Abstract
ERas, a unique member of the Ras family, was initially found only in embryonic stem (ES) cells, where it plays a crucial role in the transformation of transplanted ES cells to teratomas. ERas is involved in ES cell survival, and unlike other Ras family members, is constitutively active without any mutations. The aim of this study was to investigate the expression and role of ERas in human gastric cancer. To test whether ERas played a significant role in human cancer cells, we examined its expression and function in gastric cancer. ERas was expressed in gastric cancer cell lines at different levels. Induction of ERas expression activated the phosphatidylinositol 3 kinase (PI3K)/Akt axis and then enhanced anchorage-independent growth and ERas knockdown by siRNA suppressed cell invasion. Immunohistochemical analyses revealed that ERas was expressed in 38.7% (55/142) of human gastric carcinoma tissues, and its expression was significantly associated with metastasis to the liver (P < 0.0001) and lymph nodes (P < 0.05). ERas up-regulated transcription regulatory factors including ZFHX1A, ZFHX1B, and TCF3, which repress E-cadherin. These data suggest that ERas is activated in a significant population of gastric cancer, where it may play a crucial role in gastric cancer cell survival and metastases to liver via down-regulation of E-cadherin.
Embryonic stem (ES) cells are derived from the inner cell mass of early stage mammalian embryos. Because of their pluripotency, immortality, and rapid growth, ES cell therapies have been proposed for tissue replacement after injury or disease. However, mouse ES cells can develop into teratomas when transplanted into nude mice, which might caution against therapeutic use of ES cells. ERas (ES cell-expressed Ras), a novel member of the Ras family, was identified in mouse ES cells as a transforming oncogene accounting for the tumor-like growth properties of ES cells.1 Ras proteins are small guanosine triphosphate hydrolases (GTP)-ases that cycle between inactive guanosine diphosphate (GDP)-bound and active GTP-bound conformations.2,3 Ras proteins associate with and activate multiple downstream effectors that control diverse cellular responses involved in cell proliferation, survival, and differentiation. Point mutations of the ras gene family, including K-ras, N-ras, and H-ras, are frequently detected in human tumors, and these gain-of-function mutations lock the ras protein in GTP-bound conformations and render the protein constitutively active and oncogenic. These oncogenic Ras proteins promote tumorigenicity via interacting mainly with two of the best-characterized downstream effector targets of Ras, phosphatidylinositol-3-OH kinase (PI3K) and Raf. In contrast, ERas is constructively active without any mutations and interacts with PI3K but not with Raf.1 K-ras is the most common mutated form of Ras, and point mutations of K-ras are detected in 60 to 90% of pancreatic cancers and in more than 30% of colorectal cancers.4,5,6 In contrast, the incidence of K-ras mutation in gastric cancer is less than 10%. A correlation between K-ras mutation and pathological indices in gastric cancer has been reported,7,8 and although the roles of oncogenic Ras in gastric cancer are not well understood at the molecular level, there is some experimental evidence that aberrant Ras activation mediates malignant transformation and tumorigenesis by promoting cell proliferation, cell migration, and resistance to apoptosis.9,10,11,12 Oncogenic Ras also contributes to the epithelial to mesenchymal transition (EMT), exacerbates motility and invasiveness of many cell types, and is often considered a prerequisite for tumor infiltration and metastasis.13,14
From these previous findings, we hypothesized that ERas might play a role in cancer cell growth and metastasis. Therefore, we investigated the expression of ERas and its possible role in cell transformation and metastasis of gastric cancer.
Materials and Methods
Cell Culture and Transfection
The cell lines ISt-1, KATOIII, NUGC-4, MKN-28, MKN-45, and MKN-74 were cultured in RPMI1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS), HGC-27 was cultured in Minimum Essential Medium Eagle (MEM; Sigma-Aldrich) supplemented with 10% FBS, GCIY was cultured in MEM supplemented with 15% FBS, and AGS was cultured in Dulbecco’s minimum essential medium (Life Technologies, Rockville, CA) supplemented with 10% FBS. They were cultured under an atmosphere of 5% CO2at 37°C. Stable transfections of GCIY was performed with the expression plasmid for ERas, pCAG-hERas, as previously described,1 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. As a control, GCIY were transfected with the empty pCAG-IP plasmid (a gift from Dr. Niwa, Osaka University Graduate School of Medicine, Course of Advanced Medicine, Area of Molecular Therapeutics, Stem Cell Regulation Research15). The transfected cells were selected by growth in medium containing 5 μg/ml puromycin (Sigma-Aldrich) and subcloned to single-cell clones.
Small Interfering RNA Transfection
ERas Stealth siRNA (HSS142544, HSS179365; Invitrogen) or high GC% Negative control siRNA (Invitrogen) was mixed with Lipofectamine RNAiMAX (Invitrogen) in a OptiMEM serum-free medium (Invitrogen) for 20 minutes at room temperature and then added to cells at a final concentration of 33 nmol/L. Forty-eight hours post transfection, cells were harvested for Western blots and invasion assays.
Patient Population
Tumor specimens were obtained from 142 gastric cancer patients who had not received chemotherapy or radiotherapy before surgery. All patients underwent gastrectomy at Nagoya City University Hospital and Kasugai Municipal Hospital. Representative blocks from each specimen, which included both tumor and the adjacent normal mucosa, were taken for immunohistochemical study. Protein was extracted from tumor tissues and adjacent non-tumor tissue in 4 patients for western blots.
Production of Polyclonal Anti-Human ERas Antibody
The ERas sequences (GenBank NM_181532) were inserted into bacterial expression vector pET16b (Novagen, Madison, WI), supplying an N-terminal histidine tag and introduced into E. coli BL21. Transformed BL21 cells harboring the ERas expression plasmid were grown in Luria-Bertani medium containing 100 μg/ml ampicillin. Induction was achieved by isopropyl thio-β-D-galactoside (IPTG) supplementation to a final concentration of 0.1 mmol/L. After incubation for 16 hours at 16°C, cells were harvested by centrifugation (10,000 × g, 10 minutes, 4°C). The collected cells were suspended in 40 ml of buffer A (20 mmol/L Tris-HCl pH 7.9, 5 mmol/L imidazole, 0.5 mol/L NaCl, 8 mol/L urea, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μmol/L leupeptin, 10 μmol/L pepstain A), subjected to a single freeze/thaw cycle, and disrupted by ultrasonication on ice. Cell debris was removed by centrifugation (30,000 × g, 40 minutes, 4°C) and the supernatant was collected. His-Tag recombinant human ERas protein was purified from the supernatant using a FPLC system (GE Health care Bio-sciences, Fairfield, CA) according to manufacture’s protocol and used to raise polyclonal antisera in Japanese white rabbits using standard procedures.
Immunohistochemical Analysis
Serial 2-μm-thick sections were cut from the 10% formalin-fixed, paraffin-embedded blocks of primary gastric cancer tissue and their metastases. Sections were deparaffinized in xylene and subsequently hydrated through a graded series of ethanol. After being rinsed in PBS, endogenous peroxidase activity was blocked by treatment with 3% hydrogen peroxide in 100% methanol. Deparaffinized sections after treatment were incubated with diluted rabbit polyclonal anti-human ERas antibody (1:200) or E-cadherin antibody (Dako, Copenhagen, Denmark) (1:50), then reacted with secondary antibody. Immunodetection of signal with DAB used a NexES automatic immunohistochemical stainer (Ventana, Tucson, AZ). From five areas of each section, 100 cells were randomly selected and counted. Tumors were classified as positive for ERas when 50% of carcinoma cells were stained. The evaluation was performed using a blind experimental protocol.
Total RNA Isolation and RT-PCR
Total RNA was extracted with the Aurum total RNA fatty and fibrous tissue kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. Reverse transcription was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster, CA) according to the manufacturer’s protocol. The synthesized cDNA from each sample was subjected to polymerase chain reaction (PCR) amplification using Ex TaqDNA polymerase (Takara Bio, Shiga, Japan) and primers. The sequences of the GAPDH primers were 5′-GACCTGACTGACTACCTCAT-3′ (forward) and 5′-AGCAAGCAGGAGTAGACGA-3′ (reverse). The PCR program was as follows: 10 minutes of initial denaturation at 96°C, 10 seconds at 98°C, 30 seconds at 60°C, and 45 seconds at 72°C, repeated for 35 cycles. The ERas primers and PCR conditions have been described previously.16 To check for DNA contamination, we performed PCR without initial RT in all cell lines. Amplified products were separated by 2% agarose gel electrophoresis, and bands were visualized by ethidium bromide staining. The gels were photographed under UV illumination. The identification of PCR product was confirmed by DNA sequencing in some cell lines.
Real-Time Quantitative RT-PCR
TaqMan Gene Expression Assays for ERas (Hs01028327_ m1), Cdh-1 (Hs00170423_m1), SNAI1 (Hs00195591_m1), SNAI2 (Hs00950344_m1), ZFHX1A (Hs00611018_m1), ZFHX1B (Hs00207691_m1), TWIST1 (Hs00361186_m1), TCF3 (Hs01012685_m1), and GAPDH (Hs99999905_m1) were purchased from Applied Biosystems, and real-time quantitative RT-PCR analyses were performed in triplicate using Applied Biosystems ABI Prism 7500 according to the supplier’s recommendations. The housekeeping gene GAPDH was chosen as an endogenous control to normalize the expression data for each gene.
Gene Array Analysis
Target genes of ERas that contribute to tumor metastasis were identified by comparing mRNA expression in GCIY transfected with empty vector and GCIY overexpressing ERas using RT2 Profile PCR Array System containing tumor metastasis-related genes (APHS-028A; SABiosciences, Frederick, MD). Raw data were normalized using PCR Array analyzed software.
Immunoblots
Cells were lysed in a lysis buffer (Cell Signaling, Beverly, MA) on ice for 15 minutes, followed by sonication. An equal volume of 2× sample buffer [0.1 mol/L Tris/HCl, 4% W/V sodium dodecyl sulfate (SDS), 20% glycerol, and 100 mmol/L dithiothreitol, pH 6.8] was added. SDS-PAGE was performed using 10% polyacryl amide gels. PAGE-separated proteins were electrophoretically transferred onto nitrocellulose membranes (GE Health care Bio-sciences). The membrane filters were blocked with 5% powdered milk in TBS-T (0.1% Tween 20, 20 mmol/L Tris-HCl, 137 mmol/L NaCl, pH 7.6) for 1 hour and then incubated in rabbit anti-ERas antibody (gift from Dr. Takahashi, Kyoto University, Institute for Frontier Medical Sciences, Department of Stem Cell Biology) diluted 1:1000, rabbit anti-Akt antibody diluted 1:3000, rabbit anti-phospho-Akt antibody diluted 1:1000, rabbit anti-MEK1/2 antibody diluted 1:1000, rabbit anti-phospho-MEK1/2 antibody (Cell signaling) diluted 1:1000, mouse anti-E-cadherin antibody (BD Biosciences, San Jose, CA) diluted 1:1000, rabbit anti-Vimentin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:100, mouse anti-Fibronectin antibody (BD Biosciences) diluted 1:1000, or mouse anti-β-actin antibody (Sigma- Aldrich) diluted 1:2000 in 0.1% powdered milk in TBS-T at 4°C overnight, and then with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (1:3000; Cell Signaling) for 1 hour at room temperature. Antigens on the membrane were detected with enhanced chemiluminescense detection reagents (GE Healthcare Bio-sciences).
Immunofluorescence Microscopy
Samples were fixed with ethanol and acetone. Incubation with primary antibodies against human ERas or E-cadherin (BD Biosciences) was generally done in a solution of PBS containing 0.1% milk. Secondary antibodies were Alexa Fluor 594 goat anti-rabbit IgG or Alexa Fluor 488 goat anti-mouse IgG (H + L; Invitrogen). All sections were counterstained with 4′,6-diamidino-2-phenylindole (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Images were obtained using an Eclipse 80i fluorescence microscope (Nikon, Tokyo, Japan).
Soft Agar Assay
For soft agar assays, 2.5 × 103 cells of the stable transfectants or the parental GCIY cells were seeded in triplicate into 24-well culture plates containing 0.35% agar and MEM supplemented with 15% FBS. After 21 days, colonies were scored as positive for crystal violet staining.
MTS Assay
Cells were seeded into 96-well plates at a density of 5 × 103 cells per well in culture medium. We measured cell growth using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assays (Promega) after seeding the cells according to the manufacturer’s instructions.
Invasion Assay
Invasion assays were done in 96-well transwell plates (Corning, Lowell, MA) as described with the following modifications. Briefly, transwell inserts with 8-μm-pore-size membranes were coated with 50 μl of 50% Matrigel (BD Biosciences) and dried overnight at 37°C in a CO2 incubator. After 24 hours of serum starvation, cells (5000 cells per well) in serum-free medium were placed in the upper chamber on the membrane. Medium with 10% FBS was added to the bottom chambers. After incubation for 36 hours, the cells that invaded the lower surface were measured using calcein AM (Invitrogen) according to manufacture’s instructions.
Statistical Analysis
Unpaired Student’s t-test and Fisher’s exact test or χ2 test were used to analyze data. The level of significance was set at 5%, using two-sided analysis.
Results
ERas Is Expressed in Gastric Cancer Cells and Gastric Cancer Tissues
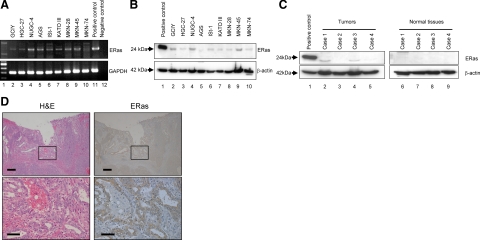
Investigation of nine gastric cancer cell lines showed various levels of expression of full-length ERas mRNA (Figure 1A). Direct DNA sequencing revealed no mutation of ERas in AGS, KATOIII, GCIY, and NUGC-4 (data not shown). To avoid contamination with genomic DNA, sample RNA without RT was subjected to PCR analysis and no bands were detected in all samples. ERas protein expression was also detected in different levels in all gastric cancer cell lines we examined (Figure 1B). Furthermore, Western blots revealed ERas expression in three of four gastric cancer samples. In contrast, ERas was not detected in normal gastric mucosa adjacent to tumor in all samples we examined (Figure 1C). Immunohistochemical staining of gastric cancer sample shows ERas expression in the tumor cells. ERas was not expressed in normal gastric mucosa (Figure 1D).
Figure 1.
ERas mRNA and protein expression in nine gastric cancer cell lines and gastric cancer tissues. A: Expression of the ERas gene in gastric cancer cell lines (Lanes 2–10) was examined by RT-PCR. The negative control (Lane 12) indicates no template in the reaction. Primers for GAPDH were used as internal control. B: Expression of ERas protein in nine gastric cancer cell lines was analyzed by Western blots with anti-ERas antibody (Lanes 2–10). C: Expression of ERas protein in four cases of human gastric cancer tissue (Lanes 2–5) and corresponding non-tumor tissue adjacent to gastric cancer (Lanes 6–9) was analyzed by Western blot with anti-ERas antibody. D: Expression of ERas in gastric cancer tissue by immunohistochemical staining. Scale bars: 200 μm (top); 50 μm (bottom).
ERas Expression Is Correlated with Liver Metastasis
We next examined ERas expression of 142 resected gastric cancer tissue samples by immunohistochemistry. In all cases, normal gastric mucosa that was adjacent to cancer cells did not express ERas. In contrast, gastric cancer cells expressed ERas protein in 55 of 142 cases (37.5%). In all cases that were positive for ERas, ERas staining was heterogeneous in the cancer tissue. To investigate the correlation between ERas expression and gastric cancer histological classification, we classified the gastric cancers into ‘differentiated’ and ‘undifferentiated’ in accordance with the criteria of the Japanese Research Society of Gastric Cancer.17 As shown in Table 1, there was no significant correlation between ERas expression and histological classification. We also analyzed for possible correlation between ERas expression and gastric cancer metastases. Interestingly, ERas expression was strongly associated with gastric cancer that was complicated with liver metastases (P < 0 0.0001). We also detected a significant correlation between ERas expression and lymph node metastases (P < 0.05). Positive ratio of ERas expression in Tis–T1 and T2–T4 were 31.5% and 43.2%, respectively (P = 0.17).
Table 1.
Correlation between Clinicopathological Factors and ERas Expression in Gastric Cancer
| ERas positive (n = 55) | ERas negative (n = 87) | Positive rate (%) | P value | |
|---|---|---|---|---|
| Age, n | ||||
| <50 years | 3 | 5 | 37.5 | |
| 51 to 70 years | 27 | 46 | 37.0 | |
| >71 years | 25 | 36 | 41.0 | 0.54 |
| Sex, n | ||||
| Male | 39 | 62 | 38.6 | |
| Female | 16 | 25 | 39.0 | 0.96 |
| Histological classification, n | ||||
| Differentiated | 32 | 53 | 37.6 | |
| Undifferentiated | 23 | 34 | 40.4 | 0.75 |
| Depth of invasion, n | ||||
| Tis to T1 | 17 | 37 | 31.5 | |
| T2 to T4 | 38 | 50 | 43.2 | 0.16 |
| Lymph node metastasis, n | ||||
| Positive | 34 | 36 | 48.6 | |
| Negative | 21 | 51 | 29.2 | <0.05 |
| Liver metastasis, n | ||||
| Positive | 14 | 2 | 87.5 | |
| Negative | 41 | 85 | 32.5 | <0.0001 |
Tis indicates carcinoma in situ; T1, lamina propria and submucosa; T2, muscularis propria and subserosa; T3, exposure to serosa; T4, invasion into serosa.
ERas Promotes Transforming Activity
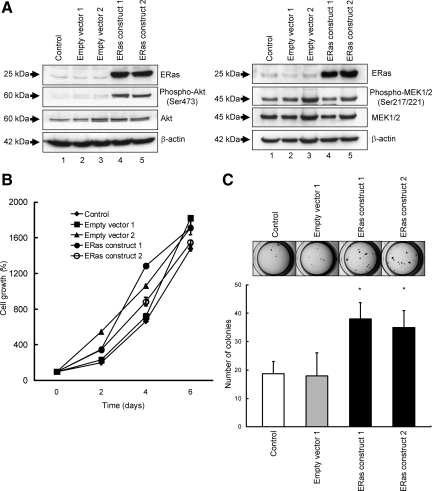
We established ERas overexpressing clones using gastric cancer cell line, GCIY, to test the effect of ERas on gastric cancer. To determine whether ERas could activate two major downstream pathways of Ras, the PI3K/Akt and the MAPK pathway in gastric cancer cells, we assessed phosphorylation of Akt and MEK by western blots analysis. As shown in Figure 2A, ERas induced phosphorylation of Akt but did not enhance MEK phosphorylation. To investigate the effects of ERas on gastric cancer cell growth, we examined cell growth using the MTS assay (Figure 2B). There were no significant differences between ERas overexpressing clones and the parental GCIY cells. To examine the potential role of ERas in the anchorage-independent growth, each clone was cultured in soft agar medium for 3 weeks and colony formation was assessed by microscopy. ERas promoted colony formation in soft agar, showing about two times the number of colonies compared with the parental GCIY cells (Figure 2C).
Figure 2.
The role of ERas on signaling pathway, cell growth, and anchorage-independent growth. A: Western blotting of total lysates of control, parental GCIY cells (Lane 1), GCIY cells transfected with empty vector (Lanes 2 and 3), and ERas-overexpressing GCIY clones (GCIY cells transfected with ERas-expressing vector) (Lanes 4 and 5) with anti-phospho-Akt and Akt antibody, or with anti-phospho-MEK1/2 and MEK1/2 antibody. The cell lysates were probed for β-actin to control for equal protein loading. B: Parental GCIY cells, GCIY cells transfected with empty vector, and ERas-overexpressing GCIY clones were plated at 103 cells per well on 96-well plates. Growth rates were measured by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetra-zolium (MTS) assay on subsequent days. This experiment was repeated three times in triplicate. C: Parental GCIY cells, GCIY cells transfected with empty vector, and ERas-overexpressing GCIY clones were plated at 2.5 × 103 cells per well on 24-well plates in soft agar. After 21 days the colonies were visualized with crystal violet and counted. Columns represent the means of three independent experiments; bars, SD. *P < 0.01 versus control.
Knockdown of ERas Inhibits Gastric Caner Cells Invasion
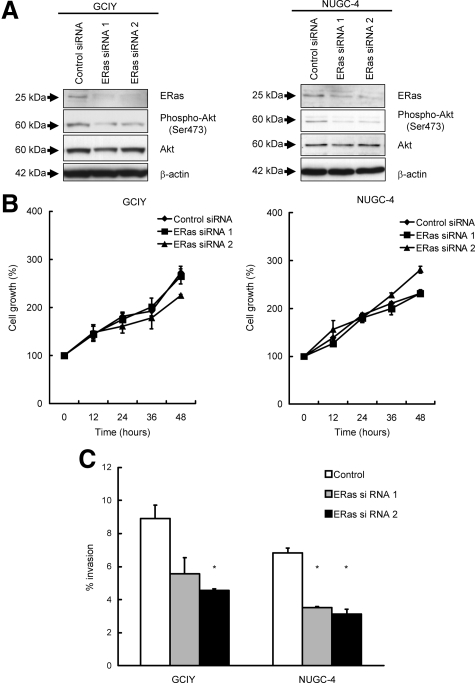
We also confirmed the effect of ERas in gastric cancer cell lines, GCIY and NUGC-4, using siRNA. As shown in Figure 3A, suppression of ERas by siRNA reduced the phosphorylation level of Akt. Knockdown of ERas inhibited the cell invasion as estimated by Matrigel assays compared with control in GCIY and NUGC-4 (Figure 3C). To check the effect of ERas knockdown on cell proliferation, we also measured cell growth after treatment with ERas siRNA by MTS assay. As shown in Figure 3B, ERas knockdown by siRNA could not change cell growth in GCIY and NUGC-4.
Figure 3.
Silencing of endogenous ERas expression suppresses invasive activity in gastric cancer cell lines. A: Western blotting of total lysates of GCIY cells and NUGC-4 cells treated with negative control siRNA (Lane 1), GCIY cells and NUGC-4 cells treated with ERas siRNA 1 (Lane 2), or ERas siRNA 2 (Lane 3) with anti-phospho-Akt and Akt antibody. The cell lysates were probed for β-actin to control for equal protein loading. B: GCIY cells and NUGC-4 cells treated with negative control siRNA, ERas siRNA 1, or ERas siRNA 2 were plated at 103 cells per well on 96-well plates. Growth rates were measured by MTS assay. C: GCIY cells and NUGC-4 cells treated with negative control siRNA, ERas siRNA 1, or ERas siRNA 2 were plated at 5 × 103 cells per well on upper chamber of membrane coated with Matrigel of 96-well Transwell plates. After 36 hours incubation, invasion of cells through Matrigel-coated membranes were measured. Columns represent the means of three independent experiments; bars, SD. *P < 0.01 versus control.
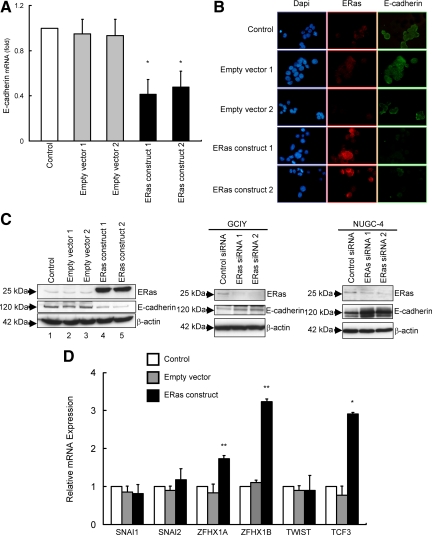
ERas Suppresses E-Cadherin Expression in Gastric Cancer
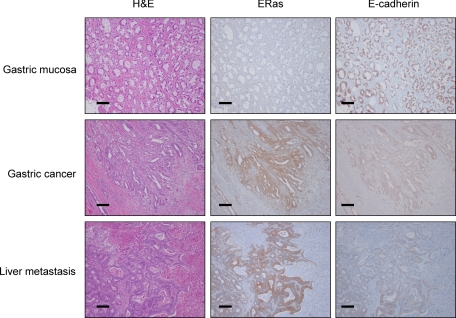
Clinicopathological analysis and functional assays suggest that ERas expression is associated with metastatic properties of gastric cancer cells. To know the mechanism whereby ERas contributes to metastasis, we analyzed the possible targets of ERas contributing to tumor metastasis using PCR Array. We focused on the result of PCR Array that ERas suppressed E-cadherin mRNA expression (Table 2), because disruption of E-cadherin is known to be associated with metastasis in gastric cancer.18,19 We performed immunohistochemical analysis of E-cadherin expression in the 142 cases of gastric cancer. E-cadherin staining was heterogeneous and frequently inversely associated with the expression of ERas in gastric cancer tissue and metastatic liver lesions (Figure 4). Next, we examined whether ERas suppressed the expression of E-cadherin in gastric cancer cells. RT-PCR showed that ERas suppressed the expression of E-cadherin mRNA in GCIY (Figure 5A). ERas suppressed the expression of E-cadherin protein (Figure 5, B and C). Conversely, siRNA for ERas resulted in increased E-cadherin expression in GCIY and NUGC-4 (Figure 5C). Next, we analyzed six known E-cadherin repressors, Snai1 (also known as Snail), Snai2 (Slug), Zfhx1a (δEF or Zeb1), Zfhx1b (SIP1 or Zeb2), Tcf3 (E12/E47), and Twist1, in GCIY transfected with ERas and parental GCIY by RT-PCR. As shown in Figure 5D, ZFHX1A, ZFHX1B, and TCF3 mRNA were significantly increased in GCIY transfected with ERas compared with parental cells.
Table 2.
Expression Profiles of Genes Related to Tumor Metastasis that Change at Least a 3-Fold in Expression by ERas Overexpressing
| Symbol | GenbankID | Increase | Gene name |
|---|---|---|---|
| CDH1 | NM_004360 | −4.65 | Cadherin 1 |
| FXYD5 | NM_014164 | −3.02 | FXYD domain containing ion transport regulator 5 |
| KISS1 | NM_002206 | −9.06 | KiSS-1 metastasis-suppressors |
| MMP10 | NM_000212 | 3.92 | Matrix metallopeptidase 10 |
| MMP2 | NM_001903 | 5.89 | Matrix metallopeptidase 2 |
| MMP9 | NM_004360 | −5.47 | Matrix metallopeptidase 9 |
| ROBO | NM_004360 | 11.82 | RAR-related orphan receptor B |
Figure 4.
Immunohistochemical analysis of ERas and E-cadherin protein expression in resected human gastric cancer tissue. Sections of normal adjacent gastric mucosa (top) did not show staining with anti-human ERas antibody but did stain with anti–E-cadherin antibody. ERas protein was expressed in gastric cancer tissue and liver metastasis. E-cadherin was not expressed in gastric cancer tissue and liver metastasis (middle and bottom). Scale bars = 50 μm.
Figure 5.
ERas suppresses the expression of E-cadherin. A: Real-time quantitative RT-PCR of E-cadherin mRNA levels in an ERas-overexpressing GCIY clones relative to parental GCIY cells. Columns represent the means of three independent experiments; bars, SD. *P < 0.01 versus control. B: Representative immunofluorescence stainings of cell lines. Nuclei stained blue with 4′,6-diamidino-2-phenylindole (DAPI) (left), ERas stained red with antibody against ERas (middle), and E-cadherin stained green with antibody against E-cadherin (right). C: Western blot analysis of total lysates of parental GCIY cells (Lane 1), GCIY cells transfected with empty vector (Lanes 2 and 3), and ERas-overexpressing GCIY clones (Lanes 4 and 5) with anti–E-cadherin antibody. Western blotting of total lysates of GCIY cells or NUGC-4 cells treated with ERas siRNA 1 or ERas siRNA 2 with anti–E-cadherin antibody. D: mRNA expression profiles of six E-cadherin repressors (SNAI1, SNAI2, ZFHX1A, ZFHX2B, TWIST1, and TCF3) in parental GCIY cells, ERas-overexpressing GCIY clone, and GCIY cells transfected with empty vector were determined by quantitative real-time RT-PCR. Columns represent the means of three independent experiments; bars, SD. *P < 0.001, **P < 0.05 versus control.
ERas Induces EMT in Gastric Cancer
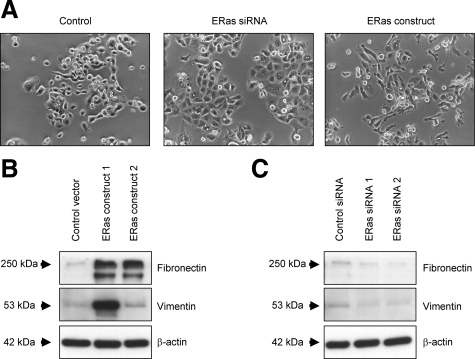
We assessed the association between ERas expression and morphological changes in GCIY. ERas transfectants disclosed spindle shape and formed irregular structures. In contrast, silencing of ERas by siRNA induced epithelial-like morphology (Figure 6A). Additionally, we examined the expression of mesenchymal markers involving Vimentin and Fibronectin by Western blotting. The expression of Vimentin and Fibronectin increased in ERas transfectants, whereas siRNA for ERas resulted in the inhibition of Vimentin and Fibronectin expression (Figure 6, B and C).
Figure 6.
ERas promotes metastasis via induction of EMT. A: Cell morphology of GCIY cells transfected with ERas-expressing vector or treated with ERas siRNA. B: Western blot analysis of total lysates of GCIY cells transfected with empty vector and ERas-overexpressing GCIY clones with anti-Fibronectin and Vimentin antibody. C: Western blotting of total lysates of GCIY cells treated with negative control siRNA, ERas siRNA 1, or ERas siRNA 2 with anti-Fibronectin and Vimentin antibody.
Discussion
We have investigated the expression and role of ERas in gastric cancer and provided the evidence of ERas expression in these cancer cells. ERas was originally found to be expressed in mouse ES cells where it plays a crucial role in tumorigenetic properties of such cells. Human ERas, previously called HRasp (Ha-Ras2), has a single open reading frame encoding a polypeptide with 76% identity to mouse ERas.1 Induced pluripotent stem (iPS) cells, generated artificially from mouse and human fibroblasts by introducing three factors, Oct3/Oct4, Klf-4, and Sox2, possess a similar phenotype to ES cells, and iPS cells generated from mouse fibroblasts express ES cell marker genes including ERas.20 However, recent reports revealed an absence of ERas gene expression in human ES cells and concluded that ERas exists as a silenced pseudogene in these cells.16,21 Here, we have revealed expression of the full-length ERas gene by RT-PCR and ERas protein by Western blots analysis in gastric cancer cell lines (Figure 1, A and B). Furthermore, we have demonstrated by Western blots and immunohistochemical staining that ERas protein is expressed in resected human gastric cancer tissues (Figure 1, C and D). Recently, another group reported ERas mRNA expression in human carcinoma including gastric cancer, colorectal cancer, pancreatic cancer, and breast cancer, and they suggested that epigenetic modification might be one of the possible mechanisms of ERas transcriptional regulation, while the expression of methyltransferases including Dnmt1, Dnmt3a, and Dnmt3b was not associated with ERas expression.22,23,24 We also confirmed that treatment with the DNA methyltransferase inhibitor 5-AzaC or the HDAC inhibitor TSA induced increased expression of ERas in some gastric cancer cell lines (data not shown). These data indicate that ERas expression might be regulated epigenetically in gastric cancer.
Western blots analysis revealed that ERas activates the PI3K/Akt pathway, but did not enhance the MAPK pathway, in gastric cancer cells (Figure 2, A and B), which was in accordance with a previous study of ERas signal transduction in mouse ES cells.1 In functional assays, we demonstrated that induction of ERas protein results in a significant increase in anchorage-independent growth but did not influence cell proliferation. Transwell invasion assays revealed that ERas positively regulated cancer cell invasion. Thus, ERas may be mainly associated with tumorigenicity.
In our sample series, 38.7% (55/142) of the stomach cancer cases expressed ERas (Table 1), a frequency that is higher than the incidence of K-ras mutation in gastric cancer.7,25 There was no significant association between ERas expression and the histological features in this study. However, we found that ERas was significantly associated with the presence of lymph node and liver metastases suggesting that ERas may serve as a new marker of poor prognosis for gastric cancer. However, in contrast to our report, good prognosis of patients with ERas-positive gastric cancer was reported, while ERas was not an independent prognostic factor.23 Differences in clinicopathological features of ERas expression in gastric cancer might be due to a higher percentage of patients with liver metastasis in our study (11.3%) compared with their report (1.6%). The indications for hepatic resection for gastric metastases have not been established and have been controversial. We performed gastrectomy for patients with liver metastasis based on previous reports.26,27 In brief, we performed gastrectomy for patients with a solitary liver metastasis who have no peritoneal dissemination.
Metastasis occur through various steps including the so-called EMT, which is an important process during tumor progression and metastasis development that causes loss of cell adhesion and increased cell motility. E-cadherin plays an important role in induction of EMT, and inactivation of E-cadherin has been reported to be relevant to invasion and metastasis of gastric cancer.18,19 The PCR Array analysis has revealed that ERas down-regulates E-cadherin (Table 2). Furthermore, we confirm directly that ERas transfection decreased mRNA and protein expression of E-cadherin, and knockdown of ERas up-regulated E-cadherin expression in gastric cancer cells (Figure 5). Immunohistochemical staining also revealed an inverse relationship between ERas and E-cadherin expression in gastric cancer tissue, in line with the results of molecular assays (Figures 4 and 5). E-cadherin loss has been reported to be associated with diffuse carcinoma.28,29 However, there have been some opposing or different reports.30,31 Moreover, Tanaka et al reported abnormalities of E-cadherin staining occur by different a manner according to histological subtypes of gastric cancer.32 In our present study, there was no significant association between histological subtype and ERas expression despite our findings that ERas repressed E-cadherin expression. Further investigation needs to clarify the association between ERas expression and histological subtype in gastric cancer. Abnormal expression and function of E-cadherin is caused by multiple mechanisms including mutation of the E-cadherin gene itself,33 transcriptional repression,30,34 hypermethylation, or chromatin rearrangement in the E-cadherin promoter.35,36 In this study, quantitative RT-PCR showed upregulation of ZFHX1A, ZFHX1B, and TCF3 in gastric cancer cells (Figure 5E). All three have been shown to bind to the proximal promoter of E-cadherin and participate in the EMT process, leading to the acquisition of invasive properties.34,37,38 Julien et al reported that activation of Akt represses the E-cadherin promoter through the upregulation of Snail and ZFHX1B.39 Although we did not observe upregulation of Snail in GCIY transfected with ERas, exogenous ERas expression could activate Akt kinase and up-regulate ZFHX1A, ZFHX1B, and TCF3. This evidence implies that ERas may up-regulate repressors of E-cadherin and induce EMT by activating Akt kinase in gastric cancer. Functionally, a positive correlation between ERas expression and invasion activity may account for the role of ERas in the EMT. In addition, morphological changes were observed in ERas-overexpressing cells, and upregulation of the mesenchymal markers by ERas reinforces the possibility that ERas induces EMT in gastric cancer cells (Figure 6).
In conclusion, we found that ERas, ES cell-specific Ras, is expressed in gastric cancer and enhances tumorigenicity by activating the PI3K/Akt pathway. ERas expression might accelerate liver metastases of gastric cancer via down-regulation of E-cadherin. ERas might be one candidate positive marker for liver metastases and a future target of antimetastasis therapy in gastric cancer.
Acknowledgments
We thank Dr. Shinya Yamanaka (Kyoto University, Institute for Frontier Medical Sciences, Department of Stem Cell Biology) for helpful advice and for providing the anti-mouse ERas antibody and plasmid for ERas. We also thank Yukimi Ito and Seizo Nagaya for technical assistance.
Footnotes
Address reprint requests to Hiromi Kataoka, M.D., Ph.D., Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601. E-mail: hkataoka@med.nagoya-cu.ac.jp.
Supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 17590666, No. 20659117, and No. 22590688), Nagono Medical Foundation, Nagoya, Japan (2007), the Aichi Cancer Research Foundation, Nagoya, Japan (2007), and Grant-in-Aid for Research in Nagoya City University (2009).
References
- Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- Sprang SR. G protein mechanisms: insights from structural analysis. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- Bos JL. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral, EMBO J. 1998;17:6776–6782. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, Kensler TW, Bose KK, Cameron JL, Bos JL. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–554. [PMC free article] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lee JS, Suh C, Kim SW, Kim SB, Lee JH, Lee MS, Park MY, Sun HS, Kim SH. Clinicopathologic significance of the K-ras gene codon 12 point mutation in stomach cancer. An analysis of 140 cases. Cancer. 1995;75:2794–2801. doi: 10.1002/1097-0142(19950615)75:12<2794::aid-cncr2820751203>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yashiro M, Nishioka N, Hirakawa K. K-ras mutation influences macroscopic features of gastric carcinoma. J Surg Res. 2005;124:74–78. doi: 10.1016/j.jss.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Eckert LB, Repasky GA, Ulku AS, McFall A, Zhou H, Sartor CI, Der CJ. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–4592. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- Giehl K. Oncogenic Ras in tumour progression and metastasis. Biol Chem. 2005;386:193–205. doi: 10.1515/BC.2005.025. [DOI] [PubMed] [Google Scholar]
- Feramisco JR, Gross M, Kamata T, Rosenberg M, Sweet RW. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984;38:109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Bifulco M. Role of the isoprenoid pathway in ras transforming activity, cytoskeleton organization, cell proliferation and apoptosis. Life Sci. 2005;77:1740–1749. doi: 10.1016/j.lfs.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Masui S, Chambers I, Smith AG, Miyazaki J. Phenotypic complementation establishes requirements for specific POU domain and generic transactivation function of Oct-3/4 in embryonic stem cells. Mol Cell Biol. 2002;22:1526–1536. doi: 10.1128/mcb.22.5.1526-1536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda T, Thomson JA. Human ERas gene has an upstream premature polyadenylation signal that results in a truncated, noncoding transcript. Stem Cells. 2005;23:1535–1540. doi: 10.1634/stemcells.2005-0054. [DOI] [PubMed] [Google Scholar]
- Japanese Gastric Cancer Association Japanese Classification of Gastric Carcinoma, 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- Cai J, Ikeguchi M, Tsujitani S, Maeta M, Liu J, Kaibara N. Significant correlation between micrometastasis in the lymph nodes and reduced expression of E-cadherin in early gastric cancer. Gastric Cancer. 2001;4:66–74. doi: 10.1007/pl00011726. [DOI] [PubMed] [Google Scholar]
- Shino Y, Watanabe A, Yamada Y, Tanase M, Yamada T, Matsuda M, Yamashita J, Tatsumi M, Miwa T, Nakano H. Clinicopathologic evaluation of immunohistochemical E-cadherin expression in human gastric carcinomas. Cancer. 1995;76:2193–2201. doi: 10.1002/1097-0142(19951201)76:11<2193::aid-cncr2820761104>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Zhan M, Miura T, Xu X, Rao MS. Conservation and variation of gene regulation in embryonic stem cells assessed by comparative genomics. Cell Biochem Biophys. 2005;43:379–405. doi: 10.1385/CBB:43:3:379. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Yashiro M, Sawada T, Ohira M, Hirakawa K. ERas oncogene expression and epigenetic regulation by histone acetylation in human cancer cells. Anticancer Res. 2007;27:4071–4075. [PubMed] [Google Scholar]
- Kaizaki R, Yashiro M, Shinto O, Yasuda K, Matsuzaki T, Sawada T, Hirakawa K. Expression of ERas oncogene in gastric carcinoma. Anticancer Res. 2009;29:2189–2193. [PubMed] [Google Scholar]
- Yashiro M, Yasuda K, Nishii T, Kaizaki R, Sawada T, Ohira M, Hirakawa K. Epigenetic regulation of the embryonic oncogene ERas in gastric cancer cells. Int J Oncol. 2009;35:997–1003. doi: 10.3892/ijo_00000414. [DOI] [PubMed] [Google Scholar]
- Ming SC. Cellular and molecular pathology of gastric carcinoma and precursor lesions: a critical review. Gastric Cancer. 1998;1:31–50. doi: 10.1007/s101200050053. [DOI] [PubMed] [Google Scholar]
- Shirabe K, Wakiyama S, Gion T, Watanabe M, Miyazaki M, Yoshinaga K, Tokunaga M, Nagaie T. Hepatic resection for the treatment of liver metastases in gastric carcinoma: review of the literature. HPB (Oxford) 2006;8:89–92. doi: 10.1080/13651820500472168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, Yamaguchi T. Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol. 2007;37:836–842. doi: 10.1093/jjco/hym113. [DOI] [PubMed] [Google Scholar]
- Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, Birchmeier W, Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–1695. [PubMed] [Google Scholar]
- Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003;200:39–46. doi: 10.1002/path.1288. [DOI] [PubMed] [Google Scholar]
- Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211:507–515. doi: 10.1002/path.2138. [DOI] [PubMed] [Google Scholar]
- Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail. SIP1, and twist in gastric cancer, Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kitajima Y, Edakuni G, Sato S, Miyazaki K. Abnormal expression of E-cadherin and beta-catenin may be a molecular marker of submucosal invasion and lymph node metastasis in early gastric cancer. Br J Surg. 2002;89:236–244. doi: 10.1046/j.0007-1323.2001.01985.x. [DOI] [PubMed] [Google Scholar]
- Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura G, Yin J, Wang S, Fleisher AS, Zou T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, Silverberg SG, Nishizuka S, Terashima M, Motoyama T, Meltzer SJ. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst. 2000;92:569–573. doi: 10.1093/jnci/92.7.569. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA, Cano A. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem. 2001;276:27424–27431. doi: 10.1074/jbc.M100827200. [DOI] [PubMed] [Google Scholar]
- Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition, Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]