Abstract
The cerebral white matter (WM) is critically involved in many bio-behavioral functions impaired in schizophrenia. However, the specific neural systems underlying symptomatology in schizophrenia are not well known. By comparing the volume of all brain fiber systems between chronic patients with DSM-III-R schizophrenia (n = 88) and matched healthy community controls (n = 40), we found that a set of a priori WM regions of local and distal associative fiber systems were significantly different in patients with schizophrenia. There were significant positive correlations between volumes (larger) in anterior callosal, cingulate and temporal deep WM regions (related to distal connections) with positive symptoms, such as hallucinations, delusions and bizarre behavior, and significant negative correlation between volumes (smaller) in occipital and paralimbic superficial WM (related to local connections) and posterior callosal fiber systems with higher negative symptoms, such as alogia. Furthermore, the temporal sagittal system showed significant rightward asymmetry between patients and controls. These observations suggest a pattern of volume WM alterations associated with symptomatology in schizophrenia that may be related in part to predisposition to schizophrenia.
Keywords: Schizophrenia, MRI-volumetry, white matter, cingulum, temporal white matter, symptoms
Introduction
Numerous neuropathological, neurogenetic and neuroimaging studies suggest a relation between schizophrenia and white matter (WM) abnormalities (see e.g., (Walterfang et al., 2006)). WM alterations are important because they directly represent one form of connectivity in the brain, thought to be abnormal in schizophrenia (Seidman, 1983; Friston & Frith, 1995; Friston, 1998; Andreasen et al., 1999; Friston, 1999; Friston, 2002, 2005; Kubicki et al., 2005a; Kubicki et al., 2005b). Given the extensive array of symptoms in schizophrenia and widespread gray and WM brain alterations, it is likely that multiple networks underlying these functional systems are impaired (Seidman, 1983; Andreasen et al., 1999). Furthermore, the volume of total WM has been shown to be smaller in schizophrenia than controls (see e.g., (Kubicki et al., 2005a; Tanskanen et al., 2009 Oct 21. [Epub ahead of print]; 2009 Nov 30). To date, several structural studies have shown WM volumetric differences in schizophrenia (see e.g., (Kubicki et al., 2005a), both smaller and larger depending on region, in either the whole brain (see e.g., (Andreasen et al., 1994; Cannon et al., 1998b; Meisenzahl et al., 1999; Sigmundsson et al., 2001; Okugawa et al., 2002; Bartzokis et al., 2003; Christensen et al., 2004a; Hulshoff Pol et al., 2004; Kubicki et al., 2005b; Mitelman et al., 2007a)) or particular regions such as frontal and/or prefrontal lobes (Breier et al., 1992; Cannon et al., 1998a; Paillere-Martinot et al., 2001; Sigmundsson et al., 2001; Bartzokis et al., 2003; Mitelman et al., 2007a), temporal lobes (Marsh et al., 1997; Okugawa et al., 2002; Bartzokis et al., 2003) or the WM within the parahippocampal gyrus (Colter et al., 1987). In addition, specific fiber tracts have been investigated such as the perforant pathway in the hippocampal formation and cingulum bundle (Benes, 1989), uncinate fasciculus, inferior longitudinal fasciculus, anterior limb of the internal capsule (Sigmundsson et al., 2001; Suzuki et al., 2002), occipitofrontal fasciculus (Suzuki et al., 2002) and corpus callosum (Downhill et al., 2000; Hulshoff Pol et al., 2004; Sun et al., 2009).
In schizophrenia, studies using DT-MRI (see e.g., (Kubicki et al., 2007)) have also shown a significant decrease in fractional anisotropy (FA) in frontal and temporal WM, specifically anterior limbs of the internal and external capsules (Buchsbaum et al., 1998; Suzuki et al., 2002), corpus callosum (Foong et al., 2000; Argartz et al., 2001), arcuate fascicle (Hubl et al., 2004), cingulum bundle (Kubicki et al., 2003a; Hubl et al., 2004), WM throughout the whole brain (“widespread”) (Mitelman et al., 2006) and prefrontal (Lim et al., 1999) and temporoparietal (Mitelman et al., 2006) region specifically. Importantly, recently WM alterations have started to be associated with symptoms of schizophrenia (Downhill et al., 2000; Hubl et al., 2004; Hulshoff Pol et al., 2004; Mitelman et al., 2006; Mitelman et al., 2007b; Rotarska-Jagiela et al., 2008).
Herein we addressed this issue using T1-weighted MRI of 88 patients with schizophrenia and 40 controls to study the variation in volume of WM fiber systems within a framework of a WM morphometric system (Makris et al., 1999; Meyer et al., 1999). This comprehensive system of quantitative WM analysis subdivides WM regionally by lobes and also radially into a comprehensive set of fine-grained regions of interest or parcellation units (PUs) (Makris et al., 1999; Meyer et al., 1999). This allowed the investigation of specific WM fiber systems from an anatomic volumetric point of view and their affiliation with different neural systems (Filipek et al., 1994; Caviness et al., 1996a; Caviness et al., 1996b; Caviness et al., 1999; Makris et al., 1999). Furthermore, the comprehensive and detailed nature of the approach adopted in this study allowed the identification of altered patterns in WM regions and, uniquely, volumetric comparisons between local connections located within superficial WM sectors and distal connections located in deep WM sectors. Volume is an evolutionary and developmentally regulated property of tissue, which is sensitive to the regularities of normal histogenetic sequence and normal systems operations (Caviness et al., 1999). Thus alterations in WM volume may be important in understanding the illness in tandem with its association with well-defined symptoms. Furthermore, findings of an alteration in normative volumetric asymmetry (Geschwind & Behan, 1984; Geschwind & Galaburda, 1985c, 1985b, 1985a) would argue for a potential genetic etiology a fact well studied in vertebrates (e.g., (Supp et al., 1997; Hyatt & Yost, 1998; Piedra et al., 1998)). In this study, in addition to performing morphometric measurements of white matter brain structures, we conducted clinical evaluations, including negative and positive symptomology. We placed special emphasis on the associative limbic and paralimbic WM, in particular WM within the cingulate gyrus, with the expectation that it would be altered volumetrically, given that limbic prefrontal connections and limbic system abnormalities, which underlie disconnection of affect and cognition, are central in schizophrenia. Furthermore, we explored the associations of WM volume alterations with positive and negative symptoms, such as hallucinations, delusions and bizarre behavior, and negative symptoms such as alogia.
Materials and Methods
Subjects
Simplex patients
Cases were recruited from three public Boston area psychiatric hospitals serving primarily psychotic patients (Goldstein et al., 1999). The sample included subjects reported in previous work (Goldstein et al., 2002; Seidman et al., 2002). Recruitment criteria consisted of subjects with ages at MRI scanning of 23-68 years, ≥ 8th grade education, English as first language, and an estimated IQ ≥ 70. Criteria required absence of: substance abuse for the past six months; history of head injury with documented cognitive sequelae or loss of consciousness >five minutes; neurologic disease or damage; medical illnesses significantly impairing neurocognitive function. Cases were DSM-III-R schizophrenia probands (n=40), based on interview by experienced diagnostic interviewers and systematic review of medical records. Senior investigators (JMG, LJS) reviewed all material to determine diagnosis (see previous work listed above for details and excellent reliability).
Multiplex probands
Were reascertained from the Harvard cohort of the NIMH Genetics Schizophrenia Initiative. Families with ≥ two persons affected with DSM-III-R schizophrenia or schizoaffective disorder, depressed type, were identified by systematic screening in psychiatric hospitals and clinics. Test-retest reliabilities were excellent (Nurnberger et al., 1994). Procedures for diagnosing multiplex probands were similar to the simplex probands described above. Re-recruitment of the Harvard cohort (NIMH MH56956, JMG, P.I.) involved recruitment letters to probands, case managers, guardians, and home visits for those without phones or listed numbers. Fifty probands were scanned, out of whom data from two were excluded due to motion artifacts.
Normal comparison subjects
(n = 40) were recruited through advertisements in the catchment areas and notices posted on hospital bulletin boards from which the patients were ascertained (Goldstein et al., 2002; Seidman et al., 2002). They were selected to be comparable to patients on age, sex, ethnicity, parental socioeconomic status (SES), and handedness and screened for current psychopathology (Vincent et al., 1984) and family history of psychoses or psychiatric hospitalizations. Potential controls were excluded if they had current psychopathology or lifetime history of any psychosis, family history of psychosis or psychiatric hospitalization, or if any MMPI clinical or validity scale, except Masculinity-Femininity, was above 70.
Blindness of assessments was maintained among MRI data and psychiatric status and subjects' sex. Written informed consent was obtained from all subjects after providing a complete description of the study, and they were compensated for their time and participation. This study was approved by Harvard Medical School and hospital (Massachusetts Mental Health Center and Massachusetts General Hospital) Human Studies committees.
Multiplex and simplex cases and normal comparisons were comparable on gender, middle- to lower-middle parental SES and right-handedness, thus Table 1 shows the combined proband characteristics. The probands' education was typically high school completion and some college. Probands primarily had undifferentiated or paranoid subtypes (see Table 1) and were clinically stable, living in the community with mild to moderate negative and positive symptomatology. Patients were a chronically disabled group with average chlorpromazine-equivalent neuroleptic daily dose not significantly different between family types. Patients and controls did not differ significantly on parental education, handedness, or past alcohol use (see Table 1). There were significant differences by education, estimated IQ, and past drug use, most likely reflecting illness effects.
Table 1. Demographic characteristics of the sample*.
| Controls | Subjects with Schizophrenia | ||
|---|---|---|---|
| n = 48 | n = 88 | ||
| Variable | Mean ± Std Dev | Mean ± Std Dev | P-value |
| Age at MRI | 40.4 ± 10.9 | 44.6 ± 9.7 | 0.02 |
| Years of Parental Education | 12.1 ± 2.3 | 12.0 ± 2.6 | 0.74 |
| Years of Subject's Education | 14.8 ± 2.3 | 12.6 ± 2.4 | <0.0001 |
| IQ Estimate1 | 112.6 ± 13.1 | 96.7 ± 16.2 | <0.0001 |
| WRAT-R Reading2 | 105.1 ± 11.7 | 98.9 ± 18.0 | 0.03 |
| Past Drug Use3 | 0.5 ± 0.8 | 0.6 ± 1.0 | 0.32 |
| Past Alcohol Use3 | 1.0 ± 0.9 | 1.0 ± 1.3 | 0.94 |
| Gender (% Male) | 56.2% | 67.1% | 0.21 |
| Ethnicity (% Caucasian) | 93.7% | 80.7% | 0.04 |
| Handedness (% Right) | 91.7% | 80.5% | 0.07 |
| Age at First Hospitalization | - | 22.9 ± 6.3 | |
| Number of Hospitalizations | - | 7.8 ± 9.4 | |
| Diagnosis: | |||
| Undifferentiated | - | 38 (3%) | |
| Paranoid | - | 30 (34%) | |
| Disorganized | - | 15 (17%) | |
| Schizoaffective | - | 5 (6%) | |
| Antipsychotic cholorpromazine equivalents4 | - | 681 ± 460 (range: 80-2250) |
|
IQ estimate derived from vocabulary and block design age scale scores (Brooker and Cyr, 1986).
WRAT-Reading=Wide Range Achievement Test–Revised (Jastak and Jastak, 1984).
Substance abuse ratings: 0=never/occasional use; 1=recreational (episodic) use; 2=regular use; 3=abuse (for a period of six months to five years); 4=sustained abuse (for greater than five years).
All subjects were clinically stable. CPZ equivalents were calculated using a standard formula for converting the specific typical & atypical antipsychotic medications.
Some of this demographic information was reported in (Goldstein et al., 1999; Goldstein et al., 2002; Seidman et al., 2002; Seidman et al., 2003).
Neuropsychological and Clinical Measures
The Vocabulary and Block Design subtests of the Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981) estimated current general intelligence (Brooker & Cyr, 1986); and the Reading subtest of the Wide Range Achievement Test – Revised (Jastak & Jastak, 1984) was used as an estimate of intellectual potential (Kremen et al., 1995). The Annett Scale (Annett, 1970) was used as a measure of handedness. Quantitative symptomatology was assessed using the Scales for the Assessment of Negative (SANS) and Positive (SAPS) Symptoms (Andreasen & Olsen, 1982). Positive symptom domains included delusions, hallucinations, positive formal thought disorder, and bizarre behavior. Negative symptom domains included flat affect, alogia, anhedonia/associability and avolition/apathy.
MRI Parameters and Segmentation Procedures
T1-weighted MRI scans were acquired at the Athinoula Martinos Biomedical Imaging Center at Massachusetts General Hospital (MGH) with a 1.5 Tesla General Electric Signa scanner. Contiguous 3.1 mm coronal (in-plane resolution = 0.9375 mm × 0.9375 mm) spoiled gradient echo images of the entire brain were obtained using the parameters: TR = 40 msec, TE = 8 msec, flip angle = 50°, field of view = 30 cm, matrix = 256 × 256, and averages = 1. MR images were analyzed at the MGH Center for Morphometric Analysis (CMA). Images were positionally normalized to adjust for variation in head position by imposing a standard 3D coordinate system on MR scans (Filipek et al., 1994; Caviness et al., 1996b), and resliced into normalized 3.1 mm coronal scans.
Scans were segmented using a semi-automated technique, described in (Rademacher et al., 1992; Filipek et al., 1994; Caviness et al., 1996b; Goldstein et al., 1999; Seidman et al., 1999), which yields separate compartments of neocortex, subcortical gray nuclei, white matter, and ventricular system subdivisions generally corresponding to natural tissue boundaries distinguished by signal intensities in the T1-weighted images. The neocortex was subdivided into bilateral parcellation units based on the system in (Caviness et al., 1996b) and applied in (Goldstein et al., 1999) to schizophrenia (see Figure 1).
Figure 1. Segmentation and Cortical and White Matter Parcellation.
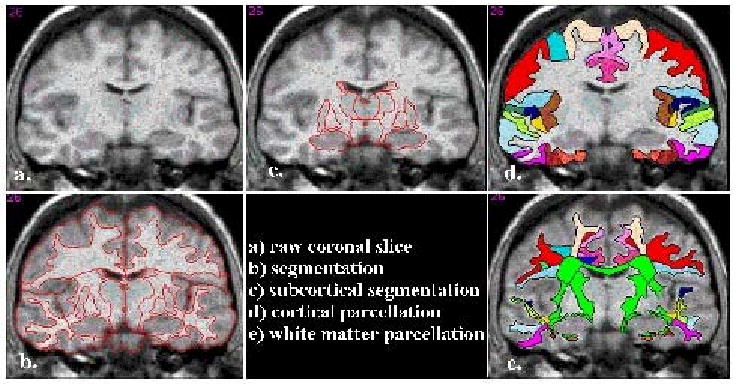
In a-c, the raw image of a coronal section (a) and the Center for Morphometric Analysis (CMA) general segmentation methodology of the cortical and subcortical structures (b, c) is shown (Filipek et al., 1994; Makris et al., 1999). The result of the detailed CMA cortical parcellation is shown in d (Rademacher et al., 1992; Caviness et al., 1996b). In e, the result of the CMA white matter parcellation technique used in the present study (Makris et al., 1999; Meyer et al., 1999) is shown.
White matter parcellation
White matter parcellation follows cortical parcellation and is a procedure that subdivides the cerebral white matter comprehensively into eighty-one (81) fine grained parcellation units based on boundaries delineated by prior segmentation and parcellation in addition to six anatomical nodal points at the corpus callosum and deep gray matter (see Figure 2) (Makris et al., 1999; Meyer et al., 1999). Operationally, this technique is semi-automated in that it consists of a combination of manual and automated routines. Once a set of anatomical landmarks has been identified and specified manually on the individual brain, the system is virtually automated (Makris et al., 1999; Meyer et al., 1999). This anatomically-specified system of analysis allows parcellation of the cerebral white matter into discrete, volumetrically quantifiable parcellation units (PUs), grouped into a superficial zone and a deep zone. The superficial zone or superficial white matter, composed of 47 PUs, contains white matter underlying the entire cerebral cortex except that subjacent to the insula, i.e., U-fibers and corona radiata. The deep zone instead is composed of 34 PUs and contains the sagittal strata systems or sagittal systems (29 PUs) and the bridging systems (5 PUs). The sagittal systems include three major classes of axonal systems: a) major cortico-cortical ipsilateral long association fiber pathways, b) projection fibers linking cortex, thalamus, basal ganglia, amygdala, hippocampus, pons, brainstem, and spinal cord, and c) the callosal commissural system. The bridging systems consist of the internal capsule, basal forebrain fiber systems such as the ventral amygdalofugal pathway, as well as the fornix, anterior commissure and dorsal and ventral hippocampal commissures (Makris et al., 1999). Although most white matter tracts are not discernable in the T1-weighted MR images (with few exceptions such as the corpus callosum), this parcellation system was originally conceived and validated for its anatomical accuracy and reliabilty. This was done on the basis of knowledge of the precise anatomy of fiber tracts using histological material (Makris et al., 1999) and DT-MRI (Makris et al., 1999) in the human brain and also compared with and extrapolating from experimental non-human primate material (Makris et al., 1999). Thus it is a strength of this method that based on T1-weighted MRI data there can be inferred locations and size of fiber tracts with notable approximation. For instance, as identified and measured by our method, the paralimbic WM (which includes the cluster of anterior cingulate white matter (wm) or CGa wm, posterior cingulate wm or CGp wm, paracingulate wm PAC wm, anterior parahippocampal wm or PHa wm, posterior parahippocampal wm or PHp wm, subcallosal wm or SC wm, temporal wm or TP wm and frontoorbital wm or FOC wm) contains a long cortico-cortical association fiber pathway, namely, the cingulum bundle, the cingulate short and medium range association fibers as well as the projection fibers by which the cingulate cortex is connected with the thalamus, the pons, the striatum, the amygdala and the hippocampus. It has, therefore, to be noted that when we use the term “connections” or “connectivity” throughout the text with respect to our results, this is only an approximation, given the limitations of our volumetric method. The corpus callosum was parcellated into seven regions, based on criteria of interhemispheric cortical connectivity (Witelson, 1989; Makris et al., 1999).
Figure 2. Topographic and Volumetric White Matter Parcellation.
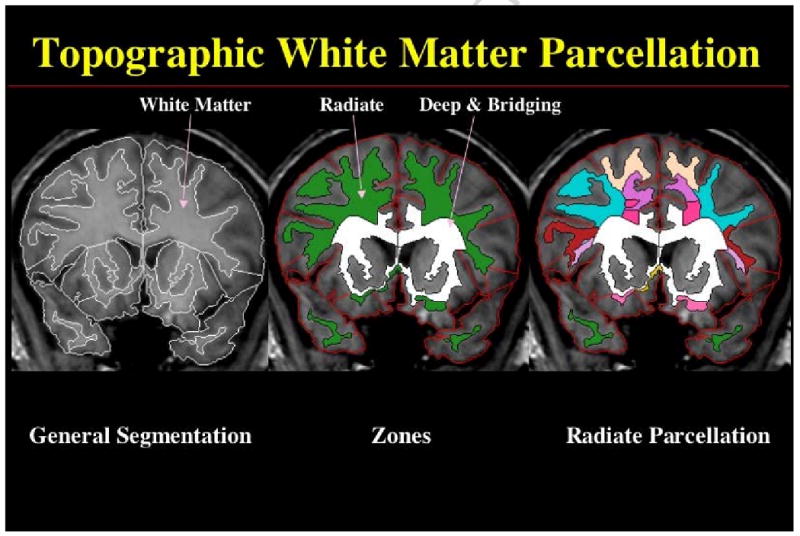
CMA white matter parcellation follows general segmentation and cortical parcellation and is a virtually an automated procedure, which requires a manual pre-processing step consisting on the identification and selection of six anatomical nodal points at the corpus callosum and deep gray matter (Makris et al., 1999; Meyer et al., 1999). Although minimal in terms of time required, this manual step is critical to guarantee the anatomic accuracy of the procedure. This anatomically-specified system of analysis allows parcellation of the cerebral white matter into a superficial zone or superficial white matter (referred in this figure as “radiate” zone) and a deep zone, which contains the sagittal strata systems or sagittal systems and the bridging systems (referred in this figure as “deep and bridging” zone). The sagittal systems include three major classes of axonal systems: a) the major cortico-cortical ipsilateral long association fiber pathways, b) the projection fibers linking cortex, thalamus, basal ganglia, amygdala, hippocampus, pons, brainstem, and spinal cord, and c) the callosal commissural system. The bridging systems consist of the internal capsule, basal forebrain fiber systems such as the ventral amygdalofugal pathway, as well as the fornix, the anterior commissure and the dorsal and ventral hippocampal commissures (Makris et al., 1999). The parcellation of the radiate zone provides 47 parcellation units of superficial white matter shown in a color-coded scheme in the plate on the far left side of this figure.
Volumes, measured in cubic centimeters (cc), were calculated for brain regions. This was done for each slice by multiplying the slice thickness by area measurements (namely, the number of pixels representing that region by the pixel in-plane resolution) on each slice. Once the volume of a region was determined on each slice the region appeared, we then added the volumes of that region in all individual slices to derive the total volume of the region. Very good inter- and intra-rater reliability of regions has been established in previous studies e.g. (Herbert et al., 2004).
Statistical Analyses
Given that volumetric findings were similar in simplex and multiplex cases, we combined the two patient groups. Parental SES and age were not significantly different between the two proband types, although we still controlled for age to insure comparability with controls. Twelve brain regions (i.e., as shown in Table 2, namely, frontal, temporal, parietal, occipital and paralimbic superficial white matter, CC, CG white matter, SS, SI, ST, basal forebrain white matter and internal capsule) were tested as components of the superficial white matter, sagittal and bridging systems. General linear model for correlated data (GLM-CD (Canaan et al., 1997)) was used to compare cases and controls across these brain regions within the white matter of the superficial and deep zones, controlled for age, sex, and total cerebral volume. GLM-CD is a multivariate model that controls for intra-person correlation across multiple brain regions, thus providing a “protected” test of group effects across multiple brain regions. The first overall test included the total corpus callosum (CC). In a second GLM-CD, we investigated group differences across the seven components of the CC, as suggested by Witelson (Witelson, 1989; Makris et al., 1999), i.e., the rostrum (sector CC1), genu (sector CC2) anterior body (sector CC3 (premotor, cingulate), CC4 (motor)) posterior body CC (sectors CC5 (somatosensory) and CC6) and splenium (sector CC7). In a third GLM-CD, we investigated whether there were laterality differences across the twelve brain regions defined as asymmetry measures of each brain region. Asymmetry of each of the brain regions was measured, as in (Geschwind & Galaburda, 1985a), using two times the difference between left minus right hemisphere volume divided by left plus right volumes. Handedness was controlled in GLMs of the white matter asymmetries. Finally, correlations of white matter abnormalities with positive and negative symptomatology were conducted to begin to investigate functional correlates of patterns of structural abnormalities. Correlational analyses were exploratory given lack of apriori hypotheses about specific white matter abnormalities and specific symptoms.
Table 2.
Group means and standard deviations, and multivariate analyses for the volumes of white matter parcellation units (in cm3).
| Controls | Patients with Schizophrenia | |||||||
|---|---|---|---|---|---|---|---|---|
| White Matter Region | Mean | Std Dev | Mean | Std Dev | Effect Size | t-value | ||
| Cerebral white matter | 434.5 | ± | 55.0 | 435.9 | ± | 60.8 | 0.02 | -0.40 |
| Superficial white matter | ||||||||
| Total | 329.2 | ± | 43.2 | 327.7 | ± | 49.0 | 0.03 | 0.86 |
| Frontal | 141.8 | ± | 19.1 | 140.7 | ± | 21.4 | 0.05 | 0.85 |
| Temporal | 55.0 | ± | 7.5 | 56.4 | ± | 9.7 | 0.15 | -1.19 |
| Parietal | 70.4 | ± | 13.0 | 71.4 | ± | 12.9 | 0.08 | -0.33 |
| Occipital | 62.0 | ± | 9.8 | 59.2 | ± | 10.3 | 0.28 | 2.39 * |
| Paralimbic | 34.2 | ± | 5.2 | 32.9 | ± | 5.7 | 0.22 | 1.72 + |
| Sagittal system | ||||||||
| Total | 88.4 | ± | 11.3 | 89.7 | ± | 12.2 | 0.11 | -0.30 |
| CC | 21.0 | ± | 3.3 | 20.7 | ± | 3.4 | 0.09 | 1.62 |
| CG | 13.8 | ± | 2.2 | 14.6 | ± | 2.6 | 0.34 | -2.09 * |
| SS | 28.4 | ± | 3.3 | 29.1 | ± | 4.4 | 0.17 | -0.41 |
| SI | 22.2 | ± | 3.4 | 22.0 | ± | 3.0 | 0.06 | 0.99 |
| ST | 3.0 | ± | 0.7 | 3.2 | ± | 0.7 | 0.39 | -1.97* |
| Bridging System | ||||||||
| Total | 19.2 | ± | 2.7 | 19.7 | ± | 2.6 | 0.19 | -1.11 |
| Basal forebrain | 3.4 | ± | 0.6 | 3.7 | ± | 0.8 | 0.30 | -1.58 |
| Internal capsule | 14.4 | ± | 2.1 | 14.7 | ± | 2.0 | 0.14 | -0.63 |
p < 0.05;
< 0.10
Abbreviations: CC = corpus callosum; CG = cingulate white matter; SI = inferior sagittal stratum; SS = superior sagittal stratum; ST = temporal sagittal stratum.
Effect size estimates are based on the raw means and pooled variances.
GLM-CD Overall F test (12 regions) controlled for age, sex and total cerebral volume: F (12,131) = 2.89, p = .001.
Within white matter domains: superficial white matter: F (5, 131) = 2.24, p = 0.05; sagittal system: F (5, 131) = 3.64, p = 0.004; bridging system: F (2, 131) = 1.25, p = 0.29.
Results
The overall multivariate test for the twelve superficial sagittal and bridging system brain regions (in Table 2) demonstrated a significant group difference between patients and healthy controls [F (12, 131) = 2.89; p = .001]. Within the superficial white matter, the occipital and paralimbic regions showed volumetric reductions in patients compared to controls (significant and at a trend level, respectively). In contrast, in the sagittal system, cingulate (SCG) and sagittal temporal (SST) regions were significantly larger in patients than controls (see Table 2 and Figure 3).
Figure 3. White Matter Abnormalities in the Cerebrum in Schizophrenia.
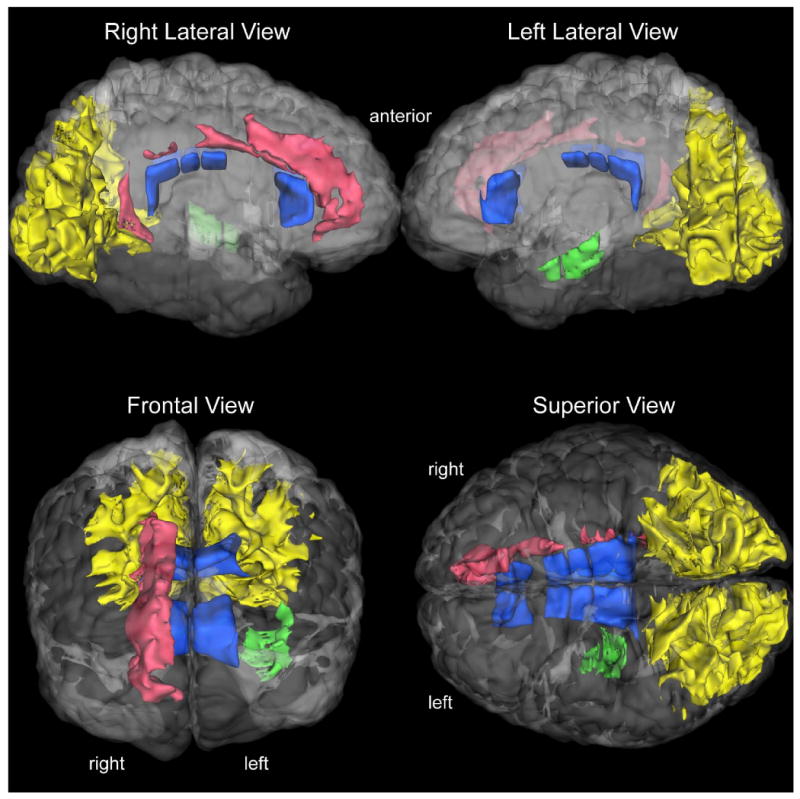
The salient results of this study are shown in a series of 3-D visualizations in a human cerebrum. The white matter regions abnormal in schizophrenia are rendered in a color-coded fashion. The occipital superficial white matter bilaterally is shown in yellow, right paralimbic white matter (including the cingulum) shown in red, the left deep temporal sagittal system white matter shown in green, and the majority of the corpus callosum shown in dark blue. The 3-D reconstructions have been done using an in-house tool called SVV (surface and volume visualizer).
The overall F test for the seven components of the corpus callosum (CC) was significant (F (7, 131) = 3.18, p = 0.004). As seen in Table 3 in patients compared to healthy controls, the rostrum (sector CC1) was significantly larger, and the posterior CC (sectors CC5 and CC6) and splenium (sector CC7) significantly smaller [with smaller genu (CC2) volume at a trend level (p<.10)] (see Table 3). The overall test of asymmetry differences of the superficial white matter, sagittal and bridging systems was not significant. In fact, the temporal sagittal system was the only region that showed a significant reduction in rightward asymmetry in patients compared to controls (controls: 10.7% rightward (SD 16.7%); patients: 3.7% rightward (SD 14.2%; t(132) = 2.5, p = 0.012). Group differences regarding the CC components were bilateral, however, more pronounced on the left. The sagittal temporal white matter region was the exception to this, which was different only in the left hemisphere.
Table 3.
Group means and standard deviations and multivariate analyses for the volumes of the corpus callosum (CC1-7) parcellation units (in cm3).
| Controls | Patients with Schizophrenia | |||||||
|---|---|---|---|---|---|---|---|---|
| Corpus Callosum Region | Mean | Std Dev | Mean | Std Dev | Effect Size | t-value | ||
| CC1 | 0.30 | ± | 0.16 | 0.44 | ± | 0.26 | 0.60 | -3.29 * |
| CC2 | 4.59 | ± | 1.05 | 4.28 | ± | 1.09 | 0.29 | 1.93 + |
| CC3 | 1.99 | ± | 0.50 | 2.13 | ± | 0.47 | 0.27 | -1.40 |
| CC4 | 1.66 | ± | 0.41 | 1.61 | ± | 0.39 | 0.12 | 1.17 |
| CC5 | 1.52 | ± | 0.35 | 1.42 | ± | 0.39 | 0.26 | 2.10 * |
| CC6 | 1.30 | ± | 0.35 | 1.14 | ± | 0.28 | 0.51 | 3.09 * |
| CC7 | 6.28 | ± | 1.14 | 5.95 | ± | 1.23 | 0.28 | 2.15 * |
p < .05;
p<.10
Each region is the sum of right and left hemisphere units. Abbreviations: rostrum (sector CC1); genu (sector CC2); anterior body (sector CC3 (premotor, cingulate), CC4 (motor)); posterior body CC (sectors CC5 (somatosensory) and CC6); splenium (sector CC7).
Effect size estimates are based on the raw means and pooled variances.
Linear mixed model for CC1-CC7 volumes, controlled for age, sex, and total cerebrum volume: F (7, 131) = 3.18, p = 0.004.
In exploratory analyses to investigate the functional correlates of the differential volumetric increases and reductions in white matter abnormalities in schizophrenia, regions were correlated with positive and negative symptomatology. Occipital, cingulate and sagittal temporal regions were positively correlated with positive symptoms, in particular hallucinations (Table 4). Bizarre behavior correlated significantly with the sagittal temporal region. Alogia, a negative symptom was significantly negatively correlated with the cingulate region (Table 4). Correlational analyses of the significant white matter regions with symptomatology showed that increased white matter volume was significantly associated with increases in positive symptoms, such as delusions and hallucinations, whereas decreased white matter volumes were significantly associated with the negative symptom, alogia.
Table 4.
Spearman Correlation Coefficients between Symptomatology and Significant White Matter Regions.
Abbreviations: CG = cingulate white matter; ST = temporal sagittal stratum.
| Region | Alogia SANS 13 | Hallucinations SAPS7 | Delusions SAPS20 |
|---|---|---|---|
| Occipital superficial white matter | -0.330 * | 0.290 * | 0.176 |
| Paralimbic superficial white matter | -0.231 * | 0.399 * | 0.233 * |
| Total sagittal system | -0.246 * | 0.284 * | 0.249 * |
| CG sagittal system | -0.245 * | 0.234 * | 0.160 |
| ST sagittal system | -0.006 | 0.344 * | 0.207 |
p < .05
Discussion
Results in this study demonstrated a characteristic pattern of WM alterations that correlated with symptoms in schizophrenia. Specifically, superficial WM underlying the cortex of the occipital and paralimbic regions was significantly smaller in patients than controls, whereas WM within the cingulate gyrus and core of the temporal lobe was significantly larger in patients than controls. The sagittal temporal WM region was larger exclusively in the left hemisphere, whereas in other regions, the effects were bilateral but more pronounced on the left. Furthermore, the callosal rostrum was significantly larger, whereas the posterior part of the body and splenium of the corpus callosum were significantly smaller in patients compared to healthy controls. Moreover, the temporal sagittal system showed a significant reduction in rightward asymmetry in patients compared to controls, potentially having etiologic implications involving the laterality hypothesis of schizophrenia (Crow, 1997a, 1997b). We understand that the voxel size used in this study is larger than what is currently used in the literature. However, this would not affect the pattern of results given that all subjects and all regions were subjected to this larger voxel size, and we would argue our findings may have been attenuated using a larger voxel size with regard to specificity of the results.
The behavioral implications of these WM abnormalities include a set of significant associations in patients with schizophrenia of significant positive correlations of WM volume with positive symptoms, such as hallucinations, delusions and bizarre behavior, and significant negative correlation between (smaller) WM volume with higher negative symptoms, such as alogia. Although this profile of abnormalities is complex, these exploratory initial findings suggest a pattern of limbic-paralimbic and temporal lobe WM abnormalities that may underlie behavioral impairments in schizophrenia. Furthermore and importantly, the identification of this complex pattern of structural WM brain volumes was made possible by the unique ability of the method used herein to quantitatively and topographically capture WM structures across all hierarchical scales of magnitude.
Previous literature on WM in schizophrenia is not entirely consistent (see e.g., (Kubicki et al., 2005a; Kubicki et al., 2005b; Kubicki et al., 2007)). This may be due to a wide range of methodological variation such as sample size, subject characteristics, MRI acquisition parameters, image analysis techniques, method of anatomic definitions of regions of interest (ROI), and statistical techniques. Significantly smaller volumes of WM in schizophrenia have been documented in studies conducted by a number of independent research groups using different MRI methodologies that principally perform segmentation using anatomic ROI-based or voxel-based procedures at the lobar or region-specific or whole brain level ((see e.g., (Colter et al., 1987; Breier et al., 1992; Andreasen et al., 1994; Marsh et al., 1997; Cannon et al., 1998a; Meisenzahl et al., 1999; Paillere-Martinot et al., 2001; Sigmundsson et al., 2001; Okugawa et al., 2002; Suzuki et al., 2002; Bartzokis et al., 2003; Christensen et al., 2004b; Hulshoff Pol et al., 2004)). Other studies, however, have shown frontoparietal WM volumetric increases which were associated with severity of illness with regard to frontal WM (Lim et al., 1999). Larger inferior frontal WM volume has also been associated with suicidality and self-aggression in schizophrenia (Rüsch et al., 2008).
The DT-MRI method is powerful in identifying characteristics of specific fiber tracts (Pierpaoli et al., 1996; Makris et al., 1997; Mori et al., 1999) and in recent years, there has been extensive work in schizophrenia using DT-MRI as reviewed previously (Kubicki et al., 2007) and in the Introduction. Importantly, studies have shown that good outcome schizophrenia patients show less extensive decrease in WM FA and also increased WM FA in regional frontal and cingulate WM (Lim et al., 1999). Although DT-MRI investigations enable us to target specific fiber tracts efficiently, a comprehensive volumetric analysis of parcels of WM throughout the cerebrum is very difficult with currently available DT-MRI methodologies. This analysis is particularly arduous for certain categories of fiber systems, such as the superficial zone of the white matter, which is populated principally by U-fibers, immediately underneath the cerebral cortex providing local connectivity.
The present study differs from other investigations in that it employs a unique volumetric method based on T1-weighted MRI that analyzes the brain white matter comprehensively, rank-orders it hierarchically in progressive scales of magnitude and subdivides it into neurobiologically meaningful parcels allowing for interpretation of results in the context of systems organization and development (Kennedy et al., 1998; Caviness et al., 1999; Makris et al., 1999). As a methodology, it has been validated and served diverse study purposes in basic and clinical neuroscience (Caviness et al., 1999). Other established methods of volumetric analysis, such as voxel-based morphometry (VBM) (Ashburner & Friston, 2000) are currently used extensively and efficiently for volumetric group comparisons. Aside from versatility, VBM is less costly than the method used in the present study. However, VBM is not optimal for investigations that aim to study a comprehensive profile of brain volumes and their covariances across the entire brain nor the course of volumetric changes from development into adulthood (Caviness et al., 1999). The volumetric method used herein is suitable for answering these types of questions regarding brain volumes across regions (Caviness et al., 1999) using appropriate statistical methods of analysis of variability (Kennedy et al., 1998) that are relevant for the study of psychiatric disorders associated with abnormal development, such as in schizophrenia. Furthermore, it is currently thought that studying anatomically organized regions in structural and functional circuits is an important avenue to identify biomarkers for a disease (Hyman & Nestler, 1993; Breiter et al., 2006) with diagnostic and treatment implications. Correlation between measures of anatomical and clinical alterations, i.e., symptoms, is an additional indication of the importance these observations may have with respect to the manifestation of the disorder.
The trend of volumetric changes in white matter across hierarchical scales and categories that we observed in patients with schizophrenia presented a profile as follows. Regions in the sagittal and bridging systems showed significant volumetric increases, whereas superficial white matter regions (i.e., occipital and paralimbic) were reduced in volume. Further, within the sagittal system, the cingulate (SCG) and sagittal temporal (SST) regions were significantly larger in patients than controls (see Table 2). These findings indicate that alterations of major limbic association fiber systems, such as the cingulum bundle, are present in schizophrenia predominantly, in the right hemisphere, emphasizing abnormalities of key neurobiological components for self-regulation and emotional processing.
Moreover, the left temporal WM sagittal system, carrying the principal association fiber tracts of the superior, middle and inferior temporal gyri and fusiform and parahippocampal gyri, was abnormal indicating abnormalities in structures supporting basic analytical processing and fundamental cognitive processes such as language and gnostic behaviors (Heilman & Valenstein, 1985). In contrast, the superficial WM of the occipital and paralimbic regions were smaller in patients than controls (see Table 2). These findings denote deficits in local fiber systems within the occipital lobe and cingulate and parahippocampal regions indicating additional abnormalities related to visual processing, self-regulative and affective behaviors.
The cingulum bundle in particular connects the cingulate cortex bidirectionally with the anterior thalamus and prefrontal, medial and lateral parietal, and mesial temporal regions (shown in Figure 3 (Mufson & Pandya, 1984; Makris et al., 2002)), with behavioral associations, such as memory, attention, learning, pain perception, motivation, emotion and autonomic function (Yakovlev & Locke, 1961; Devinsky et al., 1995; Mesulam, 2000). Its structural damage may alter communication of the cingulate cortex with prefrontal and subcortical limbic centers resulting in failure of key cortical and subcortical properties to mediate decision-making and control of drives (Cohen et al., 2000; Paus, 2001). Thus this alteration contributes to altered functioning of the limbic system (Goldstein et al., 1999; Kubicki et al., 2003b) with disconnection of affect and cognition, a hallmark of schizophrenia.
Furthermore, the corpus callosum (CC) showed significant differences in most of its seven component parts between patients and healthy controls. Rostral callosal regions, which interconnect ventral prefrontal paralimbic cortices (rostrum or sector CC1) and paracingulate, anterior cingulate, premotor and supplementary motor cortices (anterior portion of the callosal body or sector CC3) showed volumetric increases. In contrast, the rest of this commissure connecting the somatic sensory and posterior cingulate cortices, parietal and temporal heteromodal association cortical areas (posterior sectors CC5 and CC6) and visual association cortices (splenium or sector CC7) and (at a trend level of significance) frontopolar and dorsal prefrontal cortical areas (genu or sector CC2) were reduced in volume.
Our findings showing an overall volumetric decrease of the total corpus callosum reflect the general pattern reported in the literature which consist primarily of studies that have investigated callosal area as distinct from CC volume in our study (Woodruff et al., 1995). With respect to the regional changes in size of the corpus callosum (see e.g., (Walterfang et al., 2006)), surface measurements have shown reductions in regions of the corpus callosum associated with prefrontal, motor, superior temporal and inferior parietal cortices (DeQuardo et al., 1999; Keshavan et al., 2002). Our findings support reductions in volumes of callosal regions associated with prefrontal, temporal and parietal cortices. Thus, we demonstrated some consistency in CC findings comparing surface measurements with volumetric measurements. However, our volumetric findings also revealed CC increases in the rostral area (connecting ventral prefrontal paralimbc cortices) and anterior CC body (with connections to paracingulate, anterior cingulate, premotor and supplementary motor cortices), suggesting that surface and volume measurements may reflect some differences in their neurobiology. This profile of detailed callosal volumetry indicates that interhemispheric connectivity between broad regions related to cognitive and limbic-paralimbic processing is altered in schizophrenia. We suggest that the particular pattern identified in this study of intrahemispheric white matter abnormalities within the limbic-paralimbic and temporal cortical regions (i.e., local fibers within the limbic-paralimbic regions, cingulum and the long connections of the temporal lobe coursing in the temporal sagittal stratum; see Figure 3) is exacerbated by largely altered interhemispheric callosal connectivity. This may leave scarce or no available alternative pathways for normal processing of certain cognitive, affective and self-regulative behaviors in a brain affected by schizophrenia.
Volumetrically abnormal cerebral WM structures were positively correlated with positive symptoms (i.e., hallucinations, delusions and bizarre behavior) and negatively correlated with negative symptoms (i.e., alogia) (Table 4). Although these findings are exploratory and thus multiple tests performed, the pattern showed that the identical set of WM structures (i.e., paralimbic superficial WM and association fiber systems of the sagittal systems deep sector), was associated with positive symptoms when larger in volume and negative symptoms when smaller in volume. Although these findings need replicating, they indicate that negatively correlated structural abnormalities of local connections within the limbic-paralimbic system and distal connections between different multimodal limbic and non-limbic prefrontal and parietal association areas in the cerebrum may contribute to positive and negative symptoms in schizophrenia. This may lead to a breakdown in coherency of representations necessary for efficient self-regulation, emotional behavior and directed action (Spencer et al., 2003; Vierling-Claassen et al., 2008). Further, there was a disruption in the normal symmetry of the temporal sagittal system. Numerous previous studies have demonstrated disrupted asymmetry in schizophrenia particularly in the temporal lobe (Shenton et al., 1992; Crow, 1997a, 1997b). Laterality is a process that begins in fetal development, genetically and hormonally developmentally regulated, and thus may suggest that our WM results have developmental origins.
Acknowledgments
We thank the following people for their contributions to this project: Camille McPherson, Jason Tourville, Heather Sanders, Sean McInerney, Matthew Albaugh, and Joseph Normandin. Preparation of this article was supported primarily from the National Association for Research in Schizophrenia and Depression (NARSAD) to Dr. Nikos Makris and National Institute of Mental Health RO1 MH56956 to Dr. Jill M. Goldstein. In addition, subjects and technical assistance (respectively) were acquired with significant support from the NARSAD and Stanley Medical Research Institute to Dr. Larry J. Seidman and the Fairway Trust to Dr. David Kennedy. We also thank Lisa Cushman-Daly for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Arndt S, Swayze V, 2nd, Cizaldo T, Flaum M, O'Leary D, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266(5183):294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biological Psychiatry. 1999;46(7):908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative versus positive schizophrenia. Definition and validation. Archives of General Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Annett M. A classification of hand preference by association analysis. British Journal of Psychology. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Argartz I, Andersson J, Skare S. Abnormal brain white matter in schizophrenia: A diffusion tensor imaging study. Neuroreport. 2001;12(10):2251–2254. doi: 10.1097/00001756-200107200-00041. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: A magnetic resonance imaging study. Biological Psychiatry. 2003;1(53):412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- Benes F. Myelination of cortical-hyppocampal relays during late adolescence. Schizophrenia Bulletin. 1989;15(4):585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson C, Kirkpatrick B, Gelland F. Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Archives of General Psychiatry. 1992;49(12):921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Breiter BH, Gasic GP, Makris N. Imaging the neural systems formotivated behavior and their dysfunctions in neuropsychiatric illness 2006 [Google Scholar]
- Brooker BH, Cyr JJ. Tables for clinicians to use to convert WAIS-R short forms. Journal of Clinical Psychology. 1986;42:983–986. [Google Scholar]
- Buchsbaum M, Tang C, Peled S, Gudbjartsson H, Lu D, Haxlett E, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9(3):425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Canaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Statistics in Medicine. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, et al. Regional gray matter, white matter and cerebrospinal fluid distributions in schizophrenic patients, their siblings and controls. Archives of General Psychiatry. 1998a;55(12):1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Archives of General Psychiatry. 1998b;55(12):1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7-11 years: A volumetric analysis based on magnetic resonance images. Cerebral Cortex. 1996a;6(5):726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Lange NT, Makris N, Herbert MR, Kennedy DN. MRI-based brain volumetrics: Emergence of a developmental brain science. Brain and Development. 1999;21(5):289–295. doi: 10.1016/s0387-7604(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Meyer JW, Makris N, Kennedy DN. MRI-based topographic parcellation of the human neocortex: An anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience. 1996b;8(6):566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL. State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Research. 2004a;130(1):71–78. doi: 10.1016/j.pscychresns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL. State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Research. 2004b;130(1):71–78. doi: 10.1016/j.pscychresns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Cohen J, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: Who's in control? Nature Neuroscience. 2000;3:421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Colter N, Battal S, Crow TJ, Johnstone EC, Brown R, Bruton C. White matter reduction in the parahippocampal gyrus of patients with schizophrenia. Archives of General Psychiatry. 1987;44(11):1023. doi: 10.1001/archpsyc.1987.01800230103016. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Is schizophrenia the price that Homo sapiens pays for language? Schizophrenia Research. 1997a;28(2-3):127–141. doi: 10.1016/s0920-9964(97)00110-2. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends in Neurosciences. 1997b;20(8):339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- DeQuardo JR, Keshavan MS, Bookstein FL, Bagwell WW, Green WD, Sweeney JA, et al. Landmark-based morphometric analysis of first-episode schizophrenia. Biological Psychiatry. 1999;45:1321–1328. doi: 10.1016/s0006-3223(98)00181-4. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulated cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Downhill JE, Jr, Buchsbaum MS, Wei T, Spiegel-Cohen J, Hazlett EA, Haznedar MM, et al. Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophrenia Research. 2000;42:193–208. doi: 10.1016/s0920-9964(99)00123-1. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: An MRI-based morphometric analysis. Cerebral Cortex. 1994;4(4):344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- Foong J, Maier M, Clark C, Barker G, Miller D, Ron M. Neuropathological abnormalities of the corpus callosum in schizophrenia: A diffusion tensor imaging study. Journal of Neurology, Neurosurgery and Psychiatry. 2000;68(2):242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. The disconnection hypothesis. Schizophrenia Research. 1998;30(2):115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Friston K. Schizophrenia and the disconnection hypothesis. Acta Psychiatry Scandinavian Supplimental. 1999;395:68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1(2):66–71. [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Disconnection and cognitive dysmetria in schizophrenia. The American Journal of Psychiatry. 2005;162(3):429–432. doi: 10.1176/appi.ajp.162.3.429. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clinical Neuroscience. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Geschwind N, Behan P. Laterality, hormones and immunity. In: Geschwind N, Galaburda AM, editors. Cerebral lateralization: Biological mechanisms, associations, and pathology. MIT Press; Cambridge, MA: 1984. [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Archives of Neurology. 1985a;42(5):428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: II. A hypothesis and a program for research. Archives of Neurology. 1985b;42(6):521–552. doi: 10.1001/archneur.1985.04060060019009. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: III. A hypothesis and a program for research. Archives of Neurology. 1985c;42(7):634–654. doi: 10.1001/archneur.1985.04060070024012. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Siedman LJ, Kennedy DN, Makris N, Lee H. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Archives of General Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, O'Brien LM, Horton NJ, Kennedy DN, Makris N, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Archives of General Psychiatry. 2002;59(2):154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E. Clinical Neuropsychology. Oxford University Press; New York: 1985. [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55(4):530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, et al. Pathways that make voices: White matter changes in auditory hallucinations. Archives of General Psychiatry. 2004;61(7):658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Brans RG, van Haren NE, Schnack HG, Langen M, Baare WF, et al. Gray and white matter volume abnormalities in monozygotic and same-gender dizgotic twins discordant for schizophrenia. Biological Psychiatry. 2004;55(2):126–130. doi: 10.1016/s0006-3223(03)00728-5. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Yost HJ. The left-right coordinator: The role of Vg1 in organizing left-right axis formation. Cell. 1998;93(1):37–46. doi: 10.1016/s0092-8674(00)81144-7. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. The Molecular Foundations of Psychiatry. American Psychiatric Press; Washington, D.C.: 1993. [Google Scholar]
- Jastak FJ, Jastak S. Wide Range Achievement Test-Revised. Jastak Associates; Wilmington, DE: 1984. [Google Scholar]
- Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VS., Jr Gyri of the human neocortex: An MRI-based analysis of volume and variance. Cerebral Cortex. 1998;8(4):372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Diwadkar VA, Harenski K, Rosenberg DR, Sweeney JA, Pettegrew JW. Abnormalities of the corpus callosum in first episode treatment naïve schizophrenia. Journal of Neurology, Neurosurgery and Psychiatry. 2002;72:757–760. doi: 10.1136/jnnp.72.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Pepple JR, Lyons MJ, Tsuang MT. The “3 Rs” and neuropsychological function in schizophrenia: A test of the matching fallacy in biological relatives. Psychiatry Research. 1995;56(2):135–143. doi: 10.1016/0165-1781(94)02652-1. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Shenton M. Evidence for white matter abnormalities in schizophrenia. Current Opinion in Psychiatry. 2005a;18(2):121–134. doi: 10.1097/00001504-200503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Westin CF, Park HJ, Miaier S, Kikinis R, et al. A review of diffusion tensor imaging in schizophrenia. Journal of Psychiatric Research. 2007;41(1-2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin C, McCarley R, Shenton ME. The application of DTI to investigate white matter abnormalities in schizophrenia. Annals of the New York Academy of Sciences. 2005b;1064:134–148. doi: 10.1196/annals.1340.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Biological Psychiatry. 2003a;54(11):1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Society of Biological Psychiatry. 2003b;54:1171–1180. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Hedehus M, Mosley M, de Crespigny A, Sullivan E, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Archives of General Psychiatry. 1999;56(4):367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9(1):18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- Makris N, Pandya DN, Normandin JJ, Papadimitriou BS, Rauch SL, Caviness VS, et al. Quantitative DT-MRI investigations of the human cingulum bundle. CNS Spectrums. 2002;7(7):522–528. [Google Scholar]
- Makris N, Worth AJ, Sorensen AG, Papadimitriou BS, Wu O, Reese TG, et al. Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Annals of Neurology. 1997;42(6):951–962. doi: 10.1002/ana.410420617. [DOI] [PubMed] [Google Scholar]
- Marsh L, Harris D, Lim KO, Beal M, Hoff AL, Minn K, et al. Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early age at clinical onset. Archives of General Psychiatry. 1997;54(12):1104–1112. doi: 10.1001/archpsyc.1997.01830240060009. [DOI] [PubMed] [Google Scholar]
- Meisenzahl E, Frodl T, Greiner J, Leinsinger G, Maag KP, Heiss D, et al. Corpus callosum size in schizpohrenia -- A magnetic resonance imaging analysis. European Archives of Psychiatry and Clinical Neuroscience. 1999;249(6):305–312. doi: 10.1007/s004060050104. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Behavioral neuroanatomy. Large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. 2nd. Oxford University Press; Oxford; 2000. pp. 1–120. [Google Scholar]
- Meyer JW, Makris N, Bates JF, Caviness VS, Kennedy DN. MRI-Based topographic parcellation of human cerebral white matter. Neuroimage. 1999;9(1):1–17. doi: 10.1006/nimg.1998.0383. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Brickman AM, Shihabuddin L, Newmark RE, Hazlett EA, Haznedar MM, et al. A comprehensive assessment of gray and white matter volumes and their relationship to outcome and severity in schizophrenia. NeuroImage. 2007a;37(2):449–462. doi: 10.1016/j.neuroimage.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitelman SA, Newmark RE, Torosjan Y, Chu KW, Brickman AM, Haznedar MM, et al. White matter fractional anisotropy and outcome in schizophrenia. Schizophrenia Research. 2006;87(1-3):138–159. doi: 10.1016/j.schres.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophrenia Research. 2007b;92(1-3):211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PCM. Three-dimentional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. The Journal of Comparative Neurology. 1984;225:31–43. doi: 10.1002/cne.902250105. [DOI] [PubMed] [Google Scholar]
- Nurnberger JJ, Blehar MC, Kaufmann CA, WYork-Cooler C, Simpson SG, Harkavy-Friedman J. Diagnostic interview for genetic studies: Rationale, unique features, and training Archives of General Psychiatry. 1994;51:842–869. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Sedvall GC, Agartz I. Reduced grey and white matter volumes in temporal lobe of male patients with chronic schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2002;252(3):120–123. doi: 10.1007/s00406-002-0370-9. [DOI] [PubMed] [Google Scholar]
- Paillere-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophrenia Research. 2001;50(1-2):19–26. doi: 10.1016/s0920-9964(00)00137-7. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nature Reviews: Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, Rodrigues-Rey JC, Ros MA. Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell. 1998;94(3):319–324. doi: 10.1016/s0092-8674(00)81475-0. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Difussion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS., Jr Human cerebral cortex: Localization, parcellation and morphometry with magnetic resonance imaging. Journal of Cognitive Neuroscience. 1992;4:352–374. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Schönmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. NeuroImage. 2008;39(4):1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Rüsch N, Spoletini I, Wilke M, Martinotti G, Bria P, Trequattrini A, et al. Inferior frontal white matter volume and suicidality in schizophrenia. Psychiatry Research. 2008;164(3):206–214. doi: 10.1016/j.pscychresns.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Seidman L. Schizophrenia and brain dysfunction: An integration of recent neurodiagnostic findings. Psychological Bulletin. 1983;94:195–238. [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, et al. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: An MRI-based morphometric analysis. Biological Psychiatry. 1999;46(7):941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, et al. Left hippocampal volume as a vulnerability indicator for schizophrenia: An MRI morphometric study of non-psychotic first degree relatives. Archives of General Psychiatry. 2002;59:839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Pantelis C, Keshavan MS, Faraone SV, Goldstein JM, Horton NJ, et al. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophrenia Bulletin. 2003;29(4):803–830. doi: 10.1093/oxfordjournals.schbul.a007048. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. New England Journal of Medicine. 1992;327(9):604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. American Journal of Psychiatry. 2001;158(2):234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Nizikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizpohrenia. Journal of Neuroscience. 2003;23(19):7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Maller JJ, Daskalakis ZJ, Furtado CC, Fitzgerald PB. Morphology of the corpus callosum in treatment-resistant schizophrenia and major depression. Acta Psychiatria Scandanavia. 2009;120(4):265–273. doi: 10.1111/j.1600-0447.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left-right assymettry in inversus viscerum mice. Nature. 1997;389(6654):963–966. doi: 10.1038/40140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Nohara S, Hagino H, JKurokawa K, Yotsutsuji T, Kawasaki Y, et al. Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophrenia Research. 2002;55(1-2):41–54. doi: 10.1016/s0920-9964(01)00224-9. [DOI] [PubMed] [Google Scholar]
- Tanskanen P, Haapea M, Veijola J, Miettunen J, Järvelin MR, Pyhtinen J, et al. Volumes of brain, grey and white matter and cerebrospinal fluid in schizophrenia in the Northern Finland 1966 Birth Cohort: An epidemiological approach to analysis. Psychiatry Research. 2009 Oct 21;174(2):116–120. doi: 10.1016/j.pscychresns.2009.04.009. [Epub ahead of print]; 2009 Nov 30. [DOI] [PubMed] [Google Scholar]
- Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modelling GAGA alterations in schizophrenia: A link between impaired inhibition and altered gamma and beta range auditory entrainment. Journal of Neurophysiology. 2008;99(5):2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent KR, Castillo IM, Hauser RI, Zapata JA, Stuart HJ, Cohn CK, et al. MMPI-168 Codebook. Ablex Publishing Corporation; Norwood, NJ: 1984. [Google Scholar]
- Walterfang M, Wood SJ, Velakoulis D, Pantelis C. Neuropathological, neurogenetic and neuroimaging evidence for white matter pathology in schizophrenia. Neuroscience and Biobehavioral Reviews. 2006;30(7):918–948. doi: 10.1016/j.neubiorev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the corpus callosum: A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. Journal of Neurology, Neurosurgery and Psychiatry. 1995;58(4):457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Locke S. Limbic nuclei of the thalamus and connections of the limbic cortex. III. Coricocortical connections of the anterior cingulated gyrus, the cingulum, and the subcallosal bundle in monkey. Archives of Neurology. 1961;5:364–400. doi: 10.1001/archneur.1961.00450160014002. [DOI] [PubMed] [Google Scholar]


