Abstract
A symposium held at the 50th annual meeting of the Behavioral Pharmacology Society in May 2007 reviewed progress in the human behavioral pharmacology of drug abuse. Studies on drug self-administration in humans are reviewed that assessed reinforcing and subjective effects of drugs of abuse. The close parallels observed between studies in humans and laboratory animals using similar behavioral techniques have broadened our understanding of the complex nature of the pharmacological and behavioral factors controlling drug self-administration. The symposium also addressed the role that individual differences, such as gender, personality, and genotype play in determining the extent of self-administration of illicit drugs in human populations. Knowledge of how these factors influence human drug self-administration has helped validate similar differences observed in laboratory animals. In recognition that drug self-administration is but one of many choices available in the lives of humans, the symposium addressed the ways in which choice behavior can be studied in humans. These choice studies in human drug abusers have opened up new and exciting avenues of research in laboratory animals. Finally, the symposium reviewed behavioral pharmacology studies conducted in drug abuse treatment settings and the therapeutic benefits that have emerged from these studies.
Introduction, by Galen Wenger
This meeting marks the 50th anniversary of the Behavioral Pharmacology Society. As a group, the society has worked to understand the effects of drugs on behavior while simultaneously trying to understand the control of behavior. To many outside our field, these two simultaneous efforts presented an impossible challenge. Yet in practice, the two approaches have been at a minimum mutually beneficial and in some cases even symbiotic. Thus, the study of the behavioral effects of drugs has provided key insight into the control of behavior and vice versa.
Although the majority of the members of the Society have focused their research efforts on laboratory animals, the Society has also maintained a strong tradition of human behavioral pharmacology research. In large part, this research has followed the wisdom of B.F. Skinner when he said, “One moves from the experimental analysis of behavior at the lower level to the human level, not by pointing out plausible analogies, but by constructing an experimental situation in which the same kinds of variables are manipulated and the same changes in behavior measured” (Skinner, 1959). Thus, just as behavioral pharmacology set out to simultaneously study drug effects on behavior and the control of behavior, behavioral pharmacology has been interested in studying the parallels between human behavior and animal behavior for nearly 50 years.
The goal of this symposium titled, “Human Behavioral Pharmacology: Past, Present, and Future” is to highlight how research on drug abuse in human behavioral pharmacology has advanced during the last 50 years by utilizing many of the same behavioral approaches in humans as are used in laboratory animal studies. The Symposium begins with Dr. Sandra Comer discussing the evolution of human drug self-administration experiments. Then Dr. Harriet de Wit discusses individual differences in human drug abuse patterns. Dr. Warren Bickel presents some data on issues of choice in human behavioral pharmacology, and, finally, Dr. Stephen Higgins discusses the overall impact of the application of behavioral pharmacology principles to the treatment of drug dependence.
It is hoped that this symposium will provide stimulation for new work in both human and laboratory animal behavioral pharmacology. If the goals of this symposium are achieved, the tradition of behavioral pharmacology of working to provide a better understanding of the interaction between behavior and drug effects in both humans and laboratory animals will continue.
Evolution of Human Drug Self-administration Procedures, by Sandra Comer
The drug self-administration paradigm has been used for decades to help us understand the variables that may affect drug-taking behavior in the “real world.” In doing so, the hope is that this information can be used to develop strategies for reducing drug abuse and its devastating consequences. The purpose of this section of our paper is to briefly describe the historical development of the drug self-administration paradigm in humans, the uses of this procedure to illuminate the factors that may be involved in the abuse of drugs, and contemporary research that combines self-administration procedures with new technologies for elucidating the underlying factors that contribute to substance abuse.
History
A laboratory model of drug self-administration was first described in 1940 (Spragg, 1940). Chimpanzees initially were made physically dependent on morphine and then were trained to choose between two keys to receive either morphine injections or food. When deprived of morphine, chimpanzees reliably chose the drug option and when deprived of food, the animals chose the food option. For unknown reasons, a lapse occurred in the study of drug taking behavior for the next two decades. When reliable methods for studying intravenous drug self-administration were developed in rats and monkeys during the early 1960’s, however, research on the reinforcing effects of common drugs of abuse began to flourish (e.g., Weeks, 1962; Thompson and Schuster, 1964; Pickens and Harris, 1968; Deneau, et al., 1969; Goldberg, et al., 1969).
Although a case report of “re-addiction to morphine” was described as early as 1952 (Wikler, 1952), the systematic study of drug self-administration was not initiated in human research volunteers until soon after the development of intravenous drug self-administration procedures in laboratory animals. One of the first studies was that performed by Mello and Mendelson (1965) in alcoholics, where operant schedules were used to study patterns of alcohol drinking. These procedures were subsequently adapted to the study of other drugs, such as marijuana, opioids, sedatives, and stimulants (Altman, et al., 1976; Griffiths, et al., 1976; Mendelson, et al., 1976; Mello and Mendelson, 1980; Fischman and Schuster, 1982). Not surprisingly, many of these investigators initially studied the reinforcing effects of drugs using laboratory animals and so were able to adapt the rigorous procedures developed in the preclinical laboratory to the clinical laboratory. Subsequent review papers described the good concordance between drugs that are self-administered by laboratory animals and those that are abused by humans (e.g., Griffiths et al., 1980a). Several different types of self-administration procedures have been developed, as described below, to balance the need for reliable, valid data and practical considerations associated with research conducted in human volunteers.
Self-administration Procedures Used in Humans
In general, a drug is considered to be a positive reinforcer “if its presentation increases the likelihood of responses that produce it” (Catania, 1991). For example, due to societal constraints and/or fear of legal consequences, use of illegal drugs such as cocaine and heroin initially may be low. But over time, as the positive effects of the drug predominate, the behaviors leading to drug consumption may increase and the drug eventually is said to serve as a reinforcer. In some cases, such as when access to drug is unlimited, self-administration increases to the point of death (Bozarth and Wise, 1985; Johanson, et al., 1976). Because of limitations due to drug availability and/or cost, however, drug self-administration behavior often stabilizes at a particular level, and in order to verify that the drug is serving as a reinforcer, placebo or inactive drug is substituted for the active drug and responding is measured. If responding is greater when active drug is available compared to when placebo is available, then the drug is considered to be a reinforcer. The different types of procedures that have been used to measure drug taking in humans are summarized briefly below (for reviews, see Bigelow, et al., 1976; Foltin and Fischman, 1991; Henningfield, et al., 1991; Comer, et al., 2008).
Choice Procedures
Among the variety of self-administration procedures that have been used to examine the reinforcing effects of drugs, perhaps the most basic is a free access procedure in which participants simply ask for a dose of drug. For example, in studies examining the reinforcing effects of caffeine, participants resided on a hospital unit and were instructed to ask for a cup of coffee when they desired it (e.g., Griffiths, et al., 1986). Another simple type of drug self-administration paradigm is a single-choice procedure in which participants are given a sample dose of drug and then asked whether or not they would like to take the dose again. On some days, participants are given active drug and asked whether they want to take it again, and on other days, participants are given placebo and then asked whether they want to take it again. In this type of study, the behavior is a verbal response (“yes” or “no”). If participants choose the active dose on more occasions than they choose placebo, then the active dose is considered to be reinforcing. One disadvantage of the single-choice procedure, however, is that a high placebo response rate is often seen (Roehrs, et al., 1997).
A discrete-trial choice procedure is another common approach used in human self-administration studies (e.g., de Wit and Chutuape, 1993; Griffiths, et al., 1980b; Johanson and Uhlenhuth, 1980a,b; Stern, et al., 1989). During this procedure, participants receive Drug A and then, after the effects of Drug A have dissipated, they receive Drug B, where Drug A may be placebo and Drug B may be a dose of active drug. Participants are instructed to pay attention to the effects produced by Drugs A and B, and different stimuli, such as the color of the pill, are used to establish the association between the sample dose and the effects produced by that dose. After participants have sampled the available drugs, they are asked to state verbally whether they would like to ingest Drug A or Drug B. Typically, a minimum of 5 choice opportunities are provided and the number or percentage of choices of Drug A and Drug B is calculated. The drug is a reinforcer if the active dose is chosen on significantly more occasions than placebo. Another type of discrete-trial choice procedure is one in which the participant chooses between drug and money (e.g., Mello, et al., 1981; Stitzer, et al., 1983). Because participants are familiar with the value of money as an alternative reinforcer, fewer sessions generally are required in a drug versus money procedure. In addition, participants can choose between a drug and a non-drug reinforcer, which is similar to the choices that drug abusers make in the real world, thereby providing face validity to the procedure. However, a potential disadvantage of the procedure is that the value of the monetary alternative can vary across participants and some only choose the money option.
Operant Procedures
The second major type of self-administration procedure involves the use of non-verbal operant responses. These procedures most closely parallel preclinical self-administration research. Specifically, participants make responses on some form of manipulandum, such as a computer mouse, joystick, or bicycle, in order to receive drug. As in the preclinical studies, participants can respond under a wide variety of operant schedules, one of the most basic being the fixed-ratio (FR) schedule, in which participants are instructed to make a fixed number of responses in order to obtain drug. For example, drug is delivered after the participant makes 200 responses on the manipulandum. As with the laboratory animal procedures, a “timeout” period during which drug is unavailable often follows each drug delivery and a maximum number of drug deliveries that can be self administered generally is imposed for safety reasons. The rate of responding for drug, number of drug deliveries, and amount of drug received are the primary dependent variables. Other variables, such as the latency to the first response and the interdose interval are also commonly measured, particularly in research on cigarette smoking.
In addition to fixed ratio schedules, progressive ratio schedules are used frequently to examine the reinforcing effects of drugs in humans. Originally developed in rats that were trained to self-administer sweetened condensed milk (Hodos, 1961), this procedure requires progressively greater numbers of responses to be made for the same amount of drug (for detailed reviews of PR schedules, see Arnold and Roberts, 1997; Stafford, et al., 1998; Rowlett, 2000). That is, the participant is required to expend more and more effort for each drug delivery and the point at which responding stops is termed the “breakpoint value.” This procedure has been useful in examinations of the relative reinforcing effectiveness of different drugs and doses because drugs that maintain higher breakpoint values are considered to have greater abuse liability (Katz, 1990; Stafford, et al., 1998).
Behavioral Economic Analysis
Although other operant schedules have been used in humans to evaluate the reinforcing effects of drugs, the most common are the FR and PR schedules. In recent years, different ways of analyzing the data from FR and PR procedures have been developed. In particular, some investigators have used a behavioral economic approach, which takes into account the cost of the drug, income, and the presence of competing reinforcers, among other variables (e.g., Bickel, et al., 2000; Giordano, et al., 2001). In behavioral economic analyses, the unit price, or amount of effort required to obtain a given amount of the reinforcer, is plotted against the amount of drug actually consumed. A demand curve is generated from these data and several dependent variables can be derived including elasticity of demand, intensity of demand, and price at which the greatest amount of responding occurs (also termed Pmax). While several of the behavioral economic variables, such as Pmax, are analogous to traditional self-administration variables, such as progressive ratio breakpoints, the primary advantage of a behavioral economic approach is that it may provide a more precise and comprehensive description of the reinforcing effects of drugs. It is beyond the scope of the present paper to describe the nuances of behavioral economic analyses and how they compare to the more traditional measures of reinforcing effects (see p.52–54 of Bickel, et al., 2000).
Multiple-Choice Procedure
The above procedures are the most commonly used methods for examining the reinforcing effects of drugs, but the studies are often long in duration and therefore costly to conduct. In order to provide a more rapid and efficient method of estimating the reinforcing effects of a drug, a multiple-choice procedure (MCP) was developed (Griffiths, et al., 1993). As with the other procedures, the experimenter first administers the test drug to the participant, who subsequently is asked to make a series of choices on a questionnaire between either two doses of drug (Drug A versus Drug B, Drug A versus Drug C, Drug B versus Drug C, etc.) or between drug and money (Drug A versus $0.50, Drug A versus $0.75, Drug A versus $1, etc.). After the questionnaire is completed, all of the hypothetical choices are combined and one random choice is selected and given to the participant. Several investigators have now demonstrated the validity of this procedure. Drug is chosen over money, higher doses are chosen over lower doses, and active drug is chosen over placebo (e.g., Griffiths, et al., 1993; Lile, et al., 2004; Tancer and Johanson, 2003, 2007). The efficiency and cost-effectiveness of the multiple-choice procedure are some of its major advantages, but the procedure does have some disadvantages. Specifically, only one choice among many is reinforced and there is often a large delay between the time that the choices are made and the time that the reinforcer is actually delivered. Nevertheless, this can be a useful procedure for estimating the reinforcing effects of drugs.
Current Uses of the Drug Self-administration Paradigm in Humans
Medications Development for Substance Abuse
One of the most important and earliest uses of human drug self-administration procedures was for the testing of medications that may have utility as treatments for drug dependence. For example, the utility of opioid antagonists, such as naloxone and naltrexone, for treating opioid dependence was evaluated in heroin abusers who were given opportunities to self-administer heroin in controlled, inpatient laboratory settings (Altman, et al., 1976; Mello, et al., 1981). These studies showed that naltrexone was effective in virtually eliminating the reinforcing effects of heroin. Unfortunately, however, the early clinical experience with oral naltrexone was somewhat disappointing because of high dropout rates during treatment and poor compliance with medication ingestion (Callahan, et al., 1980; Kosten and Kleber, 1984; Azatian, et al., 1994). Newer, injectable sustained-release formulations of naltrexone have shown more promise for the treatment of opioid dependence and better concordance between laboratory studies of heroin self-administration and clinical outcome (Comer, et al., 2002, 2006; Sullivan, et al., 2006). Other medications that have shown good concordance between laboratory models of opioid self-administration and clinical outcome are buprenorphine and methadone (Mello and Mendelson, 1980; Johnson, et al., 1992; Ling, et al., 1996; Greenwald, et al., 1999; Comer, et al., 2001, 2005; Donny, et al., 2005). For medications development for opioid dependence, good external validity exists for the laboratory models of self-administration. Similarly, maintenance on the transdermal nicotine patch has been shown to selectively reduce the reinforcing effects of intravenous nicotine, but not cocaine or caffeine, in cigarette smokers who abused stimulants (Sobel, et al., 2004). These data also suggest a good concordance between clinical laboratory and clinical trial outcomes. The findings are complex, however, with studies showing gender differences and environmental influences on the reinforcing effects of nicotine (e.g., Perkins, et al., 1999, 2001a,b; Shahan, et al., 1999). For cocaine dependence, there are currently no effective treatments, so it is not possible to make definitive statements about the external validity of cocaine self-administration procedures, other than to note that virtually all of the medications that have been ineffective in the laboratory are also ineffective in the clinic (e.g., Fischman, et al., 1990; McDowell, et al., 2005; see Comer, et al., 2008 for a more detailed review of this literature).
Basic Research Questions
Drug self-administration is known to be malleable in that a variety of factors can influence the likelihood that a drug will be self-administered. Several interesting studies have been conducted to characterize these variables. For example, de Wit and Chutuape (1993) demonstrated that pretreatment with ethanol produced dose-related increases in the proportion of participants who subsequently chose to self-administer ethanol. These data provide some explanation for the common practice of “happy hour” drinks, in that initial ingestion of ethanol increases the likelihood of subsequent drinking. Another variable that appears to influence drug self-administration is the behavioral requirement following drug ingestion. That is, self-administration of sedative drugs decreases and of stimulant drugs increases when participants are required to perform tasks requiring vigilance, whereas the opposite occurs when participants engage in quiet, relaxing activities after drug self-administration (Silverman, et al., 1994; Stoops, et al., 2005). Yet another variable that appears to influence the propensity to self-administer drugs is the presence or absence of pain. Colpaert and colleagues (1982, 2001), for example, showed that oral fentanyl self-administration was significantly greater in “arthritic” rats that were inoculated with Mycobacterium butyricum, compared to non-arthritic control rats. Similar results were obtained when arthritic rats were given the opportunity to self-administer oral suprofen, a non-steroidal, anti-inflammatory drug (Colpaert, et al., 1980). For both fentanyl and suprofen, the time course of drug self-administration (over a period of weeks) corresponded well with the expected time course of pain produced by the inoculation. Zacny and colleagues (1996) conducted the only study of which we are aware examining the reinforcing effects of an opioid (fentanyl) in normal, healthy non-drug abusers in the presence and absence of pain. After “sampling” the effects of intravenously administered fentanyl and saline, participants were given the opportunity to choose between fentanyl and saline when their forearms were immersed in water maintained at 37°C, 10°C, or 2°C. Fentanyl choice did not differ from chance in the no pain condition (37°C), but it was chosen significantly more than chance under the pain conditions (10°C and 2°C). Taken together, these data demonstrate that context is a critical variable in drug self-administration.
In addition to studies designed to examine the influence of external variables that may influence drug self-administration, other studies have used the self-administration paradigm to try to understand the underlying physiological changes that may have occurred as a result of drug use. For example, Martinez and colleagues (2007) showed that amphetamine-induced dopamine release was substantially blunted in cocaine-dependent individuals compared to normal, healthy controls. This blunted dopamine transmission in the ventral striatum and anterior caudate was correlated with cocaine self-administration. That is, cocaine-dependent individuals who showed the lowest dopamine transmission were the ones who were most likely to self-administer cocaine. Another study used the self-administration paradigm in an attempt to quantify the apparent in vivo dissociation constant (KA) and the efficacy estimate (tau) for heroin, as well as the estimated fraction of receptors remaining (q) after treatment with buprenorphine/naloxone combination tablets (Comer, et al., 2005). These studies represent exciting new developments in the use of self-administration paradigms.
Conclusions and Future Directions
The use of drug self-administration paradigms in both laboratory animals and human research volunteers has been extremely valuable in our understanding of substance abuse. The development of new techniques and the current emphasis on translational research will only serve to further increase our understanding, and hopefully effective treatment of this complex phenomenon. Of particular interest is the use of imaging techniques to clarify the neuroanatomical processes underlying substance abuse by human research volunteers, as well as the use of pharmacological techniques that have been used in preclinical settings to examine potential changes in receptors. For both of these lines of research, the study of drug self-administration can play a critical role.
Individual differences in the reinforcing effects of drugs, by Harriet de Wit
Operant procedures assessing the positive reinforcing effects of drugs, or drug self-administration studies, represent perhaps the most influential behavioral methodology that has been developed to study drug abuse. Drug self-administration studies have greatly advanced our understanding of the patterns and environmental determinants of drug use in both non-humans and humans. Although studies conducted in the historical traditions of behavioral analysis examine behavior of individual organisms and the common effects of environmental manipulations on individual organisms, the focus of these studies has not been on preexisting individual differences. For example, most of the investigations of the positive reinforcing effects of drugs, or direct effects of drugs on behavior, implicitly assumed that all organisms respond similarly to environmental challenges. This approach has provided a rigorous assessment of the environmental influences on drug taking, but it has paid relatively less attention to the important contributions of pre-existing variations among organisms. Now, there is a growing literature on the importance of individual differences in responses to drugs, and on interactions between drugs and environmental factors. Biologically-based individual differences are now thought to play an important role in the susceptibility to use and abuse drugs, and a wide range of organismic variables are known to affect responses to drugs and drug-seeking behavior.
Although many people experiment with drugs, only a small proportion progress to abuse or dependence. For example, in 2005, 63% of women reported having used alcohol in their lifetime, but only 2.3% met criteria for Alcohol Abuse and 1.9% met criteria for Alcohol Dependence (SAMHSA, 2006). The reasons why some individuals progress to problem use are not understood, but individual differences in susceptibility likely occur at every stage in the drug use trajectory, from initiation to dependence, and they are likely to be related to both environmental and constitutional (i.e., inherent or biologically based) factors. Initiation of use is influenced by environmental factors such as social and cultural factors, as well as biologically influenced factors such as personality. Continuation of use is determined by context and consequences, as well as by genetically determined aspects of an individual’s responses to a drug. Similarly, there are likely to be biologically based individual differences in the development of tolerance, severity of withdrawal, and ability to abstain from use. There is now a growing body of literature identifying sources of biological variation that can affect the tendency to use drugs, at each of these stages in the trajectory to drug dependence.
The existing literature on biologically-based individual differences in risk for abuse can be categorized into three arbitrarily selected categories: Variables related to sex, personality (and its equivalent in nonhumans), and genotype. These categories are interconnected at every level, but they provide a convenient framework to discuss current research. For example, the prevalence of drug abuse disorders is higher in men, and there is some evidence for sex differences in drug taking in nonhumans. Certain personality variables predict the likelihood of initiation or escalation of drug use in adolescents and young adults, and comparable behavioral profiles have been proposed in laboratory animals. In addition, genetic association studies have shown that individuals with specific genotypes experience qualitatively and quantitatively different subjective or behavioral responses to drugs, which in turn may influence susceptibility to abuse drugs.
Animal models of drug taking are essential to confirm that individual differences in drug responses have a biological basis. However, such confirmations depend on the validity of the procedures to model human drug taking. Although there has been excellent concordance between human and nonhuman studies of drug taking (Johanson and Schuster, 1981), and many of the same environmental factors influence drug-taking behavior in humans and nonhumans, differences exist between human drug use and the behaviors modeled in animals. For example, human drug taking usually occurs in a social context whereas nonhumans are tested individually; humans take drugs in the context of multiple competing reinforcers whereas animals typically have access only to the drug; human drug use is usually suppressed by negative consequences whereas animals have no disincentives to take drugs; and, humans have expectancies about drug effects and their consequences before using the drugs, whereas animals learn through their own experience. These and other differences between the animal models and human drug use must be taken into account when exploring individual differences in susceptibility to drug abuse and dependence.
Another important difference between human and nonhuman studies is the type of outcome measures used. Studies with nonhumans consistently use behavioral measures of preference, self-administration or consumption as indicators of the reinforcing effects of drugs. These measures are also used in many studies with humans but in addition, many studies with humans use subjective self-report measures instead of a behavioral measure of reinforcing effects. Most drugs that are abused and that serve as positive reinforcers in humans also produce feelings of well-being or euphoria in humans (Fischman and Foltin, 1991; Jasinski, 1991), and there is a good correspondence between drugs that are reinforcing in humans and nonhumans. These correlations provide some justification for using subjective ratings as a “proxy” for reinforcing effects, when it is impractical to measure reinforcing effects. However, it should be recognized that different factors may influence reinforcing and subjective effects, compromising the apparent associations between acute subjective and reinforcing effects, and their relation to the development of abuse and dependence. Since much of our knowledge about individual differences in drug responses in humans is based on variations in their subjective effects (de Wit, et al., 1986; 1987; Holdstock and de Wit, 1999; Veenstra-Vander Weele, et al., 2006), this presents a challenge for conducting cross-species comparisons in individual differences.
Sex
Until the last decade, most drug self-administration studies with laboratory animals and most human abuse liability studies utilized exclusively male subjects. It was thought that the estrous or menstrual cycle would introduce too much variability, and in humans there were concerns about safety in using women of childbearing potential. Early in the 1990's, the field came to recognize the lack of systematic data regarding sex differences and responses to drugs in females as an important gap in our knowledge. Now, there is a rich and still developing literature on the role of sex and ovarian hormones on responses to drugs in both humans and nonhumans.
Epidemiological data show that men are more likely to use and abuse drugs. Males report higher lifetime use of alcohol, pain relievers, cocaine, hallucinogens, heroin, inhalants, marijuana and tranquilizers than women (SAMHSA, 2006). Men are also 27% more likely to meet criteria for Alcohol Dependence, and 32% more likely to become Heroin Dependent. Socio-cultural factors probably play an important role in the higher prevalence of drug use and abuse among men, but there may also be a biological basis. Studies of sex differences in animal self-administration studies have been inconsistent, and sometimes conflict with the human clinical observations (Lynch, et al., 2002; Lynch, 2007; Kantak, et al., 2007). For example, Lynch, et al. (2002) found that female rats were more likely to initiate and maintain stimulant self-administration than males, although Kantak, et al. (2007) report that females consumed less cocaine than males. Several studies have found that higher circulating levels of estrogen increase locomotor effects of stimulants and facilitate cocaine self-administration (Becker, et al., 1982; 1989; Lynch, et al., 2002), whereas others (Kantak, et al., 2007) report that females self-administer less cocaine during estrous. It is possible that the presence of estrogen makes responses more “labile” (Kantak, et al. 2007). In humans, controlled studies of sex differences in drug effects have also been inconsistent (Lynch, et al., 2002; Han and Evans, 2005). Although many studies have found no sex differences in either subjective effects or intake of drugs, some studies found pharmacokinetic differences in men and women that may influence subjective or reinforcing effects, and other studies find qualitative differences in drug effects in the absence of pharmacokinetic differences (Lukas, et al., 1996; Mumenthaler, et al., 1999). There are also differences in the relative sensitivity to other aspects of drug use: Women appear to be more sensitive to the sensory aspects of smoking, whereas men are more sensitive to the pharmacological effects of nicotine (Perkins, et al., 1999; 2001b), and women appear to experience more severe nicotine withdrawal (Leventhal, et al., 2007). The effects of some drugs also depend on the phase of the cycle in women (Terner and de Wit, 2006). Although menstrual cycle phase does not appear to affect acute responses to alcohol, benzodiazepines and opiates, it does influence responses to stimulant drugs: The effects of amphetamine and cocaine are greater during the follicular, compared to the luteal phase (Justice and de Wit, 1999; 2000; Evans, et al., 2002; White, et al., 2002). Consequently, sex differences in drug effects depend on the phase used to assess drug effects in women. These studies indicate that there are likely to be some biologically based sex differences in the propensity to self-administer drugs, experience withdrawal, or relapse, but the exact nature of these remain to be determined.
Personality
Personality has long been linked to the propensity to use drugs. Although there is little evidence for a single “addictive personality,” there is growing evidence that certain personality characteristics increase risk for drug use and abuse (Cloninger, 1987; Sher, et al., 1999; de Wit, 2005). Personality variables may influence behavior at the early phases of experimentation and initiation of drug use. Such variables may be related to subjective responses to acute drug administration, and to the ability to refrain from using drugs (Sher, et al., 1999; White, et al., 2002; Sher and Wood, 2005). For example, adolescents who are high on the trait of disinhibition/impulsivity are more likely to initiate drug use at an earlier age (Tarter, et al., 1999; Elkins, et al., 2006). White, et al. (2002) found that an oral dose of d-amphetamine produced less stimulant-like subjective effects in subjects who scored high on a measure of Harm Avoidance but greater effects in subjects who were high on Social Potency, a measure of reward sensitivity. Euphorigenic effects of amphetamine have also been linked with sensation-seeking (Kelly, et al., 2006; Stoops, et al., 2007). Considering that personality dimensions are believed to have a strong biological and genetic basis (Cloninger, 1987; Depue and Collins, 1999; Reif and Lesch, 2003), these relationships between personality and drug responses provide a key to studying neurobiological variations in predisposition to drug use.
Genes
Genetic factors have long been known to influence susceptibility to drug and alcohol use (Goldman, et al., 2005). Recently, significant advances in the techniques available for human genetic studies now allow researchers to conduct detailed analyses of the role of genotypic variation to drug responses. One approach that has particularly great potential is the study of individuals’ responses to acute administration of psychoactive drugs in relation to variations in the genes involved in their actions in the brain (e.g., Alsene, et al., 2003; Mattay, et al., 2003; Lott, et al., 2005). Because the positive reinforcing effects of drugs are highly correlated with the pleasurable subjective states they produce, and because subjective effects are easier to measure, most of the studies to date have examined genetic factors in relation to subjective feelings of euphoria and drug liking. These studies have reported interesting individual variations in acute subjective responses related to genetic variations for several drugs, including caffeine and d-amphetamine. Genetically based variations have been found not only in the positive pleasurable effects such as “euphoria,” but also in negative subjective effects such as “anxiety” and in the degree of psychomotor impairment after administration of the drug. This is a rich new area of research that promises to advance our understanding of individual differences in responses to acute and chronic drug administration, and elucidate some of the sources of variability in susceptibility to abuse drugs.
Conclusions
Research on the positive reinforcing and subjective effects of drugs in humans has taken a new direction in recent years, as researchers begin to explore sources of individual variability in the drugs’ effects. Individual differences may affect drug use at every level of drug use from initiation to dependence. Individuals are likely to vary in their reactions to environmental factors, their reactions to drugs, and probably most commonly, the interactions between environment and drug. Recent advances in genetic research have opened up a new world of opportunities to study the role of inherited sources of variation and risk for drug abuse. The following section of this paper will examine yet another factor that affects the propensity of individuals to abuse drugs, namely, decision making or “intertemporal choice,” which incorporates notions of self-control, impulsivity, delayed gratification, or delay discounting.
Inter-temporal Choice in Human Behavioral Pharmacology: Current Status and Future Opportunities, by Warren K. Bickel & Richard Yi
Inter-temporal choice refers to selection between one of two or more options available at different times. This type of choice has been referred to with a broad array of names such as impulsivity, delay discounting, self-control, and delay of gratification (note that some of these terms have been used in some cases for a variety of other types of behaviors and procedures). Examples of inter-temporal choice decisions include engaging in risky activities such as drug use (with the risk of negative life events) vs. abstaining from drug use (with greater future opportunity for a pro-social life). Similar inter-temporal choices are evident in the issues of obesity, sexually transmitted disease, the lack of personal savings in the US, and perhaps even global warming. Indeed, the ubiquity of this type of choice makes it difficult to imagine an important choice that does not entail a choice between an immediate and a later option.
Given that the importance and pervasiveness of this type of choice, it is not surprising that inter-temporal choice is receiving increasing attention by investigators. For example, Figure 1 (upper panel) displays the cumulative number of papers by year obtained by searching on delay of gratification, inter-temporal choice, and delay discounting. Overall, the clear and compelling trend is that research in this area was steady or slightly growing across the first 35 years, with a substantial increase during the last decade. Indeed, 77% of the papers on this topic have been published in that ten-year period. The topics addressed in this research focus on a variety of interests (Figure 1, lower panel). The top three topics are using inter-temporal choices to understand aspects and/or the phenotype of addiction, followed by understanding the phenotypes associated with others forms of psychopathology, with neuroscience as the third area of interest. These and the remaining topics primarily characterize contemporary considerations in this research area.
Figure 1.
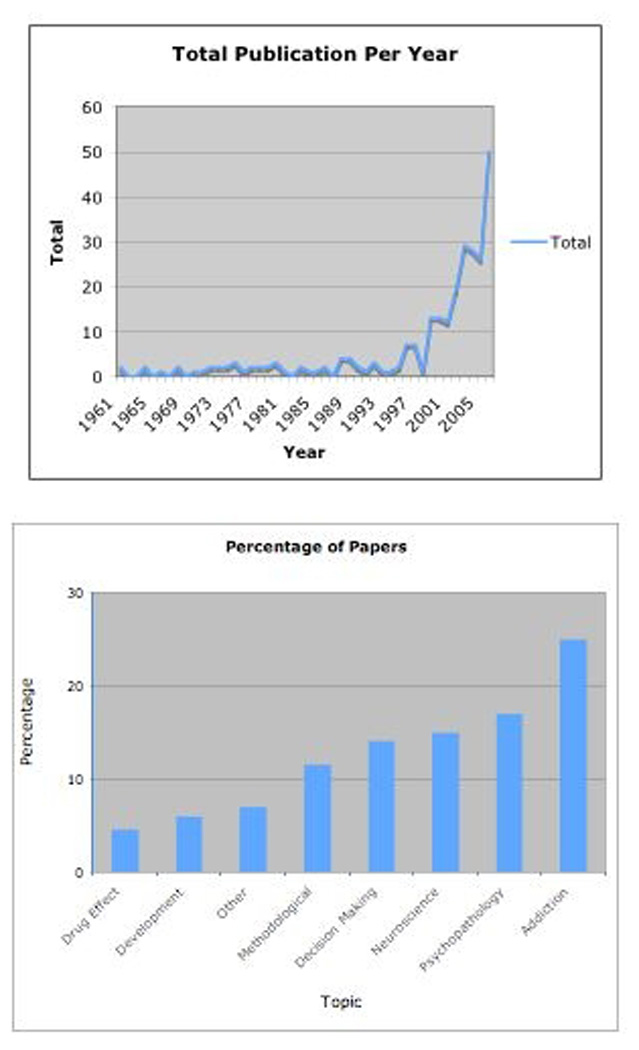
Figure 1, upper panel: Cumulative number of papers by year obtained by searching on delay of gratification, inter-temporal choice, and delay discounting. Figure 1, lower panel: Papers identified in the upper panel of Figure 1 grouped according to topic.
The purpose of this section of the paper is to review the current status and future applications of the study of inter-temporal choice within behavioral pharmacology. When we refer to behavioral pharmacology, we will use the recent definition offered by Robbins and Murphy (2006) who defined it as an “interdisciplinary field at the intersection of several research areas that ultimately leads to the development of drugs for clinical use and build understanding of how brain functions enable cognition and behavior” (p. 141). In doing so, we will first briefly review the antecedents to behavioral pharmacology’s use of inter-temporal choice, and examine its current practices. Lastly, we will speculate regarding the future of choice studies as they apply to addiction.
Inter-temporal choice: Early studies
The current status of inter-temporal choice research can be traced back at least to the first behavioral observation that distribution of choice behavior matches distribution of reinforcement. In a study of key pecking by pigeons in a concurrent variable-interval schedule of reinforcement, Herrnstein (1961) observed that the relative frequency of response on a particular key was a linear function of the relative frequency of reinforcement from that key: the Matching Law. One significant implication of this Matching Law was that differential reinforcement obtained from two concurrently available alternatives could predict the allocation of effort to each of the two sources of reinforcement. This simple observation allowed for the quantification of behavior in a realistic laboratory analog of real choice situations (Green and Hale, 1984).
The Matching Law was quickly expanded to include the match between the relative magnitude of reinforcement and relative response rate (Catania, 1963), as well as the relative immediacy of reinforcement (inverse of delay to reinforcement) and relative response rate. Applied to delayed reinforcement, the Matching Law (Chung and Herrnstein, 1967) correctly predicted that pigeons’ relative frequency of response on a particular key matched the relative immediacy of the reinforcement from that key within a concurrent choice situation, and that the function describing this relationship would become less steep per additional unit of delay.
Ainslie (1974) thereafter proposed choice of immediate rather than delayed reinforcers in concurrent situations as an issue of impulse control, as well as suggesting some initial ideas on pre-commitment as a source of impulse control. Ainslie (1975) also proposed the discounting function that would account for preference reversals (also known as dynamic inconsistency): an upward, highly concave function where discounting decreases as a function of time. This progression of ideas resulted in Mazur’s (1987) hyperbolic model of discounting, contributing to the extensive literature on temporal discounting as well research incorporating discounting constructs to examine psychological function and dysfunction.
Though early studies of temporal discounting examined pigeon and rat choice behavior, human studies of temporal discounting soon followed. Using a variation of Mazur’s (1987) adjusting procedure, Rachlin, et al. (1991) determined indifference points (the present subjective values) of hypothetical money delayed by numerous time periods (1 month – 50 years). In this procedure, participants were asked to choose between a delayed $1000 amount (delayed by 1 month, for instance) and a smaller, immediate amount. The immediate amount was systematically adjusted until preference switched from one alternative to the other (with interpolation used to determine the indifference point). Using this procedure at multiple delays allowed Rachlin to determine the indifference point of $1000 delayed by 1 month, 6 months, 1 year, etc. As expected, the indifference point for a delayed $1000 decreased as a function of the delay to it receipt. Since this study, variations of this procedure have been used to examine temporal discounting in human inter-temporal choice.
Much of the current research on human temporal discounting uses indifference points obtained from numerous delays (determined as in Rachlin, et al., 1991) to determine a single index of the rate of discounting. The most popular of these indices appears to be calculation of discounting rate (k) according to Mazur’s (1987) hyperbolic model of discounting. This index is determined by fitting indifference points to Mazur’s hyperbolic equation via nonlinear regression. The resulting parameter (k) describes the shape of the best-fitting hyperbolic function, and an estimate of the discounting rate; high values indicate greater discounting and low values indicate less discounting. Another summary measure of obtained indifference points is area-under-the-curve (AUC), an index of discounting that does not assume a particular model of discounting (Myerson et al., 2001). AUC is determined by simply calculating the area of the polygon that is created by connecting consecutive indifference points in a delay x subjective value plot. AUC is frequently used as a complement to, or in place of, the discounting rate (k).
Contemporary Status of Intertemporal Research: Characterizing Phenotypes
As indicated earlier, inter-temporal choice has been extensively examined in the context of characterizing phenotypes. In the mid-late 1990’s, temporal discounting by various groups began to be investigated with greater frequency. One important area of research was to compare addicted groups to controls in order characterize difference in inter-temporal choice. In one of the first studies of this type (Madden, et al., 1997), opioid-dependent participants were compared to control participants on temporal discounting for hypothetical $1000. Using similar procedures, opioid-dependent participants also chose between immediate and delayed heroin. The amount of delayed heroin was derived by estimating the local street value of heroin, and then determining how much heroin could be purchased with $1000. Across the opiate-dependent and control participants, the hyperbolic discounting equation accounted for 80% to 99% of the variance. Opioid-dependent participants discounted money at higher rates than controls, and discounted heroin more than money.
Another study compared discounting of heroin-dependent individuals who did and did not share injecting equipment (Odum, et al., 2000). Patients who did not share needles replicated the general observation that heroin-dependent individuals discount heroin significantly more than money. In contrast, heroin-dependent individuals who shared needles (thus exposing themselves to additional risks such as HIV transmission) discounted heroin and money more than heroin-dependent individuals who did not share needles, with no difference between discounting of money and heroin. Since these initial studies, the area has grown rapidly demonstrating that numerous addicted subtypes discount more than control (See Table 1 for a list of studies comparing drug-dependent subgroups to controls). The overwhelming preponderance of evidence suggests that addicted individuals discount the future more than controls. Abuse of a substantial variety of drugs of abuse, legal and illicit, stimulants and depressants, is associated with higher rates of temporal discounting, as is pathological gambling (see reviews in Bickel and Marsch, 2001; Reynolds, 2006). Overeating and obesity are increasingly considered within the same framework as drug dependence, and recent evidence appears to indicate this is appropriate as it relates to temporal discounting (Borghans and Golsteyn, 2006).
Table 1.
Studies that nominally compare temporal discounting by a drug-dependent group to a non-dependent group.
| STUDY | ADDICTION CRITERIA | RESULT |
|---|---|---|
| CIGARETTES | ||
| Bickel et al. (1999) | ≥ 20 cigarettes daily | Greater discounting by cigarette smokers than ex-smokers and controls |
| Mitchell (1999) | ≥ 15 cigarettes daily | Greater discounting by cigarette smokers than controls |
| Baker et al. (2003) | ≥ 20 cigarettes daily, DSM-IV for cigarettes | Greater discounting by cigarette smokers than controls |
| Reynold et al. (2004) | ≥ 20 cigarettes daily | Greater discounting by cigarette smokers than controls |
| Ohmura et al. (2005) | various | Correlation between rates of smoking and discounting |
| Heyman & Gibb (2006) | ≥ 40 cigarettes weekly | Greater discounting by regular smokers than chippers or non-smokers |
| OPIOIDS | ||
| Madden et al. (1997) | History of abuse and receiving opioid treatment |
Greater discounting by opioid dependents than controls |
| Kirby et al. (1999) | History of abuse and receiving opioid treatment |
Greater discounting by opioid dependents than controls |
| Kirby & Petry (2004) | Regular and substantial pattern of use | Greater discounting by opioid abusers than controls |
| COCAINE | ||
| Coffey et al. (2003) | DSM-IV for cocaine | Greater discounting by cocaine dependents than controls |
| Kirby & Petry (2004) | Regular and substantial pattern of use | Greater discounting by cocaine abusers than controls |
| Heil et al. (2006) | History of abuse and receiving cocaine treatment |
Greater discounting by cocaine dependents than controls |
| METHAMPHETAMINE | ||
| Hoffman et al. (2006) | DSM-IV for MA | Greater discounting by abstinent MA dependents than controls |
| Monterosso et al. (2007) | DSM-IV for MA | Greater discounting by methamphetamine users than controls |
| ALCOHOL | ||
| Vuchinich & Simpson (1998) | Khavari Alcohol Test | Greater discounting by problem / heavy drinkers than light / non-drinkers |
| Petry (2001a) | Structured Clinical Interview for DSM | Greater discounting by alcoholics than controls |
| Bjork et al. (2004) | DSM-IV for alcohol dependence | Greater discounting by abstinent alcohol dependents than controls |
| Kirby & Petry (2004) | Regular and substantial pattern of use | No difference between alcoholics and controls |
| Mitchell et al. (2005) | Self-report | Greater discounting by alcoholics than controls |
| Dom et al. (2006) | Inpatient treatment for alcohol abuse | Greater discounting by EOA than LOA to controls |
| Field et al. (2007) | Tertiary split of mean of weekly consumption | Greater discounting by heavy adolescent drinkers than light drinkers |
| PATHOLOGICAL GAMBLING | ||
| Petry (2001b) | DSM-IV for pathological gambling | Greater discounting by pathological gamblers than controls |
| Alessi & Petry (2003) | Gambling severity screen | Discount rate correlated with gambling severity screen |
| Dixon et al. (2003) | South Oaks Gambling Screen ≥ 4 | Greater discounting by pathological gamblers than non-gamblers |
A related and interesting initial area of investigation has been the examination of ex-addicted individuals. One initial study examined ex-, current-, and non-smokers (Bickel, et al., 1999b) on temporal discounting assessments for $1000 hypothetical future money. Smokers discounted the money more than controls, and ex-smokers (at least 1 year of abstinence) discounted nearly identically to the controls. Table 2 displays studies comparing individuals who are ex- or abstinent drug users to active drug users. These data are more heterogeneous, with a significance factor being duration of abstinence; short-term or more recently abstinent individuals show data most similar to current addicts. Though suggestive, these data have to be interpreted with care. Studies of this sort provide little insight into the possible causal relationship between drug dependence and rate of discounting because these results could be due to different processes. For example, ex-users may quit drug use because they discount less. Conversely, individuals may discount less once they stop engaging in their addictive activity. The validity of these two competing hypotheses has yet to be empirically determined.
Table 2.
Studies that explicitly examine effects of withdrawal/abstinence on temporal discounting.
| STUDY | DURATION OF ABSTINENCE | OUTCOME |
|---|---|---|
| CIGARETTES | ||
| Bickel et al. (1999) | ≥ 1 year | Greater discounting by active smokers than ex-smokers |
| Mitchell (2004) | ≥ 24 hours | No difference between normal smoking and acute abstinence |
| Field et al. (2006) | ≥ 13 hours | Greater discounting during abstinence than normal smoking |
| OPIOIDS | ||
| Giordano et al. (2002) | Since last buprenorphine dose | Greater discounting prior to than following dose |
| COCAINE | ||
| Heil et al. (2006) | ≥ 30 days | No difference between abstinent and non-abstinent cocaine dependents |
| ALCOHOL | ||
| Petry (2001a) | ≥ 30 days | Greater discounting by active than abstinent alcoholics |
Contemporary Status of Inter-temporal Research: Neuroscience and Neuroeconomics of Inter-temporal Choice
Neuroscientific techniques and approaches are now being applied to inter-temporal choice, and one important dimension of the neuroscience of inter-temporal choice has been in the emerging area of neuroeconomics. Neuroeconomics is a trans-disciplinary approach that combines the disciplines of neuroscience, economics, and psychology in order to examine brain mechanisms that underlie decision-making and choice (Bickel, et al., 2007). Functional magnetic resonance imaging (fRMI) is frequently used in these human studies. fRMI operates because of the different magnetic properties of oxyhemoglobin and deoxyhemoglobin. This approach assumes that there is increased oxygen utilization in those parts of the brain that are active. The blood oxygenation level dependent (BOLD) response is what is measured. A central consideration in neuroeconomic research is valuation of reinforcers. (Montague and Berns, 2002). As a result of findings in the field, neuroeconomics increasingly recognizes that choices are emergent phenomena resulting from the interaction of multiple brain regions (Sanfey, et al., 2006).
One of the first neuroeconomic studies to examine inter-temporal choice was conducted by McClure and colleagues (2004). They proposed that activity in limbic and the fronto-parietal regions might be implicated in smaller sooner vs. later larger rewards, respectively. College students performed on a delay-discounting task while being scanned with an MRI. Limbic areas showed greater activity when sooner smaller rewards were chosen and frontal and parietal regions showed greater activity when the larger later rewards were selected. Later studies have replicated and extended these operations (McClure, et al., 2007). However, one study that compared methamphetamine addicts versus controls (Monterosso, et al., 2007) failed to show differential brain activation. This negative study not withstanding, these data would tend to suggest that decreased frontal activity should be evident among addicted individuals who discount the future.
Future of Inter-Temporal Choice Research
In the following section, we offer three perspectives that are likely to influence the future of inter-temporal research, particularly as applied to substance abuse and behavioral pharmacology.
Discounting as a Measure of the Competing Neuro-Behavioral Decision Systems Hypothesis of Addiction
Recently a variety of studies have supported the notion that addiction is determined in part by the interaction between two brain regions (Jentsch and Taylor, 1999; Bechara, 2005; Daw, et al., 2005; Everitt and Robbins, 2005; Baler and Volkow, 2006; Bickel, et al., 2007; Redish and Johnson, 2007): the impulsive action and executive systems (our term).
The impulsive action system includes the amygdala, dorsolateral striatum, nucleus accumbens, insula, ventral pallidum, striatum, and related structures. Addiction and related behaviors have been closely linked to activity in these evolutionarily old mid-brain reinforcement structures. The impulsive action system may be involved in the “exaggerated processing of the incentive value of substance-related cues” (Bechara, 2005, p.1459).
The executive system includes the regions that compose the pre-frontal cortex (PFC), and is thought to be involved in actions such as working toward a defined goal, prediction and expectation of outcomes, determining future consequences of current activities, and social control (Barkley, 1997).The PFC, an evolutionarily younger brain region found in humans and higher mammals, has been recently identified in the study of addiction (Volkow, et al., 2004). Studies have decreased activity among addicts versus controls, as well as decreased activity or volumetric reduction among the addicted (Volkow and Fowler, 2000; Fein, et al., 2002; Franklin, et al., 2002).
According to this competing neuro-behavioral decision systems theory, addiction, at least in part, results from a hyperactive impulsive action system (e.g., striatal and mid-brain reinforcement structures) and a hypoactive executive system (e.g., PFC), such that the impulsive action system overwhelms the executive with corresponding emphasis on immediate outcomes and consequences. As reviewed above, the neuroimaging data suggest that discounting of delayed rewards activates many of the same brain regions implicated in this new model of addiction. Furthermore, relative brain activation appears to provide a summary measure of the “strength” of the competing regions. Thus, one future of inter-temporal choice is to measure, facilitate and understand this new hypothesis of addiction.
Discounting as a Predictor of the Therapeutic Process
If discounting is a measure of the relative control of the competing brain regions that are involved in addiction, then we would expect that it functions as a measure of severity. As such, we would expect that rate of temporal discounting would be predictive of therapeutic outcome. Two recent studies support the use of inter-temporal choice in this regard. The first study assessed monetary discounting among 30 adolescent cigarette smokers prior to a 4-week treatment consisting of cognitive-behavior therapy and contingency management (Krishnan-Sarin, et al., 2007). The 47% that failed to achieve abstinence at the end of the treatment discounted significantly more than those that were abstinent at the end of treatment. The second study (Yoon, et al., 2007) collected delay discounting of monetary reward responses during the third month of pregnancy from 48 women who had recently quit smoking. Discounting rates predicted relapse, with high rates being associated with a return to smoking at 6-months post-partum (approximately 1 year since the discounting measure was taken) and low rates being associated with maintained smoking abstinence. These two studies provide powerful initial observation of the discounting of delayed rewards as a predictor of therapeutic outcomes. However, the generality of this observation across other forms of addiction remains unknown.
Modifying Inter-Temporal Choice
One area of research that we consider an important one for the field, and one which at this point has not been addressed to our knowledge, is the manipulation of inter-temporal choice; that is, can we change the rate of discounting of delayed events within an individual? If we can, will it have an effect on therapeutic outcome? Several studies have demonstrated that discounting increases during drug deprivation, which we would presume may result in relapse or greater drug use. However, trying to produce a change in discounting that would favor the longer term and the impact of that on treatment outcome remains an important question to explore.
Conclusion
The study of inter-temporal choice is an area that has been growing. Its roots come from the experimental analysis of behavior as has many facets of behavioral pharmacology. However, this research approach has been developed into novel and interesting areas including understanding how drug-dependent individuals and other individuals burdened with psychiatric or behavioral disorders respond to inter-temporal choices. The advent of neuroimaging research has expanded opportunities to understand the brain regions associated with these behaviors. These brain regions map on to a novel hypothesis regarding addiction, and may provide a measure of the relative control of competing brain regions. The utility of discounting will expand if discounting can be used in that regard. Consistent with this view, some initial studies have demonstrated the value of discounting as a predictor of relapse. Clearly, more research is warranted. Lastly, one currently unexplored research area is the effort to produce a reduction in rate of discounting. Whether such efforts can be long lasting and therapeutic remains an interesting question of considerable importance. The last topic of this paper is devoted to a discussion of how some of the techniques developed in behavioral pharmacology have been adapted to the clinical setting.
Human Behavioral Pharmacology in Drug Abuse Treatment Settings, by Stephen Higgins
Behavioral pharmacologists have a distinguished record of treatment-outcome research in drug abuse treatment settings that spans almost 40 years. As might be expected from a discipline that was launched as a marriage between behavior analysis and general pharmacology, the contributions are behavioral and pharmacological in nature. In keeping with the spirit of the symposium that occasioned this report, these contributions are discussed in historical context. The information outlined below was obtained through informal searching of PubMed and is meant to be illustrative but by no means exhaustive. The record shows substantive contributions from behavioral pharmacologists in many different areas of treatment-outcome research, but the following three general areas particularly stand out: opioid replacement therapy for opioid dependence, nicotine replacement therapy for cigarette-smoking cessation, and contingency-management and related behavioral therapies for a wide array of different types of substance use disorders and related problems. Below we comment on each.
Opioid Replacement Therapies
Methadone Treatment
As is well known, opioid replacement therapy was launched in the mid 1960s through the seminal reports of Dole, Nyswander and Kreek, which revolutionized treatment for opioid dependence (Dole and Nyswander, 1965; Dole, et al., 1966). Behavioral pharmacologists began participating in clinic-based studies on this exciting development within five years of the publication of the seminal work (Jaffe, et al., 1970, 1972; Schuster, et al., 1971). These early reports involved double-blind, experimental comparisons of different opioids and were published in prominent, high-impact medical journals. The scientific rigor and scholarship evident in those initial studies is something that continues to distinguish many of the contributions of behavioral pharmacologists to treatment-outcome research.
Why this fruitful initial burst of scientific activity by behavioral pharmacologists on methadone therapy did not lead to a program of research is not revealed in the published record, but the work may have been too successful. Jerry Jaffe, the lead author on two of the three reports cited above, left the field to serve in the Nixon administration as the first “Drug Czar” around this same time. Bob Schuster, the best known of the behavioral pharmacologists involved, was heading a vigorous program of preclinical and later laboratory-based human behavioral pharmacology research. Whatever the reasons, the record shows an approximately 5-year hiatus in contributions of behavioral pharmacologists to this emerging area. However, this brief hiatus from research on methadone was not an idle one for behavioral pharmacologists interested in researching treatments for substance use disorders. For example, studies began appearing out of the University of Minnesota on treatments for alcoholism and sedative dependence (Pickens, et al., 1973, 1979). Around that same time, two of those investigators, George Bigelow and Roland Griffiths, took positions at Johns Hopkins University where they initially continued researching alcoholism (e.g., Bigelow, et al., 1977), including alcoholism among methadone patients (Liebson, et al., 1978). The highly influential body of experimental research on alcoholism that was being conducted by behavioral pharmacologists at Harvard University throughout this period is also important to mention (e.g., Mendelson and Mello, 1966, 1976).
Renewed interest in the establishment of the methadone clinic ended this relatively brief hiatus in research on opioid dependence and, more importantly, marked the start of a tremendously fruitful program of research in the methadone clinic at Johns Hopkins University that spans three decades and continues today. What followed was a stream of research reports examining a wide range of different aspects of methadone treatment, but particularly important to the development of this form of treatment were rigorous, experimental studies examining the influence of treatment duration and methadone dose as well as the importance of behavioral adjuvant treatments, which are discussed in the section below devoted to behavioral interventions (e.g., McCaul, et al., 1984; Stitzer, et al., 1982). Several of the studies on the effects of dose on outcome stand among the most scientifically rigorous in this area (Strain, et al., 1993, 1999; Donny, et al., 2005). The record suggests that behavioral pharmacologists will continue to make important contributions with scientifically rigorous examination of the optimal use of methadone in the treatment of opioid dependence.
Buprenorphine Treatment
Behavioral pharmacologists were centrally involved in all aspects of the clinical research that led to the approval of buprenorphine for treatment of opioid dependence, another development that is revolutionizing treatment for opioid dependence in the U.S. Much of the outstanding preclinical research and important initial clinical studies on buprenorphine related to its potential as a treatment agent were conducted by behavioral pharmacologists (e.g., Mello, 1978). In collaboration with clinical pharmacologists from the ARC/NIDA, behavioral pharmacologists were involved in many of the pivotal randomized clinical trials conducted to examine the efficacy of buprenorphine (Bickel, et al., 1988; Johnson, et al., 1995), as well as parametric studies examining different delivery schedules (e.g., Bickel, et al., 1999a) and studies important to understanding the role of drug dose (e.g., Greenwald, et al., 2003). The publication record suggests that behavioral pharmacologists continue to play important roles in the development and efficacy testing of new buprenorphine formulations (e.g., Strain, et al., 2004; Sigmon, et al., 2006) as well as the extension of buprenorphine treatment to special populations, including adolescents (Marsch, et al., 2005) and pregnant women (Jones, et al., 2005).
Nicotine Replacement Therapy for Smoking Cessation
Behavioral pharmacologists have been and continue to be leaders in the use of nicotine replacement products and other pharmacotherapies for smoking cessation. Studies by behavioral pharmacologists analyzing smoking as an orderly form of drug self-administration that produced dependence were fundamentally important to the emergence of this area of investigation, but were largely conducted in controlled laboratory environments rather than clinic settings and for that reason are not within the purview of this report (e.g., Stolerman, et al., 1973; Kozlowski, et al., 1975; Griffiths, et al., 1982).
The seminal studies characterizing the nicotine withdrawal syndrome were conducted by behavioral pharmacologists in clinical settings/populations at UCLA (e.g., Shiffman and Jarvik, 1976) and the University of Minnesota (e.g., Hughes, et al., 1984; Hughes and Hatsukami, 1986) and provided much of the grist for the development of nicotine replacement therapies. The prominence of behavioral pharmacologists in all areas of the development and testing of nicotine replacement products for smoking cessation is striking (e.g., Hughes, et al., 1984; Hughes and Miller, 1984; Rose, et al., 1984; Jarvik and Henningfield, 1988; Gross, et al., 1995; Hennigfield, 1995; Schneider, et al., 1995). Many of these same investigators remain leaders in the development and testing of pharmacotherapies for smoking cessation, which has grown well beyond the initial nicotine replacement products (e.g., Hughes, 1999; Hughes, et al., 1999). Examination of novel pharmacotherapies such as nicotine vaccines would seem likely to play a prominent role in future directions in this area of investigation (e.g., Hatsukami, et al., 2006).
Behavioral Interventions
Use of contingency management (CM) interventions in the treatment of substance use disorders is a practice that has been systematically developed by behavioral pharmacologists over the past approximately 30 years. The development of this area of research can be organized into four phases: (1) initial proof-of-concept studies with methadone patients and cigarette smokers, (2) development of voucher-based reinforcement therapy (VBRT) and the conduct of randomized clinical trials examining efficacy, (3) expansion of applications of VBRT to novel problems and special populations, and (4) conduct of multi-site trials, publication of meta-analyses, and diffusion into community settings.
Initial Studies with Methadone Patients and Cigarette Smokers
Along with the pharmacological contributions noted above, behavioral pharmacologists were involved in characterizing and attempting to treat common problems among methadone patients that were not addressed by methadone per se. For example, some methadone patients continue abusing other drugs after entering methadone treatment. Abuse of benzodiazepines is one such form of other drug abuse. In a rigorous within-subject study, contingent delivery of clinic privileges, including monetary payments, contingent on benzodiazepine-negative urine toxicology results was demonstrated to increase abstinence from drug use (Stitzer, et al., 1982). The contingent use of clinic privileges, especially medication take-home privileges, to reduce other drug abuse and promote changes in other clinical outcomes, was demonstrated further (e.g., Stitzer, et al., 1992). These same investigators also demonstrated that cigarette smokers not currently attempting to quit smoking long-term would abstain from smoking when monetary payments were available contingent upon objective evidence of recent smoking abstinence (e.g., Stitzer and Bigelow, 1984). These studies among methadone patients and cigarette smokers provided the conceptual and empirical foundation for subsequent development of CM interventions. The studies on medication take-home privileges also provided an intervention with potential for dissemination into community methadone treatment clinics.
Voucher-based Reinforcement Therapy
The U.S. cocaine epidemic of the 1980s and 1990s dramatically increased demand for effective treatments for cocaine dependence. Many different pharmacotherapies were examined, but none were shown to be efficacious. Within this context of high demand for efficacious treatments and little promise among the pharmacotherapies being examined, a programmatic series of randomized clinical trials demonstrated the efficacy of a CM intervention wherein patients could earn vouchers redeemable for retail items contingent on cocaine-negative urine toxicology results (e.g., Higgins, et al., 1991, 1994, 2000). Soon thereafter, the efficacy of VBRT was also demonstrated in randomized clinical trials with cocaine abusers enrolled in methadone treatment (e.g., Silverman, et al., 1996, 1998). These studies positioned VBRT as perhaps the most reliably efficacious treatment for cocaine dependence.
Expansion of VBRT
The success of VBRT in treating cocaine dependence led many behavioral pharmacologists and other clinical investigators to examine new applications. Space limitations preclude discussing all such new directions, but several stand out as among the more promising. Silverman and colleagues developed a program referred to as the Therapeutic Workplace (Silverman, et al., 2002). Initially directed at pregnant, opioid-dependent women but later extended to other populations, the intervention uses VBRT to promote abstinence from drug use while developing vocational/educational skills among chronically unemployed, inner-city drug-dependent patients. VBRT has been extended into the vocational rehabilitation of veterans with co-occurring substance abuse and other mental health problems (Drebing, et al., 2005). An efficacious intervention using VBRT for cigarette smoking cessation among pregnant women has been developed (Higgins, et al., 2004). A final example of new applications is a program that uses VBRT in the public school setting to promote smoking cessation among adolescents (Krishnan-Sarin, et al., 2006).
Multi-site Studies, Meta-analyses, and Diffusion into Community Settings
The reliable efficacy of VBRT discussed above led to interest in examining CM among participants in NIDA’s Clinical Trials Network. A variation of VBRT that uses retail items kept onsite at the clinics was examined in two multi-site trials conducted in community clinics throughout the U.S. Results from both trials supported the efficacy of the intervention in promoting abstinence from psychomotor stimulants and other drug use among outpatients enrolled in drug-free (Petry, et al., 2005) and methadone-maintenance (Peirce, et al., 2006) treatment clinics. Further bolstering the evidence supporting CM for treatment of substance use disorders were two independent meta-analyses that provided overwhelming quantitative evidence of efficacy for VBRT specifically (Lussier, et al., 2006) and CM more generally (Prendergast, et al., 2006). Other developments in this area were various instances of diffusion of VBRT into community settings, such as its use to treat methamphetamine abuse among gay and bisexual men in San Francisco (Strona, et al., 2006).
Conclusions
The information described above supports at least three conclusions. First, Behavioral Pharmacology as a discipline has an extraordinary record of substantive scientific contributions to the development of efficacious treatments for substance use disorders. Second, the contributions are broad, spanning licit and illicit substances, different populations and settings, and pharmacological and behavioral interventions. Third, treatment-outcome research in clinical settings is an area of behavioral pharmacology research that is growing in many different and exciting directions. Indeed, it is an area of growth where opportunities abound for making scientifically and clinically meaningful contributions.
Overall Summary
It was with great pleasure that all of the invited speakers attended the 50th Anniversary of the Behavioral Pharmacology Society. The goal of the symposium was to highlight several aspects of the behavioral pharmacology of drugs in humans, including the evolution of drug self-administration procedures in humans to examine both pharmacological and environmental variables that alter drug taking behavior, factors underlying individual differences in the reinforcing effects of drugs, analyses of inter-temporal choices as they relate to drug use (e.g., impulsivity, delay discounting, self-control, and delay of gratification), and applications of behavioral principles to drug abuse treatment settings. Hopefully, it has become clear that the roots of much of this research derive from the behavioral principles that were developed in preclinical studies. Burgeoning areas of research include the use of state-of-the-art imaging techniques to characterize the neuroanatomical factors influencing drug self-administration, and exciting new developments in genetics research that will shed light on individual variations in response to drugs of abuse. Both of these lines of research will likely contribute to an understanding of how impulsive behaviors relate to drug use. As in the past, it is likely that this understanding ultimately will find its way into the clinic, where new therapeutic techniques will improve treatment outcome. We are confident that an ongoing dialogue between preclinical and clinical researchers will continue to lead to new and exciting research endeavors in both domains.
Commentary by Chris-Ellyn Johanson
The preceding article by Comer et al. is a summary of the presentations that were given at the symposium held during the 50th Annual Meeting of the Behavioral Pharmacology Society (BPS). Both Mary Jeanne Kreek and I served as discussants following these presentations. This article is based on my comments at that time and is meant to reflect my impressions of important issues raised by these presentations about human behavioral pharmacology research past, present and future. As pointed out by several of the speakers, and eloquently stated by Skinner (as quoted by Galen Wenger), behavioral pharmacology has long been engaged in translational research, which has now become the newest hot topic targeted by the National Institutes of Health. Translational research requires that those doing the translating stay au courant with the studies being conducted in animals and likewise that basic researchers stay abreast of findings emanating from research in humans. Hopefully, the symposium served to remind us of the importance of interaction between these streams of research.
Historical Roots
Behavioral analysis (as founded by BF Skinner) is the theoretical root of behavioral pharmacology and its fundamental concept is that behavior is controlled by its consequences. Behavioral analysis, in moving away from motivational explanations of behavior, developed a compelling literature demonstrating the prediction and control of behavior by environmental contingencies. This literature continues to inform studies in behavioral pharmacology. With the observation that drugs could function as reinforcers and the development of drug self-administration procedures, a major branch of behavioral pharmacology was born, as summarized by Sandy Comer. While behavioral pharmacology as a discipline is much broader as reflected by the entire program of BPS, the drug self-administration procedure revolutionized the field. Self-administration studies demonstrated that drugs that humans abuse are self-administered by animals and drugs that humans do not abuse are for the most part not self-administered (Johanson and Balster, 1978), thus changing the conceptual basis for the addictions. This discovery allowed the principles and rigor of behavioral analysis to be used in studying and conceptualizing the etiology, prevention and treatment of drug dependence. It is in this area of behavioral pharmacology that human research has made its major impact. Much of the human research has a neuroscience basis, following the leads of animal research, but most studies are primarily behavioral in nature and furthermore have practical application. For example, the drug abuse liability evaluation studies presented by Sandy Comer aim to ultimately discover effective medications that have lower abuse potentials. Likewise, the studies she reviewed on the effects of new medications on the subjective and reinforcing effects of drugs of abuse hopefully will lead to the development of medications to treat substance abuse. These practical applications were further elaborated in Steve Higgins’ presentation, which focused on the application of human laboratory findings to both pharmacological and behavioral treatment of drug dependence in the clinic.
Models of Drug Dependence
Sandy Comer very clearly illustrated in her presentation that the paradigms used in human research to study drug dependence are founded in animal research. My own work also illustrates this point. As a graduate student, I (along with many others researchers during the early 1970s), was trying to develop self-administration methods that would obviate a unique problem of using drugs as reinforcers. Unlike food and water, the predominant reinforcers used in behavioral analysis research, drug reinforcers have profound effects on behavior, independent of their reinforcing properties. Much of the research in basic behavioral pharmacology deals with how drugs affect behavior generated by various schedules of reinforcement. In studies of drug self-administration, however, the rate-altering properties of drugs (a sort of side effect) clouded the ability to use rate as a measure of the relative reinforcing properties. Charles (Bob) Schuster liked to give the example of what occurred when 30 mg/kg intravenous pentobarbital was used as the reinforcer. After one delivery, the animal became unconscious for a long time, yielding rates of responding far lower than rates maintained by placebo (saline). This does not mean that pentobarbital at that dose did not have reinforcing properties. Rather, different approaches were required to reveal them. The approach I took to overcome rate-disrupting effects was modeled after paradigms developed by Jack Findley and colleagues (Findley, 1962; Findley et al., 1972) and involved offering animals a choice between two options. The number of choices made by the animal served as the measure of reinforcement. As an aside, I vividly recall a very prominent behavioral pharmacologist at that time telling me emphatically that I was not studying operant behavior. That is, the choice of one option over another did not mean the choice was a reinforcer. Unfortunately, this issue still plagues us. Although I was shaken by his protestation, luckily for my career I ignored him. Perhaps these studies do not show operant behavior, but at least the earliest study demonstrated that choice was lawfully related to dose (Johanson and Schuster, 1975) and subsequent studies have demonstrated that the relative reinforcing effects of drugs can be measured using choice procedures. As the animal research progressed, the issue of validity of these animal procedures to humans and their relevance to substance abuse became an issue. I decided that one way of validating the animal research was to conduct exactly the same study in humans. As in the animal studies, humans were first offered a chance to sample two different drugs and then given an opportunity to choose which one they preferred during several choice trials (Johanson and Uhlenhuth, 1980). While many variants of this basic procedure have been developed, it should be clear from all the presentations that the choice paradigm is used extensively as a means of understanding behavioral and pharmacological variables that affect drug-taking behavior in humans. By extension, these choice procedures are also important in the initiation, maintenance, and cessation of problematic drug use in humans.
Other Influences
While behavioral analysis and the self-administration paradigm are paramount in human behavioral pharmacology, there were other influences that molded the development of this discipline as we see it today. Long before behavioral pharmacologists took an interest, human research in drug dependence had a long history at such venerated institutions as the Addiction Research Center (ARC), originally located in Lexington, Kentucky, and Rockefeller University, as described below by Mary Jeanne Kreek. The Committee on Problems of Drug Dependence (now renamed the College on Problems of Drug Dependence) had been in existence for decades and its meetings were filled with studies utilizing human participants. In addition to studying the effects of drugs on physiological systems, the researchers at the ARC developed parametrically sound measures of subjective effects. This research was conducted primarily in prisoners and, in the 1970s, when this population was designated as too vulnerable, new venues were needed. This attracted the new group of addiction researchers, myself included, who used the tools of behavior analysis and also began to incorporate subjective effects measures into our studies. The purists among us referred to the reports of subjective effects as “verbal” behavior. While many studies, particularly those conducted by Marian Fischman and her colleagues (Fischman and Schuster, 1982; Foltin and Fischman, 1991), have amply demonstrated that behavioral indices of reinforcement, such as choice, and subjective effects are not always concordant, they often yield comparable results. As demonstrated in Harriet de Wit’s presentation, the use of subjective effects measures has many practical advantages and has yielded important results. Harriet de Wit’s presentation also points to another influence that is molding a great deal of today’s research. Behavior analysts are almost phobic about the notion of variation. The idea is that if we tightly control all relevant variables, we can precisely predict and control behavior. But there are variables we cannot control and this seems to be especially true for addictive behavior. Many individuals try psychoactive drugs but very few follow a trajectory to addiction. While much animal research is now focusing on individual differences, largely stimulated by the rise of genetic research, I credit human behavioral pharmacological research, and Harriet de Wit’s laboratory especially, with leading the way in the study of individual differences. Her presentation beautifully demonstrated the importance of sex and differences in behavioral repertoires (also called personality) on the subjective effects of drugs, and by extension, the reinforcing effects of drugs. She and others are clearly demonstrating that genotype and differences in brain mechanisms, which most likely underlie differences in personality, are important determinants of the effects of a drug. These findings, in turn, have important implications for prevention. If we know what leads some individuals vulnerable to becoming addicted, we may be able to change the course of their lives through prevention measures that target these risk factors.
Paradigmatic Advances
The presentation by Warren Bickel echoed the idea that individual differences underlie vulnerability to addiction. The primary focus of Bickel’s presentation was on impulsivity, a particularly important “personality” dimension. His strategy for assessing individual differences in this domain relies directly on observing differences revealed by a paradigm known as intertemporal choice (also called delay discounting and temporal discounting), in which participants choose between commodities that can be obtained now versus commodities that are available at a later time, with the magnitude of the commodities being smaller when available immediately. Once again it is important to point out that this procedure was developed in the animal laboratory but was very quickly “translated” into human studies. The popularity of this procedure in the field of the addictions is overwhelming and certainly indicates that it is tapping into a dimension that investigators consider relevant to helping to understand behaviors previously considered perplexing or irrational. It also brings the rigor of behavioral pharmacology to the study of a personality dimension, namely impulsivity. However, Dr. Bickel is very careful, and rightfully so, to remind us that impulsivity can refer to other behaviors besides delay discounting, as was evident in Harriet de Wit’s presentation. I believe these studies are very exciting but it should be remembered that the choices participants make in these studies are most of the time hypothetical and the importance of this is not clear. Efforts have been made to make the choices real but this can be very tedious for the investigator and even impossible (e.g., delivering a reinforcer 10 years from now). Nevertheless, what has emerged from these studies is the notion that addicted individuals differ from non-addicted individuals in terms of their temporal horizon, (i.e., an addicted individual’s temporal horizon is restricted to the here and now). This clearly explains why it is difficult for addicted individuals to forgo immediate drug reinforcement to avoid delayed adverse consequences. The long-term adverse consequences of continued drug use are simply beyond their horizon, so that the reinforcers in the immediate temporal environment control their behavior. Although it is clear that the inability to delay gratification is a risk factor for drug addiction, it is also likely that a history of the immediate gratification drugs provide may further intensify the problem. Treatment procedures must take into account the short temporal horizons that characterize the choices of many drug addicts and devise interventions to address this problem.
Applications
As I mentioned previously, behavioral analysis and human behavioral pharmacology in particular have always been translational in spirit and have always considered the clinical implications of the research findings. Steve Higgins’ presentation could not illustrate this point more clearly. As he notes, even in the 1960s, behavioral pharmacologists were conducting treatment-related research. In many ways, these early studies and those that followed revolutionized the treatment of addictive disorders. The rigorous analysis of behavioral outcomes combined with complementary laboratory research focusing on variables believed, but not known, to be important changed the way individuals with drug problems were treated, both with behavioral as well as pharmacological treatments. It is clear to everyone that buprenorphine would not have been marketed without the research conducted by behavioral pharmacologists.
Steve Higgins’ presentation of tobacco research deserves special emphasis because this is a political story as well. Behavioral pharmacologists have never been shy about stepping into a highly charged arena, especially with regards to the issue of tobacco dependence. In the 1980s tobacco smoking was still considered a mere habit. Many remember that at NIDAs intramural program, tobacco research had to be done almost clandestinely because the government did not support the idea that nicotine was a drug of abuse (and you can probably guess why they resisted this designation). Now it is considered the prototypic drug of abuse, largely because of the research conducted by behavioral pharmacologists demonstrating that not only was nicotine a reinforcer in both animals and humans, but also that it was possible to become physically dependent on nicotine. Studies supported this by showing that the characteristics of those who regularly smoked were similar to those who were dependent on other types of drugs. Today, tobacco dependence is a recognized disorder in the American Psychiatric Association’s Diagnostic and Statistical Manual. The consequences of this change are enormous in terms of its effect on public policy. With the implementation of new governmental regulations, we now have smoke free areas almost everywhere. The tobacco industry has been forced to make amends for hiding facts concerning the consequences of tobacco use and dramatic changes have been made in its ability to advertise and promote its products, at least in the United States. No single change in public health policy has had and will continue to have as great an impact on public health as the change in status of tobacco.
Much of the success in the area of treatment for tobacco, alcohol, and opioid dependence has been in the development of pharmacotherapies, as reviewed by both Sandy Comer and Steve Higgins. The presentation by Steve Higgins also highlighted another area where behavioral pharmacologists have been successful, namely the development of behavioral therapies. This has been particularly important for the treatment of cocaine and other stimulant addictions where effective pharmacotherapies have yet to emerge. From the roots of behavioral analysis, contingency management has been developed as one of the most successful behavioral therapies available. Again, behavioral pharmacologists have led the research in this area. Despite the overwhelming success of contingency management, this treatment strategy remains controversial, partly because it can be distorted so easily, e.g., simply paying people to stop using drugs. It is also an intensive therapy that requires careful monitoring of behavior and the expertise of well-trained therapists, both of which necessitate adequate funding for the treatment programs. If anything, money available for the treatment of the addictions is waning rather than increasing. It is also important that we further explore the lasting effects, if any, of contingency management. In most cases, when the contingencies are removed, behavior returns to “baseline”. This should be no surprise, but if we are to combat criticisms of contingency management, we must develop strategies for assuring longer-term success (even if it involves continued treatment but with less intense, and thus less expensive, monitoring). Ken Silverman and colleagues are now attempting to devise contingencies that change behavior more permanently (Donlin et al., 2008). Their treatment approach uses classic contingency management strategies to eliminate drug use, then puts into place additional contingencies to reinforce pro-social behaviors, such as employment. Not only is employment a targeted behavior, but the training needed to make someone employable is also provided, contingent on continued drug abstinence. This type of approach will make contingency management more acceptable and will hopefully lead to its widespread use.
Challenges, Foreign and Domestic
Many of the speakers in the symposium discussed challenges they saw in the future for human behavioral pharmacology and this stimulated my own thoughts in this regard. First, the tremendous growth and success of the neurosciences, along with the development of some truly remarkable technologies, is a major conceptual and practical challenge. Research funding is limited and neuroscience research using such tools as neuroimaging (is it really true that NIDA stands for the Neuroimaging Institute on Diseases of Addiction?) is expensive. The advances and implications of the findings in neurosciences are earthshaking but there is still the haunting perspective of reductionism. I contend that the organism is still always right but this is often forgotten. It is encouraging, however, that neuroscience researchers clearly appreciate the behavioral tools and paradigms that behavioral pharmacologists have developed. The challenge remains, however, to use these tools effectively and learn how to design appropriate research paradigms that capture the best of all perspectives. I was quite excited by the work that Warren Bickel presented that demonstrates that it is possible to forge these types of interdisciplinary relationships and to conduct truly innovative studies.
As described above, research on individual differences is very important to our field and any discussion of these leads one to think about the influence of genotypic variation on behavior related to drug taking. We no longer need to pit nature versus nurture but, instead, we need to focus on how a person’s genetic makeup influences the impact of environmental factors, namely the effects of drugs. In the area of the addictions, it has become increasingly clear that genes bestow vulnerability to all aspects of the trajectory from initiating recreational drug use to dependence. We know less about how these genetic variations interact with environmental risk factors. Impulsivity due to a variation in a genetic allele may be a risk factor for initiating risky drug use but this genetic trait may interact with environmental risk factors such as stress, lack of availability of other reinforcers, or response cost. Environmental risk factors can easily be studied in the laboratory using classic behavioral pharmacological paradigms. However, conducting these types of laboratory studies with the intention of understanding how the results are affected by genetic variation in the sample is a major challenge, largely because the sample sizes required at this stage of the science’s development may be very large. In order to assess the potential impact of genotypic variation on the effects of drugs that we believe are relevant to one of the transition stages of drug use, we must go beyond single site and single investigator studies. Funding mechanisms for such large-scale multi-site studies do exist but behavioral pharmacologists have yet to take advantage of these mechanisms. This will be necessary if we are going to better understand the interplay between genetic variation and other risk factors on the effects of drugs.
There are also challenges in our field related to specific types of research. One with which I am most familiar is abuse liability assessments of new compounds or, more generally, assessments of relative reinforcing efficacy, as reviewed by Sandy Comer. I find it really unfortunate that these assessments almost exclusively use only subjective effects measures, rather than drug-taking behavior. I still lament the drift away from the fundamental property of drugs as reinforcers to constructs such as craving. This strategy is driven to some extent by practical considerations. Studies involving actual drug taking are certainly more complicated and in most cases, take longer to conduct. Such complications result in increased costs and unwelcome delays. The shift away from studying drugs as reinforcers also can be attributed to the Food and Drug Administration. Despite the field’s best efforts to educate the FDA (Johanson et al., 2003), it does not seem to view self-administration studies in humans as necessary in the assessment of abuse liability (even though self-administration studies are required in animals). Furthermore, the FDA actively encourages the use of only one subjective effects measure, the so-called primary outcome variable. Even if we accept that subjective effects measures are sufficiently related to drug-taking behavior to make them acceptable as indicators, focusing on a single measure seems simplistic. Behavioral pharmacologists who use subjective effects measures like to try to characterize the abuse liability of a drug in terms of a profile and take into consideration all measures, such as the Addiction Research Center Inventory, visual analog scales, and physiological indices, to mention a few. The numbers of these measures can often be several dozen. What we have failed to do as a field is to develop sophisticated analytic methods to summarize or consolidate these different measures into a few reliable and well-validated constructs. As much as this may be needed, however, funds to support this type of research are virtually non-existent.
The final challenge I want to mention is the construct of drug dependence itself. Drug dependence has many dimensions and should be viewed developmentally starting with initiation of recreational drug use, followed by more regular use, the transition to dependence or addiction (which may involve loss of control), and finally cessation and sometimes relapse. Human behavioral pharmacologists have not investigated all of these phases, although there are trends in this direction, for instance, in the development of paradigms to study relapse. Furthermore, some dimensions, such as the transition to drug dependence or loss of control, may be very difficult, and perhaps unethical, to study in the laboratory. The field of cognitive neurosciences has also demonstrated that cognitive abilities under some conditions can be compromised and it is important to consider how these deficits interact with the variables we traditionally view as affecting drug-taking behavior. It would also be interesting to broaden the notion of medications to include those that target other behavioral changes, such as changes in cognitive abilities that may indirectly treat the addiction disorder. Most challenging is the broadening of the conceptualization of addiction to include the new theories that go far beyond the simplistic notion that addiction is based upon the “theory” that drugs control behavior because they are reinforcers. These new theories have responded to the challenge that drug-taking behavior is amazingly persistent and resistant to change and, furthermore, that there are differences in vulnerability across individuals. These new theories are based on studies that use recent technological advances, such as neuroimaging, to demonstrate changes and differences in brain function. The differences in perspective can be summarized simply as the difference in viewing drug-addiction as an instance of operant behavior that is overly controlled by drugs, or as a loss of control caused by the disease of addiction. Reconciling these two perspectives will be a major challenge for human behavioral pharmacologists.
Commentary by Mary Jeanne Kreek
I was very pleased to be one of two invited Discussants for this wonderful symposium, and honored to be part of the 50th Anniversary celebratory banquet! So many people whom I have known, worked with in the scientific arena, and a few with whom I have become very close scientific friends over the years, were feted as the scientists who conceptualized, organized and initiated the Behavioral Pharmacology Society 50 years ago. Among these, I must personally recognize Dr. Joseph Brady as a marvelous scientist in many domains of neuroscience, and especially as related to drug abuse and addiction, a committed translational scientist who has personally addressed the problems of delivery of pharmacological and behavioral treatments to those suffering from addictive diseases.
It was pointed out by several speakers in the symposium that I, in fact, had performed some of the very earliest clinical research work in “behavioral pharmacology,” although that was not the category in which we would have placed our research back in the beginning in 1964. In this brief discussion, I am going to briefly review the early history of our research, and then make three points for consideration in future research related to behavioral pharmacology. In future research, it would be of potential great importance, first, to enhance considerations of the changes with time that are observed and documented in the natural history of drug use, abuse and progression to addiction, including considerations of the different mechanisms involved at each stage and the factors contributing to the vulnerability to progression, such as the role of personality and peer pressure, along with other aspects of the environment, especially at the initiation and early progression of use along with specific aspects with further progression. Secondly, a consideration of the role of genetic factors in the vulnerability for the development of addiction at each stage, and thirdly, the further development of novel animal models, as well as human research paradigms, to increasingly define the behavioral, but also molecular neurobiological changes that occur at the various stages of drug use, abuse and addiction. Drs. Bickel and Johanson emphasized many of these needs relative to changes with progression in the natural history.
In our earliest work in 1964, possibly the major contribution was that of redefining what addiction is, that is, a paradigm shift (Dole et al., 1966a,b). We hypothesized that heroin (opiate) addiction is a disease, a metabolic disease of the brain, with resultant behaviors of “drug hunger” and drug self-administration, despite negative consequence to self and others. Further, we hypothesized that heroin addiction was not simply a criminal behavior, or due to a weak or antisocial personality. It should be noted that this specific hypothesis concerned “addiction,” not drug use or abuse. After making the decision that a new treatment approach should combine chronic pharmacological treatment, targeted, to the extent possible, to the specific site of action of heroin (then hypothesized, later proven), and coupled with behavioral treatment, we conducted a variety of studies at The Rockefeller Hospital in the first six months of 1964. The orally-effective synthetic opiate methadone had enjoyed minimal use in opiate detoxification, after early studies both at the USPHS Hospital in Lexington, KY, where it was explored simply for very short-term (7–14 day), multiple daily, tapering doses, in detoxification management of opiate addiction, and had also been studied in a few other laboratories possibly for management of pain (but in retrospect, with the unfortunate focus on acute pain, rather than chronic pain in opioid-experienced subjects.) After starting with low doses of methadone (20 to 40 mg/day), and slowly increasing single daily doses over a period of six to eight weeks, up to what we hypothesized would be a full treatment dose, offering “narcotic blockade,” that is, cross-tolerance against any effects of superimposed short-acting opiates, a dose that we then defined as 80–100mg per day orally administered (and now were defined as 80–150mg a day orally), we conducted a group of studies that certainly would be best classified as behavioral pharmacological studies. After stabilization on two different doses of methadone, a lower dose and then the higher dose, we conducted a random order, double-blinded, Latin square design series of studies in which several short- and long-acting opioids, including morphine, heroin, hydromorphone, and also methadone itself and saline, were injected intravenously against the background of daily stabilized moderate or high dose of methadone. Questions were asked of each volunteer subject after each injection, including, “What did you feel?”, “Was it a drug?”, “Did you like it?”, “Would you want it?”, and “What would you pay for it?” When the subjects were on the lower doses of methadone, we obtained responses to several of those questions. On the higher dose, the only response, on one day each week of daily double-blinded administration, was, “I feel the pins and needles; it’s like morphine, but where is the “high”? Where is the euphoria?” The cue of feeling the tingling sensation following histamine release by morphine was not sufficient to evoke any sense of high or euphoria on this one day each week, only annoyance that none occurred! Those studies were possibly the first in a university setting where a drug of abuse was given to subjects in a clinical research setting, after careful review by our local Ethics Committee (now called the Institutional Review Board). Many investigators in the Behavioral Pharmacology Society have been pioneers and strong advocates of such appropriate studies of drugs of abuse in a controlled clinical research setting.
Our very first work was published in 1966, after the presentation of the studies by Professor Vincent P. Dole, Jr., at the Association of American Physicians (Dole et al., 1966a,b). That paper, which actually reported the first work of 1964, was held until after presentation. The second set of studies, that is, translation from the Rockefeller Hospital into the “real world,” was conducted at the then Manhattan General Hospital, a local then proprietary institution, where some short-term detoxification care had been offered; these studies were conducted in early 1965 primarily by Dr. Marie Nyswander, and the publication reporting these studies included a one year follow-up of our original 1964 volunteer subjects studied in our initial research at the Rockefeller Hospital (Dole and Nyswander, 1965).
The natural history of drug abuse and addictions is extremely important to appreciate and to address in specific studies, both using appropriate animal models as well as, to the extent possible, in human research. According to the meta-analyses that have been conducted, approximately one in three to one in five who ever self-administer heroin will become opiate addicted; approximately one in eight to one in 18 who ever self-administer cocaine or alcohol will become addicted to those substances. Thus, the majority of persons do not become addicted. Primary prevention is undoubtedly most effective prior to any self-exposure to a drug of abuse. In the future, it may be possible to use targeted vaccines and selected medications in those with sporadic intermittent use who are willing to seek help at that point. Once regular use begins to occur, the molecular neurobiology of the brain begins to change, as has been shown now in human studies, as well as much more extensive animal studies and animal models. The changes that occur at this point include gene expression, with resultant changes in peptides and proteomics, integrated neurochemistry, synaptogenesis, and behavior. Further, these changes have been shown in many studies to be quite persistent, that is, they continue to be present days and weeks after final exposure to the drug of abuse in animal models, and, again, to the extent studied, in humans. It is at this point that effective treatments for each addiction will probably require a combination of targeted pharmacological treatment along with behavioral treatment. It has been repeatedly shown that less than 20% of former heroin addicts are able to be satisfactorily managed in the medication-free, abstinence-based mode with a majority first relapsing to other drugs and then to opiate use within one to two years.
Sandra Comer provided an excellent discussion of the documented effectiveness, both in animal models, and in the clinic, of opiate agonists and partial agonists, including methadone, LAAM, and buprenorphine (ideally combined with naloxone to prevent intravenous abuse; in the early 1970s, we combined naloxone with methadone, but only to find that methadone is “a boring drug” that is rarely abused by the parenteral route, although frequently used to self-maintain or self-detoxify by the oral route: see Kreek, 1973; 2000). However, Dr. Comer raised questions about the apparent paradoxical, but well-documented, findings that naltrexone, which is very effective in blocking the reinforcing effect of opiates both in animal models and in humans, has never turned out to be an effective pharmacological agent, except for modest, short-term effectiveness in treatment of special populations (e.g., parolees and health care professionals in some states only) who by law are not allowed access to agonist or partial agonist (i.e., methadone or buprenorphine) treatment. Our research, both in animal models and in humans, has documented that, although naltrexone, like methadone, buprenorphine and LAAM, was very effective in blocking the reinforcing or rewarding effects of a short-acting opiate, such as heroin, naltrexone has exactly the opposite effect on a major component of the stress-responsive system in mammals, that is, the hypothalamic-pituitary-adrenal (HPA) axis. Naltrexone activates the HPA axis, whereas methadone and buprenorphine first suppress the HPA axis and then, due to their long-acting properties, allow normalization with time in treatment. Our group and others have shown that such HPA activation is both sought by animals self-administering cocaine and alcohol, and in limited studies we have shown that it is desired by alcoholics; so perhaps an opiate antagonist such as naltrexone may be (and for alcohol has been shown to be) very effective for many alcoholics and stimulant addicts. In contrast, any activation of the HPA axis is identified by an active or former opiate addict as being the first stages of opiate withdrawal, or abstinence; thus it is considered extremely aversive and to be avoided if at all possible. Therefore, a mu opioid receptor agonist or partial agonist, especially one that allows normalization with time in treatment, through its long-acting pharmacokinetic or pharmacodynamic profile, would be expected, and has been shown, to have a beneficial effect for heroin and other opiate addicts in therapeutics, whereas an opiate antagonist, which itself activates the HPA axis directly (by blocking the usual mu opioid inhibition of the HPA axis), is rejected by the overwhelming majority of opiate addicts given such treatment (Kreek et al., 2002). Future and ongoing studies will determine whether or not steady-state sustained release administration of naltrexone by implant techniques will have a more favorable effect in heroin addicts.
The factors contributing to the vulnerability to develop a specific addiction include environmental factors of a variety of types, including pre-natal and post-natal events, contemporary issues such as setting, cues, along with psychiatric co-morbidity, and especially atypical stress responsivity. Many studies, both in animal models, and to a lesser extent in humans, have shown that chronic, but not acute or sub-acute, administration of each drug of abuse, in a mode similar to that used by addicts, will result in profound molecular neurobiological changes in the brain. Genetic factors also may play a significant role. Epidemiological studies have shown that over 25–60% of the relative risk of developing any addiction has a genetic basis, due to multiple variants of multiple different genes acting in combination to increase vulnerability for (or to increase protection against) developing an addiction when self-exposed. Each of these contributing factors needs to be addressed in behavioral pharmacological studies to the extent possible, in both in animal models as well as humans in the different stages of addiction. Further, we have hypothesized that personality factors such as impulsivity and risk-taking may play a much greater role in the initiation of drug use, and a moderate role in the progression from intermittent to regular use, whereas atypical stress responsivity may play a moderate role in the progression from intermittent drug use and a very major role in the progression to addiction. We have also hypothesized that drug-induced effects and genetic effects play a very major role in the final progression to addiction (Kreek et al., 2002, 2005, Kreek, 2006).
In 1994, we initiated collaborative work with Dr. Lei Yu to address our hypothesis that the mu opioid receptor gene might have variants, which, if functional, might in part contribute to the development of opiate addiction, as well as possibly other addictions that have been shown to involve the mu opioid receptor system. Further, we decided to study any moderate to high allelic frequency variant that involved amino acid change in the coding region of the gene, which was the initial focus of our study. We identified several gene variants (Bond et al., 1998). One of these is of relatively high allelic frequency (10.6% in an overall New York population; range from 7–20% in Caucasian populations, and higher and lower in other ethnic/cultural groups). This particular variant not only resulted in amino acid change, but was at a site of putative glycosylation. We therefore studied the receptor resulting from this A118G single nucleotide polymorphism (SNP) and compared it with the prototype receptor in specific molecular cellular constructs. We determined that this variant binds beta-endorphin three times more tightly than the prototype receptor (but only beta-endorphin, not any shorter endogenous opioids or exogenous opiates or opioids), and further, that after binding beta-endorphin, there was three-fold greater signal transduction through the G-protein coupled inwardly rectifying potassium (GIRK) channel system (Bond et al., 1998). With these findings of a functional variant, we hypothesized that specific aspects of human physiology that are under modulation by the mu opioid receptor system, might in fact be altered by the presence of one or two copies of this variant. We coined a term to describe such a phenomenon as “physiogenetics,” that is, a difference in response within an individual’s own physiological systems due to the presence of a functional gene variant, a word mimicking and paralleling “pharmacogenetics,” referring to differences in responses of some individuals and their families to specific medications due to hypothesized genetic variants; the variants causing these pharmacogenetic changes are just now being identified using molecular genetic techniques. We further hypothesized that this functional variant of the mu opioid receptor might be associated both with opiate addiction and also with alcoholism, two addictions that both have been documented to be closely related to the mu opioid receptor system, but two drugs of abuse that have opposite effects on stress responsivity, a physiological system in which we further hypothesized that this variant would play a major role because of the normal contribution of the mu opioid receptor activation in the tonic inhibition of the HPA axis (Bond et al., 1998, LaForge et al., 2000).
Finally, we hypothesized that alcoholics with one or two copies of this variant might respond more favorably to an opioid antagonist treatment. Wand and colleagues were the first to provide proof of principle of the physiogenetic hypothesis, first in a study of a very small number of subjects (7 with the A118G variant) and then a much larger study, as did other studies by Kranzler and other investigators. Each study has shown that whereas challenge with a specific mu-directed opioid antagonist will cause activation of the HPA axis in all subjects, as can be measured by ACTH or by serum cortisol levels, persons with one or two copies of the A118G variant respond with much greater activation of the HPA axis, as documented by a greater rise in serum cortisol (Wand et al., 2002, Hernandez-Avila et al., 2003, Chong et al., 2006). Further, we have recently shown that in healthy individuals with one or two copies of the variant, basal levels of serum cortisol are significantly higher than in those with the prototype variant; however, these increased levels were not elevated beyond the upper level of normal and would probably have no physiological or pathological significance, and could only be determined in a stress-minimized setting, such as our clinical research unit (Bart et al., 2006). Finally, in a very exciting study, all volunteers who had participated in the naltrexone trials for the treatment of alcoholism were invited to come back for further study; about one in six did so, and after obtaining informed consent, they were each genotyped. It was striking that the majority of those who had more days of abstinence, fewer days of drinking, and consumed less alcohol, were found to have one or two copies of the A118G variant (Oslin et al., 2003). Thus, both physiogenetics and pharmacogenetics related to this functional variant of the human mu opioid receptor gene have been documented (Kreek and LaForge, 2007; Kreek 2008).
Dr. de Wit gave an excellent presentation of some of her own exciting studies on the role of specific gene variants in specific aspects of components of drug use, abuse and addiction. Clearly, her presentation on the role of gender, personality and genetics and individual differences with respect to responses to drugs of abuse is very exciting and really emphasizes the importance in the future of routinely obtaining specific informed consents for genetics studies, even if those are not planned immediately in any human behavioral pharmacological study. Certainly, as more is understood about the natural history of the different addictions, and as the emphasis on translational research, but also as we call it, bi-directional translation research, is encouraged, it will be important to develop further novel animal models to address each of the stages of addiction, which may have different features in behavioral pharmacology, as discussed by Dr. Comer, as well as in studies of progression of drug abuse in human behavioral pharmacology, and also in behavioral economics as discussed by Dr. Bickel.
In recent years, our laboratory, along with Drs. Klaus Miczek and George Koob, has focused on extended-access self-administration models (Koob and Kreek, 2007). In one study conducted, one set of rats was given a choice to escalate or not escalate their self-administered dose of morphine, whereas another group was not given such a choice (Kruzich et al., 2003). All the animals were studied in extended 18-hour access sessions. At the end of the chronic sessions, the availability of mu opioid receptor system for activation was measured as [35S] GTPγS binding; this was found to be significantly reduced in both the thalamus and the amygdala of animals that had escalated their dose of morphine during their extended access chronic sessions, as contrasted with animals that had had a steady dose of morphine available for self-administration (Kruzich et al., 2003). Another sequence of studies examined the effects of extended access (10 hours) and also widely different cocaine doses, ranging from the usual 0.25 mg/kg per infusion up to 2.0mg/kg per infusion The cumulative amount of cocaine self-administered each day was much higher in animals given the higher doses, and over the ten hours of self-administration, there was no plateau in the amount of self-administration, despite the profound differences in doses. Further, extended-access self-administration led to disruption of HPA axis activity by the end of the first self-administration day, and even following four days of withdrawal from five days of extended-access cocaine self-administration, normalization of circadian rhythm of the HPA axis had not yet occurred (Mantsch et al., 2000). A further study examined the effects of long-access (10 hours) v. short-access over 14 days of cocaine self-administration by extinction and re-priming with cocaine. Intriguingly, pro-opiomelanocortin (POMC) mRNA, glucocorticoid receptor mRNA and memantine 2 receptor mRNA were all significantly reduced in animals that had been provided long access as opposed to those with short access (Mantsch et al, 2004). Therefore, our group and others are now finding that, in the self-administration model of addiction, long-access, mimicking the human pattern of cocaine or heroin use, causes far more extensive and dramatic molecular and cellular neurobiological changes than short-access, and further, the groups of Miczek and, especially, Koob, have shown that behaviors are profoundly different in relapse.
I want to thank the organizers again for inviting me to be part of this wonderful symposium, and to help celebrate the 50th Anniversary of the Behavioral Pharmacology Society. I think the research that has been accomplished by these investigators individually and collectively has been absolutely outstanding and has made major contributions to so many aspects related to drug use, drug abuse, and addiction, but further, as stated by Dr. Wenger in his introductory remarks, has made huge contributions to a better understanding of behavior.
Acknowledgments
This research was supported by R37 DA006526 (Bickel), DA11692 (Bickel), Tobacco Settlement Funds 2004-2005 (Bickel), DA09236 (Comer), DA16759 (Comer), DA02812 (de Wit), DA09378 (Higgins), DA14028 (Higgins), DA08076 (Higgins), DA047656 (Wenger), AA014977 (Wenger).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The following review article presents proceedings of a symposium at the 50th annual meeting of the U.S. Behavioral Pharmacology Society. The topic of the symposium was the development of human behavioral pharmacology with an eye to future challenges. This symposium appears in these pages to highlight the important role of behavioral methodology in the development of pharmacological research in human participants, especially in the United States. The symposium was followed by presentations by two discussants, Drs. Chris-Ellyn Johanson and Mary Jeanne Kreek. Both presentations are included in this article as they provide a subjective but highly informed personal sense of human behavioral pharmacology by two of the seminal contributors in this area of research.
References
- Ainslie G. Impulse control in pigeons. Journal of the Experimental Analysis of Behavior. 1974;21:485–489. doi: 10.1901/jeab.1974.21-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Proc. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Alsene KM, Deckert J, Sand P, de Wit H. Increased anxiety after acute caffeine associated with adenosine A2A receptor polymorphism. Neuropsychopharmacology. 2003;28:1694–1702. doi: 10.1038/sj.npp.1300232. [DOI] [PubMed] [Google Scholar]
- Altman JL, Meyer RE, Mirin SM, McNamee HB. Opiate antagonists and the modification of heroin self-administration behavior in man: An experimental study. Int J Addict. 1976;1:485–499. doi: 10.3109/10826087609056165. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Azatian A, Papiasvilli A, Joseph H. A study of the use of clonidine and naltrexone in the treatment of opioid addiction in the former USSR. J Addict Dis. 1994;13:35–52. doi: 10.1300/J069v13n01_04. [DOI] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting differs between current and never-smokers across commodities, sign, and magnitudes. J Abn Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12:559. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bart G, LaForge KS, Borg L, Lilly C, Ho A, Kreek MJ. Altered levels ofbasal cortisol in healthy subjects with a 118G allele in exon 1 of the mu opioid receptor gene. Neuropsycho-pharmacology. 2006;31:2313–2317. doi: 10.1038/sj.npp.1301128. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha J. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behavioural Brain Research. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economics understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Stitzer ML, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: Comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther. 1988;43:72–78. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Crean JP, Badger GJ. Buprenorphine dosing every 1, 2, or 3 days in opioid-dependent patients. Psychopharmacology. 1999a;146:111–118. doi: 10.1007/s002130051096. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999b;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measure of pharmacological reinforcement with behavioral economics: A theoretical proposal. Psychopharmacol. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and Alcohol Dependence. 2007;90S:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow G, Griffiths R, Liebson I. Experimental human drug self-administration: Methodology and application to the study of sedative abuse. Pharmacol Reviews. 1976;27:523–531. [PubMed] [Google Scholar]
- Bigelow GE, Griffiths RR, Liebsen IA. Pharmacological influences upon human ethanol self-administration. Adv Exp Med Bio. 1977;85B:523–538. doi: 10.1007/978-1-4615-9038-5_33. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type-1/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Singlenucleotide polymorphism in the human mu-opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proc. Natl. Acad. Sci. USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghans L, Golsteyn BHH. Time discounting and the body mass index: Evidence from the Netherlands. Economics & Human Biology. 2006;4:39. doi: 10.1016/j.ehb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA. 1985;254:81–83. [PubMed] [Google Scholar]
- Callahan EJ, Rawson RA, McCleave B, Arias R, Glazer M, Liberman RP. The treatment of heroin addiction: naltrexone alone and with behavior therapy. Int J Addict. 1980;15:795–807. doi: 10.3109/10826088009040057. [DOI] [PubMed] [Google Scholar]
- Catania AC. Concurrent performances: A baseline for the study of reinforcement magnitude. Journal of Experimental Analysis of Behavior. 1963;6:299–300. doi: 10.1901/jeab.1963.6-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania AC. In: Experimental Analysis of Behavior. Glossary. In Iversen and Lattal, editor. Elsevier: Science Publishers BV; 1991. pp. G32–G33. [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu- opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsycho-pharmacology. 2006;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Chung SH, Herrnstein RJ. Choice and delay of reinforcement. Journal of the Experimental Analysis of Behavior. 1967;10:67–74. doi: 10.1901/jeab.1967.10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger C. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, et al. Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping. 2005;25:409–423. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC, De Witte PH, Maroli AN, Awouters F, Niemegeers CJE, Janssen PAJ. Self-administration of the analgesic suprofen in arthritic rats: evidence of mycobacterium butyricum-induced arthritis as an experimental model of chronic pain. Life Sci. 1980;27:921–928. doi: 10.1016/0024-3205(80)90101-0. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Meert TH, De Witte PH, Schmitt P. Further evidence validating adjuvant arthritis as an experimental model of chronic pain in the rat. Life Sci. 1982;31:67–75. doi: 10.1016/0024-3205(82)90402-7. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain. 2001;91:33–45. doi: 10.1016/s0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Buprenorphine sublingual tablets: effects on IV heroin self-administration by humans. Psychopharmacol. 2001;154:28–37. doi: 10.1007/s002130000623. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacol. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Walker EA, Collins ED. Buprenorphine/naloxone reduces the reinforcing and subjective effects of heroin in heroin-dependent volunteers. Psychopharmacol. 2005;181:664–675. doi: 10.1007/s00213-005-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, et al. Injectable, sustained-release naltrexone for the treatment of opioid dependence: A randomized, placebo-controlled trial. Arch Gen Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of drugs. Drug and Alcohol Dependence. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control/Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature Neuroscience. 2005;8:1704. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Deneau GE, Yanagita T, Seevers M. Self-administration of psychoactive substances by the monkey – A measure of psychological dependence. Psychopharmacol (Berl) 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- de Wit H. Relationships between personality and acute subjective responses to stimulant drugs. In: Earleywine M, editor. Mind-Altering Drugs: The Science of Subjective Experience. New York: Oxford University Press Inc.; 2005. pp. 258–274. [Google Scholar]
- de Wit H, Chutuape MA. Increased ethanol choice in social drinkers following ethanol preload. Behav Pharmacol. 1993;4:29–36. [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Individual differences in the behavioral and subjective effects of amphetamine and diazepam. Drug Alcohol Depend. 1986;16:341–360. doi: 10.1016/0376-8716(86)90068-2. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcoholism: Clin Exp Res. 1987;11:52–59. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Marley J, Jacobs EA. Delay discounting by pathological gamblers. J App Behav Anal. 2003;36:449–458. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Nyswander ME. A medical treatment for diacetylmorphine (heroin) addiction. JAMA. 1965;193:646–650. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade: A medical technique for stopping heroin use by addicts. Trans. Assoc. Am. Phys. 1966a;79:122–136. [PubMed] [Google Scholar]
- Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch. Intern. Med. 1966b;118:304–309. [PubMed] [Google Scholar]
- Dom G, D’haene PD, Hulstijn W, Sabbe B. Impulsivity in abstinent early- and late-onset alcoholics: differences in self-report measures and a delay discounting task. Addiction. 2006;101:50–59. doi: 10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Donlin WD, Knealing TW, Silverman K. Employment-based reinforcement in the treatment of drug addiction. In: Higgins ST, Silverman K, Heil SH, editors. Contingency Management in Substance Abuse Treatment. New York: Guilford Press; 2008. pp. 314–333. [Google Scholar]
- Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction. 2005;100:1496–1509. doi: 10.1111/j.1360-0443.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- Drebing CE, VanOrmer EA, Krebs C, Rosenheck R, Rounsaville B, Herz L, et al. The impact of enhanced incentives on vocational rehabilitation outcomes for dually-diagnosed veterans. J Appl Behav Anal. 2005;38:359–372. doi: 10.1901/jaba.2005.100-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, King SM, McGue M, Iacono WG. Personality traits and the development of nicotine, alcohol, and illicit drug disorders: prospective links from adolescence to young adulthood. J Abn Psychol. 2006;115:26–39. doi: 10.1037/0021-843X.115.1.26. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacol. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioral economics of cigaette purchases in smokers: the effects of nicotine deprivation. Psychopharmacol. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Field M, Christiansen P, Cole J, Goudie A. Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction. 2007;102:579–586. doi: 10.1111/j.1360-0443.2007.01743.x. [DOI] [PubMed] [Google Scholar]
- Findley JP. An experimental outline for building and exploring multi-operant behavior repertoires. J. Exp. Anal. Behav. 1962;5 Suppl:113–166. doi: 10.1901/jeab.1962.5-s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley JP, Robinson WW, Peregrino L. Addiction to secobarbital and chlordiazepoxide in the rhesus monkey by means of a self-infusion preference procedure. Psychopharmacologia. 1972;26:93–114. doi: 10.1007/BF00422097. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Brit J Addiction. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Assessment of abuse liability of stimulant drugs in humans: A methodological survey. Drug Alcohol Depend. 1991;28:3–48. doi: 10.1016/0376-8716(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration by humans. J Pharmacol Exp Ther. 1990;253:760–770. [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Shahan TA, Badger GJ. Behavioral economics of human drug self-administration: Progressive ratio versus random sequences of response requirements. Behav Pharmacol. 2001;12:343–347. doi: 10.1097/00008877-200109000-00005. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discounting delayed heroin and money. Psychopharmacol. 2002;163 doi: 10.1007/s00213-002-1159-2. 174-12. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH, Schuster CR. Morphine: Conditioned increases in self-administration in rhesus monkeys. Science. 1969;166:1306–1307. doi: 10.1126/science.166.3910.1306. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nature Reviews: Genetics. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Green L, Hale S. Practical implications of the matching law. Journal of Applied Behavior Analysis. 1984;17:367–380. doi: 10.1901/jaba.1984.17-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Johanson CE, Schuster CR. Opioid reinforcement in heroin-dependent volunteers during outpatient buprenorphine maintenance. Drug Alc Depend. 1999;56:191–203. doi: 10.1016/s0376-8716(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Johanson CE, Moody DE, Woods JH, Kilbourn MR, Koeppe RA, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28:2000–2009. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson IA. Human sedative self-administration: Effects of interingestion interval and dose. J Pharmacol Exp Ther. 1976;197:488–494. [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Henningfield JE. Similarities in animal and human drug-taking behavior. In: Mello NK, editor. Advances in Substance Abuse. JAI Press Inc.; 1980a. pp. 1–90. [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson IA, Kaliszak JE. Drug preference in humans: Double-blind choice comparison of pentobarbital, diazepam, and placebo. J Pharmacol Exp Ther. 1980b;215:649–661. [PubMed] [Google Scholar]
- Griffiths RR, Henningfield JE, Bigelow GE. Human cigarette smoking: manipulation of number of puffs per bout, interbout interval, and nicotine dose. J Pharmacol Exp Ther. 1982;220:256–265. [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: An efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Gross J, Johnson J, Sigler L, Stitzer ML. Dose effect of nicotine gum. Addict Behav. 1995;20:371–381. doi: 10.1016/0306-4603(94)00078-d. [DOI] [PubMed] [Google Scholar]
- Han SC, Evans SM. Sex and Drugs. In: Earleywine M, editor. Mind-Altering Drugs: The Science of Subjective Experience. New York: Oxford University Press Inc.; 2005. pp. 183–216. [Google Scholar]
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther. 2006;79:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addictive Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Henningfield JE. Nicotine medications for smoking cessation. N Engl J Med. 1995;333:1196–1203. doi: 10.1056/NEJM199511023331807. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Cohen C, Heishman SJ. Drug self-administration methods in abuse liability evaluation. Brit J Addict. 1991;86:1571–1577. doi: 10.1111/j.1360-0443.1991.tb01750.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Wand G, Luo X, Gelernter J, Kranzler HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) Am. J. Med. Genet. 2003;118:60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. J Exp Anal Behav. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman GM, Gibb SP. Delay discounting in college cigarette chippers. Behav Pharmacol. 2006;17:669–679. doi: 10.1097/FBP.0b013e3280116cfe. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lissier JP, Abel RL, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchel SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacol. 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in response to ethanol and triazolam. Behav Pharmacol. 1999;10:283–295. doi: 10.1097/00008877-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Smoking cessation. N Engl J Med. 1999;341:610–611. doi: 10.1056/NEJM199908193410813. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Miller SA. Nicotine gum to help stop smoking. JAMA. 1984;252:2855–2858. [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacol. 1984;83:82–87. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Goldstein MG, Hurt RD, Shiffman S. Recent advances in the pharmacotherapy of smoking. JAMA. 1999;281:72–76. doi: 10.1001/jama.281.1.72. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Schuster CR, Smith BB, Blachley PH. Comparison of acetylmethadol and methadone in the treatment of long-term heroin users. A pilot study. JAMA. 1970;211:1834–1836. [PubMed] [Google Scholar]
- Jaffe JH, Senay EC, Schuster CR, Renault PR, Smith B, DiMenza S. Methadyl acetate vs. methadone. A double-blind study in heroin users. JAMA. 1972;222:437–442. [PubMed] [Google Scholar]
- Jasinski DR. History of abuse liability testing in humans. British J Addict. 1991;86:1559–1562. doi: 10.1111/j.1360-0443.1991.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Henningfield JE. Pharmacological treatment of tobacco dependence. Pharmacol Biochem Behav. 1988;30:279–294. doi: 10.1016/0091-3057(88)90456-x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacol. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL. A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bull. Narc. 1978;30:43–54. [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. A choice procedure for drug reinforcers: Cocaine and methylphenidate in the rhesus monkey. J. Pharmacol. Exp. Ther. 1975;193:676–688. [PubMed] [Google Scholar]
- Johanson CE, Schuster CR. Animal models of drug self-administration. Adv Subst Abuse. 1981;2:219–297. [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-Amphetamine. Psychopharmacology. 1980;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL, Bonese K. Self-administration of psychomotor stimulant drugs: The effects of unlimited access. Pharmacol Biochem Behav. 1976;4:45–51. doi: 10.1016/0091-3057(76)90174-x. [DOI] [PubMed] [Google Scholar]
- Johanson C, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacol. 1980a;71:269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: d-amphetamine. Psychopharmacol. 1980b;71:275–279. doi: 10.1007/BF00433062. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Schuster CR, Hatsukami D, Vocci F. Abuse liability assessment of CNS drugs. Drug and Alcohol Dependence. 2003;70:S1–S114. doi: 10.1016/s0376-8716(03)00095-4. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267:2750–2755. [PubMed] [Google Scholar]
- Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995;40:17–25. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- Jones HE, Johnson RE, Jasinski DR, O’Grady KE, Chisholm CA, Choo RE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: Effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacol. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJH, de Wit H. Acute effects of amphetamine during the early and late follicular phase of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Goodrich GM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Pyschopharmacol. 2007;15:37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- Katz JL. Models of relative reinforcing efficacy of drugs and their predictive utility. Behav Pharmacol. 1990;1:283–301. doi: 10.1097/00008877-199000140-00003. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, et al. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacol (Berl) 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discounting rates for delayed rewards than non-drug using controls. J Exp Psychol: Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Kleber HD. Strategies to improve compliance with narcotic antagonists. Am J Drug Alcohol Abuse. 1984;10:249–266. doi: 10.3109/00952998409002784. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Jarvik ME, Gritz ER. Nicotine regulation and cigarette smoking. Clin Pharmacol Ther. 1975;17:93–97. doi: 10.1002/cpt197517193. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Plasma and urine levels of methadone. NY State J Med. 1973;73:2773–2777. [PubMed] [Google Scholar]
- Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction:History, recent molecular and neurochemical research and the future in mainstream medicine. Ann. N.Y. Acad. Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Endorphins, gene polymorphisms, stress responsivity, and special addictions: Selected topics. In: Madras B, Colvis CM, Pollock JD, et al., editors. Cell Biology of Addiction. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. pp. 63–92. [Google Scholar]
- Kreek MJ. Role of a functional human gene polymorphism in stress responsivity and addictions. Clin. Pharmacol. Ther. 2008;83:615–618. doi: 10.1038/clpt.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS. Stress responsivity, addiction, and a functional variant of the human mu opioid receptor gene. Molec. Interv. 2007;7:74–78. doi: 10.1124/mi.7.2.7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat. Rev. Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk-taking, stress responsivity, and vulnerability to drug abuse and addiction. Nat. Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A, et al. Contingency management for smoking cessation in adolescent smokers. Exp Clin Psychopharmacol. 2006;14:306–310. doi: 10.1037/1064-1297.14.3.306. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich P, Chen ACH, Unterwald EM, Kreek MJ. Subject-regulated dosing alters morphine self-administration behavior and morphine-stimulated [35S]GTPγS binding. Synapse. 2003;47:243–249. doi: 10.1002/syn.10173. [DOI] [PubMed] [Google Scholar]
- LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: Potential implications for addictions. Eur. J. Pharmacol. 2000;410:249–268. doi: 10.1016/s0014-2999(00)00819-0. [DOI] [PubMed] [Google Scholar]
- Liebson RA, Tommasello A, Bigelow GE. A behavioral treatment of alcoholic methadone patients. Ann Intern Med. 1978;89:342–343. doi: 10.7326/0003-4819-89-3-342. [DOI] [PubMed] [Google Scholar]
- Leventhal A, Waters A, Boyd S, Moolchan E, Lerman C, Pickworth W. Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Exp Clin Psychopharmacol. 2007;15:21–36. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops W, Glaser PEA, Hays LR, Rush CR. Acute administration of the GABA reuptake inhibitor tiagabine does not alter the effects of oral cocaine in humans. Drug Alcohol Depend. 2004;76:81–91. doi: 10.1016/j.drugalcdep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, Klett J. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry. 1996;53 doi: 10.1001/archpsyc.1996.01830050035005. 410-407. [DOI] [PubMed] [Google Scholar]
- Lott DC, Kim SJ, Cook EH, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacol. 2005;30:602–609. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, et al. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacol (Berl) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to addiction. Exp Clin Psychopharmacol. 2007;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Pychopharmacol. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5(3):256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J. Pharmacol. Exp. Ther. 2000;294:239–247. [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl.) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: A randomized controlled trial. Arch Gen Psychiatry. 2005;62:1157–1164. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Nat Acad Sci. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analysis of Behavior. Vol. 5. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- McCaul ME, Stitzer ML, Bigelow GE, Liebson IA. Methadone detoxification: Effects of methadone dose versus time in treatment. NIDA Res Monograph. 1984;49:269–274. [PubMed] [Google Scholar]
- McClure SM, Laibson DL, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McDowell D, Nunes EV, Seracini AM, Rothenberg J, Vosburg SK, Ma GJ, et al. Desipramine treatment of cocaine-dependent patients with depression: A placebo-controlled trial. Drug Alc Depend. 2005;80:209–221. doi: 10.1016/j.drugalcdep.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Mello NK. Behavioral pharmacology of narcotic antagonists. NIDA Res Monogr. 1978;19:126–141. [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Operant analysis of drinking patterns of chronic alcoholics. Nature (London) 1965;206:43–46. doi: 10.1038/206043a0. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–659. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther. 1981;216:45–54. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Behavioral and biochemical interrelations in alcoholism. Annu Rev Med. 1976;27:321–333. doi: 10.1146/annurev.me.27.020176.001541. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Experimental analysis of drinking behavior of chronic alcoholics. Ann N Y Acad Sci. 1966;133:828–845. doi: 10.1111/j.1749-6632.1966.tb50930.x. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Kuehnle JC, Greenberg I, Mello NK. Operant acquisition of marihuana in man. J Pharmacol Exp Ther. 1976;198:42–53. [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and nonsmokers. Psychopharmacol. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Effects of short-term nicotine deprivation on decision-making: Delay, uncertainty, and effort discounting. Nic Tob Res. 2004;6:819–828. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D’Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcoholism: Clin Exp Res. 2005;29:2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:264–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human Brain Mapping. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, O’Hara R, Fisch HU, Yesavage JA. Effects of menstrual cycle and female sex steroids on ethanol pharmacokinetics. Alcoholism: Clin Exp Res. 1999;23:250–255. [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Badger GJ, Bickel WK. Needle sharing in opioid-dependent outpatients: Psychological processes underlying risk. Drug Alcohol Depend. 2000;60:259–266. doi: 10.1016/s0376-8716(00)00111-3. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacol. 2005;182:508–515. doi: 10.1007/s00213-005-0110-8. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, et al. A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nic Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Meeker J, White W, Wilson A. The discriminative stimulus and reinforcing effects of nicotine in humans following nicotine pretreatment. Behav Pharmacol. 2001a;12:35–44. doi: 10.1097/00008877-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nic Tob Res. 2001b;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abn Psychol. 2001a;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacol. 2001b;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A national drug abuse treatment clinical trial network study. Arch Gen Psychiatry. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Pickens R. A behavioral program for treatment of drug dependence. NIDA Res Monograph. 1979;25:44–54. doi: 10.1037/e497382006-006. [DOI] [PubMed] [Google Scholar]
- Pickens R, Harris WC. Self-administration of d-amphetamine by rats. Psychopharmacologia. 1968;12:158–163. doi: 10.1007/BF00401545. [DOI] [PubMed] [Google Scholar]
- Pickens R, Bigelow G, Griffiths RR. An experimental approach to treating chronic alcoholism: A case study and one-year follow-up. Behav Res Ther. 1973;11:321–325. doi: 10.1016/0005-7967(73)90010-7. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Johnson A. A computational model of craving and obsession. Ann New York Acad Sci. 2007;16:324–339. doi: 10.1196/annals.1390.014. [DOI] [PubMed] [Google Scholar]
- Reif A, Lesch KP. Toward a molecular architecture of personality. Behav Brain Res. 2003;139:1–20. doi: 10.1016/s0166-4328(02)00267-x. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay and probability discounting as related to cigarette smoking status in adults. Behav Proc. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Pedrosi B, Rosenthal L, Zorick F, Roth T. Hypnotic self-administration: Forced-choice versus single-choice. Psychopharmacol. 1997;133:121–126. doi: 10.1007/s002130050381. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Dissociation executive functions of the prefrontal cortex. Philosophical Transactions of the Royal Society B: Biological Sciences. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Murphy ER. Behavioural pharmacology: 40+ years of progress, with a focus on glutamate receptors and cognition. Trends Pharmacol Sci. 2006;27(3):141. doi: 10.1016/j.tips.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Jarvik ME, Rose KD. Transdermal administration of nicotine. Drug Alcohol Depend. 1984;13:209–213. doi: 10.1016/0376-8716(84)90061-9. [DOI] [PubMed] [Google Scholar]
- Rowlett JK. A labor-supply analysis of cocaine self-administration under progressive-ratio schedules: Antecedents, methodologies, and perspectives. Psychopharmacol. 2000;153:1–16. doi: 10.1007/s002130000610. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Substance Abuse and Mental Health Services Administration 2005 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2006. [Google Scholar]
- Sanfey AG, Loewenstein G, McClure SM, Cohen JD. Neuroeconomics: Cross-currents in research on decision-making. Trends in Cognitive Sciences. 2006;10(3):108. doi: 10.1016/j.tics.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Olmstead R, Mody FV, Doan K, Franzon M, Jarvik ME, et al. Efficacy of a nicotine spray in smoking cessation: a placebo-controlled double-blind trial. Addiction. 1995;90:1671–1682. doi: 10.1046/j.1360-0443.1995.901216719.x. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Smith BB, Jaffe JH. Drug abuse in heroin users. An experimental study of self-administration of methadone, codeine, and pentazocine. Arch Gen Psychiatry. 1971;24:359–362. doi: 10.1001/archpsyc.1971.01750100069010. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: A behavioral economic analysis. Psychopharmacol. 1999;147:210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Wood MD. Subjective Effects of Alcohol II. In: Earleywine M, editor. Mind-Altering Drugs: The Science of Subjective Experience. New York: Oxford University Press Inc.; 2005. pp. 135–153. [Google Scholar]
- Sher KJ, Trull TJ, Bartholow B, Vieth A. Personality and alcoholism: issues methods, and etiological processes. In: Blane H, Leonard K, editors. Psychological theories of drinking and alcoholism. 2nd ed. New York: Plenum; 1999. pp. 55–105. [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacol. 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Moody DE, Nuwayser ES, Bigelow GE. An injection depot formulation of buprenorphine: Extended bio-delivery and effects. Addiction. 2006;101:420–432. doi: 10.1111/j.1360-0443.2006.01348.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Kirby KC, Griffiths RR. Modulation of drug reinforcement by behavioral requirements following drug ingestion. Psychopharmacol. 1994;114:243–247. doi: 10.1007/BF02244844. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, et al. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Arch Gen Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CG, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Wong CJ, Hampton J, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: Three year abstinence outcomes. Exp Clin Psychopharmacol. 2002;10:228–240. doi: 10.1037//1064-1297.10.3.228. [DOI] [PubMed] [Google Scholar]
- Skinner BF. In: Animal research in the pharmacotherapy of mental disease. Cole JO, Gerard RW, editors. Washington, D.C.: National Academy of Sciences. Psychopharmacology: Problems in evalution; 1959. (NAS-NRC Publication No. 583) [Google Scholar]
- Sobel B-FX, Sigmon SC, Griffiths RR. Transdermal nicotine maintenance attenuates the subjective and reinforcing effects of intravenous nicotine, but not cocaine or caffeine, in cigarette-smoking stimulant abusers. Neuropsychopharmacol. 2004;29:991–1003. doi: 10.1038/sj.npp.1300415. [DOI] [PubMed] [Google Scholar]
- Spragg SDS. Morphine addiction in chimpanzees. Comp Psychol Monogr. 1940;15:1–132. [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: A review. Psychopharmacol. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stern KN, Chait LD, Johanson CE. Reinforcing and subjective effects of oral tripelennamine in normal human volunteers. Behav Pharmacol. 1989;1:161–167. [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for carbon monoxide reductions: Within-subject effects of pay amount. J Appl Behav Anal. 1984;17:477–483. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE, Liebson IA. Comparison of three outpatient methadone detoxification procedures. NIDA Res Monograph. 1982;41:239–245. [PubMed] [Google Scholar]
- Stitzer ML, McCaul ME, Bigelow GE, Liebson IA. Oral methadone self-administration: Effects of dose and alternative reinforcers. Clin Pharmacol Ther. 1983;34:29–35. doi: 10.1038/clpt.1983.124. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Iguchi MY, Felch LI. Contingent take-home incentives: Effects on drug use of methadone maintenance patients. J Consult Clin Psychol. 1992;60:927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Goldfarb T, Fink R, Jarvik ME. Influencing cigarette smoking with nicotine antagonists. Psychopharmacologia. 1973;28:247–259. doi: 10.1007/BF00429305. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PEA, Rush CR. Reinforcing effects of modafinil: Influence of dose and behavioral demands following drug administration. Psychopharmacol. 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: Influence of sensation-seeking status. Addictive Behav. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Dose response effects of methadone in the treatments of opioid dependence. Ann Intern Med. 1993;19:23–27. doi: 10.7326/0003-4819-119-1-199307010-00004. [DOI] [PubMed] [Google Scholar]
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate vs. high dose methadone in the treatment of opioid dependence: A randomized trial. JAMA. 1999;281:1000–1005. doi: 10.1001/jama.281.11.1000. [DOI] [PubMed] [Google Scholar]
- Strain EC, Moody DE, Stoller KB, Walsh SL, Bigelow GE. Relative bioavailability of different buprenorphine formulations under chronic dosing conditions. Drug Alcohol Depend. 2004;74:37–43. doi: 10.1016/j.drugalcdep.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Strona FV, McCright J, Hjord H, Ahrens K, Tierney S, Shoptaw S, et al. The acceptability and feasibility of the Positive Reinforcement Opportunity Project: A community-based contingency management methamphetamine treatment program for gay and bisexual men in San Francisco. J Psychoactive Drugs. 2006;3 Suppl 3:377–383. doi: 10.1080/02791072.2006.10400601. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: Antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacol. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective and physiological effects of MDMA in humans: A comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacol. 2007;189:565–573. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- Tarter R, Vanyukov M, Giancola P, Dawes M, Blackson T, Mezzich A, et al. Etiology of early age onset substance use disorder: a maturational perspective. Dev Psychopathol. 1999;11:657–683. doi: 10.1017/s0954579499002266. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Thompson T, Schuster CR. Morphine self-administration, food-reinforced and avoidance behaviors in rhesus monkeys. Psychopharmacol. 1964;5:87. doi: 10.1007/BF00413045. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vander Weele J, Qaadir A, Palmer AA, Cook EH, de Wit H. Assoication between the Casein Kinase 1 Epsilon gene region and subjective response to D-amphetamine. Neuropsychopharmacol. 2006;31:1056–1063. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacol. 2004;47 Supplement 1:3. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Vuchinich R, Simpson C. Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, et al. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: Method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weiller C, Rijntjes M. Learning, plasticity, and recovery in the central nervous system. Exp Brain Res. 1999;V128:134. doi: 10.1007/s002210050828. [DOI] [PubMed] [Google Scholar]
- White T, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol. 2007;15(2):176. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]
- Zacny JP, McKay MA, Toledano AY, Marks S, Young CJ, Klock PA, et al. The effects of a cold water immersion stressor on the reinforcing and subjective effects of fentanyl in healthy volunteers. Drug Alc Depend. 1996;42:133–142. doi: 10.1016/0376-8716(96)01274-4. [DOI] [PubMed] [Google Scholar]


