. The peak-to-peak amplitude was calculated as the sum of the n10 and p16 amplitudes. The AR was calculated using the same formula as above.
Statistical Analysis
Test-retest reliability was assessed with the intraclass correlation coefficient (ICC), calculated using a 2-way random effects, absolute agreement model with a statistical significance level of p < 0.05 (32). In the present study, the ICC is the ratio of the variance of a VEMP parameter due to subject differences divided by the sum of variances due to subject differences and measurement errors. For a VEMP test to be clinically useful, variance over repeated measures should, at minimum, reflect physiological differences between subjects more than changes in the measurement “noise” between testing sessions. That is, ICC should be ≥ 0.5. Therefore, for each ICC value we determined its significance based on this criterion using an F-test with the number of subjects, two observations, and a null-hypothesis value of ICC0 = 0.5 at an α level of 0.05. Tables 3 and 4 denote ICC values that were significant under these conditions, along with those 95% confidence intervals that were ≥ 0.5. In general, the number of observations for monaural parameters (~24) was sufficient to detect ICC values of ≥ 0.8 as significantly different than ICC0 = 0.5 (p < 0.05) with a power of 0.8. Similar to previous studies that examined VEMP test-retest reliability, we classified an ICC value of 1.00 as perfect reliability, ICC ≥ 0.75 as excellent reliability, 0.40 ≤ ICC < 0.75 as fair-to-good reliability, and ICC < 0.40 as poor reliability (30–32). ICC analysis was performed using SPSS 17.0 (Chicago, IL) and standard deviation analysis was performed using Microsoft Excel 2000 and 2007 (Seattle, WA). Age-related differences in VEMP latency, amplitude, and asymmetry ratio were analyzed using the ANOVA method with a significance level of 5%
Table 3.
ICC values for cVEMP test-retest reliability.
| cVEMP Stimulus | p13 latency | n23 latency | p13 Amplitude | Peak-to-peak Amplitude | AR for p13 amplitude | AR for peak-to-peak amplitude |
|---|---|---|---|---|---|---|
| Clicks | −0.15 | 0.38 | 0.14 | 0.40 | 0.52 | 0.65 |
| Tones | 0.33 | 0.71 | 0.43 | 0.68 | 0.068 | 0.27 |
| Hammer | 0.57 | 0.63 | 0.43 | 0.54 | 0.55 | 0.75 (−0.25, 0.96) |
| Mini-Shaker | −0.047 | 0.46 | 0.52 | 0.43 | 0.049 | 0.30 |
ICC values denoting excellent reliability are marked in bold, and the corresponding confidence intervals are shown in parentheses.
Asterisks denote excellent reliability values that furthermore were statistically significant at the 5% level when testing the null hypothesis that ICC0 = 0.5 (power = 0.80).
Table 4.
ICC values for oVEMP test-retest reliability.
| oVEMP Stimulus | n10 latency | p16 latency | n10 amplitude | Peak-to-peak Amplitude | AR for n10 amplitude | AR for peak-to-peak amplitude |
|---|---|---|---|---|---|---|
| Clicks | 0.21 | −0.41 | 0.47 | 0.84* (0.52, 0.94) | 0.055 | 0.84 (0.26, 0.97) |
| Tones | 0.17 | 0.055 | 0.81 (0.42, 0.94) | 0.79 (0.39, 0.93) | 0.44 | 0.50 |
| Hammer | −0.033 | 0.44 | 0.85* (0.57, 0.95) | 0.82* (0.57, 0.93) | 0.55 | 0.52 |
| Mini-Shaker | 0.39 | 0.55 | 0.75 (0.29, 0.91) | 0.87* (0.69, 0.95) | 0.48 | 0.51 |
ICC values denoting excellent reliability are marked in bold, and the corresponding confidence intervals are shown in parentheses.
Asterisks denote excellent reliability values that furthermore were statistically significant at the 5% level when testing the null hypothesis that ICC0 = 0.5 (power = 0.80).
RESULTS
Mean values of cVEMP test parameters
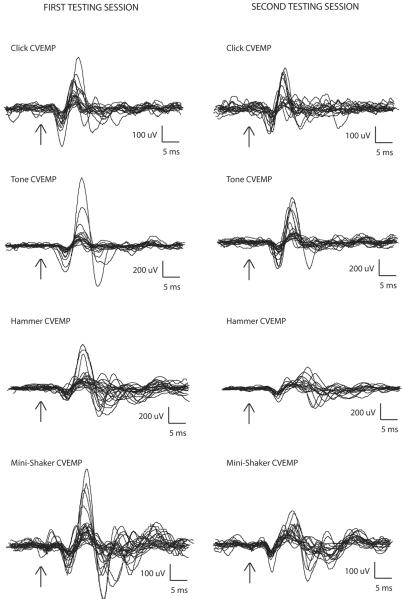
The mean values and standard deviations of the cVEMP p13 latency, n23 latency, p13 amplitude, peak-to-peak amplitude, and asymmetry ratio for the first and second testing sessions are shown in Table 1. Figure 1 shows the average cervical VEMP traces from each side of each individual obtained from both the first and second testing sessions. The arrows denote stimulus onset.
Table 1.
Means and standard deviations of cVEMP test parameters from the first and second testing sessions.
| cVEMP Stimulus | p13 latency | n23 latency | p13 Amplitude | Peak-to-peak Amplitude | AR for p13 amplitude | AR for peak-to-peak amplitude | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First session | Second session | First session | Second session | First session | Second session | First session | Second session | First session | Second session | First session | Second session | |
| Clicks | 11.54 (0.75) | 10.92 (1.03) | 19.97 (1.73) | 19.55 (3.34) | 0.79 (0.38) | 0.76 (0.41) | 1.88 (0.92) | 1.72 (1.04) | 29.9 (19.81) | 36.8 (27.09) | 28.7 (20.75) | 34.5 (12.63) |
| Tones | 13.52 (0.69) | 12.96 (1.00) | 21.87 (1.63) | 20.79 (2.10) | 0.80 (0.17) | 0.99 (0.62) | 2.14 (0.85) | 2.76 (2.08) | 22.3 (13.74) | 35.3 (19.7) | 23.1 (16.12) | 31.6 (21.80) |
| Hammer | 11.87 (2.18) | 11.02 (2.03) | 19.47 (2.33) | 19.32 (2.89) | 1.77 (2.32) | 1.26 (1.25) | 3.70 (3.46) | 2.76 (1.92) | 38.68 (27.56) | 26.91 (18.54) | 34.03 (23.18) | 32.49 (25.48) |
| Mini-Shaker | 12.23 (0.78) | 12.04 (0.86) | 21.12 (2.25) | 20.77 (2.23) | 1.04 (0.82) | 0.66 (0.33) | 1.90 (0.92) | 1.61 (0.87) | 36.21 (22.26) | 17.05 (14.02) | 29.82 (25.31) | 20.14 (15.00) |
Figure 1.
Cervical VEMP traces from each subject (averaged from 100 sweeps), in response to clicks, tones, and taps with a reflex hammer and Mini-Shaker. Traces on the left correspond to the first testing session; traces on the right correspond to the second testing session.
Two trends can be noted in Table 1 regarding the cVEMP: (1) Taps with the reflex hammer produced the highest mean p13 and peak-to-peak amplitudes, although this was not a statistically significant result; (2) Tones and taps with the Mini-Shaker produced lower asymmetry ratios for peak-to-peak amplitude than did clicks and taps with the reflex hammer.
Mean values of oVEMP test parameters
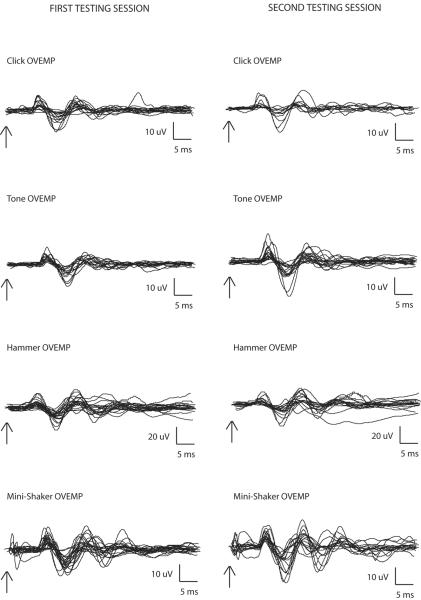
The mean values and standard deviations of the oVEMP n10 latency, p16 latency, corrected peak-to-peak amplitude, and asymmetry ratio for the first and second testing sessions are shown in Table 2. Figure 2 shows the ocular VEMP traces obtained from both the first and second testing sessions. The arrows denote stimulus onset. Table 2 demonstrates that there was a trend towards higher oVEMP amplitudes in response to vibration than to sound, which may be due to the vibration stimuli being well above vestibular threshold when compared with sound. Among the four modes of stimulation, a trend towards Mini-Shaker taps producing the lowest asymmetry ratio was also observed. However, neither of these trends was statistically significant.
Table 2.
Means and standard deviations of oVEMP test parameters from the first and second testing sessions.
| oVEMP Stimulus | n10 latency | p16 latency | n10 amplitude | Peak-to-peak Amplitude | AR for n10 amplitude | AR for peak-to-peak amplitude | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First session | Second session | First session | Second session | First session | Second session | First session | Second session | First session | Second session | First session | Second session | |
| Clicks | 8.48 (0.63) | 8.99 (1.21) | 14.01 (1.13) | 13.60 (1.21) | 4.16 (3.45) | 2.71 (2.53) | 8.45 (7.43) | 4.94 (6.35) | 26.33 (27.56) | 38.18 (22.40) | 24.80 (28.81) | 30.65 (29.29) |
| Tones | 10.35 (0.51) | 10.18 (0.56) | 15.48 (0.82) | 15.23 (0.85) | 4.22 (2.40) | 4.86 (3.62) | 8.24 (6.27) | 11.14 (8.71) | 22.8 (13.57) | 26.8 (19.79) | 30.7 (30.14) | 20.0 (15.79) |
| Hammer | 7.55 (0.54) | 7.91 (2.25) | 12.87 (0.70) | 12.94 (1.91) | 5.81 (5.38) | 6.38 (4.21) | 16.27 (11.35) | 15.11 (11.00) | 38.93 (27.38) | 41.33 (23.11) | 26.30 (26.46) | 33.18 (24.11) |
| Mini-Shaker | 9.64 (0.91) | 9.50 (1.60) | 14.54 (1.37) | 14.33 (1.57) | 7.25 (3.76) | 7.09 (3.12) | 17.32 (8.61) | 16.04 (8.67) | 24.92 (23.50) | 16.22 (11.27) | 19.93 (10.63) | 17.22 (12.23) |
Figure 2.
Ocular VEMP traces from each subject (averaged from 100 sweeps), in response to clicks, tones, and taps with a reflex hammer and Mini-Shaker. Traces on the left correspond to the first testing session; traces on the right correspond to the second testing session.
cVEMP and oVEMP test-retest reliability
Tables 3 and 4 show the ICC values for cVEMP and oVEMP test-retest reliability, respectively. ICC values indicating excellent reliability are marked with bold font. With regards to the cVEMP test, reliability was excellent for the reflex hammer asymmetry ratio for peak-to-peak amplitude; and fair to good for all peak-to-peak amplitudes; tone, hammer, and Mini-Shaker p13 amplitudes; hammer p13 latencies; tone, hammer and Mini-Shaker n23 latencies; click and hammer asymmetry ratios for p13 amplitude; and click asymmetry ratio for peak-to-peak amplitude. It is possible that the reflex hammer asymmetry ratio for peak-to-peak amplitude had high reliability because the between subject variability far exceeded the within subject variability. With regards to peak-to-peak parameters of the oVEMP test, reliability was excellent for all peak-to-peak amplitudes and click asymmetry ratio; and fair-to-good for tone, hammer, and Mini-Shaker asymmetry ratio; and hammer and Mini-Shaker p16 latency. In general, peak-to-peak reliability was better for oVEMP testing than for cVEMP testing. For n10 oVEMP response parameters, reliability was excellent for tone-, hammer-, and Mini-Shaker-evoked n10 amplitude, and fair-to-good for click-evoked n10 amplitude. Reliability was also fair-to-good for n10-calculated asymmetry ratios for all stimuli except for clicks.
Age-related trends in cVEMP and oVEMP amplitudes
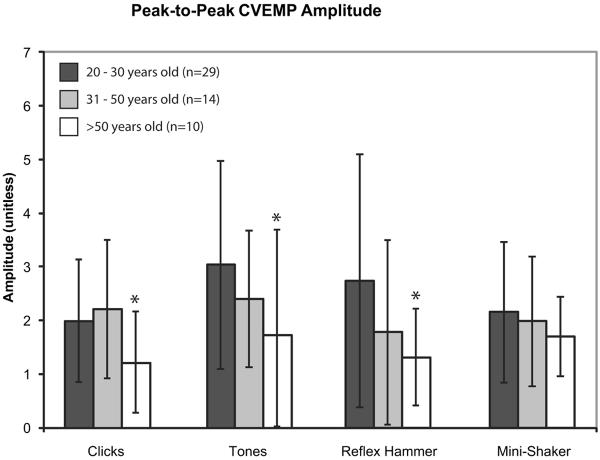
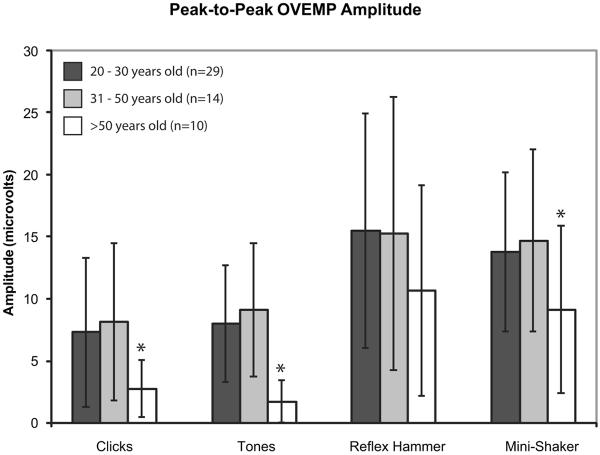
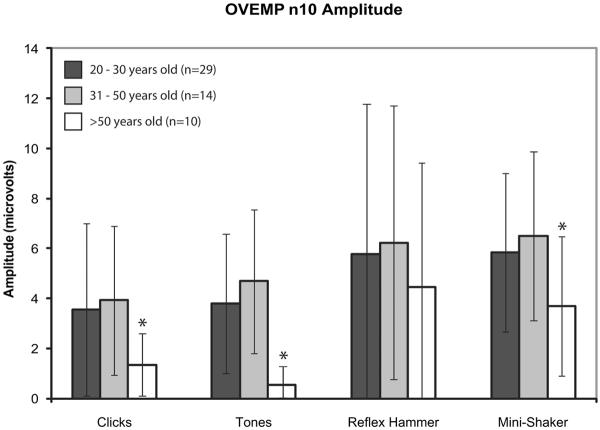
Subjects were divided into three groups based upon age: 20–30 years old, 31–50 years old, and >50 years old. Figures 3, 4, and 5 display the mean cVEMP peak-to-peak amplitudes, mean oVEMP peak-to-peak amplitudes, and mean oVEMP n10 amplitudes (± 1 SD), respectively, for each age group. For cVEMPs, amplitudes were significantly lower in subjects >50 years old in response to clicks, tones, and taps with a reflex hammer (ANOVA, p < 0.05). For oVEMPs, the age-related decrease in response was even greater; both n10 and peak-to-peak amplitudes were significantly lower in subjects >50 years old in response to clicks, tones, and taps with a Mini-Shaker (ANOVA, p < 0.05).
Figure 3.
Mean corrected peak-to-peak cVEMP amplitudes in response to clicks, tones, and taps with a reflex hammer and Mini-Shaker. Error bars represent 1 SD above and below the mean. Asterisks indicate significantly decreased amplitudes (p < 0.05).
Figure 4.
Mean peak-to-peak oVEMP amplitudes in response to clicks, tones, and taps with a reflex hammer and Mini-Shaker. Error bars represent 1 SD above and below the mean. Asterisks indicate significantly decreased amplitudes (p < 0.05).
Figure 5.
Mean n10 oVEMP amplitudes in response to clicks, tones, and taps with a reflex hammer and Mini-Shaker. Error bars represent 1 SD above and below the mean. Asterisks indicate significantly decreased amplitudes (p < 0.05).
When age was treated as a continuous variable, no significant association was found between age and either cVEMP or oVEMP amplitude in response to any stimuli (graph not shown, R2 ranged from 0.0025 to 0.15)). No significant associations were found between age and cVEMP or oVEMP latency, when age was treated as a continuous variable (R2 ranged from 0.014 to 0.38), nor when subjects were grouped according to their ages. No significant associations were found between age and cVEMP or oVEMP asymmetry ratio, when age was treated as a continuous variable (R2 ranged from 0.0070 to 0.17), nor when subjects were grouped according to their ages.
DISCUSSION
This study examined the test-retest reliability of the cervical VEMP and ocular VEMP tests in response to both sound (monaural, 0.1-ms clicks and 500-Hz tone bursts) and vibrational (midline forehead taps with a reflex hammer and Mini-Shaker) stimulation. OVEMP peak-to-peak amplitudes in response to all four modes of stimulation yielded excellent reliability, as did cVEMP peak-to-peak amplitudes in response to taps with a reflex hammer. Fair-to-good reliability was obtained for sound-induced and Mini-Shaker induced cVEMP peak-to-peak amplitudes. The click-evoked oVEMP asymmetry ratio had excellent test-retest reliability, while the tone- and vibration-evoked oVEMP asymmetry ratios and the click- and hammer-evoked cVEMP asymmetry ratios had fair-to-good reliability. Latencies to p13, n23, n10, and p16 yielded poor to good values of reliability.
Sound-evoked cVEMP test-retest reliability
There have been prior investigations of sound-evoked cVEMP test-retest reliability, mostly aimed at finding the optimal cVEMP recording procedure. Isaradisaikul, et al. evaluated tone-evoked cVEMP test-retest reliability with and without visual EMG monitoring to maintain a target tonic sternocleidomastoid activation level, and found that p13 latency, n23 latency, and asymmetry ratio had poor to good reliability (ICC values of 0.37 to 0.70), while uncorrected peak-to-peak amplitude had excellent reliability (ICC values of 0.81 to 0.86) (30). Maes, et al. also found that p13 latency, n23 latency, peak-to-peak amplitude, and asymmetry ratio had fair to excellent reliability (ICC values of 0.56 to 0.92), when subjects used a blood pressure manometer feedback mechanism to maintain adequate muscle contraction (31;34). Versino et al. found that the test-retest reliability for the monaurally-delivered, click-evoked cVEMP was fair to excellent (ICC values of 0.57 – 0.88) for p13 latency, n23 latency, and uncorrected peak-to-peak amplitude (32). Eleftheriadou et al. reported that click-evoked cVEMP parameters yielded fair to excellent reliability (ICC values of 0.60 to 0.94) (33). These reliability levels are slightly higher than the ones that we found in the present study, possibly due to differences in sample size and time elapsed between the two testing sessions.
In the present study, the second testing session occurred at a later time point (4–18 weeks, mean 10 weeks after the first session) than it did in prior studies (30 min-4 weeks after the first session). Therefore, various factors may have contributed to the increased variability in VEMP parameters. Unlike prior investigations that performed the first and second testing sessions 30 minutes apart, the present study involved tests that were performed on different days and with different electrodes. Electrode positioning may have varied slightly between testing sessions, which may have influenced both the amplitude and latency of the EMG signals picked up by the electrodes. In addition, differences in the level of effort exerted by the subject are more likely to be pronounced when more time elapses between testing sessions. Retesting subjects as soon as 30 minutes after the first testing session may artificially mask variations in testing conditions and parameters that are inherent to a test performed at multiple locations by many examiners, and may thus overestimate the “real” test-retest reliability obtained in practice. Therefore, a later re-testing session may provide a more realistic and accurate picture of clinical test-retest reliability. Additionally, it would seem that a later testing session may be more clinically relevant when studying responses to therapies, such as intratympanic gentamicin injections for Ménière's disease, that follow a more lengthy time course.
oVEMP test-retest reliability
To our knowledge, this is the first published study that examines test-retest reliability of the oVEMP. Peak-to-peak oVEMP amplitudes were found to have excellent reliability in response to all four stimuli, while n10 oVEMP amplitudes were found to have excellent reliability in response to all stimuli but clicks.
The excellent reliability of these oVEMP parameters may be due to several factors. First, the surface area of the cheek is smaller than that of the skin overlying the SCM, so there is less room for error in proper and optimal placement of the electrodes. Second, upgaze may be produce less fatigue in the muscle of interest than does flexing or rotating the neck, which may also lead to less intersession variation as well (35). Third, there may be less variability in body habitus and soft tissue depth on the cheek than on the neck. Fourth, the oVEMP response is an excitatory potential measured in the midst of relatively small background noise of extraocular muscle activation. In contrast, the cVEMP response is a small modulation in a relatively noisy background of SCM contraction (S. Rauch, personal communication). The former might be expected to be more repeatable a measure than the latter.
The present study also found that both the n10 and peak-to-peak oVEMP asymmetry ratios demonstrated fair-to-good reliability for tones, reflex hammer taps, and Mini-Shaker taps. However, click-evoked peak-to-peak asymmetry ratios had excellent reliability, while click-evoked n10 asymmetry ratios had poor reliability. This discrepancy between n10 and peak-to-peak reliability values for the click stimulus may suggest that the click-evoked p16 response has a high reliability that augments the total peak-to-peak reliability. However, the p16 component has not been systematically examined and may even not be vestibular in origin. Therefore, tones, reflex hammer taps, and Mini-Shaker taps may be more reliable stimuli for tests of vestibular function when measuring the oVEMP response.
In this study, we followed previous conventions for the classification of ICC values for test-retest reliability in VEMP testing (i.e., ICC ≥ 0.75 indicates excellent reliability and ICC < 0.40 indicates poor reliability) (30–32). However, one should keep in mind that these classifications are somewhat arbitrary. An ICC value is the ratio of the variance of a VEMP parameter due to subject differences divided by the sum of variances due to subject differences and measurement errors. We chose to set 0.5 as a minimally meaningful ICC value because this is the point at which half of the variance in a VEMP measure between testing sessions can be ascribed to subject differences. A highly conservative assumption would be that the rest of the variance is due to “noise” that is not of clinical significance. However, test-retest reliability may actually be influenced by physiological variation in a healthy subject's response at different testing sessions, which may artificially deflate the ICC. Nevertheless, we followed the conservative criterion and marked as “excellent” those reliability values in Tables 3 and 4 for which the ICC values were > 0.8 and the 95% confidence intervals were ≥ 0.5.
Advantages of the oVEMP test compared to the cVEMP test
In addition to having higher test-retest reliability, the oVEMP test offers several other advantages over the cVEMP test, especially in the diagnosis of superior canal dehiscence syndrome. Currently, evaluation of SCDS patients with the threshold cVEMP test requires multiple runs for each ear, and, thus, multiple times that prolonged SCM contraction must be maintained. Welgampola et al. reported that patients with SCDS had significantly elevated tone-evoked oVEMP amplitudes at 120 dB SPL peak intensity, suggesting that the oVEMP amplitude in response to a single standard stimulus intensity level provides useful diagnostic information (25). Therefore, using oVEMP amplitudes for the diagnosis of SCDS would shorten the testing session by requiring only one run of testing for each ear at a fixed stimulus level.
Additionally, eliciting the oVEMP does not require a baseline level of muscle activation. This is in contrast to the cVEMP, which represents an inhibitory potential and thus necessitates adequate contraction of the sternocleidomastoid muscle for proper measurement (1;36;37). Generating adequate contraction requires patient cooperation and effort to maintain contraction of the sternocleidomastoid muscle, often either by neck flexion from the supine position, or by neck rotation against tension. Verification of adequacy and symmetry of muscle contraction requires either simultaneous rectified EMG recording or a feedback mechanism such as the device reported by Vanspauwen, et al., involving a blood pressure manometer (34). Accurate calculation of the cVEMP amplitude also involves correction for background muscle activity, since the cVEMP is directly proportional to this tonic activity level. The presently accepted method for this correction is to divide the cVEMP amplitude by the rectified EMG activity measured before the stimuli are presented. However, this correction assumes a linear relationship between cVEMP amplitude and rectified SCM EMG activity, and this assumption may not be valid. This may be one of the factors behind our finding of poorer reliability of cVEMPs.
Another advantage of oVEMP recordings over cVEMP recordings is that the former does not require correction for background muscle activation as the latter does. In fact, correction for background muscle activation in the oVEMP might be more difficult than it is for the cVEMP as several muscles (orbicularis oculi, inferior oblique and inferior rectus) underlie the recording electrodes, and not all of them may contribute to the oVEMP. Therefore, the relationship between the extraocular muscle background activity and oVEMP amplitude is not known. Moreover, the modulation of oVEMP amplitude by gaze may be due to modulation of extraocular muscle activity or the position of the extraocular muscles relative to the electrodes. Fortunately, however, when oVEMPs are recorded during upgaze in the midline, the extraocular muscle activation and the relationship between the recording electrode and the relevant muscle(s) is comparable for the two sides. Therefore, symmetrical responses can be expected even without correction for background muscle activation. The result is a test that is simpler to administer for the operator.
The oVEMP test is less strenuous for the patient. Sustained gaze deviation required for the oVEMP test produces little measurable muscle fatigue (35). In contrast, subjects frequently find that the sustained neck flexion or neck rotation against resistance required for the cVEMP test is difficult, and accurate threshold measurements may be compromised by fatigue in repeated tests. This is especially true for elderly patients, who may have difficulty maintaining adequate sternocleidomastoid muscle tension required for cVEMP testing.
Finally, the better reliability for oVEMPs relative to cVEMPs suggests that changes in oVEMPs over time may better reflect actual physiological changes. Thus, oVEMPs may be a better technique for gauging the effects of therapy, disease progression, or aging than are cVEMPs.
Age-related trends in cVEMP and oVEMP response parameters
In this study, we also examined age-related trends in cVEMP and oVEMP response parameters. Subjects older than fifty years of age were found to have significantly decreased cVEMP amplitudes in response to clicks, tones, and taps with a Mini-Shaker, and significantly decreased oVEMP amplitudes in response to clicks, tones, and taps with a reflex hammer. However, when age and VEMP (cVEMP and oVEMP) amplitudes were plotted as continuous variables, no significant associations were found between these values. Additionally, we found that age did not affect cVEMP and oVEMP latencies or asymmetry ratios. This suggests that if the oVEMP test is used as a complement or an alternative to the cVEMP test, the burden of age-correction for the oVEMP would not exceed that for the cVEMP test.
In contrast to the findings of the present study, Iwasaki, et al. (19) had found that the amplitude of the n10 component of the vibration-evoked (Mini-shaker and reflex hammer) oVEMP decreased while the latency increased linearly with age. Several differences might explain why our study did not find significant age-related changes in these oVEMP parameters. Our sample population was slightly smaller (N = 53 vs. 67) and had a slightly more restricted age range (20–70 years vs. 20–83 years) than did the Iwasaki et al. study. Moreover, the mean age of the present study population was 35 years, while that of the Iwasaki et al. population was 47 years. Methodological differences might also play a role, such as differing electrode placement or differing degrees of ocular elevation at baseline (which was greater in our study). These differences draw attention to the need for each laboratory to define normal values for their specific methods.
Stretch response in cVEMP testing
It was noted that a small number of vibration-evoked cVEMP waveforms contained an overlap between the n23 response and the later-occurring, non-vestibular stretch response, making it difficult to determine the amplitude and latency of the n23 waveform. Therefore, we have included in the present study a separate analysis of the p13 response parameters, to allow for easier interpretation of p13 findings in the absence of a distinct n23 response. Such an analysis is not meant to suggest that only the p13 response should be used to evaluate otolith organ function, as both the p13 and n23 responses are known to be vestibular in origin, and together the two provide a more complete picture of otolith and vestibular function. However, our data did seem to suggest that the asymmetry ratio for p13 amplitude closely mirrors the asymmetry ratio for peak-to-peak amplitude, as the mean difference between the two values was only 10.8% (range 3.5–17.6%) for all modes of stimulation.
CONCLUSIONS
Cervical VEMPs in response to air-conducted sound are well-established as measures of saccular function. Ocular VEMPs may test a combination of utricular or saccular function, and the combination may depend on the stimulus being used. Although the exact otolith function being tested with oVEMPs is still a matter of research, it seems that oVEMPs offer several advantages over cVEMPs. These include greater overall reliability, less patient fatigue, and no need for correction for underlying muscle activity. As further research clarifies the utricular and saccular contributions to oVEMPs under specific testing conditions, the reliability and other advantages of oVEMPs should make them a useful addition to the vestibular test battery.
ACKNOWLEDGEMENTS
This study was supported by NIH R01 DC05040 (JPC) and by a grant from the Doris Duke Charitable Foundation to the Johns Hopkins University School of Medicine to fund a Clinical Research Fellow (KDN).
Footnotes
Disclosure of funding: NIH, Doris Duke Charitable Foundation
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCue MP, Guinan JJ., Jr. Sound-evoked activity in primary afferent neurons of a mammalian vestibular system. Am J Otol. 1997;18:355–360. [PubMed] [Google Scholar]
- 3.Curthoys IS, Kim J, McPhedran SK, et al. Bone conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175:256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- 4.Murofushi T, Curthoys IS. Physiological and anatomical study of click-sensitive primary vestibular afferents in the guinea pig. Acta Otolaryngol (Stockh) 1997;117:66–72. doi: 10.3109/00016489709117994. [DOI] [PubMed] [Google Scholar]
- 5.McCue MP, Guinan JJ., Jr. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994;14:6058–6070. doi: 10.1523/JNEUROSCI.14-10-06058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murofushi T, Curthoys IS, Topple AN, et al. Responses of guinea pig primary vestibular neurons to clicks. Exp Brain Res. 1995;103:174–178. doi: 10.1007/BF00241975. [DOI] [PubMed] [Google Scholar]
- 7.Uchino Y, Sato H, Sasaki M, et al. Sacculocollic reflex arcs in cats. J Neurophysiol. 1997;77:3003–3012. doi: 10.1152/jn.1997.77.6.3003. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzaki M, Murofushi T, Mizuno M. Vestibular evoked myogenic potentials in acoustic tumor patients with normal auditory brainstem responses. Eur Arch Otorhinolaryngol. 1999;256:1–4. doi: 10.1007/s004050050112. [DOI] [PubMed] [Google Scholar]
- 9.de Waele C, Huy PT, Diard JP, et al. Saccular dysfunction in Meniere's disease. Am J Otol. 1999;20:223–232. [PubMed] [Google Scholar]
- 10.Murofushi T, Matsuzaki M, Mizuno M. Vestibular evoked myogenic potentials in patients with acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1998;124:509–512. doi: 10.1001/archotol.124.5.509. [DOI] [PubMed] [Google Scholar]
- 11.Young YH, Wu CC, Wu CH. Augmentation of vestibular evoked myogenic potentials: an indication for distended saccular hydrops. Laryngoscope. 2002;112:509–512. doi: 10.1097/00005537-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Versino M, Colnaghi S, Callieco R, et al. Vestibular evoked myogenic potentials in multiple sclerosis patients. Clin Neurophysiol. 2002;113:1464–1469. doi: 10.1016/s1388-2457(02)00155-4. [DOI] [PubMed] [Google Scholar]
- 13.Itoh A, Kim YS, Yoshioka K, et al. Clinical study of vestibular-evoked myogenic potentials and auditory brainstem responses in patients with brainstem lesions. Acta Otolaryngol Suppl. 2001;545:116–119. [PubMed] [Google Scholar]
- 14.Rauch SD. Vestibular evoked myogenic potentials. Curr Opin Otolaryngol Head Neck Surg. 2006;14:299–304. doi: 10.1097/01.moo.0000244185.65022.01. [DOI] [PubMed] [Google Scholar]
- 15.Watson SRD, Halmagyi GM, Colebatch JG. Vestibular hypersensitivity to sound (Tullio phenomenon): Structural and functional assessment. Neurology. 2000;54:722–728. doi: 10.1212/wnl.54.3.722. [DOI] [PubMed] [Google Scholar]
- 16.Brantberg K, Bergenius J, Mendel L, et al. Symptoms, findings and treatment in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol. 2001;121:68–75. doi: 10.1080/000164801300006308. [DOI] [PubMed] [Google Scholar]
- 17.Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol. 2005;116:1938–1948. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki S, McGarvie LA, Halmagyi GM, et al. Head taps evoke a crossed vestibulo-ocular reflex. Neurology. 2007;68:1227–1229. doi: 10.1212/01.wnl.0000259064.80564.21. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki S, Smulders YE, Burgess AM, et al. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the mmidline forehead at Fz in healthy subjects. Clin Neurophysiol. 2008;119:2135–2147. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Todd NP, Rosengren SM, Aw ST, et al. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007;118:381–390. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki S, Smulders YE, Burgess AM, et al. Ocular vestibular evoked myogenic potentials in response to bone-conducted vibration of the midline forehead at Fz. A new indicator of unilateral otolithic loss. Audiol Neurootol. 2008;13:396–404. doi: 10.1159/000148203. [DOI] [PubMed] [Google Scholar]
- 22.Wang SJ, Jaw F-S, Young YH. Ocular vestibular-evoked myogenic potentials elicited from monaural versus binaural acoustic stimulation. Clin Neurophysiol. 2009;120:420–423. doi: 10.1016/j.clinph.2008.10.157. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki S, Chihara Y, Smulders YE, et al. The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clin Neurophysiol. 2009;120:588–593. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Rosengren SM, Aw ST, Halmagyi GM, et al. Ocular vestibular evoked myogenic potentials (OVEMPs) in superior canal dehiscence. J Neurol Neurosurg Psychiatry. 2007 doi: 10.1136/jnnp.2007.126730. [DOI] [PubMed] [Google Scholar]
- 25.Welgampola MS, Myrie OA, Minor LB, et al. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70:464–472. doi: 10.1212/01.wnl.0000299084.76250.4a. [DOI] [PubMed] [Google Scholar]
- 26.McAngus Todd NP, Rosengren SM, Coling D. A source analysis of short-latency vestibular evoked potentials produced by air- and bone-conducted sound. Clin Neurophysiol. 2008;119:1881–1894. doi: 10.1016/j.clinph.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 27.McAngus Todd NP, Rosengren SM, Coling D. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by impulsive transmastoid accelerations. Clin Neurophysiol. 2008;119:1638–1651. doi: 10.1016/j.clinph.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Wang SJ, Weng WJ, Jaw FS, et al. Ocular and Cervical Vestibular-Evoked Myogenic Potentials: A Study To Determine Whether Air- or Bone-Conducted Stimuli Are Optimal. Ear Hear. 2009 doi: 10.1097/AUD.0b013e3181bdbac0. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki S, Murofushi T, Chihara Y, et al. Ocular Vestibular Evoked Myogenic Potentials to Bone-Conducted Vibration in Vestibular Schwannomas. Otol Neurotol. 2009 doi: 10.1097/MAO.0b013e3181c0e670. [DOI] [PubMed] [Google Scholar]
- 30.Isaradisaikul S, Strong DA, Moushey JM, et al. Reliability of vestibular evoked myogenic potentials in healthy subjects. Otol Neurotol. 2008;29:542–544. doi: 10.1097/MAO.0b013e31816c7c25. [DOI] [PubMed] [Google Scholar]
- 31.Maes L, Vinck BM, De Vel E, et al. The vestibular evoked myogenic potential: a test-retest reliability study. Clin Neurophysiol. 2009;120:594–600. doi: 10.1016/j.clinph.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Versino M, Colnaghi S, Callieco R, et al. Vestibular evoked myogenic potentials: test-retest reliability. Functional Neurology. 2001;16:299–309. [PubMed] [Google Scholar]
- 33.Eleftheriadou A, Deftereos SN, Zarikas V, et al. Vestibular evoked myogenic potential eliciting in normal subjects: comparison of four different methods. J Otolaryngol Head Neck Surg. 2008;37:704–711. [PubMed] [Google Scholar]
- 34.Vanspauwen R, Wuyts FL, van de Heyning PH. Validity of a new feedback method for the VEMP test. Acta Otolaryngol. 2006;126:796–800. doi: 10.1080/00016480500527227. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs AF, Binder MD. Fatigue resistance of human extraocular muscles. J Neurophysiol. 1983;49:28–34. doi: 10.1152/jn.1983.49.1.28. [DOI] [PubMed] [Google Scholar]
- 36.Welgampola MS. Evoked potential testing in neuro-otology. Curr Opin Neurol. 2008;21:29–35. doi: 10.1097/WCO.0b013e3282f39184. [DOI] [PubMed] [Google Scholar]
- 37.Ochi K, Ohashi T, Nishino H. Variance of vestibular-evoked myogenic potentials. Laryngoscope. 2001;111:522–527. doi: 10.1097/00005537-200103000-00025. [DOI] [PubMed] [Google Scholar]