Abstract
Mesenchymal stem cells (MSCs) have emerged as a new therapeutic modality for reconstituting the hematopoietic microenvironment by improving engraftment in stem cell transplantation. However, the availability of conventional bone marrow (BM)-derived MSCs (BMSCs) is limited. Recent studies showed that a large number of MSCs can be easily isolated from fat tissue (adipose tissue-derived MSCs [ADSCs]). In this study, we extensively evaluated the hematopoiesis-supporting properties of ADSCs, which are largely unknown. In vitro coculture and progenitor assays showed that ADSCs generated significantly more granulocytes and progenitor cells from human hematopoietic stem cells (HSCs) than BMSCs. We found that ADSCs express the chemokine CXCL12, a critical regulator of hematopoiesis, at levels that are three fold higher than those with BMSCs. The addition of a CXCL12 receptor antagonist resulted in a lower yield of granulocytes from ADSC layers, whereas the addition of recombinant CXCL12 to BMSC cocultures promoted the growth of granulocytes. In vivo cell homing assays showed that ADSCs facilitated the homing of mouse HSCs to the BM better than BMSCs. ADSCs injected into the BM cavity of fatally irradiated mice reconstituted hematopoiesis more promptly than BMSCs and subsequently rescued mice that had received a low number of HSCs. Secondary transplantation experiments showed that ADSCs exerted favorable effects on long-term HSCs. These results suggest that ADSCs can be a promising therapeutic alternative to BMSCs.
Hematopoiesis is a dynamic process that involves self-renewal of hematopoietic stem cells in the bone marrow, generation of lineage-committed cells, and mobilization of mature cells into the bloodstream. Mesenchymal stem cells (MSCs) present in bone marrow (BM) are thought to give rise to cells that constitute the hematopoietic microenvironment. MSCs produce a number of cytokines and extracellular matrix proteins and express cell adhesion molecules, all of which are involved in the regulation of hematopoiesis.1 Human MSCs, when injected into the bone marrow cavity of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, differentiate into pericytes, myofibroblasts, BM stromal cells, bone osteocytes, bone-lining osteoblasts, and endothelial cells, which constitute the functional components of the hematopoietic microenvironment.2 In recent studies, cotransplantation of human MSCs and HSCs resulted in increased chimerism or accelerated hematopoietic recovery (or both) in animal models and in humans.3,4,5,6
All of the above studies used bone marrow-derived MSCs (BMSCs). However, there are several drawbacks in the use of BMSCs for clinical application, including the fact that they are only available in limited number even though large quantities of infused cells are required for treatment. In addition, there is a possibility that BMSCs might be contaminated with malignant cells when they are harvested from patients with a hematological malignancy (eg, leukemia). The discoveries that a large number of nonadipocyte stem cells exist in fat tissue (adipose tissue-derived MSCs [ADSCs]) and that these cells can be rapidly expanded ex vivo, suggested that ADSCs might be useful for clinical applications.7 Recent studies showed that ADSCs are a viable alternative to BMSCs for treatment of animal models of various kinds of diseases.8,9,10,11,12 However, it has been reported that even though ADSCs and BMSCs are very similar, ADSCs are not completely identical to BMSCs.13,14 To date, little is known concerning the ability of ADSCs to support hematopoiesis. We therefore extensively examined the hematopoiesis-supporting properties of ADSCs in vitro and in vivo and report that ADSCs possess several advantages over BMSCs.
Materials and Methods
Animal Studies
The animal experiments were approved by the institutional ethics committee for Laboratory Animal Research, Nagoya University School of Medicine, and were performed according to the guidelines of the institute.
Reagents and Cells
RPMI 1640, heat-inactivated fetal bovine serum, α-minimal essential medium, and heat-inactivated horse serum were purchased from Gibco-BRL (Invitrogen, Carlsbad, CA). Methylcellulose media containing human recombinant interleukin-3, stem cell factor, and erythropoietin (MethoCult) were purchased from Stemcell Technologies (Vancouver, BC, Canada). The mouse BM-derived stromal cell lines with capabilities to support hematopoiesis in vitro, MS-515 and S-17,16 were kind gifts from Dr. Anna C. Berardi (Ospedale Bambin Gesu, Rome, Italy) and Dr. Kenneth Dorshkind (UCLA, Riverside, CA), respectively. These cell lines express class I histocompatibility antigens but not hematopoietic cell surface markers or endothelial cell surface markers. Mouse BMSCs and ADSCs were established from C57BL/6N mice. In brief, bone marrow cells and inguinal adipose tissue were isolated from five mice at a time, mixed, respectively, and then processed as described elsewhere.17 Before experimental use, we confirmed that MSCs possessed the ability to differentiate into adipocytes and osteoblasts. Cultures of passages 4 to 8 were used. Stromal cell lines and primary MSCs were maintained in α-minimal essential medium containing 10% fetal bovine serum.
Flow Cytometry
Cells were washed twice in PBS and then stained with fluorescein isothiocyanate-labeled mouse monoclonal anti-human CD33 and CD45 antibodies or with fluorescein isothiocyanate-labeled isotype-matched antibodies (2 μg/ml at 4°C) for 45 minutes (all antibodies from Becton Dickinson, Franklin Lakes, NJ). Surface antigens were evaluated in 1 × 104 viable cells using a FACSCalibur cytofluorometer (Becton Dickinson) and were analyzed using CELLQuest software (Becton Dickinson). Background fluorescence was assessed by staining with isotype-matched antibodies.
Coculture of CD34-Positive Progenitor Cells with MS-5 Cells, S-17 Cells, BMSCs, or ADSCs
Coculture of human CD34-positive peripheral blood stem cells (PBSCs) and MSCs was performed as described previously.18,19 In brief, CD34+ PBSCs suspended in long-term culture medium (α-minimal essential medium containing 12.5% horse serum, 12.5% fetal bovine serum, 1 μmol/L hydrocortisone, and 50 μmol/L 2-mercaptoethanol) were applied (1.0 × 105 cells in 2 ml) to feeder layers comprising MS-5 cells, S-17 cells, BMSCs, or ADSCs (four independent wells per subgroup, in six-well plates). The cocultures were incubated for 4 weeks with replenishment of the culture medium twice per week. To analyze the effect of CXCL12 produced by ADSC layers, the specific CXCR4 antagonist AMD-3100 (5 μg/ml, Sigma) or recombinant CXCL12 (500 ng/ml, Peprotech) was added to the ADSC or the BMSC cocultures, respectively, twice per week. Nonadherent viable cells were counted at the indicated time points and were analyzed by fluorescence-activated cell sorting at the end of the incubation. Coculture experiments were repeated three times.
In Vitro Progenitor Assays
A colony-forming cell assay was performed to analyze the proliferative effects of stromal cells on progenitor cells. BMSCs (500 cells), ADSCs (500 cells), human CD34+ PBSCs (500 cells), PBSCs plus BMSCs (500 cells each), or PBSCs plus ADSCs (500 cells each) were individually plated in 0.5 ml of methylcellulose media containing human recombinant interleukin-3, stem cell factor, and erythropoietin (four independent wells per subgroup). The plates were incubated for 8 days, and colonies containing >50 cells were scored. Experiments were repeated three times.
Intrabone Marrow Transplantation
BMSCs (1 × 105 cells in 10 μl of RPMI 1640), ADSCs (1 × 105 cells in 10 μl of RPMI 1640), or 10 μl of RPMI 1640 were injected into the right tibiae of irradiated (9.0 Gy) C57BL/6 mice using a Hamilton syringe equipped with a 31-gauge needle,20 followed by injection of 1 × 106 bone marrow nuclear cells into the retro-orbital plexus of the mice (10 mice per subgroup). Five of the 10 mice were humanely sacrificed 6 days after cotransplantation, and the bilateral tibiae were excised for histological evaluation. The remaining mice were observed untreated for an additional 8 weeks, at which time all mice were sacrificed. Bone marrow mononuclear cells (BMNCs) were collected from the femurs and tibiae and preserved for the secondary transplantation. For other experiments, BMNCs (1 × 105 cells), a mixture of BMNCs and BMSCs (1 × 105 cells each), or a mixture of BMNCs and ADSCs (1 × 105 cells each) was injected into the bilateral tibiae of irradiated (9.0 Gy), C57BL/6 mice (seven mice per subgroup).
Histological Analysis of the Bone Marrow
Tissue processing was performed as described elsewhere.21 In brief, bone samples were fixed in 4% paraformaldehyde and equilibrated in 30% sucrose/PBS. Fixed samples were embedded in OCT medium (Sakura Finetec, Tokyo, Japan) and frozen in cooled hexane. Sections of undecalcified tibia were generated via Kawamoto’s film method (Cryofilm transfer kit; Sakura Finetec). The 7-μm-thick cryostat sections were stained with H&E. The images were captured using a Leica DM-L microscope equipped with a high-resolution digital camera system (Leica Microsystems Inc., Wetzlar, Germany). Quantitative analysis of hematopoiesis was performed with the assistance of ImageJ software (downloadable at http://rsb.info.nih.gov/ij/download/).
Secondary Transplantation
Secondary transplantation studies were performed to analyze the effects of ADSCs on long-term HSCs.22 BM cells, obtained from the tibia of primary mice that had intrabone marrow transplantation, were intravenously transplanted into irradiated secondary recipient mice. Twelve weeks after transplantation, the mice were humanely sacrificed, and the tibiae were excised for histological evaluation.
Analysis of Mouse Hematopoietic Stem Cell Homing
Analysis of cells that homed into the BM was conducted as described elsewhere.20 C57BL/6 (Ly-5.2) recipient mice received 1 × 106 MSCs (ADSCs or BMSCs, both cells were established from Ly-5.2 mice) or 10 μl of RPMI 1640 into the right tibia, followed by administration of 1 × 106 lineage−Sca-1+c-kit+ (LSK) bone marrow cells, which were isolated from the femurs and tibiae of C57BL/6 (Ly-5.1) donor mice into the retro-orbital plexus. Twenty hours after transplantation, the mice were humanely sacrificed, and BM cells were collected separately from the right tibia of each mouse. The percentage of Ly-5.1 cells was analyzed by FACS using a specific monoclonal antibody to Ly-5.1 (Becton Dickinson).
Statistical Analysis
Statistical significance of group differences was evaluated using Student’s t-test and Excel software (Microsoft, Redmond, WA).
Results
ADSCs Support Human CD34+ PBSCs to a Greater Degree Than BMSCs
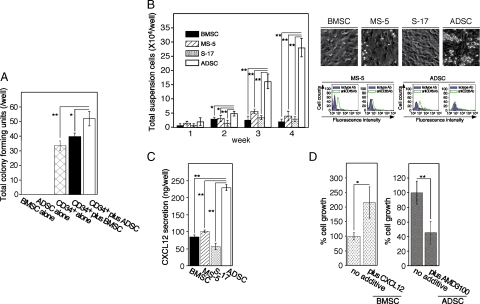
In vitro progenitor assays revealed that the total number of colonies was significantly increased when MSCs were mixed with CD34+ PBSCs. Interestingly, in three independent experiments, ADSCs promoted a higher frequency of early progenitors than BMSCs, whereas neither BMSCs nor ADSCs generated colonies (Figure 1A). The major lineages of the colonies were colony-forming unit (CFU) granulocyte and CFU granulocyte-macrophage. Few blast-forming unit-erythrocyte colonies were observed. However, there was no significant difference in the percentage of CFU granulocyte and CFU granulocyte-macrophage in CD34+ PBSCs in the presence or absence of BMSCs or ADSCs (data not shown). Representative results from three independent experiments are shown. We next performed coculture assays to analyze the ability of ADSCs to induce granulocyte differentiation from human CD34+ PBSCs. As noted previously,18 round-shaped hematic cells grew in clusters, suspended in the culture supernatant or loosely attached to supportive BM-derived stroma from which they could be easily removed (Figure 1B, right upper panel). ADSC layers not only supported CD34+ PBSCs in a manner similar to that of bone marrow-derived stromal cells (BMSCs) or cell lines (MS-5 cells and S-17 cells) but also yielded significantly more nonadherent cells than bone marrow-derived stromal cells (Figure 1B, left panel). FACS analysis of nonadherent cells showed that these cells were granulocytes derived from human CD34+ PBSCs (Figure 1B, lower right panel).
Figure 1.
In vitro comparison of the support of human HSCs by ADSCs and BMSCs. A: In vitro progenitor assays using stromal cells. BMSCs (500 cells), ADSCs (500 cells), human CD34+ PBSCs (500 cells), PBSCs plus BMSCs (500 cells each), or PBSCs plus ADSCs (500 cells each) were plated in 0.5 ml of methylcellulose media containing human recombinant interleukin-3, stem cell factor, and erythropoietin. The plates were incubated for eight days, after which progenitors were scored. The results represent the mean ± SD of four replicates. Statistical significance: **P < 0.01; *P < 0.05. Representative results from three independent experiments are shown. B: Coculture of CD34+ PBSCs with various stromal cells. Cell layers of the bone marrow-derived stromal cell lines MS-5 and S-17, BMSCs, or ADSCs were established on 0.5% gelatin-precoated 24-well plates (80% confluent). CD34+ PBSCs were applied onto the stromal layers (four wells per subgroup). Cocultures were incubated for four weeks with replenishment of the culture medium twice a week. Nonadherent viable cells were counted at the indicated time points (left panel), photographed (upper right panel), and analyzed by FACS at the end of the incubation (lower right panel). Each point represents the mean ± SD of four replicates. Statistical significance: **P < 0.01; *P < 0.05. Representative results from three independent experiments are shown. Ab, antibody. C: CXCL12 production in MS-5 cells, S-17 cells, BMSCs, and ADSCs. Similar numbers of BMSCs and ADSCs (80 to 90% confluent on six-well plates) were incubated for 72 hours. Culture supernatants were assayed for CXCL12 content using a specific enzyme-linked immunoabsorbent assay. The results represent the mean ± SD of four independent cultures. The figure is representative of three similar experiments. The asterisks denote that the CXCL12 content of ADSCs is significantly higher than those of other cells (**P < 0.01). D: Contribution of CXCL12 derived from the ADSC layer to the growth of CD34+ PBSCs. The specific CXCR4 antagonist, AMD-3100 (5 μg/ml), or recombinant human CXCL12 (500 ng/ml) was added to the CD34+ PBSC-ADSC or CD34+ PBSC-BMSC cocultures twice a week. After three weeks of coculture, viable nonadherent cells were counted. The percent growth of CD34+ PBSCs was calculated as (cell number with AMD-3100 or CXCL12/cell number with no additive) ×100 (%). The results shown reflect the mean ± SD of four independent determinations, and the results are representative of three independent experiments. The asterisks denote that the growth of CD34+ PBSCs on AMD-3100-treated ADSC layers or CXCL12-treated layers is significantly different from that on untreated ADSC layers or BMSC layers, respectively (**P < 0.01; *P < 0.05).
ADSCs Produce a Large Amount of CXCL12
To analyze the mechanism by which ADSCs support HSCs, we focused on the chemokine CXCL12, a critical regulator of hematopoiesis23,24,25 that functions through interaction with the CXCL12 receptor CXCR4. Enzyme-linked immunosorbent assay analysis showed that ADSCs secrete CXCL12 at a significantly higher level than those in MS-5 cells, S-17 cells, or BMSCs (Figure 1C). The addition of the CXCR4 antagonist AMD-3100 to HSC-ADSC cocultures significantly inhibited the growth of granulocytes, whereas the addition of recombinant CXCL12 to the HSC-BMSC cocultures significantly promoted the growth of granulocytes (Figure 1D). These data suggest that one of factors in the higher ability of ADSCs to support HSCs may be their higher expression of CXCL12.
ADSCs Positively Affected the Lodgment of Mouse HSCs in the BM
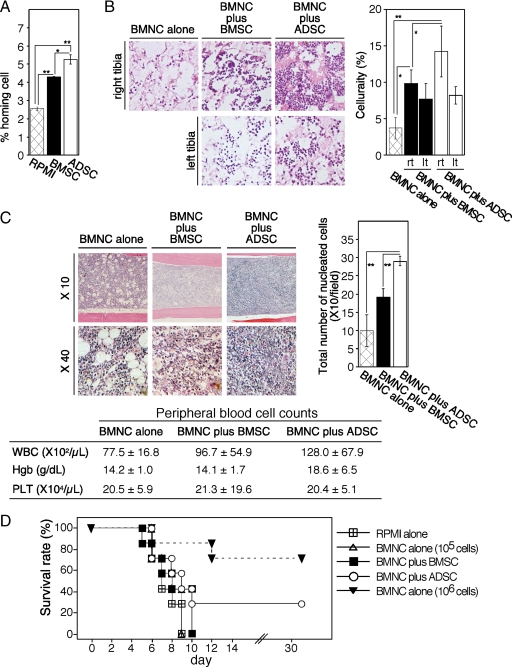
To further confirm the role of CXCL12 in ADSC support of HSCs we compared the ability of ADSCs and BMSCs to facilitate cell homing to the BM, because the CXCL12-CXCR4 axis plays a pivotal role in this phenomenon. In vivo cell homing assays showed that, when injected into the BM cavity of C57BL/6 (Ly-5.2) mice, both ADSCs and BMSCs facilitated homing of injected LSK (Ly-5.1) cells to the BM in comparison with controls. ADSCs attracted significantly more LSK cells than BMSCs (Figure 2A).
Figure 2.
In vivo transplantation studies of ADSC and BMSC support of hematopoiesis. A: Cell homing assays. C57BL/6 (Ly-5.2) mice received 1 × 106 ADSCs or BMSCs derived from Ly-5.2 mice in the right tibia, followed by intravenous administration of 1 × 106 BMNCs derived from Ly-5.1 mice. Twenty hours later, the percentage of Ly-5.1 cells in the BM was analyzed by FACS using a specific anti-Ly-5.1 monoclonal antibody. The percentage of homing cells was calculated as (the number of Ly-5.1+ cells/total cell number) ×100 (%). The results shown reflect the mean ± SD of five independent determinations, and the results are representative of three independent experiments. Statistical significance: **P < 0.01; *P < 0.05. B: Quantitative analysis of engraftment after cotransplantation. BMSCs (5 × 105 cells), ADSCs (5 × 105 cells), or 10 μl of RPMI 1640 were injected into the right tibiae of irradiated (9.0 Gy) C57BL/6 mice, after which 1 × 106 BMNCs were injected into the retro-orbital plexus of the mice (ten mice per subgroup). Five of the ten mice were humanely sacrificed six days after cotransplantation, and the right tibiae were excised for histological evaluation. Sections of the right tibiae into which BMSCs, ADSCs or RPMI 1640 were injected and the corresponding left tibiae with no additive were stained with H&E. Representative images are shown. Original magnification, ×40. For quantitative analysis of BM cellularity, ten fields were randomly selected and the area occupied by nucleated cells was automatically scored with the assistance of ImageJ software. The mean area occupied by nucleated cells per field ± SD is shown for each group. Representative results from two independent experiments are shown. Statistical significance: **P < 0.01; *P < 0.05. C: Secondary transplantation studies to evaluate the long-term effects of ADSCs on HSCs. BMNCs were obtained from the right tibia of primary mice (five mice per group) nine weeks after cotransplantation. BMNCs (1.0 × 106) were intravenously transplanted into irradiated (9.0 Gy) secondary recipient mice (five mice per subgroup). Six weeks after secondary transplantation, hematopoiesis in the BM (left panel) and peripheral blood cell counts ± SD (left panel) were evaluated. For quantitative analysis of BM cellularity, 10 fields were randomly selected and the number of nucleated cells was scored under a microscope. The mean nucleated cells per field ± SD is shown for each group. Representative results from two independent experiments are shown. Statistical significance: **P < 0.01. Representative results from two independent experiments are shown. WBC, white blood cells; Hgb, hemoglobin, PLT, platelets. D: ADSCs rescue mice that received an insufficient number of BMNCs for engraftment. BMNCs (1 × 105), a mixture of BMNCs and BMSCs (1 × 105 each), or a mixture of BMNCs and ADSCs (1 × 105 each) was injected into the bilateral tibiae of irradiated (9.0 Gy) C57BL/6 mice (seven mice per subgroup). The survival of the mice was observed for up to 30 days. The results are representative of two independent experiments.
ADSCs Facilitate Engraftment in the Setting of Bone Marrow Transplantation
Based on the results of these in vitro experiments, we performed bone marrow transplantation studies to evaluate the ability of ADSCs to facilitate hematopoiesis in vivo. BMNCs were administrated intravenously, and MSCs were injected by intramedullary injection into the right tibiae. Quantitative analysis of the engraftment after cotransplantation revealed that BM cellularities of the right tibiae, into which MSCs were injected, were significantly increased compared with controls (BMNC alone) or to the corresponding left tibiae without MSC injection (Figure 2B). Interestingly, ADSCs possessed a significantly higher ability to reconstitute hematopoiesis than BMSCs (Figure 2B). Although the BM cellularity of left tibiae, from mice that had been injected with ADSCs in the right tibiae, was significantly increased compared with that of controls; it was almost the same as that from mice that had been injected with BMSCs in the right tibiae (Figure 2B). We observed no evidence of MSC-induced toxicity (1 × 105/mouse: sufficient to support hematopoiesis) either during or after treatment.
ADSCs Exert No Harmful Effects on Long-Term HSCs
We evaluated the effects of ADSCs on hematopoiesis over a longer period by performing secondary transplantation studies. BMNCs, obtained from the right tibia of primary mice (five mice per group) 9 weeks after cotransplantation, were intravenously transplanted into lethally irradiated secondary recipient mice. There was no significant difference in the percentage of survival among subgroups even 12 weeks after secondary transplantation (data not shown). Interestingly, BM cellularity, in mice that had received BMNCs obtained from the BM of mice injected with ADSCs, was significantly higher than that of control mice and of mice that had received BMNCs derived from the BM of mice injected with BMSCs (Figure 2C, upper panels). Accordingly, white blood cell and platelet counts tended to be higher in the first group than in the other subgroups (Figure 2C, lower panel).
ADSCs Improve the Survival of Fatally Irradiated Mice That Had Received a Low Number of BMNCs
Finally, we investigated whether ADSCs could rescue fatally irradiated (9.0-Gy) mice that had received an insufficient number of engrafted BMNCs. Even though MSCs could improve the homing of HSCs to the BM (Figure 2A), neither BMSCs nor ADSCs improved the survival of mice when 1 × 105 BMNCs were administrated intravenously (data not shown), suggesting that the number of cells lodged in the BM was too low for engraftment. We next tested the effect of simultaneous bilateral injection of MSCs and BMNCs into the tibiae. Whereas intra-BM transplant of 1 × 105 BMNCs alone resulted in impaired engraftment and subsequent mortalities (Figure 2D), coinjection of BMNCs and ADSCs, but not of BMNCs and BMSCs, rescued about 30% of the mice (Figure 2D).
Discussion
MSCs are believed to be of low immunogenicity because human MSCs rarely express human leukocyte antigen major histocompatibility complex class I and lack costimulatory molecules on the surface.26,27 However, administration of allogeneic MSCs can provoke an immune response under certain conditions and can cause adverse effects,28 suggesting that it is desirable to establish MSCs from the patient to whom they will be administered. MSCs can be routinely isolated from several organs such as fetal liver, umbilical cord blood, bone marrow, and adipose tissue.14,29 The easy access to subcutaneous adipose tissue, the ability to repeatedly sample this tissue, and the uncomplicated enzyme-based isolation procedures make fat tissue the most attractive source of MSCs for clinicians. However, MSCs from different sources are not completely identical.13,14 Previous studies suggested that ADSCs can support hematopoiesis30,31,32 but that this effect may be transient.32 Thus, to date, the hematopoiesis-supporting properties of ADSCs have not been fully determined. In the present study, we observed that ADSCs exert more favorable effects than BMSCs on granulocyte differentiation from CD34+ HSCs and on the growth of progenitor cells in vitro. Accordingly, ADSCs promoted engraftment more rapidly than BMSCs in a mouse BMT model.
As reported recently, direct intra-BM transplant of HSCs is a powerful method to improve engraftment.33 However, intra-BM transplant of 1 × 105 BMNCs alone led to impaired engraftment and subsequent mortalities (Figure 2D). Surprisingly, coinjection of ADSCs, but not BMSCs, with BMNCs improved survival of the mice (Figure 2D). Transplantation of umbilical cord blood stem cells is usually applied to children or small adults because the number of umbilical cord blood cells obtained from a single donor is limited and the recommended dose ranges from 2.5 × 107 to 5.0 × 107 total nucleated cells per kilogram.34 These data suggest that co-intra-BM injection of ADSCs could allow low umbilical cord blood cell doses to be applied to normal-sized adults.
Gene expression profiling studies have not indicated any differences in gene expression linked to hematopoiesis between BMSCs and ADSCs.13,14 We found that ADSCs are rich in CXCL12, a critical regulator of hematopoiesis,23,24,25 and express CXCL12 at a statistically higher level than that in MS-5 cells, S-17 cells, or BMSCs (Figure 1C). Moreover, the addition of AMD-3100 to CD34+ PBSC-ADSC-cocultures significantly inhibited the growth of granulocytes, whereas the addition of recombinant CXCL12 to CD34+ PBSC-BMSC-cocultures significantly promoted the growth of granulocytes (Figure 1D). These results suggest that high CXCL12 expression in ADSCs is one of the factors that mediate their hematopoiesis-supporting properties. In adult BM, hematopoietic niches are thought to be located in sinusoidal perivascular areas (the vascular niche) or in the trabecular endosteum (the osteoblastic niche). Within these niches, HSCs are found in contact with cells that express high levels of CXCL12, named as CXCL12-abundant reticular cells. CXCL12-abundant reticular cells surround sinusoidal endothelial cells or are located near the endosteum.35 ADSCs have been reported to exhibit a perivascular phenotype in vitro and in vivo.36 The combined data suggest that ADSCs resemble CXCL12-abundant reticular cells in their phenotype and CXCL12 expression, which may explain why ADSCs can efficiently support hematopoiesis.
It has been reported that intravenous injection of the stromal-vascular fraction of mouse adipose tissue causes embolism in the lung and brain, and subsequent mortality in mice.31 We observed no evidence of toxicity caused by ADSCs (1 × 105/mouse: enough to support hematopoiesis) either during or after intra-bone marrow injection. Muguruma et al20 have reported that very few BMSCs migrate into the BM cavity via blood flow. Likewise in our study, the left tibiae of mice injected with ADSCs into the right tibiae exhibited modestly higher cellularities compared with controls. However, this cellularity was almost the same as that of the left tibiae of mice infected with BMSCs into the right tibiae. The combined data suggest that intrabone marrow injection but not intravenous injection is safe and, furthermore, is beneficial for eliciting superior hematopoiesis-supporting properties from ADSCs.
In summary, these data provide a rationale for the testing of ADSCs for stem cell transplantation.
Acknowledgments
We thank Drs. Takenori Ozaki, Shoichi Maruyama, Seitaro Terakura, Yoshiki Omatsu, and Takashi Nagasawa for their contributions to various aspects of this work. We also express appreciation to Dr. Giovanna Tosato for helpful suggestions.
Footnotes
Address reprint requests to Takayuki Nakayama, M.D., Ph.D., Department of Hematology, Nagoya University Graduate School of Medicine, 65 Tsuruma-cho, Showa-ku, Nagoya, Aichi 466-8550, Japan. E-mail: tnaka@med.nagoya-u.ac.jp.
Supported in part by a Japanese Grant-in-Aid for Scientific Research [(C) 20591118].
References
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Noort WA, Kruisselbrink AB, in't Anker PS, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Lowik CW, Falkenburg JH, Willemze R, Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34+ cells in NOD/SCID mice. Exp Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- in 't Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34+ cells in NOD/SCID mice. Exp Hematol. 2003;31:881–889. doi: 10.1016/s0301-472x(03)00202-9. [DOI] [PubMed] [Google Scholar]
- Bensidhoum M, Chapel A, Francois S, Demarquay C, Mazurier C, Fouillard L, Bouchet S, Bertho JM, Gourmelon P, Aigueperse J, Charbord P, Gorin NC, Thierry D, Lopez M. Homing of in vitro expanded Stro-1− or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004;103:3313–3319. doi: 10.1182/blood-2003-04-1121. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. IFATS collection: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenke-Layland K, Strem BM, Jordan MC, Deemedio MT, Hedrick MH, Roos KP, Fraser JK, Maclellan WR. Adipose tissue-derived cells improve cardiac function following myocardial infarction. J Surg Res. 2009;153:217–223. doi: 10.1016/j.jss.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, Uchino H, Mori KJ. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–153. [PubMed] [Google Scholar]
- Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987;138:1082–1087. [PubMed] [Google Scholar]
- Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- Issaad C, Croisille L, Katz A, Vainchenker W, Coulombel L. A murine stromal cell line allows the proliferation of very primitive human CD34++/CD38− progenitor cells in long-term cultures and semisolid assays. Blood. 1993;81:2916–2924. [PubMed] [Google Scholar]
- Nakayama T, Mutsuga N, Tosato G. Effect of fibroblast growth factor 2 on stromal cell-derived factor 1 production by bone marrow stromal cells and hematopoiesis. J Natl Cancer Inst. 2007;99:223–235. doi: 10.1093/jnci/djk031. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, Kato S, Ito M, Hotta T, Ando K. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Lataillade JJ, Clay D, Dupuy C, Rigal S, Jasmin C, Bourin P, Le Bousse-Kerdiles MC. Chemokine SDF-1 enhances circulating CD34+ cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768. [PubMed] [Google Scholar]
- Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1α and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Cho HH, Kim YJ, Seo SY, Kim HN, Lee JB, Kim JH, Chung JS, Jung JS. Human adipose stromal cells expanded in human serum promote engraftment of human peripheral blood hematopoietic stem cells in NOD/SCID mice. Biochem Biophys Res Commun. 2005;329:25–31. doi: 10.1016/j.bbrc.2005.01.092. [DOI] [PubMed] [Google Scholar]
- Cousin B, Andre M, Arnaud E, Penicaud L, Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun. 2003;301:1016–1022. doi: 10.1016/s0006-291x(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Corre J, Barreau C, Cousin B, Chavoin JP, Caton D, Fournial G, Penicaud L, Casteilla L, Laharrague P. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J Cell Physiol. 2006;208:282–288. doi: 10.1002/jcp.20655. [DOI] [PubMed] [Google Scholar]
- Frassoni F, Gualandi F, Podesta M, Raiola AM, Ibatici A, Piaggio G, Sessarego M, Sessarego N, Gobbi M, Sacchi N, Labopin M, Bacigalupo A. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9:831–839. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]