Abstract
The lipid transport protein apolipoprotein E (apoE) is abundantly expressed in the brain. Its main isoforms in humans are apoE2, apoE3, and apoE4. ApoE4 is the major known genetic risk factor for Alzheimer’s disease and also contributes to the pathogenesis of various other neurological conditions. In the central nervous system, apoE is synthesized by glial cells and neurons, but it is unclear whether the cellular source affects its biological activities. To address this issue, we induced excitotoxic injury by systemic kainic acid injection in transgenic Apoe knockout mice expressing human apoE isoforms in astrocytes or neurons. Regardless of its cellular source, apoE3 expression protected neuronal synapses and dendrites against the excitotoxicity seen in apoE-deficient mice. Astrocyte-derived apoE4, which has previously been shown to have detrimental effects in vitro, was as excitoprotective as apoE3 in vivo. In contrast, neuronal expression of apoE4 was not protective and resulted in loss of cortical neurons after excitotoxic challenge, indicating that neuronal apoE4 promotes excitotoxic cell death. Thus, an imbalance between astrocytic (excitoprotective) and neuronal (neurotoxic) apoE4 expression may increase susceptibility to diverse neurological diseases involving excitotoxic mechanisms.
Apolipoprotein E (apoE) is important in lipid metabolism and neuronal plasticity.1,2 Plasma apoE is primarily produced by the liver and is one of several apolipoproteins that carry cholesterol and phospholipids in very low density lipoproteins, high density lipoproteins, and large lipoprotein remnants.3 In the central nervous system, apoE is the major extracellular lipid carrier and plays a key role in neuronal protection/repair after injury.2,4,5 It is synthesized by astrocytes and, to a lesser extent, by microglia, oligodendrocytes, and ependymal cells.6,7 Under pathological conditions, apoE is also synthesized by neurons.8,9,10,11
Three apoE isoforms are found in humans: apoE2, apoE3 (the most common), and apoE4.12 ApoE4 is associated with increased risk of Alzheimer’s disease13,14 and Parkinson’s disease,15 poor outcome after head injury16 and stroke,17 accelerated progression of multiple sclerosis,18 and subtle neurological impairments in individuals without overt neurological disease.19,20 Thus, apoE4 may contribute to diverse neurological conditions.
With multiple cellular origins and isoform-specific structural properties,6 apoE4 could exert pathogenic effects through multiple pathways. In Alzheimer’s disease, apoE4 is associated with increased formation of amyloid plaques21,22,23,24 and neurofibrillary tangles.25 These effects may reflect diverse biological activities of apoE4, including its abilities to enhance the production and deposition of amyloid-β (Aβ) peptides,26,27 interfere with Aβ clearance,28 decrease cholesterol delivery to neuronal membranes,27 and increase the phosphorylation and aggregation of tau, the main constituent of neurofibrillary tangles.29,30 In neuronal cultures, apoE4 inhibits neurite outgrowth, impairs neuronal signaling pathways, disrupts cytoskeletal and mitochondrial structure and function, and potentiates Aβ-induced lysosomal leakage and apoptosis.6,31 In contrast to apoE3, apoE4 does not protect synapses from age-related, excitotoxic, or Aβ-induced degeneration in transgenic mice.32,33 In such models, apoE4 is also more susceptible to C-terminal proteolytic cleavage than apoE3,30,34,35 depletes cytosolic androgen receptor levels,36 and is associated with cognitive impairments.37,38
Interestingly, apoE4 undergoes proteolytic cleavage and increases tau phosphorylation only when produced by neurons but not when produced by astrocytes.30 To ascertain whether the cellular source of apoE also influences the modulatory effect of apoE isoforms on excitotoxicity, we analyzed neuronal integrity and death after an excitotoxic challenge in transgenic mice expressing apoE3 or apoE4 in astrocytes or neurons. Excitotoxicity involves overactivation of glutamate receptors, resulting in excessive entry of Ca2+ into neurons, activation of degrading enzymes, and destruction of essential cellular components.39,40 Surprisingly, when expressed in astrocytes, apoE3 and apoE4 were equally excitoprotective. When expressed in neurons, apoE3 was excitoprotective, but apoE4 was not. In addition, apoE4 increased neuronal susceptibility to excitotoxin-induced cell death. Thus, the cellular source of apoE4 markedly affects its biological activities in vivo.
Materials and Methods
Transgenic Mice
The mice used in this study expressed an apoE3 or apoE4 minigene under control of the neuron-specific-enolase (NSE) or the glial fibrillary acidic protein (GFAP) promoter, were deficient in murine apoE (Apoe−/−), and were generated and genotyped as described.30,33 All mice were crossed for ≥10 generations with C57BL/6J Apoe−/− mice (Jackson Laboratories, Bar Harbor, ME), housed on a 12-hour light/12-hour dark cycle at 18°C with free access to water and food (PicoLab Rodent Diet 20, number 5053, PMI Nutrition International, St. Louis, MO), and analyzed at 5 to 7 months of age.
Kainic Acid Treatment and Tissue Preparation
Mice were injected with kainic acid (KA; 18 mg/kg i.p.; Sigma, St. Louis, MI) or saline as described.33 The onset, intensity, and duration of seizures in KA-treated mice were comparable and independent of the genotype (data not shown), as expected.33 Saline-injected controls did not develop seizures. The number of mice in each group in the GFAP-apoE (GFAP-E) cohort was as follows: six saline- and five KA-injected Apoe−/− mice; eight saline- and eight KA-injected GFAP-E3 mice; and 9 saline- and 10 KA-injected GFAP-E4 mice. The number of mice in each group in the NSE-apoE (NSE-E) cohort was as follows: five saline- and four KA-injected Apoe−/− mice; 8 saline- and 13 KA-injected NSE-E3 mice; and 11 saline- and 11 KA-injected NSE-E4 mice. No deaths were observed in the KA-injected mice regardless of genotype. Six days after KA or saline injection, mice were anesthetized with choral hydrate and perfused transcardially with 0.9% saline. Brains were removed and divided sagittally. One hemibrain was postfixed in 4% phosphate-buffered paraformaldehyde, pH 7.4, at 4°C for 48 hours for vibratome sectioning. Neocortex and hippocampus were dissected from the other hemibrain, snap frozen, and stored at −70°C for protein analysis. The study was approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Western Blotting and Quantitative Analysis of Total and Fragmented ApoE
Total apoE and apoE fragmentation were measured as described.34 Brain tissues were homogenized in ice-cold lysis buffer (50 mmol/L Tris/HCl, pH 8.0, 150 mmol/L NaCl, 4% SDS, 1% Nonidet P-40, 1% sodium deoxycholate, and protease and phosphatase inhibitors) and centrifuged at 52,000 × g (35,000 rpm) for 30 minutes at 4°C in a TLA 100.3 rotor of a Beckman Optima TL Ultracentrifuge. The supernatant was subjected to SDS-polyacrylamide gel electrophoresis and analyzed by Western blotting with antibodies against human full-length apoE (Calbiochem, San Diego, CA). Bands representing full-length apoE and apoE fragments were scanned and their intensities determined by densitometry. The exposure times of the gels to film were adjusted so that all bands, including the strongest, were within the range of the standard curve. ApoE fragmentation was expressed as the ratio of truncated apoE to full-length apoE, as previously reported.30,35
Immunohistochemistry
ApoE immunohistochemistry was performed on 50-μm vibratome sections. Endogenous peroxidase was quenched by incubation in 3% H2O2/10% methanol in PBS for 15 minutes, and nonspecific binding was blocked with 10% rabbit serum, 1% nonfat dry milk, 0.2% gelatin, and 0.2% Triton-X-100. Anti-apoE (Calbiochem) was diluted 1:28,000, and biotin-labeled anti-goat secondary antibody was diluted 1:200. Secondary antibody binding was detected with the avidin-biotin complex Elite kit (Vector, Burlingame, CA) by using diaminobenzidine and H2O2 as chromagenic substrates. The immunostaining specificity has been documented.33 Photomicrographs were taken with an AxioCam digital camera (Zeiss, Jena, Germany) coupled to an Olympus BX-60 microscope (Tokyo, Japan).
For double-labeling, brain sections were incubated with anti-human apoE (1:12,000; Calbiochem) and anti-GFAP antibody (1:1000; Dako Carpinteria, CA) to identify astrocytes or anti-Neuronal Nuclei (NeuN: 1:5000; Chemicon, Temecula, CA) to identify neurons. Primary antibody binding was detected by incubation for 1 hour with Texas Red-conjugated donkey anti-goat to detect anti-human apoE or with fluorescein isothiocyanate-conjugated donkey anti-mouse to detect anti-NeuN or anti-GFAP (1:200 dilution; Jackson ImmunoResearch, West Grove, PA). Sections were viewed with a Radiance 2000 laser-scanning confocal microscope (BioRad, Hercules, CA) on an Olympus BX-60 microscope. Images were stored digitally.
Quantitative Analysis of Neurodegeneration
Synaptophysin immunoreactivity (SYN-IR) and microtubule-associated protein-2 immunoreactivity (MAP2-IR) indicate the integrity of presynaptic boutons and dendrites, respectively.33,41,42,43 The image areas occupied by SYN-IR terminals or MAP2-IR dendrites in the stratum moleculare of the hippocampus and in layers 2 to 5 of the frontal neocortex were determined by confocal microscopy of fluorescently labeled sections and computer-aided image analysis.33 Sections were blind-coded to ensure unbiased assessment. In MAP-2 stained sections, neuronal cell bodies were manually edited out to limit the quantification to MAP-2 positive dendrites. In NeuN stained sections, pyramidal neuron density in the frontal neocortex (layers 2 to 3) and the hippocampal CA1 region were estimated with a stereological optical disector probe in three randomly selected, NeuN-stained sections per mouse.44,45,46,47 NeuN-immunoreactive (NeuN-IR) nuclei were counted in a 46.3 × 31.5 × 6 μm disector. For each brain region and mouse, approximately 100 nuclei were counted in 45 disectors (15 per section). The counts for each mouse and brain region were averaged and expressed as estimated number of NeuN-positive neurons per cubic millimeter.
Statistics
Statistical analysis was done with PRISM software (GraphPad, San Diego, CA). Data were analyzed by two-way analysis of variance, followed by Bonferroni post test. P < 0.05 was considered significant.
Results
Matching Hippocampal and Neocortical ApoE Levels in GFAP-ApoE and NSE-ApoE Transgenic Lines
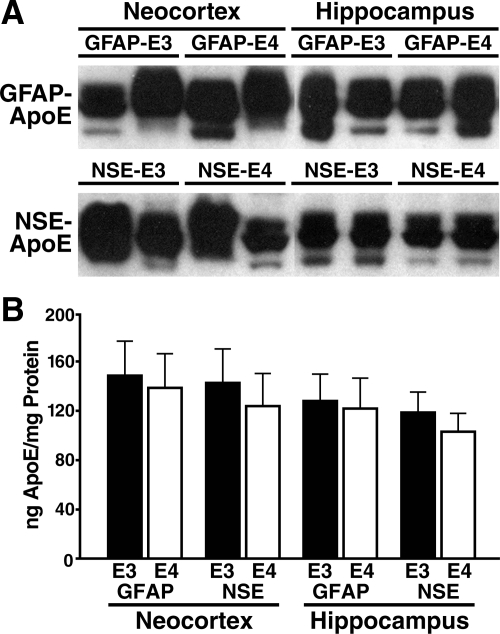
NSE-apoE mice have been described.33,38 To generate GFAP-apoE mice, the minigenes used in the NSE-apoE mice were placed downstream of the murine GFAP promoter to direct astrocyte-specific expression.48 Four founder lines were selected based on brain RNA analysis (data not shown) and crossed onto the Apoe−/− background. Brain apoE mRNA levels in one GFAP-E3 and one GFAP-E4 line matched those in the NSE-E3 and NSE-E4 lines (data not shown). Quantitative Western blot analysis confirmed that apoE levels in the hippocampus and neocortex of these four lines were comparable (Figure 1, A and B).30,33
Figure 1.
ApoE levels in the neocortex and hippocampus of GFAP-apoE and NSE-apoE mice. All brain tissues were harvested and processed in parallel. Total protein (30 μg/lane) was separated by SDS-polyacrylamide gel electrophoresis and analyzed by Western blotting with anti-apoE. A: Western blot shows apoE as a doublet in the 34 to 38 kDa range, as is typical for mouse and human brain tissues.30,33 The third lower-intensity band was probably due to partial degradation, which is often seen and was, on average, comparable across genotypes. B: ApoE levels were determined by densitometric analysis of Western blot signals, using human recombinant apoE as a standard (not shown). Results are means ± SEM; n = 4 to 5 mice per genotype.
Cell-Specific Expression of ApoE in NSE-ApoE and GFAP-ApoE Mice
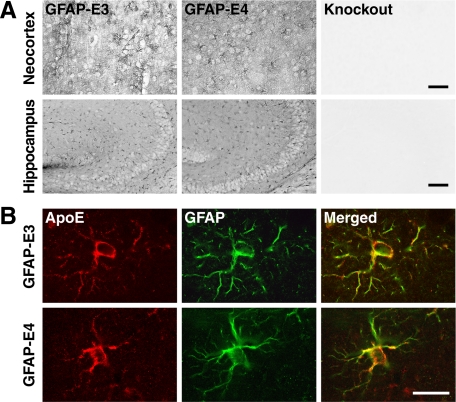
Neuron-specific expression of apoE in NSE-E3 and NSE-E4 mice has been documented.33 In GFAP-E3 and GFAP-E4 mice, apoE expression in the brain was widespread and comparable in distribution and intensity (Figure 2A). To confirm the identity of the cells expressing apoE in GFAP-apoE mice, we double-immunostained brain sections. In neocortex (Figure 2B) and hippocampus (Supplemental Figure 1 at http://ajp.amjpathol.org) of both GFAP-apoE lines, apoE immunoreactivity colocalized with GFAP-positive astrocytes, but not with NeuN-positive neurons (not shown).
Figure 2.
Transgenic apoE expression in astrocytes of GFAP-apoE mice. A: Immunoperoxidase staining of brain sections with an anti-apoE antibody revealed widespread and comparable labeling of neocortex and hippocampus in GFAP-E3 and GFAP-E4 mice. Similar comparable staining was observed in other brain regions of these two lines (not shown). No labeling was detected in apoE-deficient mice (knockout). Scale bars: 80 μm (neocortex); 200 μm (hippocampus). B: Immunofluorescence for apoE and GFAP. Neocortical sections were double-immunolabeled for apoE (red) and GFAP (green) and imaged by confocal microscopy. In GFAP-E3 and GFAP-E4 mice, apoE was found only in astrocytes. Scale bar: 10 μm.
Thus, our GFAP-E3, GFAP-E4, NSE-E3, and NSE-E4 lines express robust levels of apoE in neocortex and hippocampus, two brain regions vulnerable to Alzheimer’s disease and other neurodegenerative diseases. ApoE is expressed primarily, if not exclusively, by astrocytes in GFAP-apoE mice and by neurons in NSE-apoE mice.
Cellular Source of ApoE4 Determines How It Modulates Excitotoxin-Induced Neurodegeneration
Excitotoxicity resulting from the excessive stimulation of glutamate receptors is one of the main mechanisms of neuronal injury in neurodegenerative diseases.40 Previously, we reported that NSE-E4 mice are more susceptible to KA-induced excitotoxicity than NSE-E3 mice.33,49 To determine whether this apoE isoform-specific effect depends on the cellular source of apoE, we treated the mice with KA (18 mg/kg i.p.) and analyzed the neocortex and hippocampus for SYN-IR presynaptic terminals, MAP2-IR dendrites, NeuN-IR neurons, and apoE fragments.
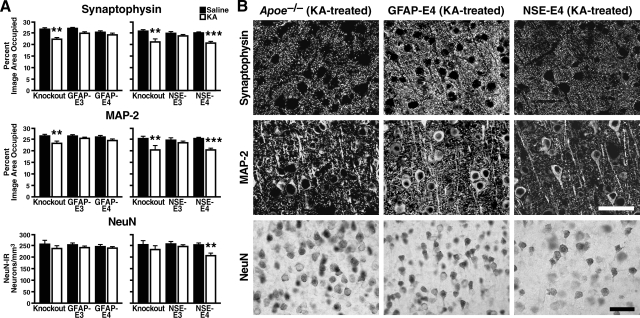
ApoE-deficient controls from the different NSE-apoE and GFAP-apoE transgenic lines showed similar excitotoxin-induced losses of SYN-IR presynaptic terminals and MAP2-IR dendrites in the neocortex (Figure 3, A and B) and hippocampus (Supplemental Figure 2 at http://ajp.amjpathol.org). ApoE3 protected synapses and dendrites against excitotoxic injury in these regions regardless of its cellular source, whereas apoE4 did so only when it was produced by astrocytes (Figure 3, A and B, and Supplemental Figure 2 at http://ajp.amjpathol.org).
Figure 3.
Neuronal integrity in the neocortex of GFAP-apoE versus NSE-apoE mice six days after intraperitoneal injection of KA (18 mg/kg) or saline (n = 4 to 13 mice per group). A: Neocortical brain sections were immunostained for synaptophysin, MAP-2, or NeuN, and immunoreactive structures were quantified as described in Materials and Methods. KA impaired synaptic and dendritic integrity in NSE-E4 mice and apoE-deficient mice, but not in GFAP-E3, NSE-E3, and GFAP-E4 mice. Loss of NeuN-positive pyramidal neurons was significant only in NSE-E4 mice. Thus, neuron-produced apoE4 increases vulnerability to excitotoxic injury, whereas astrocyte-produced apoE4 is as excitoprotective as apoE3. Results are means ± SEM; **P < 0.01; ***P < 0.001 versus control of the same genotype (Bonferroni post test). B: Photomicrographs depict examples of SYN-IR presynaptic terminals (top), MAR2-IR dendrites (middle), and NeuN-IR neuronal nuclei (bottom). Astrocyte-derived, but not neuron-derived, apoE4 protected neurons against excitotoxic injury. Apoe−/− control mice showed no loss of pyramidal neurons, although their loss of cortical SYN-IR terminals and MAP-2 dendrites was similar to that seen in NSE-apoE4 mice. Scale bars: 40 μm (upper rows); 75 μm (bottom row).
Estimation of neuronal density showed no significant loss of NeuN-positive pyramidal neurons in neocortex of KA-treated NSE-E3, GFAP-E3, or GFAP-E4 mice and apoE-deficient controls (Figure 3, A and B), consistent with the resistance of wild-type C57BL/6J mice to excitotoxin-induced neuronal loss.50 However, KA-treated NSE-E4 mice had a significant loss of neocortical and hippocampal pyramidal neurons (Figure 3, A and B, Supplemental Figure 2 at http://ajp.amjpathol.org), suggesting that neuronal expression of apoE4 overcomes this resistance and that apoE4 may be a critical mediator of neuronal death in neurodegenerative disease. NSE-E4 mice were more susceptible to KA-induced loss of neocortical and hippocampal pyramidal neurons than apoE-deficient mice. KA-treated apoE-deficient controls showed a trend toward loss of pyramidal neurons in the hippocampus that did not reach statistical significance (Supplemental Figure 2 at http://ajp.amjpathol.org).
As reported previously,30 apoE fragmentation was more prominent in NSE-E4 than NSE-E3 mice and absent in GFAP-E3 and GFAP-E4 mice (Supplemental Figure 3 at http://ajp.amjpathol.org). KA challenge increased apoE fragment levels only in NSE-E4 mice.
Discussion
This study demonstrates that both apoE3 and apoE4 are able to protect presynaptic terminals and neuronal dendrites against excitotoxicity when expressed by astrocytes. However, when expressed by neurons, only apoE3 was excitoprotective, whereas apoE4 was not and actually promoted excitotoxin-induced neuronal death, even relative to apoE-deficient mice.
Our finding that astrocytic apoE4 is excitoprotective in vivo contrasts with in vitro studies, which have consistently shown that apoE4 impairs neurons in culture.6 Previous in vivo studies in independent GFAP-apoE transgenic lines reported no obvious neuropathological alterations in GFAP-E4 mice at baseline,51,52 consistent with our observations, although in one of these studies52 GFAP-E4 mice showed a working memory impairment. Another study reported no protective effects of apoE3 or apoE4 in neonatal GFAP-apoE mice after hypoxic-ischemic insults.53 Conceivably, the severity of the injury combined with the immaturity of the brain obscured some of the biological activities of apoE, which appear to be of greater relevance to the aging than the developing central nervous system.
Excitotoxicity and other injuries trigger neuronal production of apoE,8,9,10,11 a response that would be beneficial to neurons producing apoE3 but detrimental to those producing apoE4. After an excitotoxic challenge, it was worse for neurons to express apoE4 than to express no apoE at all, consistent with other data suggesting that apoE4 has an adverse gain-of-function compared with apoE3 and apoE2.7,37,49 ApoE4 is more susceptible than apoE3 to cleavage by a neuron-specific protease that appears to impart an adverse gain-of-function on apoE4 through C-terminal truncation.30,34,35 KA increased the levels of apoE4 fragments, which might perpetuate a vicious cycle of injury-induced neuronal apoE4 production and impairments of critical subcellular compartments.
Indeed, expression of C-terminally truncated apoE4 is highly toxic in cultured neurons and causes neurodegeneration and behavioral deficits in transgenic mice.34,35,54 It leads to hyperphosphorylation of tau and to the formation of neurofibrillary tangle-like inclusions in some neurons30,34,35 and also causes mitochondrial dysfunction.54 Because brains of GFAP-E4 mice showed no evidence of apoE truncation, the pathogenic truncation of apoE4 likely requires production of apoE4 within neurons and does not occur after neuronal uptake of astrocyte-produced apoE4. These findings are consistent with evidence that apoE4 truncation is tightly linked to neuronal apoE synthesis.30
Our study suggests that astroglial production of apoE may be excitoprotective in both APOE ε3 carriers and APOE ε4 carriers and that APOE ε4 carriers, but not APOE ε3 carriers, may benefit from suppression of apoE expression specifically in neurons. Recent studies have identified unique mechanisms by which neuronal apoE expression is regulated11 that might be exploited to selectively suppress expression of apoE4 by neurons.
Best known as a risk factor for Alzheimer’s disease, apoE4 also seems to have a negative impact on the risk, age of onset, progression, or outcome of Parkinson’s disease,15 fronto-temporal dementia,55 multiple sclerosis,18 neuromuscular disease,56 and traumatic brain injury.16,17 Excitotoxicity has been implicated in all of these conditions,39,57 whereas increased accumulation of Aβ is seen mainly in Alzheimer’s disease.58 Therefore, although apoE4 clearly promotes the buildup of Aβ in the brain,22,26 enhancement of excitotoxic injury would seem a more parsimonious mechanism for apoE4 to promote the development of diverse neurological disorders. Our findings suggest that this pathogenic effect may depend on neuronal production of apoE4.
Acknowledgments
We thank Brian Jones for technical assistance, John Carroll and Chris Goodfellow for preparation of graphics, Stephen Ordway and Gary Howard for editorial review, and Monica De La Cruz for administrative assistance.
Footnotes
Address reprint requests to Lennart Mucke, M.D., or to Manuel Buttini, Ph.D., Elan Pharmaceuticals, 800 Gateway Blvd., South San Francisco, CA 94080 Gladstone Institute of Neurological Disease, 1650 Owens St, San Francisco, CA 94158-2261. E-mail: lmucke@gladstone.ucsf.edu or manuel.buttini@elan.com.
Supported by NIH grants AG011385 and NS041787 (L.M.) and AG022074 (Y.H.).
Y.H. has received funding from Merck for other research on apolipoprotein E4 and its role in neurodegenerative disorders. L.M. is a consultant for Merck. None of the other authors declare any relevant financial relationships.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of M.B.: Elan Pharmaceuticals, South San Francisco, CA; of T.W.-C.: Department Neurology, Stanford University School of Medicine, CA.
References
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009;50 Suppl:S183–S188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein E: from atherosclerosis to Alzheimer’s disease and beyond. Curr Opin Lipidol. 1999;10:207–217. doi: 10.1097/00041433-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Ignatius MJ, Gebicke-Harter PJ, Skene JH, Schilling JW, Weisgraber KH, Mahley RW, Shooter EM. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci USA. 1986;83:1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD. Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol. 1995;136:107–122. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- Huang Y, Weisgraber KH, Mucke L, Mahley RW. Apolipoprotein E: diversity of cellular origins, structural and biophysical properties, and effects in Alzheimer’s disease. J Mol Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- Huang Y. Molecular and cellular mechanisms of apolipoprotein E4 neurotoxicity and potential therapeutic strategies. Curr Opin Drug Discov Devel. 2006;9:627–641. [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris FM, Tesseur I, Brecht WJ, Xu Q, Mullendorff K, Chang S, Wyss-Coray T, Mahley RW, Huang Y. Astroglial regulation of apolipoprotein E expression in neuronal cells: implications for Alzheimer’s disease. J Biol Chem. 2004;279:3862–3868. doi: 10.1074/jbc.M309475200. [DOI] [PubMed] [Google Scholar]
- Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S. Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol Dis. 1999;6:508–514. doi: 10.1006/nbdi.1999.0251. [DOI] [PubMed] [Google Scholar]
- Xu Q, Walker D, Bernardo A, Brodbeck J, Balestra ME, Huang Y. Intron-3 retention/splicing controls neuronal expression of apolipoprotein E in the CNS. J Neurosci. 2008;28:1452–1459. doi: 10.1523/JNEUROSCI.3253-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannis VI, Just PW, Breslow JL. Human apolipoprotein E isoprotein subclasses are genetically determined. Am J Hum Genet. 1981;33:11–24. [PMC free article] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis; APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Li YJ, Hauser MA, Scott WK, Martin ER, Booze MW, Qin XJ, Walter JW, Nance MA, Hubble JP, Koller WC, Pahwa R, Stern MB, Hiner BC, Jankovic J, Goetz CG, Small GW, Mastaglia F, Haines JL, Pericak-Vance MA, Vance JM. Apolipoprotein E controls the risk and age at onset of Parkinson disease. Neurology. 2004;62:2005–2009. doi: 10.1212/01.wnl.0000128089.53030.ac. [DOI] [PubMed] [Google Scholar]
- Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- Slooter AJ, Tang MX, van Duijn CM, Stern Y, Ott A, Bell K, Breteler MM, Van Broeckhoven C, Tatemichi TK, Tycko B, Hofman A, Mayeux R. Apolipoprotein E epsilon4 and the risk of dementia with stroke: a population-based investigation. JAMA. 1997;277:818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- Pinholt M, Frederiksen JL, Christiansen M. The association between apolipoprotein E and multiple sclerosis. Eur J Neurol. 2006;13:573–580. doi: 10.1111/j.1468-1331.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Sunderland T, Friz JL, Parasuraman R. Genetics and visual attention: selective deficits in healthy adult carriers of the epsilon 4 allele of the apolipoprotein E gene. Proc Natl Acad Sci USA. 2000;97:11661–11666. doi: 10.1073/pnas.97.21.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, West HL, Rebeck GW, Buldyrev SV, Mantegna RN, Ukleja M, Havlin S, Stanley HE. Quantitative analysis of senile plaques in Alzheimer disease: observation of log-normal size distribution and molecular epidemiology of differences associated with apolipoprotein E genotype and trisomy 21 (Down syndrome). Proc Natl Acad Sci USA. 1995;92:3586–3590. doi: 10.1073/pnas.92.8.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000;47:739–747. [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm TG, Scharnagl H, Marz W, Bohl J. Apolipoprotein E isoforms and the development of low and high Braak stages of Alzheimer’s disease-related lesions. Acta Neuropathol. 1999;98:273–280. doi: 10.1007/s004010051080. [DOI] [PubMed] [Google Scholar]
- Bales KR, Dodart JC, DeMattos RB, Holtzman DM, Paul SM. Apolipoprotein E, amyloid, and Alzheimer disease. Mol Interv. 2002;2:363–375, 339. doi: 10.1124/mi.2.6.363. [DOI] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Goedert M, Weisgraber KH, Dong LM, Jakes R, Huang DY, Pericak-Vance M, Schmechel D, Roses AD. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:11183–11186. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Dee Fish J, Wyss-Coray T, Buttini M, Mucke L, Mahley RW, Huang Y. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation. J Neurosci. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahley RW. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J Neurosci. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, Weisgraber KH, Mucke L, Mahley RW, Huang Y. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000;404:352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor–still lethal after eight years. Trends Neurosci. 1995;18:57–58. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- Olney JW. Excitatory transmitter neurotoxicity. Neurobiol Aging. 1994;15:259–260. doi: 10.1016/0197-4580(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32:321–329. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Masliah E, Barbour R, Grajeda H, Motter R, Johnson-Wood K, Khan K, Seubert P, Freedman S, Schenk D, Games D. Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer’s disease. J Neurosci. 2005;25:9096–9101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Qyang Y, Kelsoe JR, Masliah E, Geyer MA. Impaired postnatal development of hippocampal dentate gyrus in Sp4 null mutant mice. Genes Brain Behav. 2007;6:269–276. doi: 10.1111/j.1601-183X.2006.00256.x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L. Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia. 1995;13:174–184. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
- Buttini M, Akeefe H, Lin C, Mahley RW, Pitas RE, Wyss-Coray T, Mucke L. Dominant negative effects of apolipoprotein E4 revealed in transgenic models of neurodegenerative disease. Neuroscience. 2000;97:207–210. doi: 10.1016/s0306-4522(00)00069-5. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Sikes J, Levin JA. Human apolipoprotein E allele-specific brain expressing transgenic mice. Neurobiol Aging. 1998;19:407–413. doi: 10.1016/s0197-4580(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer’s-like neuropathology. Exp Neurol. 2001;170:326–344. doi: 10.1006/exnr.2001.7715. [DOI] [PubMed] [Google Scholar]
- Lendon CL, Han BH, Salimi K, Fagan AM, Behrens MI, Muller MC, Holtzman DM. No effect of apolipoprotein E on neuronal cell death due to excitotoxic and apoptotic agents in vitro and neonatal hypoxic ischaemia in vivo. Eur J Neurosci. 2000;12:2235–2242. doi: 10.1046/j.1460-9568.2000.00113.x. [DOI] [PubMed] [Google Scholar]
- Chang S, ran Ma T, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelborghs S, Dermaut B, Marien P, Symons A, Vloeberghs E, Maertens K, Somers N, Goeman J, Rademakers R, Van den Broeck M, Pickut B, Cruts M, Van Broeckhoven C, De Deyn PP. Dose dependent effect of APOE epsilon4 on behavioral symptoms in frontal lobe dementia. Neurobiol Aging. 2006;27:285–292. doi: 10.1016/j.neurobiolaging.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Bedlack RS, Strittmatter WJ, Morgenlander JC. Apolipoprotein E and neuromuscular disease: a critical review of the literature. Arch Neurol. 2000;57:1561–1565. doi: 10.1001/archneur.57.11.1561. [DOI] [PubMed] [Google Scholar]
- Olney JW, Wozniak DF, Farber NB. Excitotoxic neurodegeneration in Alzheimer disease: new hypothesis and new therapeutic strategies. Arch Neurol. 1997;54:1234–1240. doi: 10.1001/archneur.1997.00550220042012. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]