Abstract
Mast cells affect growth in various human tumors, but their role in prostate cancer (PC) is unclear. Here, we identify mast cells as independent prognostic markers in PC using a large cohort of untreated PC patients with a long follow-up. By analyzing mast cells in different tissue compartments, our data indicate that intratumoral and peritumoral mast cells have anti- opposed to protumor properties. Intratumoral mast cells negatively regulate angiogenesis and tumor growth, whereas peritumoral mast cells stimulate the expansion of human prostate tumors. We also observed mast cell recruitment particularly to the peritumoral compartment in men during the formation of castrate-resistant prostate tumors. In our ortothopic rat model, mast cells accumulated in the peritumoral tissue where they enhanced angiogenesis and tumor growth. In line with this, prostate mast cells expressed high levels of the angiogenic factor FGF-2. Similar to the situation in men, mast cells infiltrated rat prostate tumors that relapsed after initially effective castration treatment, concurrent with a second wave of angiogenesis and an up-regulation of FGF-2. We conclude that mast cells are novel independent prognostic markers in PC and affect tumor progression in animals and patients. In addition, peritumoral mast cells provide FGF-2 to the tumor micro environment, which may contribute to their stimulating effect on angiogenesis.
Cancer growth is dependent on the reciprocal interaction between tumor cells and their microenvironment. Cancer-associated fibroblasts,1 vascular cells, and inflammatory cells such as macrophages2,3,4 have been shown to promote prostate tumor growth. Mast cells are also of importance for tumor growth and have been shown to affect angiogenesis5,6,7 but are also potent regulators of inflammation and thus their specific function during tumor progression is complex and shows significant plasticity.8,9,10 Their role in prostate cancer (PC) in patients was recently explored in two separate studies using tryptase and c-Kit as markers for mast cells. These studies lacked the discrepancy between intra- and peritumoral mast cells and did not identify mast cells as independent prognostic variables. One of the studies showed that tryptase-positive mast cells were related to poor outcome in PC patients.12 The other study analyzed c-Kit-positive cells in tumor samples from PC patients and found an association between increased mast cell numbers and a favorable prognosis.13
Castration therapy is the gold standard for treatment of patients with metastatic PC, but in the majority of cases the formation of castrate-resistant prostate tumors is inevitable. The mechanisms behind the relapse are not fully understood but could be related to changes in the androgen receptor and the tumor stroma.14 Identifying pathways and cell types that support the relapse of the tumor offers therapeutic opportunities to enhance the effects of castration. Interestingly, mast cell density is increased in the normal prostate stroma after castration,15 but the functional significance of this is unknown.
The aim of this study was to explore the role of mast cells in relation to clinopathological variables in men and during the formation of castrate-resistant tumors. We analyzed a large cohort of untreated PC patients with a follow-up of more than 20 years and subsequent samples from those men who developed castrate-resistant tumors. Our results identify mast cells for the first time as independent prognostic markers. Furthermore, our study shows that prostate peritumoral mast cells, in part by providing FGF-2 to the tumor micro environment, stimulate angiogenesis and prostate tumor growth.
Materials and Methods
Castration Therapy of Dunning H Tumors
To investigate mechanisms for the formation of castrate-resistant prostate tumors we used the Dunning H tumor model, which shows a clear cut initial response to castration followed by a relapse.16,17 Immunocompetent Copenhagen rats with subcutaneous (s.c.) Dunning H tumors were left intact or surgically castrated. Six to eight animals were used in all groups and the tumors analyzed after one and four weeks. The tissues were handled as described below.
Ortothopic Implantation of AT-1 Tumor Cells and Treatment with Sodium Cromoglycate, Imatinib Mesylate, and Compound 48/80
One group of Copenhagen rats received 2000 prostate tumor cells (AT-1) injected into the ventral prostate (VP) lobe and the animals were examined 7, 10, and 14 after, as described earlier.18 Another group was treated as above and castrated at day 7 and examined at day 14. Two additional groups were injected with phosphate-buffered saline (PBS) or pretreated with the inhibitor of mast cell degranulation, Sodium Cromoglycate (SDG, Sanofi-Aventis AB, Stockholm, Sweden) at a dose of 100 mg/kg/day intraperitoneal (i.p.) for two days, followed by an injection of AT-1 tumor cells as described above. During 10 days, the rats received a daily dose of 50 mg/kg/day i.p. of SDG or an equal volume of PBS. And finally, two groups received 2000 AT-1 cells injected into the VP diluted in growth factor reduced matrigel (BD Biosciences, CA) and PBS or 1 μmol/L of the mast cell degranulation agent, compound 48/80,19 (n = 6) respectively. Due to the high risk of anaphylactic shock when this substance is injected systemically during a prolonged period of time, we used intraprostatic administration of compound 48/80. All animals were injected with bromodeoxyuridine (BrdU, 50 mg/kg intraperitoneally, Sigma, St. Louis, MO) one hour before sacrifice to label proliferating cells. The animals treated with SDG also received an injection of the tissue hypoxia marker pimonidazole to stain tissues with a pO2 less than 10 mm Hg (Hypoxyprobe 100 mg/kg, Chemicon, Temecula, CA) as earlier described.18 Another way to target mast cells is by using the c-Kit inhibitor Imatinib Mesylate20 (kindly donated by Novartis Pharma, AG, Basel, Switzerland). Thus, we pretreated immunocompetent Copenhagen rats orally with 125 mg/kg/day of Imatinib (n = 8). The controls received PBS (n = 10) during the same time period. After 17 days the rats received an ortothopic injection of 2000 AT-1 cells. The two groups were subsequently treated with Imatinib or PBS during 10 days. The tumors were dissected, weighed, fixed in formalin, and embedded in paraffin. All animal experiments were approved by the local ethic committee in Umeå, Sweden.
Patients
Our patient samples are thoroughly described elsewhere.21 In brief, tissue specimens were obtained from patients who underwent transurethral resection of the prostate due to obstructive voiding problems and subsequent histological analysis showed cancer. The patients had not received any anticancer therapy before transurethral resection of the prostate and had tumors with Gleason Score (GS) ranging from 4 to 10. This study includes 394 patients, of which 295 patients were followed with watchful waiting and thus received no treatment. From these formalin-fixed tissue specimens, we constructed tissue micro arrays (TMAs) using a Beecher Instrument (Sun Prairie, WI). The TMAs contained five to eight samples (cores with a diameter of 0.6 mm, tissue area = 0.28 mm2) of tumor tissue and four samples of nonmalignant tissue from each patient. Only intact spots were used in the analysis. To examine whether castration therapy resulted in an influx of mast cells in tumors, we used a set of tissue sections from TUR-diagnosed PCs relapsing after surgical castration treatment.22 The local human investigations committee approved the study.
Protein Analysis
Protein extraction and Western blot were performed as previously described.23 Primary antibodies to detect FGF-2 (147: sc-79, Santa Cruz, Santa Cruz, CA) and actin (A2066, Sigma) were followed by secondary anti-rabbit (Amersham Biosciences, GE Healthcare, Uppsala, Sweden) incubation. Blocking experiment to verify the specificity of the 16-kDa band observed with the FGF-2 antibody was performed by preincubating the antibody with a 50× excess of the FGF-2 peptide (sc 79 P, Santa Cruz).
Tissue Staining and Stereology
Human tissue samples including the TMA were stained with an antihuman tryptase antibody (DAKO, Stockholm, Sweden), and the number of mast cells per spot was recorded. Sections from animal tumor tissues were stained with toluidine blue and the volume densities of mast cells determined using a light microscope with a square-lattice mounted in the eye-piece. The number of grid intersections that fell on mast cells versus other areas was used to determine the volume density. Blood vessel density was measured in a similar manner. The mean value of the number of mast cells present in each tumor (n = 5–8) and nonmalignant (n = 4) TMA core per patient was used in survival and correlation analysis. The few mast cells present in the epithelial compartment were excluded. Immunohistochemical staining of the platelet-derived growth factor receptors (PDGFRs, R&D Systems, Abingdon, UK) FGF-2 (Santa Cruz) and Factor VIII (Dako) were performed on paraffin-embedded tissues.20 CD68 (ED1, Serotec, Oxford, UK) was used to recognize tissue-resident macrophages.15 Desmin was detected using a monoclonal antibody (Dako) on paraffin sections and immunoreactivity detected with 3,3′ diaminobenzidine (DAB, Dako). For assessing proliferation, animal tissues were stained with an antibody recognizing BrdU (Becton Dickinson AB, Stockholm, Sweden), and human samples were stained for the cellular proliferation marker Ki-67 (Dako).
Laser Microdissection and Pressure Catapulting and cDNA Synthesis
Six-micrometer frozen sections from four different control Dunning H tumors, intact VP, and AT-1 tumors were mounted on PALM Membrane Slides (P.A.L.M Microlaser Technologies AG, Bernried, Germany). Mast cells were micro dissected and used for RNA extraction with Trizol (Invitrogen, Stockholm, Sweden). The RNA was used in a first-strand cDNA synthesis using Superscript II (Invitrogen) according to protocol.
RNA Extraction from Dunning H Tumor Tissue and cDNA Array Analysis
Total RNA was prepared using Trizol reagent (Invitrogen), and 1 μg of RNA from four to seven animals were pooled. The RNA was transcribed into cDNA using RT2 PCR First strand kit (C-02, SABiosciences, Bioscience Corporation, by way of MedProbe, Lund, Sweden) according to instructions. The cDNA was used in two arrays, a rat angiogenesis array RT2 PCR Array (APRN-024A, SABiosciences) and a chemokine array (PARN-022A, SABiosciences) using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Stockholm, Sweden) and ABI Prism 7900 SDS Software 2.1. Data were analyzed with PCR Array Data Analysis template from SABiosciences.
RT-PCR Procedures
mRNA levels of GAPDH and FGF-2 were examined by real-time PCR using the Light Cycler SYBR Green 1 technology (Roche Diagnostics, Bromma, Sweden). All samples were run on a 2% agarose gel to verify the specificity of the PCR product.
Cell Culture
AT-1 cells (ATCC, Manassas, VA) were grown in RPMI (ATCC) with supplements according to protocol. The cells were subjected to Imatinib (range 0.5–3.0 μmol/L), 3 μmol/L SDG, or compound 48/80 (range 1–3 μmol/L) dissolved in PBS. A cell proliferation kit (Cell Proliferation Kit, Roche) was used to determine the amount of metabolic activity according to manufacturer’s protocol.
Statistics
Bivariate correlations were calculated using the Spearman’s rank correlation test. Correlations between nominal variables and continuous variables were analyzed using the Kendall’s tau b correlation test. Data used in the correlation analysis was collected at the time of PC diagnosis. Groups were compared with the Mann–Whitney U-test. Probability of event-free survival (P-EFS) is presented ± SE. The level of statistical significance was defined as P < 0.05 (two-sided). Statistical analysis was performed using the SPSS 14.0.0 software for Windows (SPSS Inc., Chicago, IL).
Survival Analysis
Patients included in the survival analyze using Kaplan–Meier and Cox regression were followed with watchful waiting and thus were left untreated. The duration of EFS is defined as the time from transurethral resection of the prostate until the date of PC death, death of other causes, or if no death occurred, until the date of last follow-up. Differences in outcome between groups were tested with the log-rank test. The prognostic relevance of the number of mast cells per tissue array core was examined by Cox regression analysis alone and together with GS and local tumor stage.
The cut-offs for the average number of mast cells in tumor and nonmalignant prostate tissue cores were obtained using receiver operating curve (ROC) analysis. These showed areas under the curve of 0.42 and 0.59 for mast cells in the tumor and nonmalignant tissue respectively [95% confidence interval (CI): 0.34−0.49 and 0.51–0.67]. Cut-offs obtained (13 and 20 mast cells in the tumor stroma and nonmalignant stroma respectively, Figure 1, A and B) were used in correlation and survival analyses (Cox regression and Kaplan–Meier).
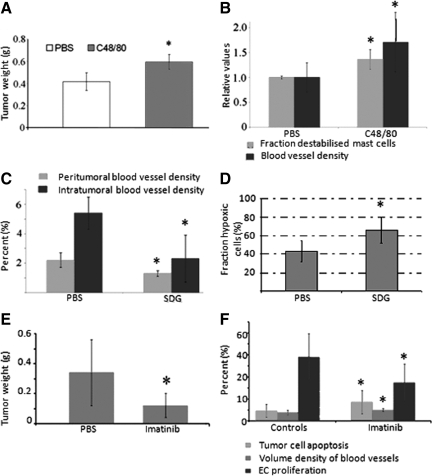
Figure 1.
A: Coinjection of AT-1 cells with compound 48/80 stimulated tumor growth compared with PBS. *P < 0.05. B: Compound 48/80 stimulated mast cell degranulation (determined on toluidine blue staining and defined as mast cells with a discontinuous membrane) and increased the volume density of blood vessels. Values in A and B, are shown as means ± SD with six to ten animals in each group. *P < 0.01. C: SDG reduced the volume density of blood vessels both in the nonmalignant stroma surrounding the tumor and in the tumor stroma. *P < 0.05. D: SDG induced tumor hypoxia compared with PBS. The fraction hypoxic cells increased from 43 ± 11% to 66 ± 14%. *P < 0.05. Data from SDG experiment represent one experiment with three to seven animals in each group. E: Imatinib reduces growth of AT-1 tumors compared with controls. *P < 0.05. F: Significant effects on tumor cell apoptosis, volume density of blood vessels, and endothelial proliferation were observed after treatment with Imatinib. Values from experiment using Imatinib are shown as means ± SD with eight to ten animals in each group. *P < 0.05.
Results
Peritumoral Mast Cells Stimulate Peritumoral Angiogenesis and Prostate Tumor Growth
To investigate the significance of mast cells, we used the ortothopic AT-1 model where implantation of tumor cells resulted in a recruitment of mast cells into the peritumoral tissue (see Supplemental Figure S1A at http://ajp.amjpathol.org). Very few mast cells migrated into the tumor. Vascular density and vascular BrdU labeling index increased with time in the peritumoral VP tissue, as described earlier.4,18 The gradual increase in mast cell density correlated with vascular BrdU labeling index, vascular density in the surrounding prostate tissue (and in the noninjected VP, data not shown), and importantly with tumor size (see Supplemental Figure S1A at http://ajp.amjpathol.org).
To examine the importance of peritumoral mast cell accumulation in more detail we used compound 48/80 to locally stimulate mast cell degranulation. Coinjection of compound 48/80 with AT-1 cells resulted in significantly larger tumors compared with PBS (Figure 1A). This was accompanied by a significant increase in the percentage of destabilized mast cells and an increase in the volume density of blood vessels (Figure 1B) supporting a proangiogenic role for peritumoral mast cells in PC. Compound 48/80 did not affect AT-1 cells growing in vitro (see Supplemental Figure S1B at http://ajp.amjpathol.org) suggesting that the effect of this compound in vivo is not mediated by direct effects on the tumor cells.
Next, we investigated whether SDG, a stabilizer of mast cell degranulation, could impair tumor angiogenesis and growth. The rats were pretreated with SDG or PBS two days before tumor cell injection as previously described.6 SDG significantly stabilized mast cells (see Supplemental Figure S1C at http://ajp.amjpathol.org) and tumor take was reduced (7/9 for PBS and 3/8 for animals treated with SDG). Importantly, we also observed inhibitory effects on angiogenesis, both in the nonmalignant tissue surrounding the tumors and within the tumors (Figure 1C). In line with this, hypoxia was up-regulated after SDG treatment (Figure 1D). In contrast, SDG did not have any effect on AT-1 tumor cells in vitro (see Supplemental Figure S1D at http://ajp.amjpathol.org).
Previously, we have recognized the potential of Imatinib to reduce mast cell numbers in prostate tumors.20 This is of major importance because Imatinib is already used successfully in the clinic for several mast cell-related disorders such as mastocytosis24 and gastrointestinal tumors.25 Therefore we wanted to evaluate the potential of Imatinib to reduce mast cell numbers and inhibit tumor growth in our ortothopic tumor model. Notably, Imatinib is not specific for mast cells but has inhibitory effects on vascular support cells as well as macrophages. Interestingly, however, we found that Imatinib completely eradicated mast cells from the normal VP after 17 days of treatment (see Supplemental Figure S2A at http://ajp.amjpathol.org), and subsequent injection of tumor cells resulted in an increased rate of tumor cell apoptosis, inhibitory effects on EC proliferation, and a threefold reduction in tumor weight (Figure 1, E–F). Surprisingly, vessel density was slightly albeit significantly increased after Imatinib treatment (Figure 1F). We hypothesized that this was due to more dilated vessels rather than an actual increase in vessel numbers, and in line with this we found a strong down-regulation of the pericyte marker desmin (see Supplemental Figure S2B at http://ajp.amjpathol.org). The fraction of α smooth muscle cell actin (SMA)-covered vessels nearly reached statistical significance (the fraction of SMA-double stained vessels decreased from 40.0 ± 8.73% to 32.7 ± 5.67%, P = 0.083), but there were no major changes in the expression of PDGFR after Imatinib treatment and there was no obvious difference in the number of tissue-resident CD68+ macrophages (data not shown). In line with a previous study,26 Imatinib reduced the well-known angiogenic factor FGF-2 (see Supplemental Figure S2, C and D at http://ajp.amjpathol.org). However, Imatinib had inhibitory effects on the tumor cells in vitro especially at higher concentrations, although the PDGFRs were not expressed by tumor cells in vivo (see Supplemental Figure S2, E and F at http://ajp.amjpathol.org). Hence, we cannot determine the exact contribution of the inhibitory effects on mast cells exerted by Imatinib in our experiment.
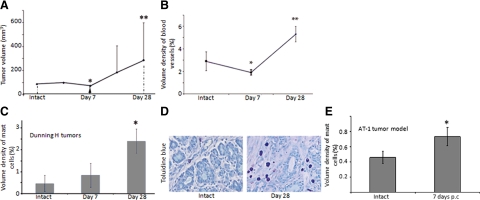
To investigate whether mast cells contribute to the formation of castrate-resistant tumors, we analyzed prostate tumors with a clear-cut initial inhibitory response to castration followed by a clear-cut relapse (Figure 2A). Seven days postcastration (p.c.), vascular density was reduced but the number of mast cells largely unaffected in the Dunning H tumor model (Figure 2, B and C). However, four weeks after treatment, tumor volume and vascular density had significantly increased (Figure 2, A and B). This was accompanied by a significant accumulation of mast cells in the tumor stroma compartment (Figure 2, C and D). In addition, several chemokines that induce mast cell migration and or trigger their activation27,28 were up-regulated after castration therapy (see Supplemental Table 1 at http://ajp.amjpathol.org). Castration also induced an influx of mast cells into the peritumoral tissue in AT-1 tumors one week p.c. (Figure 2E).
Figure 2.
A: Dunning H tumor volumes at the start of the experiment (intact) were set to 100%, and all other values were compared with this day. Seven days p.c. tumor volume was significantly reduced (arrowhead), *P < 0.05 compared with intact but four weeks p.c. there was a significant increase, **P < 0.05 compared with one week p.c. This occurred concurrently with an increase in the volume density of factor VIII positive blood vessels (B; *P < 0.05 compared with intact, **P < 0.05 compared with one week p.c.). C: Castration therapy recruited mast cells to the tumor stroma after four weeks (*P < 0.05 compared with intact). Values are shown as means ± SD with six to eight tumors in each group. D: Illustrative picture of the increase in toluidine blue-positive mast cells in the tumor stroma after castration therapy. E: Mast cells were also recruited to AT-1 tumors 7 days p.c. (*P < 0.05 compared with intact).
Mast Cells Express High Levels of FGF-2 in Dunning H and AT-1 Tumors
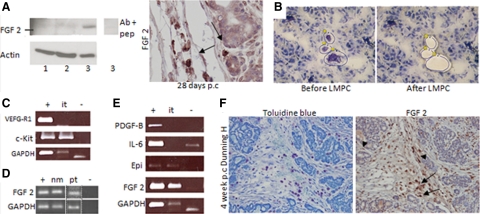
Next, we performed an array of angiogenic regulators in Dunning H tumors to find factors that could support the vascular relapse after castration therapy (see Supplemental Table 2 at http://ajp.amjpathol.org). In this context, the fourfold induction of the well-known angiogenic factor FGF-2 was particularly interesting. Western blot and immunohistochemical analysis confirmed a strong expression of FGF-2 in 28 days p.c. Dunning H tumors (Figure 3A). Mast cells have been found to release FGF-2 by degranulation and drive tumor angiogenesis in other models,5,6 and therefore we analyzed the expression of FGF-2 in microdissected mast cells from malignant and nonmalignant compartments in Dunning H and AT-1 tumors (Figure 3, B–E; see also Supplemental Table 2 at http://ajp.amjpathol.org). The expression of c-Kit and VEGF-R1 was used to verify the accuracy of the laser microdissected material (Figure 3C). RT-PCR demonstrated a strong expression of FGF-2 in mast cells from the nonmalignant (nm) VP as well as when mast cells were located in the stroma surrounding AT-1 tumors (pt, Figure 3D). In the Dunning H tumor model, where mast cells were found intratumoral (it), FGF-2 was strongly expressed by mast cells (Figure 3E). Epiregulin was also found to be expressed by mast cells but was not further investigated. In contrast, the other factors that were found to be up-regulated by castration therapy on our array of Dunning H tumors did not show a comparatively strong expression by mast cells (Figure 3E). Immunohistochemistry on consecutive sections from a relapsing 4-week-p.c. Dunning H tumor showed a strong expression of FGF-2 in cells that were also positive for toluidine blue and a weaker staining in fibroblasts and tumor cells (Figure 3F). We conclude that prostate mast cells express FGF-2 regardless of their localization within the prostate microenvironment. Another possible source of FGF-2 difficult to distinguish from mast cells on tissue sections stained with FGF-2 are macrophages. Although macrophages were not up-regulated after castration therapy (data not shown), we cannot exclude the possibility that they contribute to the increase in FGF-2 levels by other mechanisms.
Figure 3.
A: Western blot of FGF-2 in Dunning H tumor lysates showing intact tumors (lane 1), 7 days p.c. (lane 2), and 28-days p.c. (lane 3). The band detected 28 days p.c. was subsequently blocked using a control peptide at 50× excess (lane 3 inset). The samples represent a pool of four to six tumors in each group, and actin was used as a loading control. Right: An illustrative immunohistochemical staining of FGF-2 on a 28-day p.c. Dunning H tumor showing strong FGF-2 staining by cells that by morphological criteria appeared to be mast cells (arrows). B: Extraction of pooled mast cells from relapsing 28 days p.c. Dunning H tumors using laser microdissection and pressure catapulting (LMPC). C: RT-PCR on LMPC extracted mast cells showed, as expected, that the mast cell marker c-Kit was strongly expressed by intratumoral (it) mast cells whereas the hematopoietic marker VEGF-R1 was not expressed. D: Also mast cells from the nonmalignant (nm) stroma (from VP) and in the peritumoral stroma (pt) from the AT-1 model expressed FGF-2. The pt-isolated mast cells were run in a separate analysis from the nm extracted mast cells (dashed lines) but with the appropriate controls. E: Whole tumor homogenates from 4 weeks p.c. tumors were used as positive control (denoted +) and water as a PCR negative control (−). GAPDH was used as a loading control. The lower band of GAPDH in the negative sample (E) probably represents primer dimer. PDGF-B and IL-6 were up-regulated on the angiogenesis array comparing intact and 28 days p.c. Dunning H tumors but were not found to be expressed by infiltrating intratumoral mast cells. In contrast, epiregulin (Epi) was expressed by mast cells as well as FGF-2. Importantly, compare the expression of FGF-2 in relation to GAPDH in corresponding sample for the whole tumor homogenates and for the isolated mast cell fraction. F: Consecutive sections of a 28-day p.c. Dunning H tumor showing toluidine blue (left) staining to detect mast cells and FGF-2 (right). FGF-2 was expressed in mast cells (arrows), but there was also a weaker staining of fibroblasts (arrowhead) and possibly tumor cells.
Mast Cells Are Located in the Prostate Stroma in Men
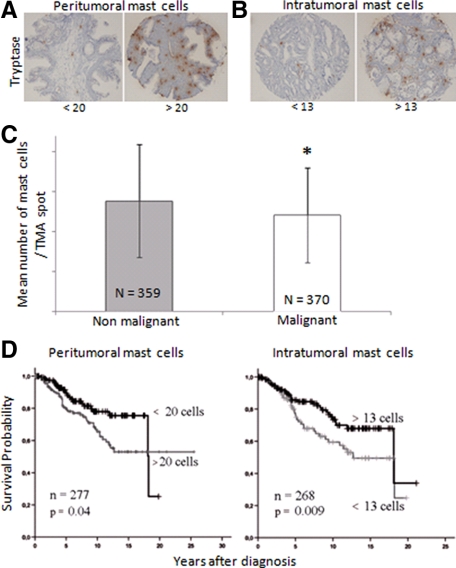
We then turned to our clinical samples to evaluate the importance of our findings from our animal models and to examine the contradictory findings from previous studies.12,13 In both nonmalignant and malignant human prostate tissue, mast cells were found principally in the stroma. In prostate tumors only a few mast cells were detected in the epithelial compartment (Figure 4, A and B). We also found that the mean number of mast cells in the nonmalignant stroma was significantly higher than in the tumor stroma (Figure 4C, 13.9 ± 7.1 versus 12.1 ± 5.9, n = 359 and 370, respectively, P < 0.05).
Figure 4.
Illustrative immohistochemical staining with a low versus high number of tryptase-positive mast cells in TMA cores from nonmalignant (A) and malignant (B) prostate tissue. The few mast cells that were located in the epithelial compartment were excluded from the analysis. Only intact cores were used in the analysis. C: Our data set, using a large number of PC patients, showed a significant difference between the numbers of mast cells in the nonmalignant tissue compartment versus in the malignant stroma. *P < 0.05. D: Kaplan-Meier analysis on untreated patients with a high or low number of tryptase-positive mast cells in the nonmalignant versus malignant stroma showed a remarkable discrepancy. Mast cells in the surrounding peritumoral stroma related to a poor prognosis (left) in contrast to the scenario within the tumors, where stromal mast cells served a protective role and related to a favorable outcome (right).
Mast Cells Are Related to Clinical Outcome
Patients with a high number of mast cells in the resected nonmalignant stroma had significantly shorter cancer-specific survival than patients with low number of mast cells in this prostate compartment (15-year P-EFS was 46 ± 10% and 67 ± 4% in the two groups; Figure 4D, left). To elucidate the strength of this association, we used multivariate Cox regression analysis and included the strong prognostic markers GS and local tumor stage. Interestingly, we found that a high number of mast cells in the nonmalignant stroma were significantly associated with poor prognosis and an independent prognostic marker (Table 1, top). High mast cell density in the nonmalignant part of the prostate was related to high GS (Table 2), but in contrast to the findings in the AT-1 model, it was not correlated with vascular density in this tissue compartment.
Table 1.
Cox Regression Analysis of Mast Cells in the Tumor and Nonmalignant Tissue of Patients Followed with Watchful Waiting
| Variable* | n | RR | P value | 95% CI | |
|---|---|---|---|---|---|
| Multivariate analysis | |||||
| Gleason score | 4–5 | 84 | 1** | ||
| 6 | 91 | 13.3 | 0.013 | 1.74–101.33 | |
| 7 | 46 | 30.8 | 0.001 | 4.00–237.06 | |
| 8–10 | 54 | 66.4 | <0.001 | 8.59–513.38 | |
| Local tumor stage | T1 | 175 | 1** | ||
| T2 | 66 | 1.7 | 0.130 | 0.86–3.26 | |
| T3 | 31 | 2.8 | 0.009 | 1.29–6.02 | |
| T4 | 3 | 5.1 | 0.122 | 0.65–40.92 | |
| Mast cells in nonmalignant stroma | <20 | 227 | 1** | ||
| ≥20 | 48 | 2.2 | 0.005 | 1.28–3.91 | |
| Multivariate analysis | |||||
| Gleason score | 4–5 | 80 | 1** | ||
| 6 | 81 | 17.8 | 0.005 | 2.34–135.89 | |
| 7 | 47 | 36.4 | 0.001 | 4.77–278.54 | |
| 8–10 | 57 | 81.5 | <0.001 | 10.73–618.21 | |
| Local tumor stage | T1 | 163 | 1** | ||
| T2 | 68 | 1.8 | 0.067 | 0.96–3.39 | |
| T3 | 31 | 1.9 | 0.081 | 0.92–3.90 | |
| T4 | 3 | 4.8 | 0.142 | 0.59–38.19 | |
| Mast cells in tumor stroma | <13 | 148 | 1** | ||
| ≥13 | 117 | 0.6 | 0.037 | 0.32–0.97 |
Cox regression analysis using Gleason score, local tumor stage, and number of mast cells in the tumor and nonmalignant tissue as categorical variables.
Reference value.
RR, relative risk; CI, confidence interval.
Table 2.
Correlation Analysis Using Kendall’s Tau and Spearman’s Rank Correlation Test
| Method | Variable | Intratumoral mast cells | Peritumoral mast cells | |
|---|---|---|---|---|
| Kendall’s tau | Presence of metastasis* | Correlation coefficient (N) | −0.157§ | NC |
| 289 | 277 | |||
| Spearman’s rank test | Tumor stage | Correlation coefficient (N) | −0.147§ | NC |
| 362 | 352 | |||
| GS | Correlation coefficient (N) | −0.113‡ | 0.152§ | |
| 369 | 358 | |||
| Density of bv in the nonmalignant tissue† | Correlation coefficient (N) | −0.217§ | NC | |
| 148 | 145 | |||
| Tumor cell proliferation | Correlation coefficient (N) | −0.177§ | NC | |
| 363 | 343 |
(N) equals number of patients analyzed. Note the protective role of intratumoral mast cells and the lack of, or even protumor role of, peritumoral mast cells. NC indicates not correlated.
According to bone scan at the time of diagnosis.
No correlation between mast cells and intratumoral blood vessels (bv, data not shown).
P < 0.05,
P < 0.01.
In contrast, patients with a low number of mast cells in the tumor compartment had, as described previously in surgically treated patients,13 significantly shorter cancer-specific survival than patients with high number of mast cells in the tumors (15-year P-EFS was 51 ± 6% and 71 ± 6% in the two groups; Figure 4D, right). Because mast cells were found primarily in the stroma and high GS tumors contain a relatively low fraction of stroma compared with tumor cells, we repeated the multivariate analysis including GS and local tumor stage. This showed that intratumoral mast cell number was an independent prognostic marker (Table 1, bottom). Poor survival in patients with few intratumoral mast cells can consequently not be explained only by an association with high GS. Low number of intratumoral mast cells was also related to markers of unfavorable prognosis such as high tumor stage, presence of metastases, and high tumor cell proliferation (Table 2).
Castration Therapy of Men Stimulates Mast Cell Recruitment
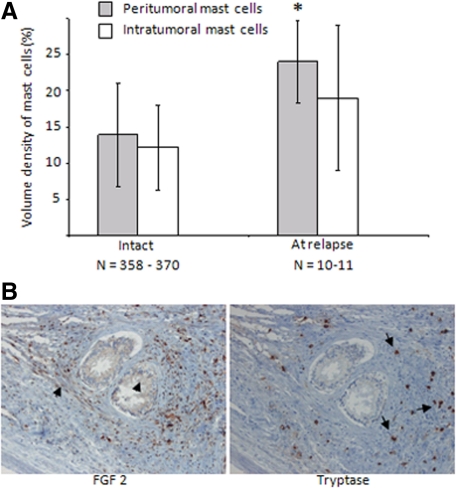
Mast cells are recruited to the rodent nonmalignant prostate after castration therapy,15 and in this study we discovered an influx of mast cells to the malignant prostate in our animal models. Having found that peritumoral mast cells stimulate tumor growth and are correlated with poor prognosis in men, we explored whether mast cells are recruited to human prostate tumors after castration therapy. Indeed, castration therapy increased the number of mast cells primarily in the peritumoral tissue compartment in samples taken from locally relapsing human prostate tumors but not significantly within the tumor (Figure 5A).
Figure 5.
A: Similar to our animal models, tryptase-positive mast cells infiltrated primarily the peritumoral compartment after castration therapy in men, and a trend was observed for tumoral mast cells. *P < 0.05. GS was similar for both groups. B: Consecutive sections of FGF-2 and mast cell-specific tryptase showed that FGF-2 was indeed expressed in mast cells also in human tissues (arrows) and also in lower levels in various fibroblasts and epithelial cells (arrowheads). Pictures are shown at ×20 magnification.
And finally we also explored whether human mast cells express FGF-2 and therefore analyzed consecutive sections for their expression of FGF-2 and tryptase. In line with our data from our animal models, we found that several tryptase-positive cells were positive for FGF-2 but FGF-2 was also expressed in lower levels in fibroblasts and possibly in the epithelial cells (Figure 5B).
Discussion
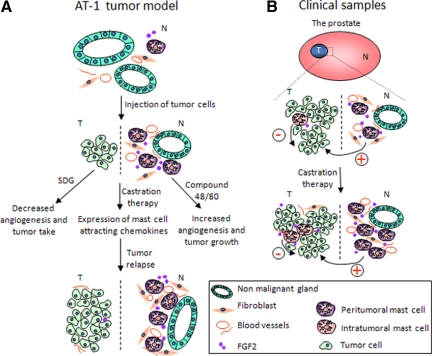
In these experiments we explore the role of mast cells, in particular peritumoral mast cells in PC (Figure 6, A and B). We identify for the first time mast cells as independent prognostic variables, and, importantly, we found that mast cell function is related to the local tumor microenvironment as peritumoral and intratumoral mast cells are differently related to prognosis in PC (Figure 6).
Figure 6.
A schematic illustration of the role of mast cells in PC. Dashed bar indicates the margin between malignant (T = tumor) and nonmalignant (N) tissue. A: In the AT-1 ortothopic tumor model a few mast cells can be seen in the normal VP (top) expressing FGF-2. After the injection of tumor cells (middle), FGF-2-expressing mast cells are recruited to the surrounding tumor microenvironment (but not within the tumor) supporting tumor angiogenesis. When the tumor is small and surrounded by nonmalignant tissue, tumor growth and angiogenesis can be modulated by the use of inhibitors (ie, SDG) and stimulators (ie, compound 48/80) of mast cell degranulation. In addition, castration therapy that temporarily inhibits tumor growth and angiogenesis up-regulates mast cell-attracting chemokines, such as for example TNF-α which may assist the second wave of mast cell recruitment, thus possibly support the ensuing angiogenesis (bottom). B: In a cohort of untreated PC with a long follow-up, the accumulation of intratumoral mast cells serves a protective role and relates to a favorable prognosis as well as to a number of well-known prognostic factors such as the presence of metastasis at diagnosis, tumor stage, and GS. On the contrary, mast cells in the nonmalignant tissue (ie, peritumoral) relate to a poor prognosis and correlate with GS. In line with the data from the animal models, human mast cells also express FGF-2, and the accumulation of mast cells particularly to the nonmalignant tissue after castration therapy (bottom) hence may contribute to the subsequent vascular and tumor relapse.
Compound 48/80 is the most potent inducer of histamine release from mast cells in vitro and in vivo.19,29 Degranulation of mast cells can be induced by several mechanisms, for instance by the crosslinking of Immunoglobulin E receptors on their cell surface or in a nonimmunological fashion by the stimulation of G proteins,30 both relying on an influx of extracellular Ca2+. The exact mechanism by which compound 48/80 stimulates mast cell degranulation is not known in great detail, other than that it inhibits the calcium-binding protein calmodulin31 and regulates certain G proteins and the effector molecule phospholipase C.32
Stimulation of peritumoral mast cell degranulation with compound 48/80 enhanced angiogenesis and tumor growth compared with PBS without having any stimulatory effects on the tumor cells in vitro. Similarly, inhibition of mast cell degranulation with SDG decreased angiogenesis and tumor growth. These observations and the correlations between peritumoral mast cell numbers, angiogenesis, and tumor growth indicate that peritumoral mast cells stimulate PC progression. This is in line with other animal tumor models where mast cells drive tumor angiogenesis and growth.5,6,33 We also used Imatinib to eradicate prostate mast cells before tumor cell injection and found that it reduced peritumoral mast cells, angiogenesis, FGF-2, and tumor growth in an ortothopic PC-model. In support of our findings, Imatinib is successful in the treatment of aggressive systemic mastocytosis24 and is now considered a standard first-line therapy for GIST tumors25 as well as to enhance the effects of chemotherapy in a mouse model of PC metastasis.34 Unfortunately, the use of Imatinib to specifically evaluate the importance of mast cells in a preclinical setting is limited. Based on our findings, however, it seems plausible that the reduction of mast cells after Imatinib treatment could be of therapeutic value. Previous studies have shown that growth of the vasculature in the surrounding nonmalignant prostate tissue is of major importance for prostate tumor growth.4,18 The present study indicates that this vascular growth is in part due to the accumulation of mast cells in this tissue compartment.
Mast cells have been shown to regulate vascular function.5,6,33,35,36 FGF-2 has previously been identified as a growth factor released by mast cells by degranulation.36 FGF-2 is known to be produced in the prostate stroma and acts together with its receptor FGF-R1 as stimulators of epithelial as well as vascular growth and are of major importance in PC in both animal models and patients.37,38,39,40 Our study shows that prostate mast cells, in addition to stroma fibroblasts, could be a major source of FGF-2 in PC. Interestingly, FGF-2 is also a mediator of the second wave of angiogenesis after initially successful antiangiogenic treatment,41 and the inhibitory effects of castration are in part mediated by vascular regression.15,20,23 It would therefore be interesting to determine whether FGF-2 is the major stimulator of angiogenesis released from the mast cells accumulating in castration-resistant prostate tumors or is the major stimulator of angiogenesis during tumor relapse. Recently, a report showed that Imatinib inhibits angiogenesis and tumor growth by reducing FGF-2 levels in fibroblasts in the cervix stroma.26 Importantly, we also observed that Imatinib potently inhibits the stromal expression of FGF-2. We have previously shown that Imatinib enhances the effect of castration in Dunning H tumors.20 The present study suggests that this effect could be accomplished by multiple effects in the microenvironment, including depletion of mast cell-derived FGF-2.
In patients, we observed that mast cell density in different tissue compartments apparently had different impact on outcome. This could explain the contradictory findings regarding mast cell function in PC by other researchers12,13 and are in line with the described plasticity of the role of mast cells in tumor biology.8,9,10,11 Mast cells produce a broad repertoire of cytokines to regulate inflammatory responses, growth factors to stimulate blood vessels and fibroblasts, and enzymes capable of releasing growth factors from extracellular matrix.5,8,9,10,11,27,28,42 In other human tumors studies it has been shown that mast cell function is complicated with both pro - and antitumor effects,35,43,44,45,46 but to the best of our knowledge no studies have previously described differential roles of mast cells depending on the local microenvironment within the same clinical material. It is well established that inflammatory cell types within or surrounding tumors such as tumor-associated macrophages47 and more lately also neutrophils48 can be educated by growth factors and cytokines within the tumor microenvironment to acquire pro- or antitumor properties.49 Whether or not mast cells can be polarized in a similar manner remains to be elucidated. In this study, we found that mast cells lying in the peritumoral tissue appear to be related to vascular and tumor growth in animal models and hence offers an opportunity as a therapeutic target. Similarly, in men, they relate to a poor prognosis and correlates with GS. In contrast, intratumoral mast cells correlate negatively with the presence of metastasis, tumor stage, GS, peritumoral blood vessel density, and tumor cell proliferation as well as relates to a good prognosis in PC, similar to results from a large cohort of breast43 and prostate cancer13 patients. The mechanism behind this is not evaluated in this study but could speculatively relate to their ability to regulate immunity.10,11 Surprisingly, in men, intratumoral mast cells did not correlate with blood vessel density within the tumor despite their expression of FGF-2 suggesting that other cell types or growth factors are of importance for blood vessel density within human prostate tumors.
We also observed that mast cells were recruited to relapsing prostate tumor in men as well as in our Dunning H tumors, in the latter, concurrent with an induction of FGF-2 and tumor angiogenesis. Because the expression of FGF-2 was found also in mast cells derived from nonmalignant VP tissue, it is probable that the increased levels of FGF-2 after castration therapy is mainly due to the increased recruitment of mast cells into the peritumoral stroma and not an increased synthesis. We did not investigate the importance of inhibiting mast cells to enhance the effects of castration in this study, but we identify a possible contribution of mast cells to relapsing prostate tumors in rodents and more importantly, in men. Stroma epithelial interactions have been shown to be of major importance for prostate development,50 prostate tumor progression,4,18,51,52 and during the formation of castrate-resistant tumors,14 and thus mast cells could represent a therapeutic target also under these settings.
To summarize, our study show for the first time that peritumoral and intratumoral mast cells are differential independent prognostic markers in a large cohort of untreated PC patients. In addition, our study shows that prostate peritumoral mast cells regulate angiogenesis in animal models of PC, possibly by secreting FGF-2. Future studies should clarify the context-dependent role of mast cells in PC and how mast cells could be used as a novel target for therapy during prostate tumor progression or to further enhance the effects of castration therapy.
Acknowledgments
We thank Birgitta Ekblom, Pernilla Andersson, Elizabeth Dahlberg, and Sigrid Kilter for technical assistance, Nina Norgren for her help quantifying mast cells, and A/Prof. Ruth Ganss at Western Australian Institute for Medical Research, Perth, WA, for valuable comments.
Footnotes
Address reprint requests to Dr. Anna Johansson, Western Australian Institute for Medical Research (WAIMR), 50 Rear Murray Street, 6000 Perth, WA. E-mail: ajohansson@waimr.uwa.edu.au.
Supported by grants from the Swedish Cancer Society, Umeå University Hospital, Umeå, Sweden, The Swedish Research Council, Stockholm, Sweden, and the Lions Research Foundation, Umeå University, Sweden.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of PC growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human PC: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- Halin S, Häggström SR, Rooijen NV, Bergh A. Extratumoral macrophages promote tumor and vascular growth in an orthotopic rat prostate tumor model. Neoplasia. 2009;11:177–186. doi: 10.1593/neo.81338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, Weissleder R, McNagny KM, Khazaie K. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky DS, Nechushtan H. Mast cells and cancer - no longer just basic science. Crit Rev Oncol Hematol. 2008;68:115–130. doi: 10.1016/j.critrevonc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Wasiuk A, de Vries VC, Hartmann K, Roers A, Noelle RJ. Mast cells as regulators of adaptive immunity to tumors. Clin Exp Immunol. 2009;155:140–146. doi: 10.1111/j.1365-2249.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura N, Takayama H, Nishimura K, Oka D, Nakai Y, Shiba M, Tsujimura A, Nakayama M, Aozasa K, Okuyama A. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of PC. Br J Cancer. 2007;97:952–956. doi: 10.1038/sj.bjc.6603962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A, Schlomm T, Köllermann J, Sekulic N, Huland H, Mirlacher M, Sauter G, Simon R, Erbersdobler A. Immunological microenvironment in prostate cancer: high mast cell densities are associated with favorable tumor characteristics and good prognosis. Prostate. 2009;15:976–981. doi: 10.1002/pros.20948. [DOI] [PubMed] [Google Scholar]
- Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–1671. doi: 10.1158/1078-0432.CCR-06-0067. [DOI] [PubMed] [Google Scholar]
- Lissbrant IF, Häggström S, Damber JE, Bergh A. Testosterone stimulates angiogenesis and vascular re growth in the ventral prostate in castrated adult rats. Endocrinology. 1998;139:451–456. doi: 10.1210/endo.139.2.5683. [DOI] [PubMed] [Google Scholar]
- Smolev JK, Heston WD, Scott WW, Coffey DS. Characterization of the Dunning R3327 H prostatic adenocarcinoma: an appropriate animal model for prostatic cancer. Cancer Treat Rep. 1977;61:273–287. [PubMed] [Google Scholar]
- Isaacs JT, Coffey DS. Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res. 1981;41:5070–5075. [PubMed] [Google Scholar]
- Halin S, Hammarsten P, Wikström P, Bergh A. Androgen-insensitive PC cells transiently respond to castration treatment when growing in an androgen-dependent prostate environment. Prostate. 2007;67:370–377. doi: 10.1002/pros.20473. [DOI] [PubMed] [Google Scholar]
- Sullivan TJ, Parker CW. Pharmacologic modulation of inflammatory mediator release by rat mast cells. Am J Pathol. 1976;85:437–464. [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Jones J, Pietras K, Kilter S, Skytt A, Rudolfsson SH, Bergh A. A stroma targeted therapy enhances castration effects in a transplantable rat PC model. Prostate. 2007;67:1664–1676. doi: 10.1002/pros.20657. [DOI] [PubMed] [Google Scholar]
- Chung SC, Hammarsten P, Josefsson A, Stattin P, Granfors T, Egevad L, Mancini G, Lutz B, Bergh A, Fowler CJ. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in PC. Eur J Cancer. 2009;45(1):174–82. doi: 10.1016/j.ejca.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Wikström P, Ohlson N, Stattin P, Bergh A. Nuclear androgen receptors recur in the epithelial and stromal compartments of malignant and non-malignant human prostate tissue several months after castration therapy. Prostate. 2007;67:1277–1284. doi: 10.1002/pros.20569. [DOI] [PubMed] [Google Scholar]
- Johansson A, Häggström Rudolfsson S, Wikström P, Bergh A. Altered levels of Angiopoietin 1 and Tie 2 are associated with androgen-regulated vascular regression and growth in the ventral prostate in adult mice and rats. Endocrinology. 2005;146:3463–3470. doi: 10.1210/en.2004-1480. [DOI] [PubMed] [Google Scholar]
- Hungness SI, Akin C. Mastocytosis: advances in diagnosis and treatment. Curr Allergy Asthma Rep. 2007;7:248–254. doi: 10.1007/s11882-007-0037-8. [DOI] [PubMed] [Google Scholar]
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D, Wehre E, Nikolova Z, Joensuu H. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signalling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:e19. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004;114:21–27. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Brzezińska-Błaszczyk E, Pietrzak A, Misiak-Tłoczek AH. Tumor necrosis factor (TNF) is a potent rat mast cell chemoattractant. J Interferon Cytokine Res. 2007;27:911–919. doi: 10.1089/jir.2006.0158. [DOI] [PubMed] [Google Scholar]
- Rothschild AM. Mechanisms of histamine release by compound 48/80. Br J Pharmacol. 1970;38:253–262. doi: 10.1111/j.1476-5381.1970.tb10354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YX, McCloskey MA. Activation of mast cell K+ channels through multiple G proteinlinked Receptors. Proc Natl Acad Sci USA. 1993;90:7844–7848. doi: 10.1073/pnas.90.16.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietzen K, Adamczyk-Engelmann P, Wüthrich A, Konstantinova A, Bader H. Compound 48/80 is a selective and powerful inhibitor of calmodulin-regulated functions. Biochim Biophys Acta. 1983;736:109–118. doi: 10.1016/0005-2736(83)90175-x. [DOI] [PubMed] [Google Scholar]
- Bronner C, Wiggins C, Monté D, Märki F, Capron A, Landry Y, Franson RC. Compound 48/80 is a potent inhibitor of phospholipase C and a dual modulator of phospholipase A2 from human platelet. Biochim Biophys Acta. 1987;920:301–305. doi: 10.1016/0005-2760(87)90108-1. [DOI] [PubMed] [Google Scholar]
- Starkey JR, Crowle PK, Taubenberger S. Mast cell deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988;42:48–52. doi: 10.1002/ijc.2910420110. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Uehara H, Yazici S, Langley RR, He J, Tsan R, Fan D, Killion JJ, Fidler IJ. Simultaneous blockade of platelet-derived growth factor-receptor and epidermal growth factor-receptor signalling and systemic administration of Paclitaxel as therapy for human prostate cancer metastasis in bone of nude mice. Cancer Res. 2004;64:4201–4208. doi: 10.1158/0008-5472.CAN-03-3763. [DOI] [PubMed] [Google Scholar]
- Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:195–199. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Crivellato E, Candussio L, Vacca A, Nico B, Benagiano V, Roncali L, Dammacco F. Angiogenic activity of rat mast cells in the chick embryo chorioallantoic membrane is down-regulated by treatment with recombinant human alpha-2a interferon and partly mediated by fibroblast growth factor-2. Haematologica. 2002;87:465–471. [PubMed] [Google Scholar]
- Doll JA, Reiher FK, Crawford SE, Pins MR, Campbell SC, Bouck NP. Thrombospondin-1, vascular endothelial growth factor and fibroblast growth factor-2 are key functional regulators of angiogenesis in the prostate. Prostate. 2001;49:293–305. doi: 10.1002/pros.10025. [DOI] [PubMed] [Google Scholar]
- Polnaszek N, Kwabi-Addo B, Peterson LE, Ozen M, Greenberg NM, Ortega S, Basilico C, Ittmann M. Fibroblast growth factor 2 promotes tumor progression in an autochthonous mouse model of PC. Cancer Res. 2003;63:5754–5760. [PubMed] [Google Scholar]
- Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human PC. Clin Cancer Res. 1999;5:1063–1071. [PubMed] [Google Scholar]
- Acevedo VD, Gangula RD, Freeman KW, Li R, Zhang Y, Wang F, Ayala GE, Peterson LE, Ittmann M, Spencer DM. Inducible FGF-R1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell. 2007;6:559–571. doi: 10.1016/j.ccr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signalling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput AB, Turbin DA, Cheang MC, Voduc DK, Leung S, Gelmon KA, Gilks CB, Huntsman DG. Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: a study of 4,444 cases. Breast Cancer Res Treat. 2008;107:249–257. doi: 10.1007/s10549-007-9546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Nico B, Quondamatteo F, Ria R, Minischetti M, Marzullo A, Herken R, Roncali L, Dammacco F. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer. 1999;79:451–455. doi: 10.1038/sj.bjc.6690070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Ennas MG, Vacca A, Ferreli F, Nico B, Orru S, Sirigu P. Tumor vascularity and tryptase-positive mast cells correlate with a poor prognosis in melanoma. Eur J Clin Invest. 2003;33:420–425. doi: 10.1046/j.1365-2362.2003.01152.x. [DOI] [PubMed] [Google Scholar]
- Yano H, Kinuta M, Tateishi H, Nakano Y, Matsui S, Monden T, Okamura J, Sakai M, Okamoto S. Mast cell infiltration around gastric cancer cells correlates with tumor angiogenesis and metastasis. Gastric Cancer. 1999;2:26–32. doi: 10.1007/s101200050017. [DOI] [PubMed] [Google Scholar]
- Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, DeNardi DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. Mesenchymal-epithelial interactions: past, present, future. Differentiation. 2008;76:578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- Memarzadeh S, Xin L, Mulholland DJ, Mansukhani A, Wu H, Teitell MA, Witte ON. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson N, Bergh A, Stattin P, Wikström P. Castration-induced epithelial cell death in human prostate tissue is related to locally reduced IGF-1 levels. Prostate. 2007;67:32–40. doi: 10.1002/pros.20480. [DOI] [PubMed] [Google Scholar]