Abstract
Vertebrate Shroom proteins define cytoskeletal organization and cellular architecture by binding directly to F-actin and Rho-kinase and spatially regulating the activity of non-muscle myosin II (myosin II). Here we report characterization and gain-of-function analysis of Drosophila Shroom. The dShrm locus expresses at least two protein isoforms, dShrmA and dShrmB, which localize to adherens junctions and the apical membrane, respectively. dShrmA and dShrmB exhibit differing abilities to induce apical constriction that are based on their subcellular distribution and the subsequent assembly of spatially and organizationally distinct actomyosin networks that are dependant on the ability to engage Rho-kinase and the activity of myosin II. These data show that the differential subcellular distribution of naturally occurring isoforms of Shroom proteins can define both the position and organization of actomyosin networks in vivo. We further hypothesize that differentially positioned contractile arrays have distinct effects on cellular morphologies and behaviors.
Keywords: Shroom, Rock, myosin, actin, epithelia, constriction
Introduction
Maintaining proper epithelial cell shape is of fundamental importance during embryo and organ morphogenesis. These morphogenetic processes are often controlled by the actin cytoskeleton in conjunction with the contractile activity of non-muscle myosin II (myosin II) (Lecuit and Lenne, 2007; Quintin et al., 2008). Contraction of the actin cytoskeleton generates tension that can define shape, adhesion, and migration on the individual cell level. When these changes in behavior occur synchronously in a groups of cells, they can promote alterations in stiffness or architecture that facilitate tissue invagination, convergent extension, or compartmentalization (Franke et al., 2005; Lee and Harland, 2007; Landsberg et al., 2009; Zhou et al., 2009). Myosin II activity is regulated by phosphorylation of the associated myosin regulatory light chains (MRLC). This is typically mediated by Rho-kinase (Rock or Rok), which acts by directly activating the MRLC or by inhibiting the myosin phosphatase (Giuliano et al., 1992; Amano et al., 1996; Kawano et al., 1999; Totsukawa et al., 2000). Rock appears to be activated primarily by release of an auto-inhibitory intramolecular interaction that can be achieved by binding to activated Rho (Ishizaki et al., 1996; Leung et al., 1996; Matsui et al., 1996; Amano et al., 1999).
Results from multiple experimental approaches have shown that the Rho-Rock-myosin II pathway is conserved throughout animal evolution and controls cellular behaviors during many diverse processes (Winter et al., 2001; Vaezi et al., 2002; Kim and Han, 2005; Lai et al., 2005; Shimizu et al., 2005; Verdier et al., 2006; Diogon et al., 2007; Ybot-Gonzalez et al., 2007; Skoglund et al., 2008; Rolo et al., 2009). In Drosophila specifically, actomyosin networks generate tension that is required for germ band extension, convergent extension, dorsal closure, invagination, and boundary formation (Winter et al., 2001; Bertet et al., 2004; Nikolaidou and Barrett, 2004; Zallen and Wieschaus, 2004; Franke et al., 2005; Padash Barmchi et al., 2005; Blankenship et al., 2006; Simoes et al., 2006; Verdier et al., 2006; Landsberg et al., 2009; Martin et al., 2009; Sawyer et al., 2009).
Apical constriction of epithelial cells is a recurring theme during embryonic development (Pilot and Lecuit, 2005; Quintin et al., 2008). Apical constriction occurs when the localized contraction of actomyosin networks induces a decrease in apical area. In Drosophila, apical constriction is essential for the formation of grooves and furrows, invagination of the salivary glands and spiracles, and tissue folding (Costa et al., 1994; Nikolaidou and Barrett, 2004; Dawes-Hoang et al., 2005; Franke et al., 2005; Simoes et al., 2006; Corrigall et al., 2007; Kolesnikov and Beckendorf, 2007; Mulinari et al., 2008; Xu et al., 2008; Martin et al., 2009). Several signaling pathways and cytoskeletal proteins, including Fog, Hedgehog, Jak/Stat, Dpp, RhoGef2, and RhoGef64C, regulate apical constriction by temporally and spatially controlling the assembly and activity of contractile actomyosin networks (Nikolaidou and Barrett, 2004; Dawes-Hoang et al., 2005; Padash Barmchi et al., 2005; Simoes et al., 2006; Corrigall et al., 2007; Bertet et al., 2009; Widmann and Dahmann, 2009). A unifying feature of these pathways is that they all utilize Rho1 and dRok to trigger contraction of actin networks via myosin II. Apical constriction is also essential for the morphogenesis of several vertebrate tissues, including the neural tube, eye, intestine, lateral line, and bottle cells (Haigo et al., 2003; Lee and Harland, 2007; Choi and Sokol, 2009; Hava et al., 2009; Rolo et al., 2009; Chung et al.; Plageman et al., 2010).
While the Rho-Rock pathway is indispensable for many morphogenic processes, in vertebrates there is an additional essential pathway for regulating myosin II that is dependent on Shroom (Shrm) proteins (Hildebrand and Soriano, 1999; Haigo et al., 2003; Hildebrand, 2005; Fairbank et al., 2006; Yoder and Hildebrand, 2007; Nishimura and Takeichi, 2008; Taylor et al., 2008; Lee et al., 2009; Chung et al., 2010). The Shrm family is comprised of four members, Shrm1-Shrm4, which exhibit varying degrees of homology and conservation of function (Hagens et al., 2006). Using multiple animal models, it has been shown that Shrm3 is required for neural tube, intestine, and eye morphogenesis (Haigo et al., 2003; Hildebrand, 2005; Nishimura and Takeichi, 2008; Chung et al., 2010; Plageman et al., 2010). Additionally, blocking Shrm2 function in Xenopus has been shown to impair retinal development and epithelial thickening (Fairbank et al., 2006; Lee et al., 2009). Shrm3 binds to Rock and recruits it to the tight junction (TJ) where it facilitates the assembly of a circumferential actomyosin network. The defects observed in Shrm3 mutants likely result from the loss of apical contractility and tension which impairs cell wedging and cellular rearrangements (Hildebrand, 2005; Nishimura and Takeichi, 2008). The localization of Shrm3 is mediated by binding to F-actin via the centrally localized Shrm Domain 1 (SD1, previously called ASD) (Hildebrand and Soriano, 1999; Dietz et al., 2006). The Shrm3-Rock interaction is mediated by the SD2 of Shrm and a Shrm Binding Domain (SBD) found in the central part of Rock, just upstream of the Rho-binding domain (Nishimura and Takeichi, 2008). It does not appear that Rho activity is required for Shrm3-induced apical constriction, suggesting that the Shroom-Rock interaction may represent an alternative pathway to regulate the localization, and perhaps activation, of Rock and actomyosin networks (Haigo et al., 2003; Hildebrand, 2005).
Until now it was not known if the functions of Shrm proteins are conserved in invertebrate organisms. We have characterized and assayed the functionality of different Drosophila Shrm (dShrm) isoforms and show that they exhibit distinct subcellular distributions and subsequently induce the formation of contractile actomyosin networks with differential localization, organization, and activity in polarized epithelia. We also show that one isoform of dShrm retains all of the properties attributed to vertebrate Shrm3, including binding to both F-actin and dRok. Taken together, our results indicate that the Shrm-Rock-myosin II pathway has been conserved across animal evolution to regulate epithelial cell morphology.
RESULTS
dShrm expression and subcellular localization
Based on homology to the C-terminal SD2, we previously identified the Drosophila ortholog of vertebrate Shrm genes and designated it dShrm (Dietz et al., 2006). The dShrm locus is predicted to express three different protein products, Shroom-PD (AAF58260), Shroom-PF (AAF58259), and Shroom-PG (AAM70999) that we have designated dShrmA, B, and C, respectively. When we first identified dShrm, only isoforms B and C were predicted and of these only dShrmB was represented by a full-length cDNA. The genome has since been re-annotated to include dShrmA, which is also represented by a full-length cDNA. The protein products of dShrmA, dShrmB, and dShrmC are predicted to be 1576, 669, 669 amino acids in length, respectively (Fig. 1A). All three proteins have 659 amino acids of identical sequence at the C-terminus and this contains the SD2 motif, suggesting they should all be capable of binding to dRok. The three proteins are predicted to differ only at their N-termini (Fig. 1A). It should be noted that mouse Shrm2, 3, and 4 all possess one N-terminally located PDZ domain and this domain is not conserved in dShrm.
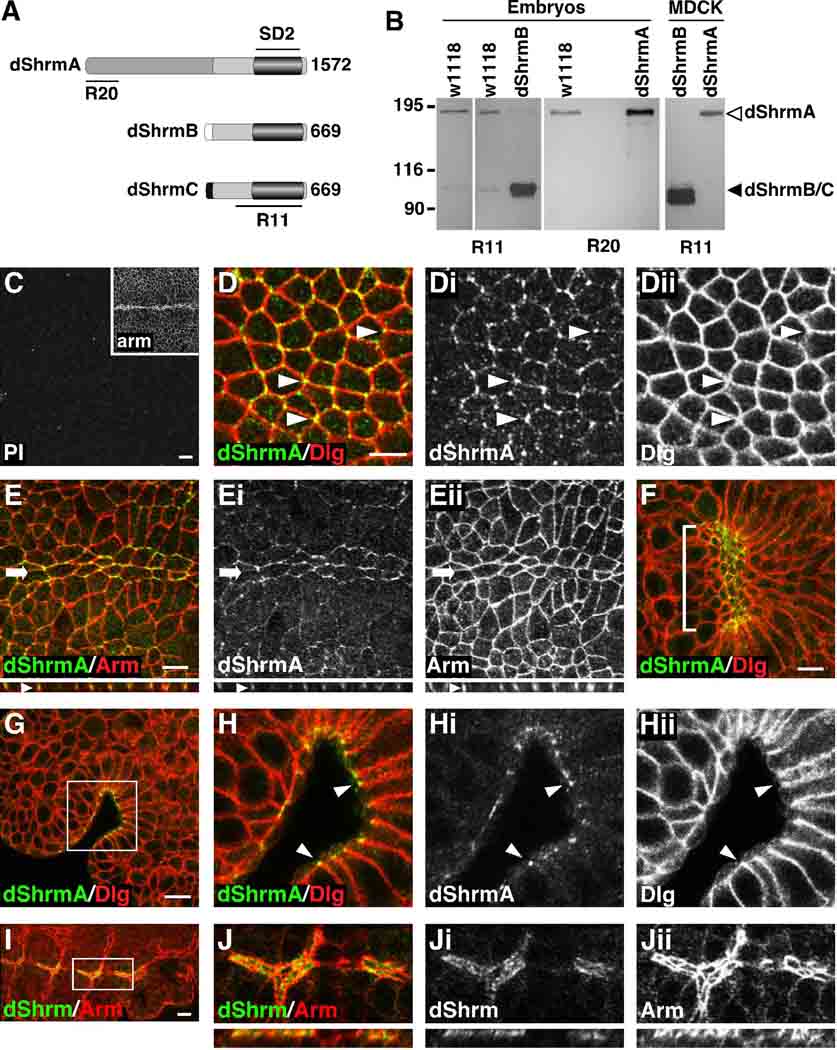
Figure 1. Expression and distribution of dShrm.
(A) Predicted proteins encoded by the dShrm locus. Lines indicate the regions used to generate antibodies. Numbers indicate the amino acid length of each protein.
(B) Western blot of endogenous and ectopic dShrm isoforms. Lysates from the indicated sources were subjected to Western blot analysis using R20 (dShrmA) or R11 (all isoforms).
(C) Stage 10 w1118 embryo stained with pre-immune (PI) serum and anti-Arm (inset).
(D-Eii) Stage 10 w1118 embryos stained to detect dShrmA and Dlg (D-Dii) and dShrmA and Arm (E-Eii). Arrows and arrowheads; the ventral midline and tricellular junctions, respectively. x–z projections are shown below.
(F) Ventral view of a stage 11 w1118 embryo stained to detect dShrmA and Dlg shows expression in invaginating foregut (bracket).
(G-Hii). A lateral view of dShrmA and Dlg in the invaginating foregut shows dShrmA positioned apically to Dlg (arrowheads). Boxed region in G is enlarged in H-Hii.
(I and J) Staining of a stage 12 w1118 embryo to detect all dShrm isoforms (R11) and Arm shows expression dShrmB in the dorsal trunk of the trachea. Boxed region in I is enlarged in J-Jii, x–z projections shown below.
Scale bar: 5 µm.
Western blot analysis of embryo lysates using antibodies that should recognize all of the isoforms (R11) detects proteins of 180 kDa and 100 kDa, while antibodies specific to dShrmA (R20) detect only the 180 kDa protein (Figure 1B). Similar analysis of either Drosophila embryos or cultured cells that ectopically express dShrmA or dShrmB detects proteins of the same Mr as seen in wild type embryos. Based on these data, we predict that dShrmA is the most abundant isoform expressed in the developing embryo. While we cannot distinguish if the 100kDa species is dShrmB or dShrmC, based on subcellular distribution (discussed below) we predict that this species may correspond to dShrmB.
Because vertebrate Shrm proteins are expressed in epithelial cells undergoing apical constriction, invagination, or thickening during vertebrate embryogenesis, we hypothesized that dShrm may be expressed in similar populations of cells and tissues during Drosophila embryogenesis. To test this, we looked for expression of dShrm in the embryonic ectoderm and in other epithelial tissues undergoing morphogenesis. In wild-type embryos dShrm staining is undetectable prior to gastrulation (data not shown). In stage 7 embryos (shortly after the start of gastrulation) dShrmA expression is detected in the ectoderm in a pattern that is consistent with cell-cell junctions (data not shown). By stage 10 (germ band extended), dShrmA is seen in cell-cell junctions of the epithelial ectoderm and is enriched in tricellular junctions (arrowheads, Fig. 1D-Dii). To more accurately define the localization of dShrmA, embryos were co-stained to detect dShrmA and Armadillo (Arm), a marker of the adherens junctions (AJ). In these embryos dShrmA co-localizes with Arm in AJ, particularly in cells of the ventral midline (arrow, Fig. 1E-Eii). Consistent with the idea that these proteins are involved in epithelial morphogenesis, dShrmA is enriched in the invaginating foregut (Fig. 1F-Hii) and hindgut (data not shown). In these cells dShrm is apically positioned relative to Dlg, further indicating that dShrmA localizes to AJs (Fig. 1H-Hii). Using antibodies that detect all dShrm isoforms, staining is also detected in the dorsal trunk of the developing tracheal system (Fig. 1I and 1J). In these cells, dShrm protein is restricted to the apical plasma membrane of the epithelial cells. This staining pattern is seen only with antibodies to the C-terminus, suggesting that it represents either dShrmB or dShrmC.
dShrmA and dShrmB exhibit different localization and activity
We hypothesized that dShrmA localizes to the AJ while dShrmB localizes to the apical plasma membrane of polarized cells. To test this, arm-GAL4;UAS-dShrmA or arm-GAL4;UAS-dShrmB embryos were stained to detect dShrm. Ectopic dShrmA co-distributes with Arm in the AJ (Fig. 2A) and is apically positioned relative to Dlg in the epithelium of the hindgut (Fig. 2B), while ectopic dShrmB localizes to the apical plasma membrane of the ectoderm (Fig. 2C-Dii). These results indicate that dShrmA and dShrmB exhibit different localization. They also suggest that the two populations of endogenous dShrm we observe likely correspond to dShrmA and dShrmB.
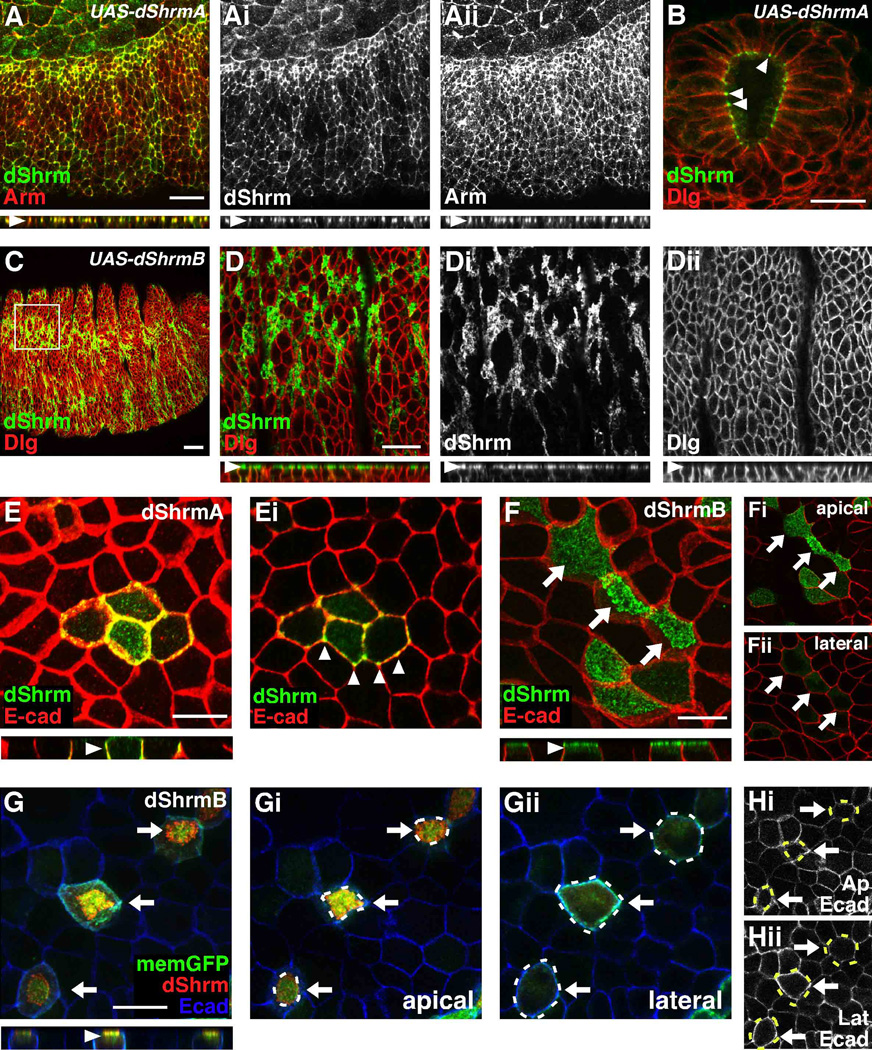
Figure 2. Differential distribution and activity of dShrmA and dShrmB.
(A-Dii) Stage 11 Arm-GAL4;UAS-dShrmA (A and B) and stage 14 Arm-GAL4;UAS-dShrmB (C and D-Dii) embryos stained to detect dShrm and Arm (A) or dShrm and Dlg (B-Dii). In the ectoderm (A) and hindgut epithelium (B) dShrmA is restricted to the AJ while dShrmB is enriched at the apical membrane (C-Dii). Boxed region in C is enlarged in D-Dii. D-Dii, apical optical sections; x–z projections of the confocal series are shown beneath.
(E) MDCK cells transiently expressing dShrmA stained as indicated. Panel E is a projection while Ei is a lateral section. Arrowheads, dShrmA localization; x–z projection shown below panel E.
(F-Hii) MDCK cells transiently expressing dShrmB stained to detect dShrm and E-cadherin. Arrows denote apically constricted cells. Panels Fi and Fii show single optical sections from the apical and lateral regions, respectively. (G-Hii) MDCK cells expressing membrane-GFP and dShrmB were stained to detect dShrm and E-cadherin. Panel G is a projection while Gi and Gii are optical sections from the apical and lateral regions, respectively. Hi and Hii are apical and lateral optical sections of E-cadherin staining. Arrows, constricted cells; dotted lines, outline of apically constricted cells, x–z projections shown beneath F and G; scale bar: 20 µm.
To further investigate dShrmA and dShrmB localization, we expressed these proteins in polarized MDCK cells (Fig. 2E-Hii). Similar to what was observed in embryos, ectopic dShrmA co-localizes with E-cadherin in AJ (Fig 2E and 2Ei). It should be pointed out that the spatial organization of cell-cell adhesion structures is not conserved across all animal phyla, such that AJ are located along the entirety of the lateral membrane in mammalian epithelia but are restricted to the apical-most portion of the lateral membrane in Drosophila epithelia. In the AJs, dShrmA is not uniformly distributed, but is enriched in discrete spots and in tricellular junctions (arrowheads, Fig. 2Ei). MDCK cells expressing dShrmA are normal and show no changes in cell morphology.
Like dShrmA, dShrmB localization is also faithfully recapitulated in MDCK cells such that it localizes to the apical plasma membrane and is excluded from the lateral membrane (Fig. 2F-Fii). This distribution was confirmed by co-expressing dShrmB with a membrane-targeted GFP and observing the localization of dShrm, GFP, and E-cadherin (Fig 2G). GFP and dShrmB co-distribute at the apical plasma membrane (Fig. 2Gi) but only GFP is seen at the lateral membrane (Fig. 2Gii). It was also noted that cells expressing dShrmB typically have a smaller apical area as assessed by both membrane GFP and E-cadherin staining (Fig. 2F-2Hii, arrows and cell outlines). Quantification of this phenotype shows that dShrmB expression causes a 23% decreases in apical area. In these experiments, control cells exhibit an average apical area of 125 ±18.5 µm2 while cells expressing dShrmB have an average apical area of 96 ±21 µm2 (p < 0.01, 3 experiment, n = 50 cells/experiment). These data indicate that in polarized epithelial cells dShrmA and dShrmB have different activities. In conjunction with our previous studies showing that Shrm3 is localized to the TJ and can cause apical constriction, we hypothesize that the lateral surfaces of MDCK cells may be refractory to Shrm activity or that the apical domain of the cell is permissive to Shrm activity.
Distinct mechanisms control dShrmA and dShrmB localization
We next wanted to test if the localization of dShrmA to discrete spots and tricellular junctions is mediated by F-actin. Staining of MDCK cells expressing dShrmA shows significant co-distribution of dShrmA and F-actin (Fig. 3A and 3E). To determine if dShrmA localization is dependant on F-actin, cells were treated with cytochalasin D (CD). CD treatment causes the redistribution of both dShrmA and F-actin from AJs into large cytoplasmic aggregates (Fig. 3A vs. Fig. 3B). This treatment does not disrupt AJs, as β-catenin localization is unaffected (Supp. Fig. S2A). Treatment of cells with latrunculin B (lat B) gives a similar result, while nocodazole treatment has no effect (Supp. Fig. S2A and B). Based on the above results, we tested if dShrmB localization is also controlled by F-actin. Detection of both dShrmB and F-actin in MDCK cells suggests that there is minimal co-distribution of dShrmB and the actin cytoskeleton (Fig. 3C). Consistent with this observation, dShrmB remains apically localized following CD, lat B, or nocodazole treatment (Fig. 3D and Supp. Fig. S2C). These data indicate that dShrmA and dShrmB use different mechanisms for localization.
Figure 3. Different sequences mediate dShrmA and dShrmB localization.
(A–D) dShrmA (A and B) or dShrmB (C and D) were transiently expressed in MDCK cells grown on transwell filters and either left untreated (A and C) or treated with cytochalasin D prior to staining to detect dShrm and F-actin (phalloidin). In A and B, lateral optical sections are shown, inset show enlargement of dShrmA and F-actin colocalization, and arrowheads denote dShrmA. In C and D, apical optical sections are shown and boxed regions are enlarged in the insets.
(E–L) Indicated regions of dShrmA were transiently expressed in either MDCK (E–H) or Cos7 (I–L) cells and co-stained to detect either dShrmA (R20) or the Myc tag (9E10) and F-actin. For MDCK cells, laterally positioned optical sections are shown; for Cos7 cells, insets show dShrm staining alone. Black oval represents the Myc tag.
(M–P) Indicated dShrmB proteins were expressed in MDCK cells grown on transwell filters and the cells stained to detect either dShrm (R11) or the KT3 epitope tag (open oval; M–O) and F-actin (phalloidin). Panels M and N are apical optical sections while O and P are lateral optical sections. x–z projections of the confocal series are shown below each panel.
Scale bar, 20 µm.
Previous work on vertebrate Shrm proteins has shown that while Shrm2, 3, and 4 exhibit different subcellular distributions, their localizations are all mediated by direct interactions with F-actin. Additionally, Shrm2 and Shrm3 appear to utilize the same core SD1 motif to bind F-actin (Hildebrand and Soriano, 1999; Dietz et al., 2006; Etournay et al., 2007; Yoder and Hildebrand, 2007). We hypothesized that the different localization of dShrmA and dShrmB could either be mediated by distinct sequence motifs and molecular mechanisms or that they could utilize a common sequence element that has different activity depending on the context. To distinguish between these possibilities, regions of dShrmA and dShrmB were expressed in either polarized MDCK cells or Cos7 cells and assayed for subcellular localization. Full-length dShrmA localizes to the AJ in MDCK cells (Fig. 3E) and to cortical actin in Cos7 cells (Fig. 3I). A protein consisting of amino acids 1–920, which represents the sequences unique to dShrmA, mimics the full-length protein (Fig. 3F and 3J). To further map this domain, amino acids 1–482 or 445–920 were assayed for localization in both Cos7 and MDCK cells. Amino acids 445–920 (Fig. 3G and 3K) localize correctly while amino acids 1–482 (Fig. 3H and 3L) are found in the cytoplasm. This indicates that dShrmA and dShrmB utilize distinct sequences for localizations.
To map the sequences that mediate localization of dShrmB to the apical plasma membrane, N-terminal portions containing amino acids 1–411 or 1–134 were expressed in MDCK cells. Both of these fragments recapitulate the subcellular distribution of the full-length protein, suggesting that the unique N-terminus of the protein might mediate targeting to the apical membrane (Fig. 3C vs. Fig. 3M and 3N). Results obtained using two additional variants support this hypothesis. First, a protein consisting of amino acids 35–411 is largely found in the cytoplasm with some enrichment toward the apical region of the cell (Fig. 3O). Secondly, placement of a 6x myc tag at the N-terminus completely abolishes dShrmB localization to the apical surface (Fig. 3P). To quantify the degree of localization to the apical plasma membrane versus the cytoplasm, we measured the ratio of fluorescent intensities of dShrmB staining at the apical plasma membrane relative to the basal cytoplasm for the various proteins. In dShrmB, 1–411, and 1–134 the ratios are 15.6:1, 17.4:1 and 11.5:1, respectively, indicating that the majority of these proteins are at the apical membrane. In contrast, for 35–411 and myc-ShrmB the ratios are 2.2:1 and 1.4:1, suggesting that while there may be some enrichment at the plasma membrane, these proteins are largely mislocalized to the cytoplasm. Collectively, these data indicate that N-terminal sequences are required for dShrmB localization to the apical plasma membrane.
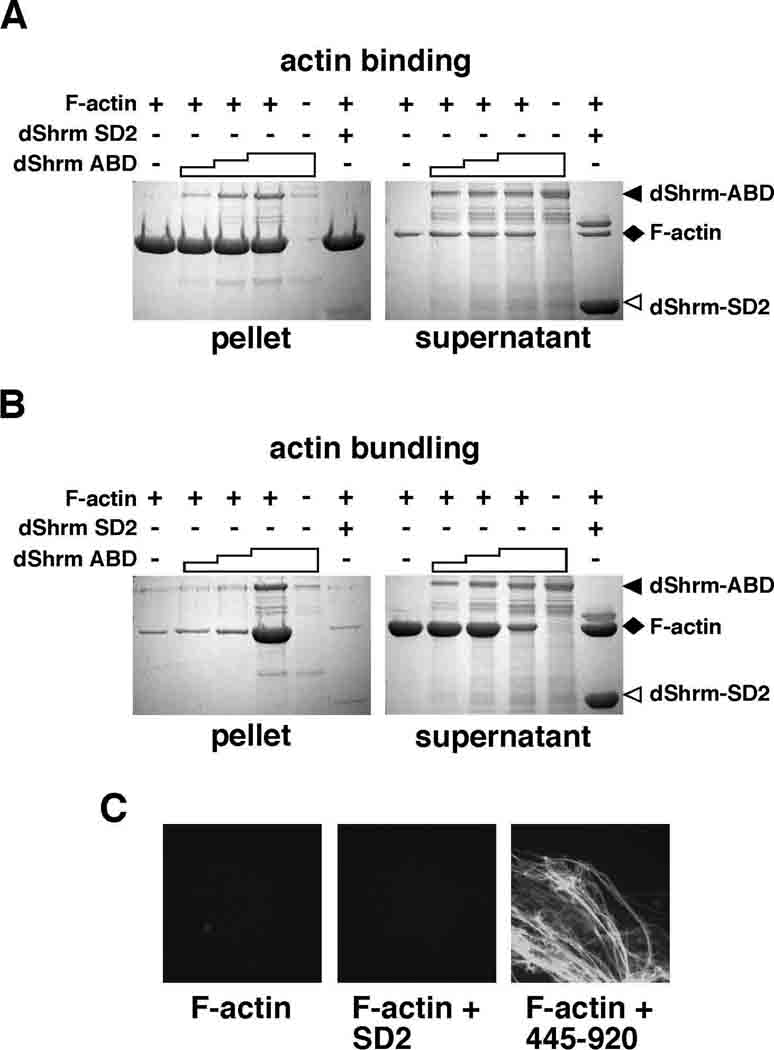
The data presented above suggests that dShrmA can bind directly to F-actin. To test this possibility, we expressed and purified amino acids 445–920 (termed the Actin Binding Domain, ABD) from bacteria and assayed it for the ability to directly bind F-actin in a co-sedimentation assay. Results from these experiments show that dShrmA-ABD is able to both bind directly to actin filaments (Fig. 4A) and induce the formation of actin aggregates that sediment at low speed (Fig. 4B). A negative control, the dShrm SD2 purified under identical conditions, does not co-sediment with actin or induce aggregation. Based on phalloidin staining and confocal analysis, the F-actin aggregates induced by dShrmA appear to be bundles (Fig. 4C). Sequence analysis of amino acids 445–920 identified a 116 amino acid region (residues 482–598) that is highly conserved in other insect species (Supp. Fig. S1A). A psi-blast search (Altschul et al., 1997) using this region of dShrmA identified limited homology (26% identity) to the SD1 motif found in vertebrate Shrm3, the region that we have shown to bind directly to F-actin and induce the formation of actin bundles (Hildebrand and Soriano, 1999; Dietz et al., 2006). These data suggest that this sequence may represent an evolutionarily conserved, yet degenerate, actin-binding module. Taken together, we predict that dShrmA localization is mediated by direct binding to F-actin and that the actin binding activity of Shrm proteins has been conserved despite limited sequence conservation.
Figure 4. dShrmA directly binds to F-actin.
(A) For actin binding assays, increasing amounts of dShrmA proteins consisting of amino acids 445–920, containing the putative Actin Binding Domain (dShrm-ABD) was mixed with purified F-actin and then F-actin was centrifuged at high speed to pellet all actin filaments and any associated proteins. Supernatant and pellet fractions were resolved by SDS-PAGE and detected by Coomassie staining. Staircase denotes increasing amounts of dShrm-ABD added while + and − signs denote the presence or absence of the indicated protein in the reaction. dShrm-SD2, dShrm-ABD alone, and F-actin alone are shown as controls.
(B and C) dShrmA can induce the formation of actin aggregates. Actin binding experiments were repeated as in A, but F-actin was centrifuged at low speed to pellet only actin aggregates. In C, samples were removed prior to centrifugation, incubated with FITC-phalloidin, and visualized by confocal microscopy.
dShrm controls epithelial cell morphology in vivo
Previous work has shown that Shrm3 can induce apical constriction. To test if dShrmA also possesses this ability, we ubiquitously over expressed dShrmA in embryos using the arm-GAL4 driver. This results in lethality of virtually all embryos due to multiple defects in morphogenesis (Supp. Fig. S3B). These phenotypes all appear to result from excessive apical constriction and subsequent disruption of the AJ in the embryonic ectoderm (Supp. Fig. S3C and S3D). Expression of dShrmB results in approximately 28% lethality due to defects in the ectoderm. However, these are less severe than those caused by over expression of dShrmA (Supp. Fig. S3E).
Because of the above results, we expressed dShrm proteins in just the dorsal third of the ectoderm using the pnr-GAL4 driver. This allowed for more accurate characterization and analysis of phenotypes that result from dShrm expression. Over-expression of dShrmA in the dorsal ectoderm causes embryonic lethality due to defects in dorsal closure (Fig. 5). This dorsal open phenotype correlates with the inability of epidermal cells within the pnr-GAL4 expression domain, including the leading edge (LE) cells, to elongate along the dorsal-ventral axis (Fig. 5A vs. 5B). This may in turn inhibit the dorsal movement of the lateral ectoderm that is required for dorsal closure. Observation of cells within the pnr-GAL4 expression domain by SEM shows that dShrmA-expressing cell have a rounded apical surface and a smaller circumference then those in control embryos (bracket, Fig 5Ci vs. Fig. 5D). Note that lateral cells outside of the pnr-GAL4 expression domain are normal. This phenotype does not appear to be the result of defects in cell-cell adhesion since the expression level and localization of Dlg, phosphotyrosine (pTyr, a marker of the AJ), dE-cadherin, and Arm appear to be unchanged (Fig. 5H and 5K, data not shown). Expression of dShrmB also causes defects in dorsal closure but these defects are significantly less pronounced and most embryos complete the process. However, many of the embryos that complete dorsal closure show defects at the cellular level such that the LE cells are not elongated along the D–V axis (Fig. 5E vs. 5F) and the segments are often not matched correctly at the midline (data not shown). As a result, approximately 45% of stage 15 pnr-GAL4; UAS-dShrmB embryos (n=150) exhibit variable defects at the dorsal midline, ranging from completely open to small holes or puckering (Fig. 7I).
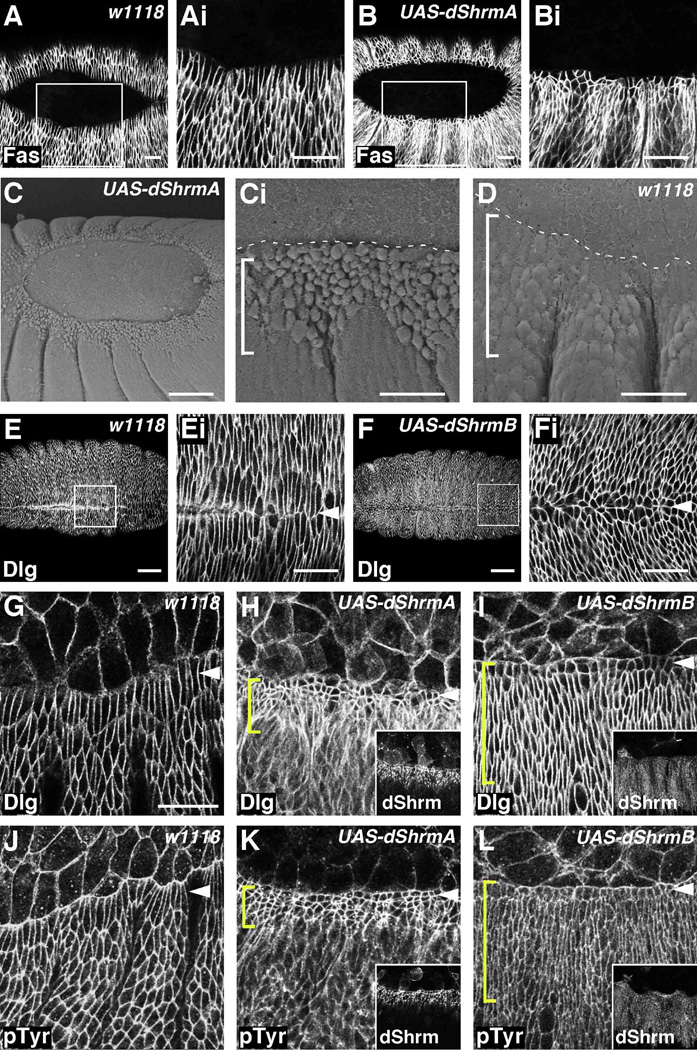
Figure 5. dShrmA and dShrmB exhibit different activities in vivo.
(A and B) Embryos of the indicated genotypes were stained to detect Fasciclin III (Fas). pnr-GAL4;UAS-dShrmA embryos exhibit defects in dorsal closure (B). Boxed regions in A and B are enlarged in Ai and Bi. Dorsal views of stage 14 embryos are shown.
(C and D) SEM of pnr-GAL4;UAS-dShrmA and control embryos. Dotted line, boundary between the amnioserosa (AS) and leading edge (LE).
(E-Fi) Control (E) and pnr-GAL4;UAS-dShrmB (F) stage 15 embryos stained to detect Dlg. Boxed regions in E and F are enlarged in Ei and Fi. Arrowheads denote dorsal midline. Control (G and J), pnr-GAL4;UAS-dShrmA (H and I) and pnr-GAL4;UAS-dShrmB (I and L) stage 13 embryos stained to detect dShrm and either Dlg (G–I) or pTyr (J–L). In pnr-GAL4;UAS-dShrmA embryos (H and I) cells are constricted and disorganized while in pnr-GAL4;UAS-dShrmB embryos (I and L), only the LE cells (arrowheads) fail to elongate. Insets in H, I, K, and L show dShrm expression.
In all panels, anterior to the left, dorsal to the top; brackets denote pnr-GAL4 expression domain; scale bars: 10 µm (Ci and D), 20 µm (A, B, Ei, Fi, G–L), 50 µm (E and F).
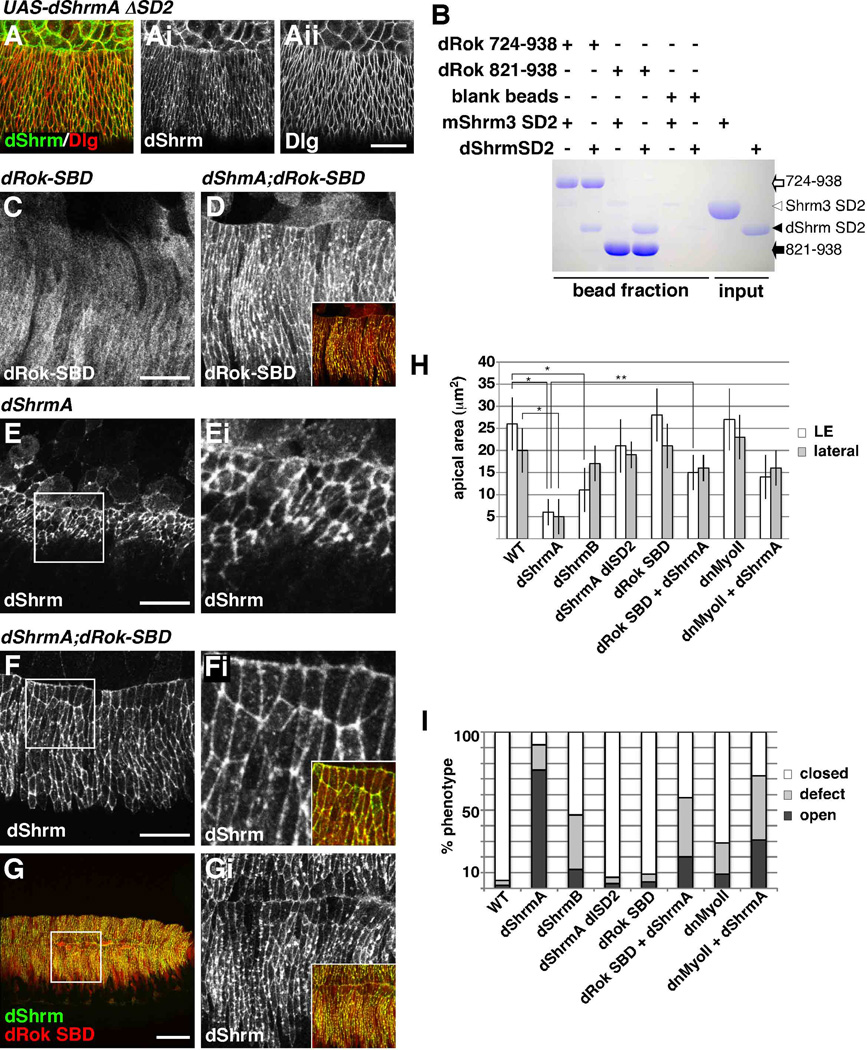
Figure 7. dShrmA requires dRok and myosin II to cause apical constriction.
(A) Stage 13 pnr-GAL4; UAS-dShrmA-ΔSD2 embryo stained to detect dShrmA and Dlg.
(B) Ni-beads or Ni-beads coated with either dRok 724–938 or dRok 834–938 were mixed with purified dShrm or mShrm3 SD2 and the protein complexes detected by Coomassie staining. + and − denote presence or absence of indicated proteins in the reaction.
(C–G) pnr-GAL4;UAS-dRok 724–938 (dRok-SBD, C), pnr-GAL4;UAS-dShrmA;UAS-dRok 724–938 (D, F, and G), or pnr-GAL4;UAS-dShrmA (E) stage 13 embryos stained to detect dShrmA and dRok-SBD (9E10). Boxed regions in E, F, and G are shown enlarged in Ei, Fi, and Gi. Insets in D, Fi, and Gi show merged images of dShrmA and dRok-SBD. Scale bar; 50 µm (G), 20 µm (A, C, E, F, Gi) or 5 µm (Ei and Fi).
(H and I) Quantification of apical constriction (H) and dorsal closure defects (I) caused by dShrmA, dShrmB, or dShrmA in combination with either dRok-SBD or dn-myoII expressed using the pnr-GAL4 driver. LE, leading edge cells; “lateral”, cells within the Pnr expression domain not adjacent to the amnioserosa; “defect”, puckering, small holes, or misaligned segments. p values < 0.005 (*) and 0.01, (**).
The inability of dShrmA and dShrmB expressing embryos to undergo dorsal closure prompted further analysis of cell morphology by staining embryos to detect either Dlg or pTyr. In control embryos, cells of the dorsal ectoderm exhibit the typical elongated morphology with the long axis parallel to the dorsal-ventral axis (Fig. 5G and 5J). Expression of dShrmA causes a dramatic change in cell morphology such that these cells appear disorganized, have significantly smaller apical membrane domains, and are reminiscent of cells that have undergone apical constriction (Fig. 5H and 5K). Cell measurements within the pnr-GAL4 expression domain show that dShrmA expression causes a roughly 70% decrease in apical area. In contrast, dShrmB expression causes only the LE cells to lose their elongated shape and appear apically constricted, exhibiting an approximate 50% decrease in apical area (arrowheads, Fig. 5I and 5L). Apical measurements for dShrmA and dShrmB expressing cells are quantified in Figure 7H. The different activities displayed by dShrmA and dShrmB are not due to expression level, as dShrmB is more highly expressed than dShrmA (data not shown). These data show that both dShrmA and dShrmB can induce apical constriction but they have significantly different efficacy in vivo.
dShrmA and dShrmB have different effects on cytoskeletal organization
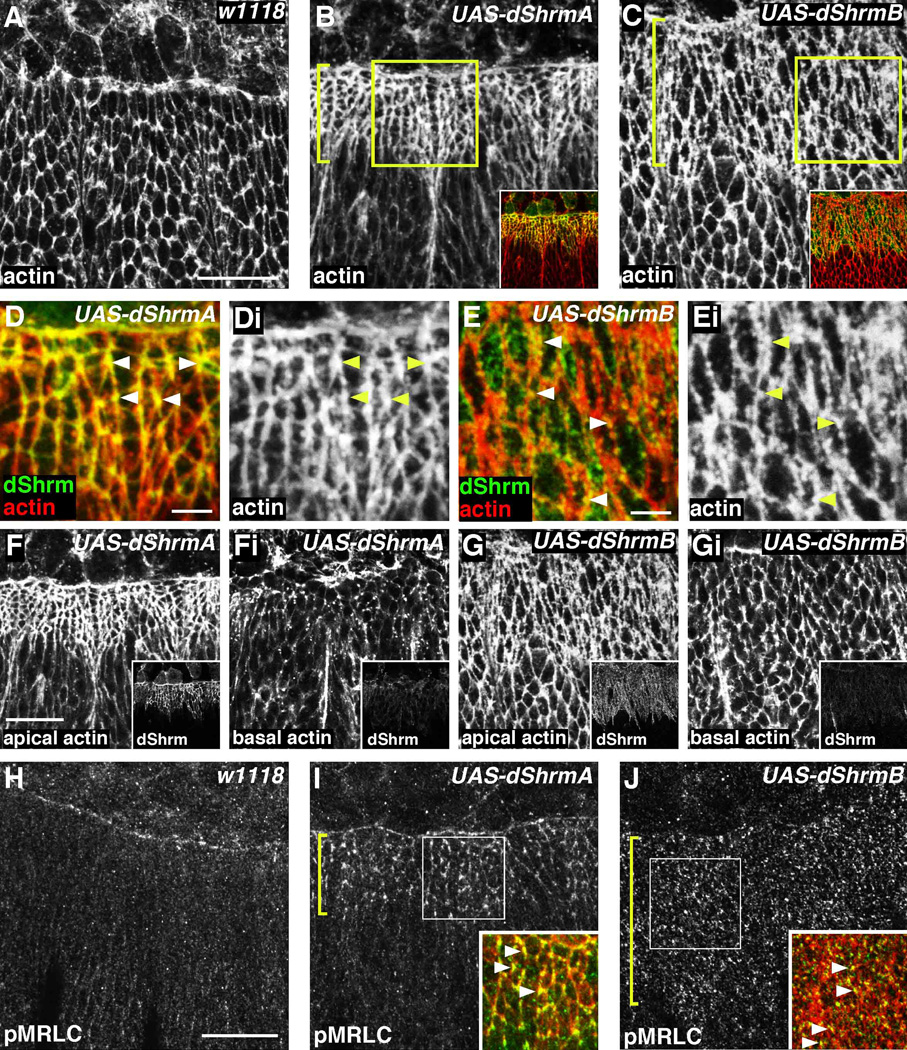
Previous studies showed that Shrm3-induced apical constriction correlates with the assembly of a circumferential contractile actomyosin network positioned at the level of the TJ (Hildebrand, 2005). We tested if dShrm proteins exhibit a similar activity by observing the organization of F-actin in Pnr-GAL4;UAS-dShrm embryos. Compared to control cells, dShrmA expressing cells exhibit an increase in the concentration of F-actin associated with the AJ (Fig. 6B). Based on measured fluorescent intensity, there is an approximate 2.1 ± 0.7 fold increase in F-actin in the AJ in dShrmA expressing cells. This increase is limited to the AJ as the fluorescent intensity of F-actin in the basal region of the cell is unchanged (Fig. 6F). Similar analysis shows that dShrmB induces reorganization of F-actin within the apical domain and this is limited to just those cells that express dShrmB (Fig 6C). The ectopic F-actin induced by dShrmB is found in aggregates that are associated with the AJs and the apical membrane and these tend to contain dShrmB (Fig. 6E, Ei, G, and Gi). We conclude that dShrm-induced changes in cell morphology are due to alterations in cytoskeletal organization in the AJ and apical domain of the cell.
Figure 6. dShrmA and dShrmB induce the formation of apical actomyosin networks.
(A–G) Control (A), pnr-GAL4;UAS-dShrmA (B, D, and F), and pnr-GAL4;UAS-dShrmB (C, E and G) stage 13 embryos were stained to detect dShrm and F-actin (phalloidin). In B and C, brackets denote the pnr-GAL4 expression domain and boxed regions are shown enlarged in D and E, respectively. Merged images of dShrm and phalloidin staining for B and C are shown as insets. Panels F and Fi and G and Gi show F-actin distribution in the apical (F and G) and basal (Fi and Gi) domains. Insets for F-Gi show the corresponding dShrm expression and localization.
(H–J) Control (H), pnr-GAL4;UAS-dShrmA (I), and pnr-GAL4;UAS-dShrmB (J) stage 13 embryos were stained to detect singly phosphorylated myosin regulatory light chain (pMRLC) and dShrm. Insets in I and J corresponded to the boxed regions and show the merged images of pMRLC and dShrm staining. The bracket denotes the pnr-GAL4 expression domain and the arrowheads denote colocalization of dShrm and pMRLC.
Scare bar; 5 µm (D, E) or 20 µm).
Virtually all cases of apical constriction described to date, including those involving Shrm3, require the localization of activated myosin II to the apical domain of the cell. To test if dShrmA and dShrmB induces the apical re-distribution of activated myosin II, embryos were stained to detect singly phosphorylated MRLC (pMRLC). The expression of dShrmA induces an approximate 1.8 ± 0.5 fold increase in the amount of pMRLC in the AJ (p < 0.01, Fig. 6I). Redistribution of pMRLC is also seen in embryos expressing dShrmB, but to a lesser extent (1.4 ± 0.2 fold, p < 0.01, Fig. 6J). Importantly, the re-distribution of pMRLC induced by either dShrmA or dShrmB faithfully mirrors the localization of F-actin and the dShrm protein being expressed. This is particularly clear in cells expressing dShrmA, such that all three components are highly enriched in tri-cellular junctions (arrowhead, Fig. 6D and 6I). These data support the hypothesis that different dShrm isoforms can dictate where actomyosin networks are assembled and what organization these networks will adopt. Furthermore, our results suggest that even within the apical compartment, different actomyosin networks can induce alternate outcomes in cell morphology and behavior.
dShrmA utilizes the dRok-myosin II pathway to control cell morphology
To test if the phenotypes caused by dShrmA result from engaging dRok to activate myosin II, we expressed a version of dShrmA that lacks SD2 (dShrmA-ΔSD2), the region of dShrm that should bind to dRok and trigger apical constriction. While this protein is expressed at high levels and localizes to AJs it does not cause any overt phenotypes (Fig. 7A-Aii). This result also indicates that the phenotypes induced by dShrmA are not secondary to defects in AJs or the cytoskeleton that might be caused by dShrmA over expression. To further address the role of dRok in dShrmA activity, we expressed and purified the dShrm SD2 and regions of dRok spanning amino acids 724–938 or 834–938, both of which are predicted to contain the highly conserved Shrm Binding Domain (SBD) of dRok (Nishimura and Takeichi, 2008) and Supp. Fig. S1B). In vitro binding assays show that dShrm SD2 can bind to dRok (Fig. 7B). Our results also show that there is species specificity to this interaction as dRok can only bind to the SD2 from dShrm and not to that from Shrm3.
If dShrmA works through dRok and Myosin II, then disrupting this pathway should abrogate the activity of dShrmA in vivo. To test this hypothesis we co-expressed dShrmA with either the SBD of dRok (amino acids 724–938) or a dominant negative myosin II (dn-myosin II, (Dawes-Hoang et al., 2005) in the dorsal ectoderm under control of the Pnr-GAL4 driver. We opted to use this approach as the SBD of dRok should function as a specific inhibitor of dRok-dShrm interactions and should not perturb other cellular processes that are dRok-dependant but dShrm-independent. Similar Rock constructs have been shown to block Shrm3 activity in both neural tube morphogenesis and neurite extension (Nishimura and Takeichi, 2008; Taylor et al., 2008). When expressed alone, dRok-SBD is localized primarily to the cytoplasm and does not cause any overt phenotype (Fig. 7C, 7H, and 7I). When co-expressed with dShrmA, dRok-SBD becomes enriched in AJs (Fig. 7D). In addition to co-localizing with dShrmA, dRok-SBD significantly blocks apical constriction (Fig. 7F, Fi and 7H) and ameliorates the dorsal open phenotype caused by dShrmA over expression (Fig. 7G, 7Gi, and 7I). These experiments were repeated using dShrmB and the results recapitulate those seen with dShrmA (data not shown). To verify the role of myosin II in the pathway, we co-expressed dShrmA and dn-myosin II and assayed for apical constriction and dorsal closure defects. As seen for the dRok-SBD, dn-myosin II also causes significant rescue of the dShrmA phenotype (Fig.7H and 7I). From these results we conclude that dShrmA utilizes the dRok-myosin II pathway to control cell morphology.
DISCUSSION
Previously, it was not clear how well the basic characteristics and functions of Shrm proteins were conserved in invertebrates. This was particularly true in Drosophila as the lone identifiable Shrm-related protein exhibited only limited sequence homology. While dShrm has been identified in genetic screens as a potential regulator of longevity and architecture of the neuromuscular junction, no studies on dShrm function in flies has been performed (Seong et al., 2001; Chang et al., 2008). We show here that the dShrm locus encodes at least two protein isoforms that have different activities in vivo. We predict that dShrmA is the most functionally similar to Shrm3 as it is expressed in epithelia, is spatial restricted to the same relative position in polarized cells, directly interacts with F-actin and dRok, and can induce robust apical constriction.
Our results show that dShrmA and dShrmB are not functionally equivalent when expressed in the same cellular contexts, such that dShrmA triggers the assembly of a circumferential contractile network while dShrmB induces the formation of a more disorganized actin network at the apical surface. Importantly, these alternate contractile networks seem to exhibit profoundly different activities in vivo. In a side-by-side comparison, the circumferential network appears to be more efficient at causing apical constriction than the apically positioned network. This is an interesting observation in light of the elegant studies showing that contractile actomyosin networks that span the apical plasma membrane are more critical to the apical constriction that drives ventral furrow formation (Martin et al., 2009; Sawyer et al., 2009). We suggest that dShrmA and dShrmB may participate in distinct morphogenic processes and their function may be dependant on the cellular context in which they are expressed.
The apically targeted dShrmB isoform appears to be specific to Drosophila and its localization seems to be mediated by a unique mechanism. In contrast to dShrmA, the mechanism by which dShrmB is targeted to the apical plasma membrane is currently unknown, but is independent of both the actin and microtubule cytoskeletons. However, the molecular basis for this localization is conserved since dShrmB is apically targeted in vertebrate epithelia. Sequence analysis of dShrmB does not indicate the presence of any signal peptides, transmembrane segments, or lipid modifications that could target it to the plasma membrane. These results indicate that dShrmB may directly interact with a protein or lipid constituent of the apical plasma membrane.
There are several explanations as to why dShrmA and dShrmB have different activity in vivo. First, it is possible that the SD2-dRok interaction is either inhibited or less efficient in the apical plasma membrane as opposed to the AJ. This could be achieved by a currently unknown mechanism, such as phosphorylation, which regulates the interaction specifically within the apical compartment. Alternatively, the ability to assemble a robust apical actomyosin network could be inhibited by another pathway. This concept is supported by the recent characterization of Jak signaling as a negative regulator of apical actomyosin networks in the lateral ectoderm (Bertet et al., 2009). Alternatively, there may be more total Rok or active Rok available in the AJ than at the apical plasma membrane. Interestingly, only dShrmB causes constriction in MDCK cells. This is likely due to the intrinsic differences in the organization in the cell-cell junctions between the species, where in vertebrate cells the TJs occupy the same relative position along the apical-basal axis as AJs in Drosophila cells. This is particularly intriguing in light of our previous observations that mShrm3 is specifically localized to TJs and can induce apical constriction in MDCK cells (Haigo et al., 2003; Hildebrand, 2005). In contrast, dShrmA localizes to the AJ and does not cause changes in cell morphology in MDCK cells, suggesting that there are strict requirements for where Shrm proteins can be localized and still induce the formation of actomyosin networks.
Initial sequence analysis indicated that the SD2 was the only motif conserved between vertebrate and Drosophila Shrm proteins. However, our analysis of dShrmA localization and actin-binding activity identified sequences that are highly conserved in other insect species and have low homology to the actin-binding region of Shrm3. It appears that this functional motif is conserved across animal phyla and is probably the primary mechanism by which both dShrmA and Shrm3 are targeted to the correct cellular locale. While these proteins share a common actin binding activity, the characteristic that is more stringently conserved between these proteins is their spatial distribution within polarized epithelial cells. We predict that there may have been selective pressure to retain the localization of mShrm3 and dShrmA proteins to the same relative position along the apical-basal axis. This has occurred even though the apical compartment of the lateral membrane is comprised of TJ in vertebrate cells and AJ in Drosophila cells. Our results indicate that while a conserved actin-binding activity targets both dShrmA and mShrm3 to their respective domains in the cell, this property has been modified to convey specific localization to distinct actin populations in vivo.
In summary, our data suggest that the Shrm-Rock pathway has been conserved during animal evolution. We also propose that naturally occurring protein isoforms that exhibit differential subcellular localization are important for determining the spatial distribution of contractile networks and this specifies developmentally regulated changes in cell morphology.
EXPERIMENTAL PROCEEDURES
Fly stocks used
Flies carrying the UAS-dShrmA, UAS-dShrmB, UAS-dShrmA ΔSD2, or UAS-dRok-SBD (containing the dRok Shrm Binding Domain, SBD) transgenes were generated by Genetic Services Inc (Sudbury, MA). Driver lines pnr-GAL4, arm-GAL4, and C381-GAL4 were originally from the Bloomington Stock Center (Department of Biology, Indiana University) and were obtained from Dr. Beth Stronach (University of Pittsburgh). UAS-dn-myoII (UAS mYFP-myosin IIDN, (Dawes-Hoang et al., 2005) was obtained from Dr. Brooke McCartney (Carnegie Mellon University). In all experiments, females carrying the GAL4 driver were crossed to males carrying the UAS transgene and all GAL4/UAS crosses and embryo collections were performed at 25°C.
Molecular biology
cDNAs encoding dShrmA (LP13775), dShrmB (GH26230), and dRok (LD15203) were obtained from the DGRC. For expression in Drosophila, cDNAs encoding dShrmA, dShrmA-ΔSD2 (amino acids 1–1441), dShrmB, and dRok-SBD (Shroom Binding Domain, amino acids 724–938 with an N-terminal myc tag) were cloned into pUAST and used for the production of transgenic flies. For expression in cells, cDNA sequences were cloned into pCS2 or pCS3mt (containing an N-terminal 6x myc tag). For C-terminal epitope tagged dShrmB, cDNAs encoding amino acids 1–411, 1–134, or 35–411 were amplified by PCR, cloned into pBS-ctag (Hildebrand et al., 1993) which contains sequences from large T antigen that are recognized by mouse monoclonal antibody (mAb) KT3 (MacArthur and Walter, 1984), and then cloned into pCS2. For expression in bacteria, DNA encoding the desired protein products were cloned into pET151 (Invitrogen).
Cell biology
MDCK and Cos7 cells were maintained at 37°C, 5% CO2 in EMEM or DMEM, respectively, supplemented with 10% FBS, pen/strep, and L-glutamine. Cells were transiently transfected in suspension with expression vectors using Lipofectamine 2000 and plated onto either fibronectin-coated coverslips (Cos7) or onto transwell filters (MDCK). Cells were allowed to grow for 24 hours and then processed for either Western blotting or immunofluorescence analysis. Treatments with cytochalasin D (CD, 1 or 2 µM), latrunculin B (0.5 µM), or nocodazole (3 µM) were carried out for 90 minutes at 37°C.
Immunofluorescence and scanning electron microscopy (SEM) analysis
Cells were fixed with either 4% paraformaldehyde or −20°C methanol and stained as described previously (Hildebrand, 2005). Drosophila embryos were collected after 4–12 hours collections and dechorionated in 50% bleach for 3 minutes. Embryos were rinsed with water, collected in heptane, and fixed in a 1:1 mixture of 37% formaldehyde and heptane for five minutes at RT with shaking. Embryos were devitellinized via vigorous shaking in a 1:1 mixture of methanol and heptane for 1 minute. Embryos were rehydrated in PBS + 0.1% Triton (PBST) for 30 minutes, blocked in PBST + 4% goat serum, and incubated in primary antibody overnight at 4°C. Embryos were washed for 6 times for 10 minutes in PBST, incubated in secondary Ab at RT for 2hrs, washed, and mounted on microscope slides in Vectashield (Vector Labs). For phalloidin staining, embryos were dechorionated and fixed as above, hand devitellinized, and stained as above. Images were acquired using a Biorad Radiance 2000 Laser Scanning System and a Nikon E800 microscope with 40 and 60X oil objectives and processed using either ImageJ or Photoshop. For cell measurements, control and experimental embryos were stained to detect dShrm, Arm, dE-cadherin, or membrane GFP and subjected to confocal microscopy in which imagining parameters were maintained constant across all samples. Statistical significance was determined using a Student’s T test on the average values obtained from 75 cells from at least three stage-matched embryos from three different collections. The fluorescent intensity of actin or pMRLC was determined using straight-line selections drawn perpendicular to the cell membrane at the level of the AJ in ImageJ. When possible, we determined changes in fluorescent intensity of phalloidin or pMRLC staining as ratios relative to the fluorescent intensity of Dlg or pTyr staining. For SEM, embryos were collected as above and fixed as described (Mulinari et al., 2008). Fixed embryos were dehydrated through a grades series of EtOH washed through a graded series of EtOH: hexamethyldisilazane (HMDS) into a final of 100% HMDS. Embryos were air-dried, sputter coated, and observed using a Jeol JSM6390LV SEM.
Antibodies
dShrm antisera were produced in rats using bacterially expressed proteins consisting of amino acids 1–183 (R20, R21, and R22) and 1144–1576 (R11 and R12) of dShrmA. Antibodies were affinity purified and all sera gave similar results. Other reagents: anti-Disc Large (Dlg) mAb 4F3 (1:400, DSHB), anti-Armadillo (Arm) mAb N2 7A1 (1:100, DSHB), anti-Fasciclin III (Fas) mAb 7G10 (1:400, DSHB); anti-Myc mAb 9E10, (1:100, a gift from Dr. Ora Wiesz, University of Pittsburgh); anti-SV40 T antigen mAb KT3, (1:200), anti-E-cadherin mAb (1:400, BD Bioscience); anti-phosphotyrosine mAb 4G10, ( 1:1000, Upstate), rabbit anti-phospho Ser19 MRLC (1:50, Cell Signaling), anti-phospho Ser19 MRLC mAb (1:50, Cell Signaling), TRITC-phalloidin (Sigma), To-Pro 3 (Invitrogen), goat anti-mouse, goat anti-rat, or goat anti-rabbit secondary antibodies conjugated to Alexa-488, Alexa-568, or Alexa-633 (1:400, Invitrogen).
Western blotting and in vitro binding assays
Embryos or cells were lysed in RIPA buffer, proteins resolved by SDS-PAGE, transferred to nitrocellulose and probed with specific antibodies overnight at 4°C. Antibodies were detected with HRP-conjugated secondary antibodies (1:2500, GE Health Systems), and detected using ECL. For in vitro binding assays, His-tagged proteins were purified from bacteria with Ni-NTA sepharose following induction with either IPTG or by autoinduction (Studier, 2005) and lysis in 500 mM NaCl, 20 mM Tris pH 8.0, 10% glycerol, 1 µM β-ME, 5mM imidazole. Soluble proteins were obtained by elution with lysis buffer supplemented with 500 mM imidazole. SD2 motifs were further purified by treatment with TEV protease (provided by Dr. Andrew VanDemark, University of Pittsburgh) and binding to Ni-NTA sepharose to remove TEV, the free His-tag, and uncleaved protein. In vitro actin binding and bundling experiments were performed as previously described (Dietz et al., 2006) using purified dShrm 445–920 or dShrm SD2 (negative control). For dRok binding assays, His-tagged dRok 724–938 or dRok 824–938 bound to Ni-NTA sepharose were mixed with untagged SD2 motifs from dShrm or mShrm3, incubated at RT for 1 hr with rocking, washed, resolved by SDS-PAGE, and proteins detected by Coomassie staining.
Supplementary Material
(A) Alignment of conserved sequences that are found in the Actin Binding Domain (ABD) of dShrmA, the putative Shrm proteins from other insect species, and the SD1 from vertebrate Shrm3. Xenopus is shown because it has the highest similarity, but Shrm3 from other species show similar degrees of sequences conservation.
(B) Alignment of the conserved Shrm Binding Domain (SBD) of Rock from multiple animal species.
(A) T23 cells transfected with dShrmA were grown on transwell filter, treated with the indicated compounds, and stained to detect either dShrmA or β-catenin.
(B) Cos7 cells transfected with dShrmA were treated with the indicated compounds and stained to detect dShrmA.
(C) T23 cells transfected with dShrmB were grown on transwell filter, treated with the indicated compounds, and stained to detect dShrmB.
(A and B) Control (A) and arm-GAL4;UAS-dShrmA (B) embryos stained to detect dShrmA and Arm. Arrows, dorsal open phenotype; arrowhead, failure in germ band retraction; bracket, exposed endoderm.
(C-Diii) Stage 11 arm-GAL4;UAS-dShrmA embryos stained to detect dShrmA and Arm (C) or dShrmA and Dlg (D). Boxed region in C is shown enlarged in Ci-Ciii. In Ci-Ciii, dark blue brackets denote defects in AJ integrity while light blue brackets indicate regions that have intact AJ and appear to be apically constricted. In D-Diii, D is a projection, while Di-Diii are optical sections. Dark blue brackets denote regions in which AJ integrity appears to be disrupted (based on dShrmA staining) but cell adhesion is retained (based on Dlg staining). Cells appear to be stretched between regions that are apically constricted and have intact AJ (light blue brackets). In both C and D, anterior is to the left. Panel C is a lateral view and D is a ventral-lateral view with the ventral midline at the bottom.
(E-Eii) Stage 14 arm-GAL4;UAS-dShrmB embryo stained to detect dShrmB and Dlg. Boxed region in panel E is shown enlarged in panels Ei and Eii. Panels Ei and Eii show apical and basal optical sections respectively. Bracket denotes defect in the ectoderm.
Acknowledgements
We thank Drs. Brooke McCartney and Beth Stronach for providing reagents and advice and Dr. Deborah Chapman, Dr. McCartney, and lab members for critical reading and discussion of this manuscript. The antibodies obtained from the Developmental Studies Hybridoma Bank were developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology. This work was supported by grant NIH GM67525 to J.D.H.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Bertet C, Rauzi M, Lecuit T. Repression of Wasp by JAK/STAT signalling inhibits medial actomyosin network assembly and apical cell constriction in intercalating epithelial cells. Development. 2009;136:4199–4212. doi: 10.1242/dev.040402. [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Chang HC, Dimlich DN, Yokokura T, Mukherjee A, Kankel MW, Sen A, Sridhar V, Fulga TA, Hart AC, Van Vactor D, Artavanis-Tsakonas S. Modeling spinal muscular atrophy in Drosophila. PLoS One. 2008;3:e3209. doi: 10.1371/journal.pone.0003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SC, Sokol SY. The involvement of lethal giant larvae and Wnt signaling in bottle cell formation in Xenopus embryos. Dev Biol. 2009;336:68–75. doi: 10.1016/j.ydbio.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MI, Nascone-Yoder NM, Grover SA, Drysdale TA, Wallingford JB. Direct activation of Shroom3 transcription by Pitx proteins drives epithelial morphogenesis in the developing gut. Development. 2010;137:1339–1349. doi: 10.1242/dev.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev Cell. 2007;13:730–742. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76:1075–1089. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- Dietz ML, Bernaciak TM, Vendetti F, Kielec JM, Hildebrand JD. Differential ctin-dependent localization modulates the evolutionarily conserved activity of Shroom family proteins. J Biol Chem. 2006;281:20542–20554. doi: 10.1074/jbc.M512463200. [DOI] [PubMed] [Google Scholar]
- Diogon M, Wissler F, Quintin S, Nagamatsu Y, Sookhareea S, Landmann F, Hutter H, Vitale N, Labouesse M. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development. 2007;134:2469–2479. doi: 10.1242/dev.005074. [DOI] [PubMed] [Google Scholar]
- Etournay R, Zwaenepoel I, Perfettini I, Legrain P, Petit C, El-Amraoui A. Shroom2, a myosin-VIIa- and actin-binding protein, directly interacts with ZO-1 at tight junctions. J Cell Sci. 2007;120:2838–2850. doi: 10.1242/jcs.002568. [DOI] [PubMed] [Google Scholar]
- Fairbank PD, Lee C, Ellis A, Hildebrand JD, Gross JM, Wallingford JB. Shroom2 (APXL) regulates melanosome biogenesis and localization in the retinal pigment epithelium. Development. 2006;133:4109–4118. doi: 10.1242/dev.02563. [DOI] [PubMed] [Google Scholar]
- Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–2221. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- Giuliano KA, Kolega J, DeBiasio RL, Taylor DL. Myosin II phosphorylation and the dynamics of stress fibers in serum-deprived and stimulated fibroblasts. Mol Biol Cell. 1992;3:1037–1048. doi: 10.1091/mbc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagens O, Ballabio A, Kalscheuer V, Kraehenbuhl JP, Schiaffino MV, Smith P, Staub O, Hildebrand J, Wallingford JB. A new standard nomenclature for proteins related to Apx and Shroom. BMC Cell Biol. 2006;7:18. doi: 10.1186/1471-2121-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–2137. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Hava D, Forster U, Matsuda M, Cui S, Link BA, Eichhorst J, Wiesner B, Chitnis A, Abdelilah-Seyfried S. Apical membrane maturation and cellular rosette formation during morphogenesis of the zebrafish lateral line. J Cell Sci. 2009;122:687–695. doi: 10.1242/jcs.032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118:5191–5203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J. Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:486–497. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. Embo J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- Kolesnikov T, Beckendorf SK. 18 wheeler regulates apical constriction of salivary gland cells via the Rho-GTPase-signaling pathway. Dev Biol. 2007;307:53–61. doi: 10.1016/j.ydbio.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Chang CN, Wang PJ, Lee SJ. Rho mediates cytokinesis and epiboly via ROCK in zebrafish. Mol Reprod Dev. 2005;71:186–196. doi: 10.1002/mrd.20290. [DOI] [PubMed] [Google Scholar]
- Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Julicher F, Dahmann C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–1955. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Lee C, Le MP, Wallingford JB. The shroom family proteins play broad roles in the morphogenesis of thickened epithelial sheets. Dev Dyn. 2009;238:1480–1491. doi: 10.1002/dvdy.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Harland RM. Actomyosin contractility and microtubules drive apical constriction in Xenopus bottle cells. Dev Biol. 2007;311:40–52. doi: 10.1016/j.ydbio.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur H, Walter G. Monoclonal antibodies specific for the carboxy terminus of simian virus 40 large T antigen. J Virol. 1984;52:483–491. doi: 10.1128/jvi.52.2.483-491.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Mulinari S, Barmchi MP, Hacker U. DRhoGEF2 and diaphanous regulate contractile force during segmental groove morphogenesis in the Drosophila embryo. Mol Biol Cell. 2008;19:1883–1892. doi: 10.1091/mbc.E07-12-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidou KK, Barrett K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr Biol. 2004;14:1822–1826. doi: 10.1016/j.cub.2004.09.080. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–1502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- Padash Barmchi M, Rogers S, Hacker U. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J Cell Biol. 2005;168:575–585. doi: 10.1083/jcb.200407124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685–694. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- Plageman TF, Jr, Chung MI, Lou M, Smith AN, Hildebrand JD, Wallingford JB, Lang RA. Pax6-dependent Shroom3 expression regulates apical constriction during lens placode invagination. Development. 2010;137:405–415. doi: 10.1242/dev.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin S, Gally C, Labouesse M. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet. 2008;24:221–230. doi: 10.1016/j.tig.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Rolo A, Skoglund P, Keller R. Morphogenetic movements driving neural tube closure in Xenopus require myosin IIB. Dev Biol. 2009;327:327–338. doi: 10.1016/j.ydbio.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer JK, Harris NJ, Slep KC, Gaul U, Peifer M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J Cell Biol. 2009;186:57–73. doi: 10.1083/jcb.200904001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong KH, Ogashiwa T, Matsuo T, Fuyama Y, Aigaki T. Application of the gene search system to screen for longevity genes in Drosophila. Biogerontology. 2001;2:209–217. doi: 10.1023/a:1011517325711. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes S, Denholm B, Azevedo D, Sotillos S, Martin P, Skaer H, Hombria JC, Jacinto A. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development. 2006;133:4257–4267. doi: 10.1242/dev.02588. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development. 2008;135:2435–2444. doi: 10.1242/dev.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Taylor J, Chung KH, Figueroa C, Zurawski J, Dickson HM, Brace EJ, Avery AW, Turner DL, Vojtek AB. The scaffold protein POSH regulates axon outgrowth. Mol Biol Cell. 2008;19:5181–5192. doi: 10.1091/mbc.E08-02-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Verdier V, Guang Chao C, Settleman J. Rho-kinase regulates tissue morphogenesis via non-muscle myosin and LIM-kinase during Drosophila development. BMC Dev Biol. 2006;6:38. doi: 10.1186/1471-213X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann TJ, Dahmann C. Dpp signaling promotes the cuboidal-to-columnar shape transition of Drosophila wing disc epithelia by regulating Rho1. J Cell Sci. 2009;122:1362–1373. doi: 10.1242/jcs.044271. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Xu N, Keung B, Myat MM. Rho GTPase controls invagination and cohesive migration of the Drosophila salivary gland through Crumbs and Rho-kinase. Dev Biol. 2008;321:88–100. doi: 10.1016/j.ydbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, Greene ND, Copp AJ. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder M, Hildebrand JD. Shroom4 (Kiaa1202) is an actin-associated protein implicated in cytoskeletal organization. Cell Motil Cytoskeleton. 2007;64:49–63. doi: 10.1002/cm.20167. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136:677–688. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Alignment of conserved sequences that are found in the Actin Binding Domain (ABD) of dShrmA, the putative Shrm proteins from other insect species, and the SD1 from vertebrate Shrm3. Xenopus is shown because it has the highest similarity, but Shrm3 from other species show similar degrees of sequences conservation.
(B) Alignment of the conserved Shrm Binding Domain (SBD) of Rock from multiple animal species.
(A) T23 cells transfected with dShrmA were grown on transwell filter, treated with the indicated compounds, and stained to detect either dShrmA or β-catenin.
(B) Cos7 cells transfected with dShrmA were treated with the indicated compounds and stained to detect dShrmA.
(C) T23 cells transfected with dShrmB were grown on transwell filter, treated with the indicated compounds, and stained to detect dShrmB.
(A and B) Control (A) and arm-GAL4;UAS-dShrmA (B) embryos stained to detect dShrmA and Arm. Arrows, dorsal open phenotype; arrowhead, failure in germ band retraction; bracket, exposed endoderm.
(C-Diii) Stage 11 arm-GAL4;UAS-dShrmA embryos stained to detect dShrmA and Arm (C) or dShrmA and Dlg (D). Boxed region in C is shown enlarged in Ci-Ciii. In Ci-Ciii, dark blue brackets denote defects in AJ integrity while light blue brackets indicate regions that have intact AJ and appear to be apically constricted. In D-Diii, D is a projection, while Di-Diii are optical sections. Dark blue brackets denote regions in which AJ integrity appears to be disrupted (based on dShrmA staining) but cell adhesion is retained (based on Dlg staining). Cells appear to be stretched between regions that are apically constricted and have intact AJ (light blue brackets). In both C and D, anterior is to the left. Panel C is a lateral view and D is a ventral-lateral view with the ventral midline at the bottom.
(E-Eii) Stage 14 arm-GAL4;UAS-dShrmB embryo stained to detect dShrmB and Dlg. Boxed region in panel E is shown enlarged in panels Ei and Eii. Panels Ei and Eii show apical and basal optical sections respectively. Bracket denotes defect in the ectoderm.