Abstract
Objective
In a murine model of antibiotic-refractory Lyme arthritis, the numbers of T regulatory cells (Treg) are dramatically reduced. Our goal was to examine Treg numbers and function in human patients with antibiotic-refractory Lyme arthritis.
Methods
CD4+ T cell subsets were enumerated in peripheral blood (PB) and synovial fluid (SF) in 12 patients with antibiotic-refractory arthritis and 6 with antibiotic-responsive arthritis. Treg function was examined using Borrelia-specific and non-specific Treg proliferation assays.
Results
In both patient groups, IFN-γ+ TH1 cells in SF were abundant and enriched (~50% of CD4+ T cells). In patients with antibiotic-refractory arthritis, the median percentages of FoxP3+ Treg were significantly higher in SF than PB (12% versus 6%) (P<0.01) or in SF in patients with antibiotic-responsive arthritis (12% versus 5%) (P=0.04). Moreover, in the refractory group, a higher percentage of Treg in SF correlated with a shorter duration to resolution of arthritis (r = −0.74, P = 0.006). In contrast, patients with fewer Treg had suboptimal responses to DMARDs and longer duration of arthritis after antibiotics, and they often required synovectomies for arthritis resolution. In each group, Treg in SF dampened B. burgdorferi-specific proliferative responses, and in 2 patients with refractory arthritis, Treg were functional in non-specific suppression assays.
Conclusions
Treg were functional in patients with antibiotic-refractory arthritis, and in some patients, large numbers of these cells in SF appeared to participate in arthritis resolution. However, as in the murine model, patients with refractory arthritis and low numbers of Treg seemed unable to resolve synovial inflammation.
Lyme arthritis, which is caused by the tick-borne spirochete Borrelia burgdorferi, is characterized by intermittent or persistent inflammation in a few large joints, especially the knee, over a period of several years (1). In most patients, the joint infection can be treated successfully with oral antibiotic therapy for 1 or 2 months, or intravenous (IV) antibiotics for 1 month, and the arthritis resolves (2). However, in about 10% of patients, particularly in those infected with highly inflammatory B. burgdorferi strains (3), the arthritis in one or both knees persists for more than 3 months after 2–3 months of oral or IV antibiotics, or both, called antibiotic-refractory Lyme arthritis.
In such patients, polymerase chain reaction (PCR) results for B. burgdorferi DNA in synovial fluid (SF) are usually negative after oral and IV antibiotic therapy (3), cellular and humoral immune responses to B. burgdorferi antigens decline (4,5), and break-through cases of active infection have rarely been observed during the post-antibiotic period (2). Thus, synovitis in patients with antibiotic-refractory arthritis may persist after the near or total eradication of spirochetes from the joint with antibiotic therapy.
The duration of antibiotic-refractory arthritis is variable. In a previous analysis of 67 patients, the median duration from the initiation of antibiotics to the resolution of arthritis was 11 months (range, 4–44 months) (2). In the post-antibiotic period, we usually treat with a non-steroidal anti-inflammatory agent (NSAID) and a disease modifying anti-rheumatic drug (DMARD) (2). If patients have only a minimal-to-moderate response after 12–18 months, we consider arthroscopic synovectomy.
Antibiotic-refractory Lyme arthritis shares certain pathogenetic themes with other forms of chronic inflammatory arthritis, particularly rheumatoid arthritis (RA). These include similar synovial histology (6,7), HLA-DR associations with the DRB1*0401 and 0101 alleles (8–10), a dominant TH1 response in SF and synovial tissue (11,12), and high SF levels of pro-inflammatory cytokines and chemokines (13–15), especially CXCL9 and CXCL10, which are strong chemoattractants for CD4+ and CD8+ T effector cells (Teff). We have postulated that antibiotic-refractory arthritis may result from infection-induced, tissue-specific autoimmunity within affected synovia (16).
The autoimmunity hypothesis has been reinforced recently by the development of a murine model (17). In this model, both the human HLA-DR4 transgene, which is associated with antibiotic-refractory arthritis, and lack of the CD28 co-receptor, which leads to dramatically reduced numbers of T regulatory cells (Treg) (18), are necessary for persistent synovitis after antibiotic therapy. Mice that lack only the CD28 co-receptor, without the HLA-DR4 transgene, do not develop persistent synovitis after treatment (19); and similarly, mice that lack the CD28 co-receptor and have the human HLA-DR11 transgene, which is associated with antibiotic-responsive arthritis, do not develop post-treatment synovitis (20). These outcomes in mice support the HLA-DR findings in human patients with Lyme arthritis (8), but Treg numbers and function have not yet been examined in human Lyme arthritis.
In this study, we enumerated CD4+ T cell subsets, including Treg, in peripheral blood (PB) and SF in 18 patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis. In those with antibiotic-refractory arthritis, a higher percentage of Treg correlated with a shorter duration to the resolution of arthritis. However, as in the murine model, patients with refractory arthritis and lower numbers of Treg seem unable to resolve synovial inflammation.
PATIENTS AND METHODS
Patients
During a 22-year period, from November 1987 through January 2009, we evaluated 192 patients with Lyme arthritis. The Human Investigations Committees at Tufts Medical Center (Boston, MA) (1987–2002) and Massachusetts General Hospital (2002–2009) approved the study, and all patients (or the parents of patients who were minors) provided written informed consent.
For this study, large numbers of concomitant peripheral blood mononuclear cells (PBMC) and synovial fluid mononuclear cells (SFMC) were available from 18 patients, 12 with antibiotic-refractory arthritis and 6 with antibiotic-responsive arthritis. All 18 patients met the Centers for Disease Control and Prevention (CDC) criteria for the diagnosis of Lyme disease (17); they were entered into a study called “Immunity in Lyme Arthritis”. PCR testing for B. burgdorferi DNA and serum antibody responses to B. burgdorferi were determined as previously described (18,19). They received antibiotic therapy according to the guidelines of the Infectious Diseases Society of America (IDSA) (20). As in the past (2,4,5), antibiotic-responsive arthritis was defined as resolution of arthritis within 3 months after treatment with no more than 4 weeks of IV antibiotics or 8 weeks of oral antibiotics, and antibiotic-refractory arthritis was defined as persistent joint swelling for >3 months after the start of ≥4 weeks of IV antibiotics or ≥8 weeks of oral antibiotics, or both.
Isolation and quantification of PBMC and SFMC
To collect PBMC and SFMC, heparinized peripheral blood and synovial fluid were centrifuged at 2100 rpm in the Lymphocyte Separation Medium (MP Biomedicals) for 30 min. The total number of mononuclear cells per ml of joint fluid was calculated by dividing the total cells recovered after Ficoll-Hypaque separation by the volume of joint fluid. A fraction of cells in each blood or synovial fluid sample was stained with anti-CD3 and anti-CD4 monoclonal antibodies (BD Bioscience). The percentage of monocytes, CD4+T cells and non-CD4+ T cells was determined by flow cytometer (BD) using CD3- CD4low, CD3+CD4+, and CD3+CD4- as markers, respectively.
Intracellular staining of T cell subsets in PBMC or SFMC
Intracellular staining of FoxP3 (eBiosciences) or the cytokines, IFN-γ, IL-4, or IL-17 (BD Biosciences) was done according to the manufacturer’s instructions. Frozen cells were quickly thawed and washed in PBS. For FoxP3 determinations, the cells were first stained for surface expression of CD3, CD4, and CD25 (BD Biosciences); they were then fixed with intracellular fixation buffer, washed with permeabilization buffer, and stained with anti-FoxP3 antibody. For cytokine staining, washed cells were first stimulated with phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, 50ng/ml) and ionomycin (Sigma-Aldrich, 1 µmg/ml) in the presence of Golgi Stop (BD Biosicences) for 5 hours at 37 °C in 5% CO2. Stimulated cells were collected, washed with FACS buffer and stained for surface expression with anti-CD4 and anti-CD3 or antibody isotypes (mouse IgG, BD Biosciences) as a negative control. After fixation and washing, cells were stained intracellularly for expression of IFN-γ, IL-17 and IL-4. The stained samples were analyzed by flow cytometry.
Immunofluorescent staining of T cells in synovial tissue
Sections (8 µm) of synovium or tonsil were stained with hematoxylin and eosin. Adjacent slides were blocked with 5% donkey serum for 30 min at room temperature, and stained with mouse-anti-human anti-CD3 antibody (1:100) (DAKO) and goat-anti-human anti-FoxP3 antibody (1:2,500) (Novus Biologicals) at 4°C overnight. The slides were then stained with Cy2-conjugated donkey-anti-mouse IgG (1:100) and with Cy3-conjugated donkey-anti-goat IgG (1:250) (Jackson ImmunoResearch) at room temperature for 1 hour.
Borrelia-specific proliferation assay
SFMC were stained with either anti-CD25 monoclonal antibody (FITC, BD Biosciences) or anti-CD25 (APC, BD Biosciences) monoclonal antibody as a control, followed by anti-FITC microbeads (Miltenyi). Cells were then passed through a negative selection column (LD column, Miltenyi) under a magnetic field (Miltenyi). The flow-through was collected as Treg-depleted SFMC (cells stained with anti-CD25 FITC antibody), or Treg-retained SFMC (cells stained with anti-CD25 APC antibody). The depletion efficiency was more than 90%.
Treg-depleted SFMC or Treg-retained SFMC were cultured at 1 × 105 cell/well in 96-well round bottom plate in complete RMPI-1640 medium containing 10% human AB serum (heat-inactivated, Mediatech), 1mM sodium pyruvate, 2mM L-glutamine, 0.1 mM non-essential amino acid, 100U/ml penicillin and 50 µg/ml streptomycin (Gibco), and 5 × 10−5M 2-mercarptoethanol (Sigma) at 37°C in a CO2 incubator for 5 days. B. burgdorferi sonicate (strain G39/40) was added into each well at a final concentration of 25 µg/ml. After 5 days in culture, cells were pulsed with 0.5 µCi 3H-thymidine, and thymidine incorporation was measured 16 hours later with a TopCount instrument (Packard).
Non-specific Treg suppression assay
To isolate CD4+ T cells, PBMC or SFMC were stained with a panel of antibodies, including anti-CD8, CD14, CD19, and CD56 (APC, BD bioscience) followed by anti-APC microbeads (Miltenyi). Cells were passed through a LD depletion column attached to a magnetic field (Miltenyi). The flow-through had >90% purity of CD4 T cells. To isolate CD4+CD25+ and CD4+CD25-cells, the CD4+ T cells obtained with negative selection were stained with anti-CD25 antibody (FITC, BD Bioscience) and anti-FITC microbeads (Miltenyi). Cells were then passed through a positive selection column; the flow-through contained CD4+CD25- cells, and CD4+CD25+ cells retained in the column were washed out. For the suppression assay, 1 × 104 CD4+CD25- cells were cultured in the presence or absence of 1 × 104 CD4+CD25+ cells in 96-well, round-bottom plates. Each well contained 2 × 104 irradiated (3,500 rad) PBMC and 0.3 µg/ml of anti-CD3 antibody. After 5 days, cells were pulsed with 0.5 µCi 3H-thymidine, and thymidine incorporation was measured 16 hours later. For comparison, PBMC from a healthy human donor, obtained from the Massachusetts General Hospital Blood-Component Laboratory, from which CD4+CD25+ T cells were depleted or retained, were also stimulated with anti-CD3 antibody with irradiated PBMC.
Statistics
Categorical variables were compared in 2 × 2 contingency tables by chi-square analysis. The distribution of values between groups was compared by Mann-Whitney rank sum test. Correlations between percentage of Treg cells and duration of arthritis were sought by Pearson correlation test. All P values are 2-tailed. A value of <0.05 was considered statistically significant.
RESULTS
Clinical characteristics and treatment of patients
The clinical characteristics of the 18 study patients are shown in Table 1. Among the 6 patients with antibiotic-responsive arthritis, who were treated with oral doxycycline for 1 or 2 months, joint fluid samples were only available prior to or soon after the start of antibiotics because of the rapid resolution of joint inflammation. In contrast, the 12 patients with antibiotic-refractory arthritis were usually referred during or after 2–4 month courses of both oral doxycycline and intravenous (IV) ceftriaxone. In 6 of the 12 patients, joints were aspirated during antibiotic therapy, and in 5, PCR testing for B. burgdorferi DNA in joint fluid was still positive. The remaining 6 patients were referred after treatment with 3–9 months of antibiotics; and in these patients, PCR results were negative. Altogether, in the refractory group, the median duration from the start of antibiotics to the sample date was 4 months (range, 0.2–12 months), a date near or soon after the completion of antibiotics.
Table 1.
Clinical and demographic characteristics and treatment regimens of the patients with Lyme arthritis.
| Patients with | ||
|---|---|---|
| Antibiotic- Responsive Arthritis (N=6)* |
Antibiotic- Refractory Arthritis (N=12)* |
|
| Age, years | 26 (18–59) | 29 (11–54) |
| Sex, No. of men/women | 3/3 | 8/4 |
| Year of onset of arthritis | 2004 (1994–2007) |
2005 (1996–2007) |
| Duration (mos.) of arthritis prior to start of antibiotics | 0.25 (0–2) | 1 (0–5) |
| Duration (mos.) from start of antibiotics to sample date | 0 (0–0.25)† | 4 (0.2–12)† |
| Findings on the sample date | ||
| Degree of joint swelling (ml) | 50 (30–70) | 40 (10–70) |
| PCR results for B. burgdorferi DNA in joint fluid, no. positive / total tested |
3/5§ | 5/12§ |
| Duration (mos.) from | ||
| start of antibiotics to resolution of arthritis | 2 (1–3) | 14 (5–32) |
| sample date to resolution of arthritis | 2 (1–3) | 5.5 (1–28) |
Age, year of onset, duration of arthritis, and degree of joint swelling are shown as median (range). All other values are numbers of patients.
Patients with antibiotic-responsive arthritis were referred prior to or soon after the initiation of antibiotic therapy, whereas those with antibiotic-refractory arthritis were most commonly referred near or soon after the conclusion of 2–4 month courses of antibiotics. By definition, the 2 groups also differed in the duration of arthritis after treatment. There were no other significant differences between the 2 groups.
Patients with positive PCR results were seen prior to antibiotics in the responsive group and during 2–4 month courses of antibiotics in the refractory group. No patient had a positive PCR result in the post-antibiotic period.
During the post-antibiotic period, 11 of the 12 patients were treated with NSAIDs and DMARDs, usually naproxyn and hydroxychloroquine, which in 3 cases was changed to methotrexate. Because of incomplete responses, 4 patients, including the 3 treated with methotrexate, had arthroscopic synovectomies, with successful results. The final patient, who was referred after 9 months of antibiotic therapy, elected to have a synovectomy, which also resulted in the resolution of arthritis.
Total number of mononuclear cells in SF
The concentration of mononuclear cells and the percentages of monocytes and T cells were similar in SF in patients with antibiotic-responsive or antibiotic-refractory arthritis (Figure 1). CD3-CD4low monocytes comprised a median of ~20% of the total mononuclear cells; CD3+CD4+ T cells made up a median of ~40% of the total cells, and CD3+CD4- T cells comprised a median of 15–20% of the total cells.
Figure 1.
The total concentration of mononuclear cells in SF and the percentages of cell types in PB and SF in patients with antibiotic-responsive or antibiotic-refractory arthritis. In panel A, the concentration of mononuclear cells in SF and the percentages of monocytes and CD4+ and non-CD4+ T cell are shown. In 1 of the 6 patients with antibiotic-responsive arthritis, the number of mononuclear cells was not available. In panel B, the percentages of each CD4+ T cell subset are compared in PB and SF in the 2 patient groups. The horizontal bar represents the median value. Resp or responsive = patients with antibiotic-responsive arthritis, refr or refractory = patients with antibiotic-refractory arthritis, PB = peripheral blood, SF = synovial fliud.
Percentages of CD4+ T cell subsets in SF and PB
In patients with antibiotic-responsive or antibiotic-refractory arthritis, pro-inflammatory IFN-γ+CD4+ TH1 cells were the most abundant T cell subset in SF (50–55% of CD4+ T cells), and they were abundant and enriched in SF compared with PB (15–20%) (Figure 1). In contrast, in both patient groups, the median percentages of pro-inflammatory IL-17+CD4+ T cells were low in PB and SF (~5%), but a few patients in both groups had percentages in SF as high as 10–25%. Similarly, in both patient groups, the median percentages of IL-4+CD4+ TH2 cells were low in both PB and SF (~5%), but the range was again large (2–22%). Moreover, in the refractory group, the percentage of TH2 cells was significantly higher in SF than in their PB or in SF in the responsive group (for both comparisons, P=0.03). Finally, in patients with antibiotic-refractory arthritis, the median percentages of FoxP3+ Treg were significantly higher in SF than in PB (12% versus 6%) (P<0.01) or in SF in the responsive group (12% versus 5%) (P = 0.04). This was the case whether Treg were identified with the transcriptional factor FoxP3 or the surface marker CD25. As expected, most of the CD4+CD25+ cells co-expressed the FoxP3 marker (data not shown).
Correlation of CD4+ T cell subsets in SF and duration of arthritis
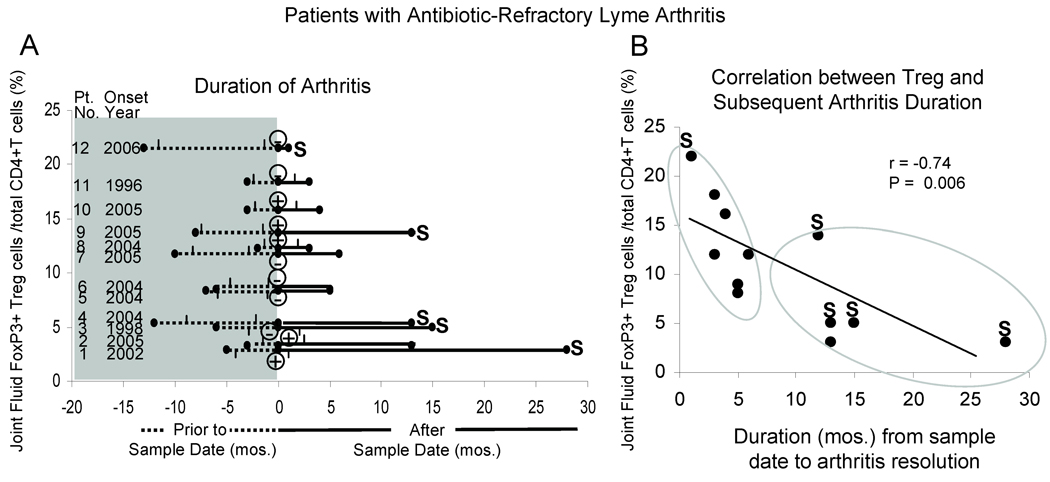
In Figure 2A, the year of onset, duration of joint swelling prior to, during and after antibiotics, and the results of PCR testing for B. burgdorferi DNA on the sample date are shown for each of the 12 patients with antibiotic-refractory arthritis, arranged according to the percentage of Treg. On the sample date, which was usually near the completion of antibiotics or early in the post-antibiotic period, a higher the percentage of Treg in SF correlated with a shorter duration to resolution of arthritis (r = −0.74, P = 0.006) (Figure 2B). In contrast, Treg percentages did not correlate with the duration of arthritis prior to the sample date, with how long the cells had been frozen prior to testing (r = −.12, P = 0.6) or with positive or negative PCR results (median Treg percentage, 12.2% in PCR-positive samples versus 8.5% in PCR-negative samples).
Figure 2.
Duration of arthritis and the percentages of FoxP3+ Treg in patients with antibiotic-refractory arthritis. In panel A, the year of onset and the duration of arthritis in each of the 12 patients is displayed; the 2 tick marks indicate the period of antibiotic therapy; the heavy line shows the period after the sample date (primarily the post-antibiotic period); and the S indicates that the patient’s arthritis was terminated with arthroscopic synovectomy. On the sample date (time 0), the patient had a positive ⊕ or ⊖ PCR result for B burgdorferi DNA. In panel B, the percentage of FoxP3+ T reg on the sample date is correlated with the duration of arthritis after the sample date. The patients could be divided into 2 groups, as shown with the large elliptical circles, according to the duration of arthritis after the sample date.
Among the 7 patients in whom arthritis resolved within 6 months after the sample date, the median percentage of Treg in SF was 12% (range, 8–22%) (Figure 2B). Of these 7 patients, 6 had the resolution of arthritis while being treated with NSAIDs and hydroxychloroquine; and the seventh patient, who had the highest percentage of Treg (22%), chose to have a synovectomy after 9 months of antibiotics. In contrast, the remaining 5 patients had lower percentages of Treg (median value, 5%; range, 3–14%) (P = 0.02), incomplete responses to DMARDs (including methotrexate in 3), and longer duration of arthritis after antibiotics (10–28 months); and 4 of the 5 patients had synovectomies.
In the 6 patients with antibiotic-responsive arthritis, the percentage of Treg in SF did not correlate with either the prior or subsequent duration of arthritis (data not shown). Rather, arthritis resolution in this group occurred with spirochetal killing during or near the end of antibiotic treatment.
In the 12 patients with antibiotic-refractory arthritis, higher percentages of TH2 cells in SF tended to correlate with faster resolution of arthritis. Among the 7 patients whose arthritis resolved within 6 months after the sample date, the median percentage of TH2 cells in SF was 9% (range, 4–22%), whereas the 5 patients with longer courses had a median percentage of 6% (range, 3–9%) (P=0.04). The percentages of TH1 or TH17 cells in SF did not correlate with the duration of arthritis prior to or after the sample date. Patients with a higher percentage of TH1 cells tended to have a lower percentage of Treg cells, but this inverse correlation was not statistically significant (r = −0.4, P = 0.16). PCR positivity did not correlate with the percentages of any of the CD4+ T cell subsets.
Serial determinations of CD4+ T cell subsets in SF
In 2 of the 4 patients (patients 1 and 4, Figure 2A) who had synovectomies because of incomplete responses to DMARDs, 4 serial joint fluid samples were obtained during the course of the illness (Figure 3). In patient 1, samples 1 and 2 were obtained during treatment with oral doxycycline and then IV ceftriaxone when PCR tests for B. burgdorferi DNA in SF were positive. In patient 4, all 4 samples were obtained in the post-antibiotic period when PCR results were negative. During methotrexate therapy, when the third sample was obtained, both patients had a >4-fold decline in IgG antibody responses to B. burgdorferi. Because of incomplete responses to methotrexate, both patients underwent arthroscopic synovectomies. When methotrexate was stopped prior to the procedure, both patients had increases in the size of joint effusions and small rebounds in antibody responses to B. burgdorferi, but PCR results remained negative in synovial fluid and synovial tissue.
Figure 3.
Correlation of clinical and laboratory results in 2 patients with prolonged antibiotic-refractory arthritis who underwent arthroscopic synovectomies. In panel A, the size of joint effusions and the type of treatment regimens are displayed. In panel B, serum antibody titers to B. burgdorferi are shown. In panel C, the percentages of IFN-g+ and FoxP3+ T cells in SF are shown. Dox = oral doxycycline, Cef = IV ceftriaxone, DMARDs = hydoxychloroquine or methotrexate, Syn = all medications were stopped prior to arthroscopic synovectomy, and Bb = B. burgdorferi.
In both patients, the percentages of IFN-γ+ TH1 cells in the first 1 or 2 samples were exceedingly high in SF (~70%), and the percentages of FoxP3+ Treg were very low (2–5%) (Figure 3). While taking methotrexate, the percentages of IFN-γ+ TH1 cells were less, and the percentages of FoxP3+ Treg were slightly higher. When all medications were stopped prior to synovectomy, the percentages of IFN-γ+ TH1 cells were almost as high and the percentages of FoxP3+ Treg were only slightly higher than in the initial samples. In both patients, synovial tissue samples from the procedure contained scattered clusters of infiltrating mononuclear cells. These clusters consisted primarily of CD3+ T cells, whereas very few FoxP3+ Treg were seen (Figure 4).
Figure 4.
Histologic sections of synovial tissue obtained from the synovectomies of the same 2 patients with antibiotic-refractory arthritis whose courses are shown in Figure 3. In panel A, the synovial samples were stained with hematoxylin and eosin (original magnification X 200). In panel B, the sections were stained with immunofluorescent-tagged anti-CD3 antibody (green) and anti-FoxP3 antibody (red) (original magnification X 400). In panel C, as a positive control, a section of human tonsil was stained with anti-CD3 and anti-FoxP3 antibodies (original magnification X 400).
Borrelia-specific proliferation assay
Eight patients had sufficiently large numbers of SFMC for B. burgdorferi-specific proliferation assays in which Treg were depleted or retained. Of the 8 patients, 3 had antibiotic-responsive arthritis, 3 had antibiotic-refractory arthritis that resolved within months, and 2 had antibiotic-refractory arthritis with prolonged courses ending in synovectomies. Because it was not possible to sort cells using the intracellular FoxP3 marker, Treg were depleted using the surface marker CD25; the percentages of Treg among the 8 patients varied from 3% to 13%. Teff were defined as those that responded to either an antigen-specific or non-specific stimulus, as measured by proliferation assay. Although B. burgdorferi-specific Teff were not enumerated, the percentages of these cells were surely variable as well.
Among the 8 patients with antibiotic-responsive or antibiotic-refractory arthritis, B. burgdorferi-specific responses were significantly lower when CD25+ Treg were retained than when they were depleted (P = 0.01) (Figure 5A). The median percentage of suppression was similar in patients with responsive or refractory arthritis (32% versus 38%) and in those with positive or negative PCR results (35% versus 34%). Thus, Treg in SF were functional in suppressing B. burgdorferi-specific proliferation responses. However, the percentage of Treg did not correlate with the degree of Borrelia-specific suppression. For example, in patient 3, a Treg frequency of 5% suppressed proliferation by 59%, whereas Treg frequencies of 12% in patients 7 and 8 suppressed proliferation by only 35% and 32%, respectively. This was presumably due to variation in the numbers of Borrelia-specific Teff in patients’ samples.
Figure 5.
Treg function in patients in whom enough cells were available for testing. In panel A, B. burgdorferi-specific proliferation assays in which CD25+ Treg were depleted or retained are shown in 3 patients with antibiotic-responsive arthritis, 3 patients with antibiotic-refractory arthritis that resolved within 1–6 months, and 2 with antibiotic-refractor arthritis that lasted for more than one year. In panel B, mixing experiments are shown with anti-CD3 antibody-stimulated Teff cells from PB or SF cultured alone or with purified Treg (1:1 ratio) from either SF or PB in 1 patient with antibiotic-refractory arthritis whose arthritis resolved within 4 months after the sample date (refractory arthritis of short duration) and another whose arthritis was terminated by synovectomy 18 months after the sample date (refractory arthritis of long duration). The histogram shows the average of triplicate values, and the I-bar shows the standard error of the mean.
Non-specific Treg suppression assay with stimulation with anti- CD3 mAb
To control for variability in the numbers of Borrelia-specific Teff and Treg in different patients, we conducted crossover suppression assays with PB and SF in which equal numbers of Teff and Treg (1:1 ratio) were stimulated with irradiated syngeneic PBMC and soluble anti-CD3 monoclonal antibody, which activates all T cells. Sufficiently large numbers of SFMC and PBMC were available in only 2 patients with antibiotic-refractory arthritis (patients 4 and 10, Figure 2A); patient 10 had the resolution of arthritis within 4 months while taking hydroxychloroquine, whereas patient 4 had incomplete responses to DMARDS, including methotrexate, and underwent a synovectomy 13 months after the sample date.
In both patients, Treg from either PB or SF suppressed Teff from PB by about 80% (Figure 5B). Although Teff from SF were less susceptible to suppression compared with their PB counterparts (50% versus 80%), Treg from PB and SF appeared similarly functional. Moreover, Treg from PB of a normal human donor also suppressed autologous Teff from PB by 80% (data not shown).
DISCUSSION
We characterized the CD4+ T cell subsets in PB and SF from 18 patients with antibiotic-responsive arthritis or antibiotic-refractory Lyme arthritis. Even though the samples in the antibiotic-responsive group were obtained prior to or soon after the start of antibiotic therapy and those in the refractory group were usually obtained near or soon after the beginning of the post-antibiotic period, the most abundant CD4+ T cell subset in SF in both groups was IFN-γ+ TH1 cells. The prominence of IFN-γ+ Borrelia-specific TH1 cells has been noted in PB in patients with erythema migrans early in the infection (25), and in PB and SF in patients with Lyme arthritis (26–28). Borrelia-specific TH1 responses decline with spirochetal killing prior to the resolution of arthritis (4). However, as shown previously (28) and again here, TH1 responses in these patients persist at high levels in the post-antibiotic period, and the antigen specificity of these cells may include T cells that react with currently unidentified autoantigens.
In contrast, the percentage of TH17 cells was low in each patient group, though several patients had percentages as high as 10–25%. TH17 cells were originally identified in the SF of a patient with Lyme arthritis (29), and neutrophil-activating protein A (NapA) of B. burgdorferi induced T cell lines derived from the SF of patients with Lyme arthritis to secrete IL-17 in culture (30). Thus, these cells may also play a role in control of the spirochete in Lyme arthritis, at least in some patients. However, high levels of IFN-γ, as seen in SF in patients with Lyme arthritis (15), may often serve as a negative regulator of TH17 cell differentiation (31).
In both patient groups, the percentage of IL-4+ TH2 cells was usually low in both PB and SF. However, compared with patients with antibiotic-responsive arthritis, those with antibiotic-refractory arthritis had significantly higher levels of TH2 cells in SF, and there was a trend toward higher percentages of TH2 cells and faster resolution of arthritis. Thus, TH2 cells may have a role in resolution of the post-infectious phase of antibiotic-refractory arthritis. Although TH1 and TH2 responses are more polarized in mice than in humans, the switch from TH1 to TH2 responses in B. burgdorferi-infected BALBc mice is accompanied by arthritis resolution (32).
The major difference in CD4+ T cell subsets here was the higher median percentage of FoxP3+ Treg in SF in patients with antibiotic-refractory arthritis than in those with antibiotic-responsive arthritis. However, the Treg percentage in SF varied greatly, from 3% to 22%. Moreover, in patients with antibiotic-refractory arthritis, there appeared to be 2 groups. One consisted of patients with higher percentage of Treg who resolved their arthritis within several months after the completion of antibiotics either because of the natural history of the illness or hydroxychloroquine therapy, or both. The second group had persistent lower percentages of Treg in the post-antibiotic period, as in the murine model (17), less response to DMARDs, and longer courses of arthritis, which often ended with synovectomy. In patients with antibiotic-responsive arthritis, it was not possible to determine whether the percentages of Treg increased prior to arthritis resolution, but these patients clearly down-regulated synovial inflammation along with spirochetal killing.
Although the number of patients was small in whom enough cells were available for functional assays, Treg suppression seemed similar in the various patient groups. In patients with antibiotic-responsive arthritis or antibiotic-refractory arthritis of short or long duration, Treg from SF repressed Teff similarly in B. burgdorferi-specific assays. In addition, in 1 patient each with antibiotic-refractory arthritis of short or long duration, non-specific, crossover suppression assays showed that Treg from either PB or SF were equally effective in suppressing Teff, although the level of suppression exerted on Teff from SF (50%) was lower than PB (80%). The difference between Treg suppression of SF and PB may be explained simply by differences in cell composition in the 2 compartments, since SF presumably contained more activated T cells than PB, and activated cells are relatively more resistant to Treg than naïve T cells (33,34). However, it is also possible that Teff were functionally altered by the local inflammatory environment in the joint (35). Although these factors need to be determined in larger numbers of patients, current observations suggest that patients with antibiotic-refractory arthritis are more likely to have functional differences in Teff than intrinsic defects in Treg.
As in antibiotic-refractory Lyme arthritis, a dominant TH1 response (13,14) and increased numbers of Treg have been found in SF in a number of types of chronic inflammatory arthritis, including RA (35–41) and juvenile idiopathic arthritis (JIA)(42). Moreover, in RA patients, anti-TNF-α therapy (a biologic DMARD) increases the numbers of Treg (43,44), as was seen here in patients treated with synthetic DMARDs. In one study of untreated patients with RA, Treg from PB appeared to be defective due to their inability to suppress Teff inflammatory cytokine production (43). However, in another study in which most patients were treated with methotrexate, Treg from PB were as functional as cells from normal donors (35), as seen here. Furthermore, Treg from SF had enhanced suppressive abilities, but this enhanced ability was offset by heightened Teff function. Thus, even in RA, the relative contribution of Teff or Treg to persistent synovial inflammation is not yet clear, and may not be the same in all patients.
In antibiotic-refractory Lyme arthritis, the correlation between higher percentages of Treg and faster resolution of arthritis suggests that the determination of Treg numbers in the post-antibiotic period may be a useful prognostic marker. It will be important to learn prospectively whether a high percentage of Treg, determined at the completion of antibiotics, predicts a favorable course, whereas a low percentage indicates a longer and more difficult course. Such information may be valuable in treatment decisions.
AKNOWLEDGMENTS
We thank personnel in the laboratory of Dr. Cathryn Nagler for help with purification of T cell subsets, Dr. Diana Londono for help with H & E staining of synovia, and Colleen Squires and Thomas Anderson for help with the figures.
Supported in part by grant AR-20358 from the National Institutes of Health, the Arthritis Foundation, the English, Bonter, Mitchell Foundation, the Eshe Fund, and the Lyme/Arthritis Research Fund at Massachusetts General Hospital. Dr. Shen was supported by a scholarship from the Lillian B. Davey Foundation. Drs. Shin and Strle were supported by scholarships from the Walter J. and Lille A. Berbecker Foundation, and Dr. Shin was also supported by NIH training grant (AR-007258). Dr. Li received support from a Claflin Distinguished Scholar Award at Massachusetts General Hospital.
REFERENCES
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Angelis SM. Therapy for Lyme arthritis; strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–3085. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 3.Jones KL, McHugh GA, Glickstein LJ, Steere AC. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: High frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum. 2009;60:2174–2182. doi: 10.1002/art.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannian P, Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Decline in the frequencies of Borrelia burgdorferi OspA161-175-specific T cells after antibiotic therapy in HLA-DRB1*0401-positive patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis. J Immunol. 2007;179:6336–6342. doi: 10.4049/jimmunol.179.9.6336. [DOI] [PubMed] [Google Scholar]
- 5.Kannian P, McHugh G, Johnson BJ, Bacon RM, Glickstein LJ, Steere AC. Antibody responses to Borrelia burgdorferi in patients with antibiotic-refractory, antibiotic-responsive, or non-antibiotic-treated Lyme arthritis. Arthritis Rheum. 2007;56:4216–4225. doi: 10.1002/art.23135. [DOI] [PubMed] [Google Scholar]
- 6.Steere AC, Duray PH, Butcher EC. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis: comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988;31:487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 7.Akin E, Aversa J, Steere AC. Expression of adhesion molecules in synovia of patients with treatment-resistant Lyme arthritis. Infect Immun. 2001;69:1774–1780. doi: 10.1128/IAI.69.3.1774-1780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–971. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis: an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 10.Weyand CM, Hicok KC, Conn DL, Goronzy JJ. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992;117:801–806. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- 11.Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000;43:617–627. doi: 10.1002/1529-0131(200003)43:3<617::AID-ANR19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Canete JD, Martinez SE, Farres J, Sanmarti R, Blay M, Gomez A, Salvador G, Munoz-Gomez J. Differential Th1/Th2 cytokine patterns in chronic arthritis: interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann Rheum Dis. 2000;59:263–268. doi: 10.1136/ard.59.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arend WP. Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Rheum. 2001;45:101–106. doi: 10.1002/1529-0131(200102)45:1<101::AID-ANR90>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 15.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 16.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 17.Iliopoulou BP, Alroy J, Huber BT. Persistent arthritis in Borrelia burgdorferi-infected HLA-DR4-positive CD28-negative mice post-antibiotic treatment. Arthritis Rheum. 2008;58:3892–3901. doi: 10.1002/art.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 19.Iliopoulou BP, Alroy J, Huber BT. CD28 deficiency exacerbates joint inflammation upon Borrelia burgdorferi infection, resulting in the development of chronic Lyme arthritis. J Immunol. 2007;179:8076–8082. doi: 10.4049/jimmunol.179.12.8076. [DOI] [PubMed] [Google Scholar]
- 20.Iliopoulou BP, Guerau-de-Arallano M, Huber BT. HLA-DR alleles determine responsiveness to Borrelia burgdorferi antigens in a mouse model of self-perpetuating arthritis. Arthritis Rheum. 2009;60:3831–3840. doi: 10.1002/art.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. Case definitions for public health surveillance. MMWR Morb Mortal Wkly Rpts. 1990;39:1–43. [PubMed] [Google Scholar]
- 22.Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in joint fluid in patients with Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 23.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis. 2008;47:188–195. doi: 10.1086/589242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 25.Glickstein L, Moore B, Bledsoe T, Damle N, Sikand V, Steere AC. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect Immun. 2003;71:6051–6053. doi: 10.1128/IAI.71.10.6051-6053.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yssel H, Shanafelt MC, Soderberg C, Schneider PV, Anzola J, Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Experi Med. 1991;174:593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross DM, Steere AC, Huber BT. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J Immunol. 1998;160:1022–1028. [PubMed] [Google Scholar]
- 28.Chen J, Field JA, Glickstein L, Molloy PJ, Huber BT, Steere AC. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer-surface protein A (OspA) of Borrelia burgdorferi. Arthritis Rheum. 1999;42:1813–1822. doi: 10.1002/1529-0131(199909)42:9<1813::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 30.Codolo G, Amedei A, Steere AC, Papinutto E, Cappon A, Polenghi A, et al. Borrelia burgdorferi NapA-driven Th17 cell inflammation in Lyme arthritis. Arthritis Rheum. 2008;58:3609–3617. doi: 10.1002/art.23972. [DOI] [PubMed] [Google Scholar]
- 31.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 32.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T helper cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baeccher-Allan C, Viglietta V, Hafler D. Inhibition of human CD4+CD25+high regulatory T cell function. J Immunol. 2002;169:6210–6217. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Brook MO, Carvalho-Gaspar, Zang J, Ramon HE, Sayegh MH, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 36.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–223. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 37.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:R335–R346. doi: 10.1186/ar1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao D, Borjesson O, Larsson P, Rudin A, Gunnarsson I, Klareskog L, et al. FOXP3 identifies regulatory CD25bright CD4+ T cells in rheumatic joints. Scand J Immunol. 2006;63:444–452. doi: 10.1111/j.1365-3083.2006.001755.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN. The presence of cytokine-suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol. 2005;62:312–317. doi: 10.1111/j.1365-3083.2005.01656.x. [DOI] [PubMed] [Google Scholar]
- 40.Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, et al. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford) 2006;45:1210–1217. doi: 10.1093/rheumatology/kel089. [DOI] [PubMed] [Google Scholar]
- 42.de Kleer IM, Wedderburn LR, Taams LS, Patel A, Varsani H, Klein M, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–6443. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 43.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNF alpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204(1):33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]