Abstract
Tendency to mimic others’ emotional facial expressions predicts empathy and may represent a physiological marker of psychopathy. Anatomical connectivity between amygdala, cingulate motor cortex (M3, M4), and facial nucleus demonstrates a potential neuroanatomical substrate for mimicry, though pharmacological influences are largely unknown. Norepinephrine modulation selectively impairs negative emotion recognition, reflecting a potential role in processing empathy-eliciting facial expressions. We examined effects of single doses of propranolol (beta-adrenoceptor blocker) and reboxetine (selective norepinephrine reuptake inhibitor) on automatic facial mimicry of sadness, anger, and happiness, and the relationship between mimicry and empathy. Forty-five healthy volunteers were randomized to 40 mg propranolol or 4 mg reboxetine. Two hours after drug subjects viewed and rated facial expressions of sadness, anger, and happiness, while corrugator, zygomatic, and mentalis EMG were recorded. Trait emotional empathy was measured using the Balanced Emotional Empathy Scale. EMG confirmed emotion-specific mimicry and the relationship between corrugator mimicry and empathy. Norepinephrine modulation did not alter mimicry to any expression or influence the relationship between mimicry and empathy. Corrugator but not zygomaticus mimicry predicts trait empathy, consistent with greater anatomical connectivity between amygdala and M3 coding upper facial muscle representations. Although influencing emotion perception, norepinephrine does not influence emotional facial mimicry or its relationship with trait empathy.
Keywords: Norepinephrine, Mimicry, Empathy, Emotion
INTRODUCTION
Human social interactions are critically dependent on emotional communication and, in particular, emotional signals portrayed in the face (Darwin, 1872). The primacy of facial expressions to social interaction is demonstrated by primate studies which show cross-cultural (Izard 1971) and cross-species (Burrows, Waller, Parr, & Bonar, 2006) consistency of facial muscle configurations for specific emotional expressions, suggesting an evolutionary conserved neuroanatomical basis. Spontaneous mimicry of observed emotional facial expressions by human newborns extends this position and suggests “hard-wired” connections between facial emotion perception and emotional expression (Field, Woodson, Greenberg, & Cohen, 1982).
Tract tracing studies that show connectivity between lateral-basal amygdala nucleus, cingulate motor cortex (M3, M4) and brainstem facial motor nucleus suggest a potential anatomical basis for this mimicry (Morecraft et al. 2007; Morecraft, Lousie, Herrick, & Stilwell-Morecraft, 2001). For example, the lateral-basal amygdala nucleus, which together with the lateral amygdala nucleus is believed to play a key role in extracting emotionally salient sensory information across modalities (LeDoux, 2000), shows dense bidirectional connectivity to the rostral cingulate motor cortex (M3) which codes a representation predominantly of the upper face. Less dense projections from lateral-basal amygdala also occur to more caudal M4, which itself codes a representation predominantly of the lower face. Both M3 and M4 show projections to the facial motor nucleus in the brainstem, potentially providing a two- or three- synapse bidirectional anatomical framework for emotional facial mimicry.
Consistent with a pivotal role in healthy human social interaction, emotional facial communication is impaired across the breadth of human psychopathology. For example, in depression, depressed individuals have a tendency to misclassify neutral or ambiguous emotional facial expressions as negative (Rubinow & Post, 1992). This effect correlates with increased amygdala activity and is reversed after treatment with antidepressant medication (Sheline et al., 2001). Antidepressants such as the selective norepinephrine reuptake inhibitor Reboxetine also induce a positive emotional perceptual bias in healthy subjects (Harmer, Shelley, Cowen, & Goodwin, 2004; Norbury, Mackay, Cowen, Goodwin, & Harmer, 2008), suggesting that norepinephrine positively colors the emotional perception of facial expressions in humans. Recognition that norepinephrine regulates neuronal excitability in the basolateral amygdala by facilitation of GABA (Roniadou-Anderjaska, Qashu, & Braga, 2007) release demonstrates a potential neuroanatomical locus for this effect.
Although the majority of studies investigating the role of emotional facial expressions in human social communication have focused on mechanisms of emotion perception, theoretical considerations suggest similar importance for mechanisms of emotional expression. Lipps (1907) first proposed that observation of emotional displays leads to mimicry and, via a feedback process, a convergence of observed and observer's emotional feeling states. This tendency to automatically mimic and synchronize expressions, postures, and vocalizations with another person and consequently converge emotionally has subsequently been termed “emotional contagion” (Hatfield, Cacioppo, & Rapson, 1994) and argued to form the basis for emotional empathy (de Wied, van Boxtel, Zaalberg, Goudena, & Matthys, 2006).
Supporting this position, healthy individuals observing emotional expressions automatically produce congruent facial responses (Dimberg & Karlsson, 1997) even when unaware of the emotion displayed (Dimberg, Thunberg, & Elmehed, 2000). Strikingly, individuals high in trait empathy show the greatest mimicry of others’ emotional expressions (Sonnby-Borgstrom 2002; Sonnby-Borgstrom, Jonsson, & Svensson, 2003) and further, report a greater change in their own emotional state when their facial expressions are surreptitiously modulated (Andreasson & Dimberg, 2008). These findings suggest that more empathetic individuals show a greater sensitivity to others’ emotional facial displays, expressed as an increase in mimicry and likely in experienced congruent emotion (Andreasson & Dimberg 2008). Whether this results from enhanced emotion perception (Harrison, Wilson, & Critchley, 2007), coupling of perception to facial responses (Sonnby-Borgstrom, 2002), feedback from motor responses, or interaction between these factors is currently unclear.
In addition to an effect on emotion perception, depression also biases emotional expression, in particular responses in the upper face. Depressed individuals display less spontaneous happy facial expressions and less mimicry of observed happiness indexed not by a reduction in zygomaticus major (ZM) smiling responses but by greater corrugator supercilli (CS) frowning activity (Schwartz et al., 1978; Schwartz, Fair, Salt, Mandel, & Klerman, 1976). Emotional facial mimicry indexed by CS responses is also impaired in autism (Hermans, van Wingen, Bos, Putman, & van Honk, 2009), oppositional defiant disorder (ODD) and conduct disorder (CD) (de Wied et al., 2006), conditions characterized by poor empathetic skills and poor ability to understand and share another's emotional state (Cohen & Strayer, 1996). Together these findings highlight contributions of perception and reciprocation of emotional facial expressions, in particular upper facial features, to adaptive human social interaction. Anatomical studies suggest the amygdala is a critical substrate mediating the perception and, through connectivity to M3 and to a lesser extent M4, expression and mimicry of emotional facial expressions. Pharmacological influences on perceptual biases are well described, but their role in expressive components is currently unclear.
We addressed this issue by investigating the effect of norepinephrine modulation on implicit mimicry of both upper and lower facial muscles and the relationship between facial mimicry and emotional empathy. In a double-blind study, healthy volunteers received either reboxetine, a selective norepinephrine reuptake inhibitor, or propranolol, a centrally acting beta-adrenergic antagonist, before observing randomized positive (happy) and negatively (sad and angry) valenced facial expressions. Each expression was rated for subjective arousal/intensity and valence. Mimetic upper (CS) and lower (ZM) facial responses were simultaneously recorded using electromyography (EMG). We predicted that if norepinephine selectively modulates emotional expression pathways, this would be expressed in differential mimetic responses between groups and as differential relationships between facial mimicry and emotional empathy scores.
METHODS
Subjects and pharmacological manipulation
Forty-five participants were recruited from a web-based volunteer recruitment site at University College London. Five participants were excluded from the analysis because of technical problems, leaving 40 subjects (14 female), median 24 years (range 18-34). Twenty-one subjects received reboxetine and 19 propranolol. Participants were screened by clinical interview to exclude those with a history of asthma, psychiatric or significant medical illness, or current medication use. All participants also underwent a 12-lead electrocardiograph (ECG) which was reviewed by a registered doctor (NAH) to exclude participants with evidence of cardiac conduction abnormalities. All participants gave their written consent to participate in this study, which was approved by the joint National Hospital for Neurology and Institute of Neurology research ethics committee.
Volunteers were randomly allocated to receive either reboxetine 4 mg, a selective norepinephrine reuptake inhibitor, or propranolol 40 mg, a centrally acting nonselective B1 and B2 adrenergic receptor blocker. Both experimenter (RM) and subject were blind to drug administration. A further experimenter (NAH) was aware of drug administration to ensure participant safety but played no role in direct data acquisition. Timing of the behavioral task was informed by the pharmacokinetics of reboxetine and propranolol, which show peak plasma levels at 1.5-2 h after oral drug administration.
Stimuli and testing procedure
Participants were individually tested 2 h after drug administration in a dark, electrically isolated room, with stimuli displayed in a 400 × 400 pixel array on a 21-inch Sony GDM-F520 CRT. Images of facial expressions were taken from the Karolinska Directed Emotional Faces (KDEF) series (Lundqvist, Flykt, & Öhmann, 1998). Eight male and eight female facial identities were used, each displaying one of three emotional expressions (happy, sad, and angry). Each identity expression combination was shown three times.
To ensure that participants attended to the face stimuli and to minimize startle responses, we preceded each trial with a 2000 ms duration scrambled image of equal luminance to the ensuing emotional facial expression. A visual analog scale (VAS) represented by a 200 mm horizontal line was presented below the image 1500 ms after face presentation. Subjects successively rated the valence (−10 to +10), intensity/arousal (0 to +10), and attractiveness (0 to +10) of each emotional face using a mouse-controlled cursor. Each facial expression was displayed until all face ratings were completed.
EMG reactions
Facial EMG activity was measured using miniature bipolar Ag/AgCl electrodes coated with high-conductance gel placed according to guidelines for EMG research (Fridlund & Cacioppo, 1986) on the left side of the face. The subjects’ skin was cleansed and lightly abraded with disposable pads (70% alcohol and pumice) before the electrodes were applied. Inter-electrode distance was approximately 1.5 cm. Activity of the CS (FACS AU 4), which shows an increase in activity in anger and sadness and a decrease in happiness, was employed to assess activity in the upper face. ZM (FACS AU 12), which shows the opposite pattern of activity, was employed to assess responses in the lower face. We also recorded mentalis (ME: FACS AU 17), a lower facial muscle, to attempt to differentiate responses to sadness and anger. However, due to technical difficulties associated with recording from this small muscle in the majority of subjects, these data were not further analyzed. EMG signals were recorded at a sampling rate of 1 kHz using a CED 1902 preamplifier and CED Power 1401 data acquisition interface and stored on a laptop computer running Spike2 software.
To conceal recording of facial muscle activity we adopted the established strategy of informing participants that their sweat gland activity was being measured (Sonnby-Borgstrom, Jonsson, & Svensson, 2003). At post-testing debriefing no participant realized the true purpose of the electrodes, implying that they were not aware that their facial muscle activity was of interest until the true purpose of the study was explained.
EMG data were analyzed in Matlab using purpose-written routines. Data were first filtered using a sixth-order Butterworth filter (bandwidth 10-480 Hz), then rectified and smoothed using the root mean squared (rms) technique with a time constant of 200 ms. Following Dimberg (1982), facial EMG responses in the 500 ms period 500-1000 ms after stimulus onset were determined and expressed as the change in activity from a 1000 ms pre-stimulus baseline. Individual EMG responses were Z-normalized within muscle sites and participants to reduce participant and site-specific variability (Bush, Hess, & Wolford, 1993). These were then used to produce participant-specific mean, normalized responses to each emotional expression in each facial muscle.
Questionnaires
Participants completed the Mehrabian Balanced Emotional Empathy Scale (BEES) (Mehrabian & Epstein, 1972) after exposure to the facial expressions. The BEES is a widely used and validated measure of emotional empathy with a high internal consistency (Cronbach alpha = 0.87), suggesting that it measures a single, unidimensional latent construct (Mehrabian, 1997). Beck's Depression Inventory revised (BDI-I) (Beck, Steer, Ball, & Ranieri, 1996) and the Toronto Alexithymia Scale (TAS-20) (Bagby, Taylor, & Parker, 1994) were also completed. Questionnaires were completed after the emotional facial processing task to minimize the risk of behavior in the experiment being influenced by the content of the questionnaires.
Statistical analysis
All statistical analyses were performed in SPSS v. 16.0 (SPSS, Inc). Effects of observed emotional expression, noradrenergic manipulation and empathy scores on mean subjective ratings of emotional intensity/arousal and valence were analyzed in separate mixed measures ANOVAs.
Effects of observed emotional expression and noradrenergic modulation on muscle-specific EMG responses were determined using a 3 (expression) x 2 (drug) x 2 (muscle) mixed-measures ANOVA. Interaction of each of these factors with emotional empathy was determined by repeating this analysis with the addition of empathy score (BEES) as a continuous between-subjects factor. Inhomogeneity of variance was controlled as appropriate by reducing the degrees of freedom using the Greenhouse-Geisser correction.
RESULTS
Questionnaires
All subjects bar one scored in the minimal severity range (1-13) on the BDI. One subject in the Reboxetine group scored in the mild severity range (14-19) without meeting criteria for ICD-10 depressive episode. All subjects scored below the recommended threshold for alexithymia (Taylor et al., 1988) of 74 on the TAS. Subject demographics are given in Table 1.
TABLE 1.
Demographics of participants
| Age | BEES | TAS | BDI | |
| Propranolol | 24.9 (3.8) | 31.8 (33.2) | 54.6 (6.4) | 4.1 (4.2) |
| Reboxetine | 25.8 (4.8) | 34.4 (20.5) | 54.3 (7.2) | 5.2 (4.6) |
| T score | 0.65 | 0.30 | 0.14 | 0.79 |
| P value | .52 | .76 | .89 | .44 |
Notes: BEES, Balanced Emotional Empathy Scale score; TAS, Toronto Alexithymia Scale; BDI, Beck's Depression Inventory. Mean (standard deviation) shown.
Subjective ratings of emotional facial expressions
There was a significant main effect of observed emotion on both intensity/arousal and valence ratings, F(2) = 33.75, p < .001; F(2) = 20.54, p < .001, respectively, with significant differences (p < .002) between all pairs of contrasts (angry > happy > sad) for intensity/arousal and (happy > angry > sad) for valence. Noradrenergic modulation had no significant effect on either rating, F(1) = 0.41, p > .1; F(1) = 0.39, p > .1, and there were no significant interactions between drug and ratings, F(2) = 0.47, p > .1; F(2) = 1.1, p > .1. There was also no significant main effect of emotional empathy on overall emotion rating scores. Interestingly, however, we did observe a significant empathy x expression interaction on arousal ratings, F(2) = 4.13, p < .02, driven by a tighter correlation between empathy and arousal to sadness than happy or angry expressions, F(1) = 4.45, p < .04; F(1) = 10.13, p < .003. Analysis of this interaction showed that more empathetic individuals rated the sad facial expressions as more arousing than low-empathy participants.
EMG responses to observation of emotional facial expressions
Time course of EMG activity in CS and ZM muscles to each observed emotional facial expression is shown in Figure 1. There was no significant main effect of facial muscle, F(1) = 3.57, p = ns, or norepinephric manipulation, F(1) = 0.10, p = ns, on EMG responses, although similarly to our analysis of arousal and valence ratings we did observe a significant main effect of emotional expression, F(2) = 3.41, p < .04.
Figure 1.
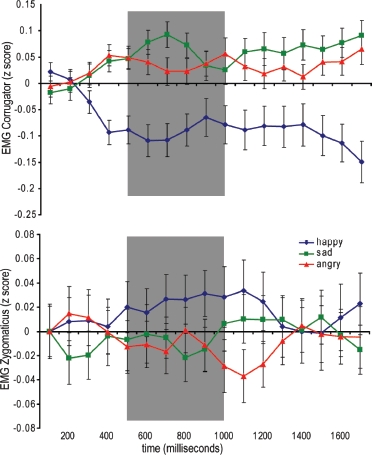
Mean corrugator (upper) and zygomaticus (lower) responses to observation of happy, sad, and angry facial expressions. The area in gray denotes the period of EMG responses used in all subsequent analyses.
Importantly, there was also a significant emotion x muscle interaction, F(2) = 8.02, p < .002, demonstrating mimicry of observed emotional expression (Figure 2). Post-hoc contrasts showed significantly greater CS response to both sad, F(1) = 9.81, p < .003, and angry, F(1) = 7.31, p < .01, than happy expressions. Conversely, ZM responses were significantly greater to observation of happy than angry or sad expressions, F(1) = 4.81, p < .03. There was no significant interaction between responses to sad and angry expressions for either muscle.
Figure 2.
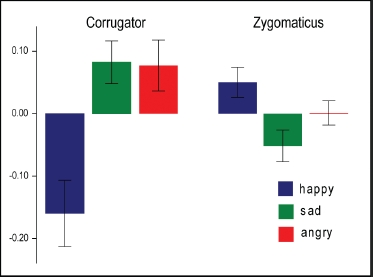
Mean normalized EMG responses (± SE) in CS and ZM muscles to observation of happy, sad, and angry facial expressions (500-1000 ms after stimulus onset).
Norepinephric modulation again showed no interaction with muscle-specific EMG responses across emotions or overall EMG responses to individual emotions. There was no significant three-way interaction between norepinephric modulation, observed facial expression, and observers’ own facial muscular response. This finding confirms firstly that norepinephric modulation does not result in a general change in facial motor responses to observed emotional expressions. More importantly, it also demonstrates that norepinephric modulation does not have a differential emotion-specific influence on mimetic responses to observed sadness, anger, or happiness. This implies that previously reported effects of propranolol on speeded motor responses to expressions of sadness do not extend to facial motor responses. To further explore the robustness of this result, we also investigated individual post-hoc contrasts. Again these analyses confirmed that norepinephric modulation had no significant effect on any emotion-specific EMG response described above (p > .05 for all post-hoc analyses).
Correlation of EMG responses with BEES
Previous studies have reported a differential CS and ZM mimetic response to happy and angry faces in high-and low-empathy subjects, with high-empathy subjects showing a greater mimetic response at least in brief exposures (Sonnby-Borgstrom, 2002; Sonnby-Borgstrom et al., 2003). We explored this effect in our current data by including BEES as a between-subjects factor in our previous 3 (emotion) x 2 (muscle) x 2 (norepinephric manipulation) ANOVA. Inclusion of BEES in this model identified a significant emotion x BEES interaction, F(2) = 16.87, p < .0001, and muscle x emotion x BEES interaction, F(2) = 6.15, p < .003. Post-hoc regression analyses of these interactions revealed that they were driven by differential correlations between BEES and CS activity to happy, sad, and angry facial expressions: in particular, differences in correlations between BEES and happy and sad, F(1) = 6.70, p < .02, and happy and angry, F(1) = 8.76, p < .005, facial expressions (Figure 3). There were no significant correlations between ZM responses to any emotional expression and BEES.
Figure 3.
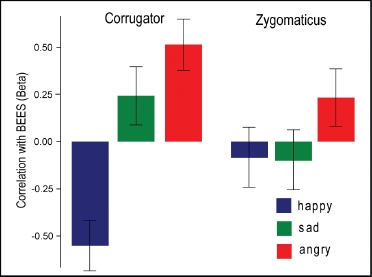
Correlation between EMG responses in CS and ZM muscles to observation of happy, sad, and angry facial expressions and BEES. Mean beta (± SE) displayed.
Finally, to determine the relative contributions of arousal ratings and EMG responses to emotional empathy scores, we performed a multiple regression analysis including all factors significantly predicting BEES score in the above analyses (arousal ratings of sad expressions and CS responses to happy, sad, and angry expressions). A backwards removal method was adopted (consistent with the exploratory nature of this analysis). Mimetic CS responses to observed angry expressions, i(1) = 4.56, p < .0001, and arousal ratings of sad expressions, /(1) = 3.38, p < .002, explained over 40% of the variance in individuals’ emotional empathy score, F(2) = 14.4, p < .00003, adjusted R2 = .41. To confirm our reported lack of effect of noradrenergic modulation on mimetic responses, we also repeated this regression analysis as a general linear model incorporating norepinephric modulation as a random between-subjects factor. Again, this analysis confirmed (1) correlation between CS mimicry to anger and empathy score and (2) correlation between arousal ratings of sad expressions and empathy score. There were no significant interactions between any mimetic response and norepinephric modulation (p > .1 all interactions).
DISCUSSION
Despite the proposed central role of facial mimicry to emotion contagion and emotional empathy, relatively few studies have investigated the mechanisms mediating this relationship (Hess, Philippot, & Blairy, 1999). To our knowledge this is the first study to investigate the effects of norepinephrine on spontaneous facial mimicry and its relationship with trait emotional empathy. Our study provides several new findings.
Firstly we replicated the emotional facial imitation phenomenon in 40 healthy subjects treated with propranolol or reboxetine, with differential modulation of CS and ZM to observed happy vs. sad and angry facial expressions. These data build on previous observations of facial mimicry to static (Dimberg, 1982; Dimberg & Lundquist, 1988) and dynamic emotional facial expressions (Achaibou, Pourtois, Schwartz, & Vuilleumier, 2008). Onset of the mimetic response 300-500 ms after stimulus presentation supports the automatic nature of the mimetic response we observed. This is also substantiated by the fact that none of the subjects was aware of the true nature of the study. Similarly to Achaibou et al., we used a large number of stimuli indicating that facial mimicry can be elicited over many successive trials, a finding that again supports the automaticity of the mimetic response.
Our data also provide the first independent replication of Sonnby-Borgstrom's report of a relationship between intensity of facial mimicry and trait emotional empathy (Sonnby-Borgstrom, 2002; Sonnby-Borgstrom et al., 2003). Using a multiple regression analysis we extend this finding to show a specific relationship between upper (CS) but not lower (ZM) facial mimicry and trait emotional empathy. Interestingly, these findings also applied to expressions of happiness where a reduction of CS activity was a stronger predictor of empathy than increase in ZM activity. Effects of depression on facial expressions of experienced emotion (Schwartz et al., 1976) and mimicry of observed emotions (Schwartz et al., 1978) have also previously reported a selective effect on upper rather than lower facial features. Similarly, individuals with disorders of social communication such as Asperger's syndrome (Hermans et al., 2009) and conduct disorder (de Wied et al., 2006) have been reported to show a selective impairment in mimetic responses in the upper face.
Explanation for these findings may rest on the anatomy of facial mimicry. Emotion perception across modalities activates lateral and basal amygdala nuclei (LeDoux, 2000), the latter of which shows strong bidirectional connectivity with cingulate motor cortices representing upper facial features (M3) but only weak connectivity with areas representing the lower face (M4). Basolateral amygdala, however, also shows strong connectivity to the central amygdala nucleus and from there to the hypothalamus, cingulate, insula, and other regions mediating physiological, behavioral, and subjective emotional responses. It is therefore interesting to speculate that mimetic responses in the upper face may perhaps provide a more direct representation of efferent amygdala responses to emotional stimuli. Alternately, following the logic of the facial feedback hypothesis, feedback to the amygdala from upper facial features may play a more prominent role in mediating subjective emotional responses.
Interestingly, the empathy scale employed in our study, the BEES, is a refinement of the earlier QMEE scale (Mehrabian & Epstein, 1972) used by Sonnby-Borgstrom et al. to show a relationship with emotional empathy. In this regard, previous investigators using other measures of empathy such as the emotional quotient (EQ) have failed to show a relationship with mimicry (Achaibou et al., 2008). It has been argued that this may be because the QMEE is a broad measure of emotional empathy sensitive to general emotional arousability (Chlopan, McCain, Carbonell, & Hagen, 1985). This raises the issue of the range of elicitors of emotionally congruent facial responses. De Gelder and colleagues have demonstrated emotionally congruent facial EMG responses to emotional bodily postures, which occur even when the stimuli are presented outside of the subjects’ awareness (Tamietto and de Gelder 2009). These data suggests a more general linkage between emotion perception and congruent facial responses and raise the question of whether highly empathetic individuals on the BEES would also show strong congruent facial responses to emotional stimuli across sensory modalities.
Finally, we show that norepinephric modulation, in contrast to its effects on emotion perception, does not modulate emotional mimicry or its relationship with emotional empathy. It should be noted, however, that this conclusion is drawn by comparing effects of reboxetine vs. propranolol without an additional placebo arm. Though propranolol has known j31 and j32 blocking activity (compared to the norepinephrine increasing effects of reboxetine), its pharmacology is complex. For example, some studies have shown that propranolol increases synaptic norepinephrine (Tackett, Webb, & Privitera, 1981), suggesting that it may additionally have indirect a1 agonist activity. Future studies should also consider the addition of a placebo arm to investigate this potential effect. Our finding of an absence of an effect of norepinephine modulation on emotional mimicry should be interpreted in the context of emotion perception studies, many of which have shown an effect of norepinephrine only on weakly arousing negatively valenced emotional stimuli, with negligible effect on more potent stimuli (Harmer, Perrett, Cowen, & Goodwin, 2001). These findings suggest a potential sensory threshold effect, whereby norepinephrine modulates the limit above which perceived stimuli are labeled as negatively valenced. To minimize potential sensory threshold effects we therefore utilized only highly arousing facial stimuli in the current study. Consistent with the potential sensory threshold effect of norepinephrine, there were no significant differences in arousal or valence ratings for positive or negatively valenced stimuli between groups in our current study.
In contrast to our current findings, Hermans et al. showed that acute administration of testosterone to healthy females was associated with a significant reduction in differential corrugator responses to happy compared to angry facial expressions (Hermans, Putman, & van Honk, 2006) (a trend-level effect in the same direction was also found in ZM responses). They argued that the influence of testosterone on mimetic responses underpinned the effects of high testosterone on empathy.
In conclusion, we have shown that differential CS but not ZM responses to emotional facial stimuli predict emotional empathy. Despite influencing negative emotion perception, acute norepinephrine modulation does not modulate facial mimetic responses.
Contributor Information
Neil A. Harrison, University of Sussex, Falmer, and University College London, London, UK.
Robert Morgan, University College London, London, UK.
Hugo D. Critchley, University of Sussex, Falmer, UK
REFERENCES
- Achaibou A. Pourtois G. Schwartz S. Vuilleumier P. Simultaneous recording of EEG and facial muscle reactions during spontaneous emotional mimicry. Neuropsychologia. 2008;46:1104–1113. doi: 10.1016/j.neuropsychologia.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Andreasson P. Dimberg U. Emotional empathy and facial feedback. Journal of Nonverbal Behavior. 2008;32:215–224. [Google Scholar]
- Bagby R. M. Taylor G. J. Parker J. D. A. The 20-Item Toronto-Alexithymia-Scale. 2. Convergent, discriminant, and concurrent validity. Journal of Psychosomatic Research. 1994;38:33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Beck A. T. Steer R. A. Ball R. Ranieri W. F. Comparison of Beck Depression Inventories-IA and - II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Burrows A. M. Waller B. M. Parr L. A. Bonar C. J. Muscles of facial expression in the chimpanzee (Pan troglodytes): Descriptive, comparative and phylo-genetic contexts. Journal of Anatomy. 2006;208:153–167. doi: 10.1111/j.1469-7580.2006.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush L. K. Hess U. Wolford G. Transformations for within-subject designs: A Monte Carlo investigation. Psychological Bulletin. 1993;113:566–579. doi: 10.1037/0033-2909.113.3.566. [DOI] [PubMed] [Google Scholar]
- Chlopan B. E. McCain M. L. Carbonell J. L. Hagen R. L. Empathy: Review of available measures. Journal of Personality and Social Psychology. 1985;48:635–653. [Google Scholar]
- Cohen D. Strayer J. Empathy in conduct-disordered and comparison youth. Developmental Psychology. 1996;32:988–998. [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. London: John Murray; 1872. [Google Scholar]
- de Wied M. van Boxtel A. Zaalberg R. Goudena P. P. Matthys W. Facial EMG responses to dynamic emotional facial expressions in boys with disruptive behavior disorders. Journal of Psychiatric Research. 2006;40:112–121. doi: 10.1016/j.jpsychires.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dimberg U. Facial reactions to facial expressions. Psychophysiology. 1982;19:643–647. doi: 10.1111/j.1469-8986.1982.tb02516.x. [DOI] [PubMed] [Google Scholar]
- Dimberg U. Karlsson B. Facial reactions to different emotionally relevant stimuli. Scandinavian Journal of Psychology. 1997;38:297–303. [Google Scholar]
- Dimberg U. Lundquist L. O. Facial reactions to facial expressions: Sex-differences. Psychophysiology. 1988;25:442–443. [Google Scholar]
- Dimberg U. Thunberg M. Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Field T. M. Woodson R. Greenberg R. Cohen D. Discrimination and imitation of facial expression by neonates. Science. 1982;218:179–181. doi: 10.1126/science.7123230. [DOI] [PubMed] [Google Scholar]
- Fridlund A. J. Cacioppo J. T. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Harmer C. J. Perrett D. I. Cowen P. J. Goodwin G. M. Administration of the beta-adrenoceptor blocker propranolol impairs the processing of facial expressions of sadness. Psychopharmacology. 2001;154:383–389. doi: 10.1007/s002130000654. [DOI] [PubMed] [Google Scholar]
- Harmer C. J. Shelley N. C. Cowen P. J. Goodwin G. M. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Harrison N. A. Wilson C. E. Critchley H. D. Processing of observed pupil size modulates perception of sadness and predicts empathy. Emotion. 2007;7:724–729. doi: 10.1037/1528-3542.7.4.724. [DOI] [PubMed] [Google Scholar]
- Hatfield E. Cacioppo J. T. Rapson R. L. Emotional contagion. Cambridge, UK: Cambridge University Press; 1994. [Google Scholar]
- Hermans E. J. Putman P. van Honk J. Testosterone administration reduces empathetic behavior: A facial mimicry study. Psychoneuroendocrinology. 2006;31:859–866. doi: 10.1016/j.psyneuen.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Hermans E. J. van Wingen G. Bos P. A. Putman P. van Honk J. Reduced spontaneous facial mimicry in women with autistic traits. Biological Psychology. 2009;80:348–353. doi: 10.1016/j.biopsycho.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Hess U. Philippot P. Blairy S. Mimicry: Facts and fiction. In: Philippot P., editor; Feldman R. S., editor; Coats E. J., editor. The social context of nonverbal behavior: Studies in emotion and social interaction. New York: Cambridge University Press; 1999. pp. 213–241. [Google Scholar]
- Izard C. E. The face of emotion. New York: Appleton-Century-Crofts; 1971. [Google Scholar]
- LeDoux J. E. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lipps T. Das Wissen von fremden Ichen. In: Lipps T., editor. Psychologische Untersuchungen. Vol. 1. Leipzig, Germany: Engelmann; 1907. pp. 694–722. [Google Scholar]
- Lundqvist D. Flykt A. Öhmann A. The Karolinska Directed Emotional Faces (KDEF) Stockholm: Karolinska Institute; 1998. [Google Scholar]
- Mehrabian A. Relations among personality scales of aggression, violence and empathy: Validational evidence bearing on the Risk of Eruptive Violence Scale. Aggressive Behavior. 1997;23:433–445. [Google Scholar]
- Mehrabian A. Epstein N. A measure of emotional empathy. Journal of Personality. 1972;40:525–543. doi: 10.1111/j.1467-6494.1972.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Morecraft R. J. Louie J. L. Herrick J. L. Stilwell-Morecraft K. S. Cortical innervation of the facial nucleus in the non-human primate: A new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- Morecraft R. J. McNeal D. W. Stilwell-Morecraft K. S. Gedney M. Ge J. Schroeder C. M., et al. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. Journal of Comparative Neurology. 2007;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- Norbury R. Mackay C. E. Cowen P. J. Goodwin G. M. Harmer C. J. The effects of reboxetine on emotional processing in healthy volunteers: An fMRI study. Molecular Psychiatry. 2008;13:1011–1020. doi: 10.1038/sj.mp.4002091. [DOI] [PubMed] [Google Scholar]
- Roniadou-Anderjaska V. Qashu F. Braga M. F. Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: Implications for epilepsy and anxiety disorders. Amino Acids. 2007;32:305–315. doi: 10.1007/s00726-006-0415-x. [DOI] [PubMed] [Google Scholar]
- Rubinow D. R. Post R. M. Impaired recognition of affect in facial expression in depressed patients. Biological Psychiatry. 1992;31:947–953. doi: 10.1016/0006-3223(92)90120-o. [DOI] [PubMed] [Google Scholar]
- Schwartz G. E. Fair P. L. Mandel M. R. Salt P. Mieske M. Klerman G. L. Facial electromyography in the assessment of improvement in depression. Psychosomatic Medicine. 1978;40:355–360. doi: 10.1097/00006842-197806000-00008. [DOI] [PubMed] [Google Scholar]
- Schwartz G. E. Fair P. L. Salt P. Mandel M. R. Klerman G. L. Facial muscle patterning to affective imagery in depressed and nondepressed subjects. Science. 1976;192:489–491. doi: 10.1126/science.1257786. [DOI] [PubMed] [Google Scholar]
- Sheline Y. I. Barch D. M. Donnelly J. M. Ollinger J. M. Snyder A. Z. Mintun M. A. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sonnby-Borgstrom M. Automatic mimicry reactions as related to differences in emotional empathy. Scandinavian Journal of Psychology. 2002;43:433–443. doi: 10.1111/1467-9450.00312. [DOI] [PubMed] [Google Scholar]
- Sonnby-Borgstrom M. Jonsson P. Svensson O. Emotional empathy as related to mimicry reactions at different levels of information processing. Journal of Nonverbal Behavior. 2003;27:3–23. [Google Scholar]
- Tackett R. L. Webb J. G. Privitera P. J. Cerebroventricular propranolol elevates cerebrospinal fluid norepinephrine and lowers blood pressure. Science. 1981;213:911–913. doi: 10.1126/science.7256285. [DOI] [PubMed] [Google Scholar]
- Tamietto M. Castelli L. Vighetti S. Perozzo P. Geminiani G. Weiskrantz L., et al. Unseen facial and bodily expressions trigger fast emotional reactions. Proceedings of the National Academy of Sciences. 2009;106(42):17661–17666. doi: 10.1073/pnas.0908994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. J. Bagby R. M. Ryan D. P. Parker J. D. Doody K. F. Keefe P. Criterion validity of the Toronto Alexithymia Scale. Psychosomatic Medicine. 1988;50:500–509. doi: 10.1097/00006842-198809000-00006. [DOI] [PubMed] [Google Scholar]


