Abstract
This commentary discusses the role of caveolin-1 in atherosclerosis.
Caveolin-1 and Caveolae
The term caveolae was defined more than fifty years ago by Yamada as a “vesicle, cave, or recess communicating with the outside of a cell, and extending inward, indenting the cytoplasm and the cell membrane.”1 A few years earlier, Palade had identified the same structures as plasmalemmal vesicles.2 Indeed, Palade and collaborators were the first to propose a function for these structures in the transport of macromolecules from the lumen of blood vessels to peripheral tissues.3,4 These studies suggested a role for caveolae in the development of atherosclerosis.
The main protein component of caveolae was later identified as caveolin-1,5 which was found to be necessary for caveolae formation. However, until recently, very little work had been performed to examine the importance of caveolin-1 in the development of atherosclerosis. Given the relative cholesterol enrichment of caveolae compared with the rest of the plasma membrane, a few studies have proposed that caveolae and caveolin-1 might be involved in the regulation of intracellular cholesterol homeostasis. Moreover, when caveolin-1 expression was examined in numerous organs and cell types, it was found that caveolin-1 was highly expressed in terminally-differentiated cells, with endothelial cells and adipocytes expressing the highest levels.
Atherosclerosis is a disease of the blood vessel characterized by the presence of lesions formed by the initial buildup of cholesterol-enriched lipoproteins followed by the accumulation of macrophages and smooth muscle cells. Interestingly, endothelial cells, macrophages, and smooth muscles cells, which are involved in the progression of this disease, all express caveolin-1 and display caveolae, as observed by electron microscopy. In a previous study using caveolin-1–deficient mice, we have shown that a complete caveolin-1 deficiency is associated with a major reduction in the development of atherosclerosis.6 These findings have independently been confirmed by others.7 However, the role of caveolin-1 in the development of atherosclerosis is highly dependent on the cell type in which this protein is expressed.8
Evidence for a Role of Caveolin-1/Caveolae in Atherosclerosis
Caveolin-1 is a 178-aa plasma membrane protein with a unique conformation. It adopts a hairpin-like structure with both N and C termini in the cytoplasm, and its central domain is included in the plasma membrane. Numerous studies have shown that caveolin-1 can regulate cellular signaling pathways in various cell types. Previous work has identified a domain—termed the caveolin-scaffolding domain—responsible for the protein’s ability to regulate signal transduction. This region of caveolin-1 (residues 82-101) can bind signaling molecules such as EGF-R or other proteins like endothelial nitric oxide synthase (eNOS) and maintain them in an inactive state.9
Because caveolin-1 is particularly abundant in endothelial cells, it is believed to have a critical function in this cell type. Initial studies have indicated that endothelial caveolin-1 may act as a proatherogenic protein.8 The basis for this assertion came from the postulated role of caveolae in the transcytosis of low-density lipoprotein (LDL) particles.10 In addition, several putative atherogenic proteins colocalize with caveolae in endothelial cells. Some of these proteins are involved in lipoprotein metabolism (SR-BI, CD36, RAGE), cellular proliferation and migration (PDGF-R, VEGF-R), inflammation (TNFR), or the regulation of various signaling pathways (Src, G proteins).11,12 Taken together, these data suggest that caveolae and caveolin-1 must play an important role in the regulation of endothelial function and in the development of atherosclerosis.
Macrophage involvement in the development of atherosclerosis has been well established. Important studies have demonstrated macrophage uptake of modified lipoproteins at the sites of inflammation. If not properly controlled, macrophages are thought to further amplify the inflammation process and therefore aggravate the atherosclerotic development of the plaque. Recent studies have indicated that caveolin-1 could regulate the inflammation process in macrophages.13 Moreover, its function in the regulation of cellular cholesterol homeostasis may be important to limit foam cell formation.14 The proper regulation of this pathway is especially important during cholesterol accumulation in macrophages because it escalates the inflammatory response in atherosclerotic lesions.
Caveolin-1 has also been shown to control vascular smooth muscle function. In particular, it has been shown that elimination of vascular smooth muscle caveolin-1 is associated with increased cellular migration and proliferation.15 In vivo studies have confirmed these findings using a mouse model of arterial restenosis. In these cases, it has been shown that the absence of caveolin-1 can increase neointimal formation after carotid artery blood-flow cessation.15 Consistent with these findings, previous investigations have shown that caveolin-1 expression is decreased in neointimal smooth muscle cells present in atherosclerotic lesions.16
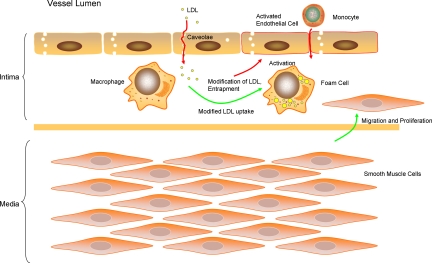
Taken together, these data suggest that while endothelial caveolin-1 may be proatherogenic, macrophage and smooth muscle cell caveolin-1 may play an inhibitory role in the development of atherosclerosis (Figure 1).
Figure 1.
Identification of the role of caveolin-1/caveolae in atherosclerosis. In this figure, the beneficial roles of caveolin-1 in atherosclerosis have been highlighted with green arrows. They are primarily limited to functions affecting macrophages (cholesterol metabolism and inflammation) and smooth muscle cells (inhibition of migration and proliferation). While caveolin-1 may have beneficial functions in these cell types, its effect is detrimental to endothelial cell function during atherogenesis (red arrows). Endothelial caveolin-1 may promote the transcytosis of LDL into the arterial intima, the activation of endothelial cells, and the subsequent migration of monocytes into the subendothelial space.
An Essential Role for Endothelial Caveolin-1 in the Development of Atherosclerosis
In their study published in the current issue of The American Journal of Pathology, Fernández-Hernando et al17 have demonstrated the important if not essential function of caveolin-1 in the development of atherosclerosis. Their data confirmed previous findings they had obtained using caveolin-1–deficient mice that were bred with mice overexpressing caveolin-1 specifically in endothelial cells. However, the current study extends these findings in the apoe−/− mouse background. Fernández-Hernando et al show that increased caveolin-1 expression in endothelial cells alone is associated with increased atherosclerosis in this mouse model.
Two important roles for caveolin-1 and caveolae have been proposed during the development of atherosclerosis: inflammation and LDL transfer into the intima. These findings clearly highlight the importance of the endothelium in the development of atherosclerosis. As previously suggested, caveolae/caveolin-1 may be necessary for the transfer of LDL in the intima, where lipoproteins are retained and modified.8 Caveolin-1 may also control the modification of lipoproteins, because the production of reactive oxygen subspecies has already been shown to be regulated by caveolin-1.18 However, at this point, we can only speculate as to whether caveolin-1 is involved in the retention of lipoproteins in the subendothelial space.
During the inflammatory process, endothelial vascular cell adhesion molecule 1 (VCAM-1) is thought to allow the adhesion of monocytes to the activated endothelium. After adhesion to the endothelium, monocytes migrate into the subendothelial space where they eventually differentiate into macrophages. As previously suggested,6,7 Fernández-Hernando et al also show that increased caveolin-1 is responsible for an increased expression of VCAM-1, which may be a direct consequence of the reduced NO production observed in endothelial cells overexpressing caveolin-1. Caveolin-1 expression in endothelial cells may also regulate inflammation in the vasculature via other proinflammatory molecules, such as TNF-α, by regulating their receptor expression.19,20
Finally, Fernández-Hernando et al also show reduced proliferative and migrating properties of caveolin-1 overexpressing endothelial cells. The latter findings may be critical for a better understanding of the disease. Caveolin-1 overexpression may reduce the capacity of endothelial cells to regenerate an intact lining of the vessel wall. Therefore, the proliferative and migrating ability of endothelial cells may limit lipid and lipoprotein accumulation at lesion sites.
Conclusions
Importantly, this study may allow for the investigation of new drugs that specifically target caveolin-1 in endothelial cells. In this regard, Brouet et al21 have shown that endothelial cell exposure to atorvastatin can decrease caveolin-1 expression in macrovascular but not microvascular cells. Conversely, exposure of macrophages to atorvastatin is associated with increased caveolin-1 expression.22 However, these effects may need to be confirmed in whole organisms. Moreover, results obtained in animal models may not be reproducible in humans. For example, recent studies by Patel et al23 have indicated that idiopathic hypertension (IPAH) in mice and humans may have different etiologies and that caveolin-1 expression levels may have different implications in these two systems. In this disease, pulmonary artery smooth muscle cells have been shown to play an essential role in the development of this illness. In their study, Patel et al have shown that increased smooth muscle caveolin-1 expression is observed in pulmonary arteries from patients with IPAH. However, very different observations have been made in caveolin-1–deficient mice and in a rat model for pulmonary hypertension, where it has been shown that caveolin-1 is protective against the development of the disease.24,25
Footnotes
Address reprint requests to Philippe G. Frank, Ph.D., Department of Cancer Biology, 233 South 10th Street, Room 933, Philadelphia, PA 19107. E-mail: philippe.frank@jefferson.edu.
See related article on page 998
Supported by grants from the W.W. Smith Charitable Trust Fund, the Susan G. Komen Foundation, and the Jane Barsumian/Mary Lyons Trust fund.
References
- Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE. Fine structure of blood capillaries. J Appl Physics. 1953;24:1424. [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries. II Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol. 1968;37:277–299. doi: 10.1083/jcb.37.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns RR, Palade GE. Studies on blood capillaries. I General organization of blood capillaries in muscle, J Cell Biol. 1968;37:244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia TV, Dupree P, Monier S. VIP21-Caveolin, a protein of the trans-Golgi network and caveolae. FEBS Lett. 1994;346:88–91. doi: 10.1016/0014-5793(94)00466-8. [DOI] [PubMed] [Google Scholar]
- Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, Sessa WC. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank PG, Lisanti MP. Caveolin-1 and caveolae in atherosclerosis: differential roles in fatty streak formation and neointimal hyperplasia. Curr Opin Lipidol. 2004;15:523–529. doi: 10.1097/00041433-200410000-00005. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low density lipoprotein in the arterial endothelium in situ. J Cell Biol. 1983;96:1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- von Eckardstein A, Rohrer L. Transendothelial lipoprotein transport and regulation of endothelial permeability and integrity by lipoproteins. Curr Opin Lipidol. 2009;20:197–205. doi: 10.1097/MOL.0b013e32832afd63. [DOI] [PubMed] [Google Scholar]
- Wang XM, Kim HP, Song R, Choi AM. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol. 2006;34:434–442. doi: 10.1165/rcmb.2005-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank PG, Cheung MW, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol. 2006;291:H677–H686. doi: 10.1152/ajpheart.01092.2005. [DOI] [PubMed] [Google Scholar]
- Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry. 2004;43:8312–8321. doi: 10.1021/bi049609t. [DOI] [PubMed] [Google Scholar]
- Schwencke C, Schmeisser A, Walter C, Wachter R, Pannach S, Weck B, Braun-Dullaeus RC, Kasper M, Strasser RH. Decreased caveolin-1 in atheroma: loss of antiproliferative control of vascular smooth muscle cells in atherosclerosis. Cardiovasc Res. 2005;68:128–135. doi: 10.1016/j.cardiores.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Yu J, Davalos A, Prendergast J, Sessa WC. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2010;177:998–1003. doi: 10.2353/ajpath.2010.091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta. 2008;1783:1866–1875. doi: 10.1016/j.bbamcr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio A, Al-Lamki RS, Bradley JR, Pober JS. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am J Pathol. 2005;166:1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzin C, Brouet A, De Vriese J, Dewever J, Feron O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J Immunol. 2007;178:1505–1511. doi: 10.4049/jimmunol.178.3.1505. [DOI] [PubMed] [Google Scholar]
- Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- Gargalovic P, Dory L. Cellular apoptosis is associated with increased caveolin-1 expression in macrophages. J Lipid Res. 2003;44:1622–1632. doi: 10.1194/jlr.M300140-JLR200. [DOI] [PubMed] [Google Scholar]
- Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970–2979. doi: 10.1096/fj.07-8424com. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006;114:912–920. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]