Abstract
Angioimmunoblastic T-cell lymphoma (AITL) is the most frequent nodal T-cell lymphoma and is characterized by a polymorphic lymph node infiltrate, various dysimmune disorders, and a poor prognosis. Regulatory T-cells (Treg) play an emerging role in the prognosis of non-Hodgkin B-cell lymphoma and mediate significant autoreactive T-cell suppression. In this report, we demonstrate that numbers of Treg are significantly decreased in AITL lymph nodes [n = 30, 91 (40–195) per high power fields] compared with follicular lymphoma [n = 19, 179 (86–355)] and reactive lymph nodes [n = 8, 186 (140–265)]. Moreover, the few Treg in lymph nodes of AITL are resting Treg (rTreg) and have a naive CD45RA+, PD1−, and ICOS− phenotype [n = 5, 57% of Treg are CD45RA+ (16–96)], in contrast to the Treg in follicular lymphomas [n = 5, 7.4% (1–13)] or reactive lymph nodes [n = 7, 18.6% (6–48)]. Interestingly, Treg depletion was not observed in AITL peripheral blood at diagnosis. Altogether, these data suggest that Treg depletion could contribute to the nodal neoplastic TFH expansion and dysimmune symptoms in AITL.
Angioimmunoblastic T-cell lymphoma (AITL), one of the most frequent entities among peripheral T-cell lymphoma,1 is characterized by lymphadenopathy, B-symptoms, and an aggressive behavior.2,3 Its natural history has been the subject of controversy, having been considered for many years to be a nonmalignant disorder or a dysimmune disease, until the clonal nature of AITL was proven by molecular studies.4
The neoplastic cells in AITL show an immune profile closely related to that of follicular T-helper cells (TFH), characterized by the expression of markers such as CD4, CD10, CXCL13, ICOS, and PD1.5,6,7 The putative derivation of AITL from TFH cells has also been demonstrated recently by gene profiling studies.8 Because AITL is characterized by a polymorphic proliferation and a dysimmune disorder, a pathogenic role for the peritumoral immune cells is possible. Regulatory T-cells (Treg) CD4+ CD25hi FOXP3+ CD127low in the tumoral microenvironment play an emerging role in lymphoma growth regulation.9 They are also responsible for autoreactive T-cell suppression in T-cell lymphopoiesis, and they can control inappropriate autoimmune responses.10 Furthermore, they comprise different subsets referred to as CD45RA− activated Treg (aTreg) and CD45RA+ resting Treg (rTreg).11 Several recent studies underline the prognostic impact of Treg lymph node involvement in B-cell non-Hodgkin lymphoma and Hodgkin lymphoma.12,13,14 However, Treg activity in T-cell non-Hodgkin lymphoma has not been evaluated. We report here quantitative and qualitative Treg evaluation in involved lymph nodes and blood samples of AITL patients, which suggest that Treg alteration could contribute to tumor cell development and autoimmune associated symptoms.
Materials and Methods
Lymph Nodes and Peripheral Blood
Twenty fresh lymph node biopsies or cryopreserved lymph node cell suspensions from AITL patients (n = 7), follicular lymphoma (n = 6), and reactive lymph nodes (n = 7) were analyzed. We used a distinct series of 57 formalin-fixed paraffin-embedded lymph node biopsies from patients with AITL (n = 30), follicular lymphoma (n = 19), and reactive lymph nodes (n = 8). Eighteen blood samples from follicular lymphoma (n = 6), AITL (n = 7), or healthy donors (n = 5) were also analyzed. Experimental procedures for handling human samples were performed according to European Union guidelines with respect of the declaration of Helsinki.
Antibodies and Flow Cytometry (FC)
Treg are historically defined by high expression of CD25 and FOXP3, but these markers used separately become insufficient, because activated T-cells also express CD25 and FOXP3.15 CD127 weak expression was considered to be a preferred alternative for identification of Treg.16,17 In a multiparametric FC approach, we gated Treg on CD4+, CD25hi, FOXP3+, CD127low expression, and AITL cells on CD4+ and CD10+ expression (see supplemental Figure S1 at http://ajp.amjpathol.org). Cells were stained with monoclonal antibodies by an eight-color immunostaining panel, acquired on a CANTO II cytometer, and analyzed with FACS DIVA software (Becton-Dickinson). Antibodies used to phenotype Treg and AITL T-cells in peripheral blood and lymph node cell suspension were as follows: CD127 (R34.34, PE, Immunotech), CD45RA (L48, FITC, Becton-Dickinson), CD10 (HI10a, PE, DAKO), CD25 (2A3, PE-Cy7, Becton-Dickinson), CD8 (SK1, Percp-Cy5.5, Becton-Dickinson), FOXP3 (206D, Alexa Fluor 647, Biolegend) used after cell permeabilization, CD3 (UCHT1, Alexa Fluor 700, Becton-Dickinson), CD4 (RPA-T4, Blue Pacific, Becton-Dickinson), CD45 (2D1, Amcyan, Becton-Dickinson).
We estimated Treg as the CD25hi FOXP3+ CD127low percentage of the total CD4+ T-cell population, as previously described.18
Histology and Immunohistochemistry (IHC)
All diagnostic samples of AITL, follicular lymphomas, and reactive lymph nodes were reviewed by the same hematopathologist (D.C.). Immunohistochemistry was performed on fixed tissues by the peroxidase-based method (Dako, Ely, UK). Double stainings were performed as previously described19 (contact Dr T. Marafioti for details). Antibodies used were FOXP3 (clone 236A/E7 Abcam, Cambridge, UK), PD1 (clone NAT Abcam, Cambridge, UK), and ICOS (provided by Dr T. Marafioti). Results were scored as the mean number of FOXP3+ nuclei per high-power fields (HPF ×400) evaluated in five fields. Counts were performed in the interfollicular zones in reactive lymph nodes and FL cases.
Statistical analysis was performed with Prism software (GraphPad), using nonparametric Mann–Whitney and paired Student’s t-test. P < 0.05 was considered significant.
Results
Treg Depletion in AITL Lymph Nodes
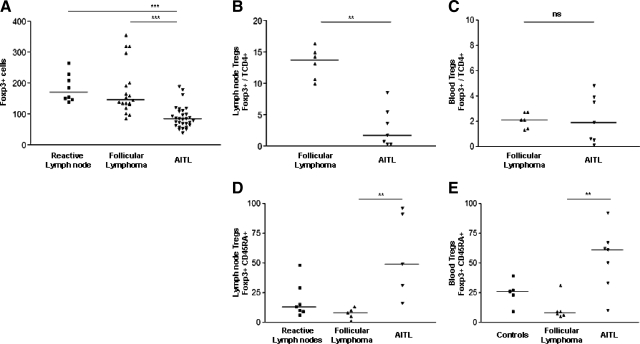
By IHC, FOXP3+ cells were distributed in a scattered pattern in the T-cell zones of reactive lymph nodes (Figure 1, A and D), whereas they usually predominated around the neoplastic follicles in follicular lymphoma (FL) (Figure 1, B and E). In contrast, FOXP3+ cells were rare and scattered in AITL (Figure 1, C and F). As shown in Figure 2A, the FOXP3+ cells were significantly less numerous in AITL [n = 30, 91 (40-195) per HPF] compared with reactive lymph node [n = 8, 186 (140-265)] or FL [n = 19, 179 (86-355)]. We then evaluated lymph node Treg infiltration by eight-color FC on cell suspensions (Figure 2B). In keeping with the IHC results, CD4+, CD25hi, FOXP3+, CD127low Treg were significantly decreased in AITL [n = 7, 3% of CD4+ T cells (0,4–8,6)] compared with FL [n = 6, 13.3% (10–16.4)].
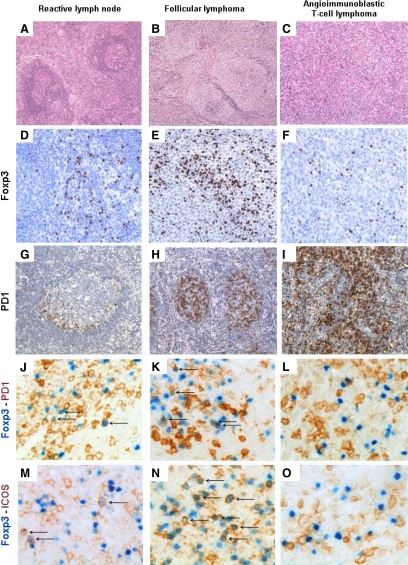
Figure 1.
Immunohistochemistry in lymph node biopsies. Reactive lymph node: A (×100) and D (×200): FOXP3+ cells are scattered within the interfollicular zones. G (×100): PD1+ cells are mostly located in the germinal center. J and M (×400): Double staining with FOXP3 (nuclear) and PD1 (membrane) or FOXP3 and ICOS (membrane) show few cells expressing both markers (arrows). Follicular lymphoma: B (×100) and E (×200): Numerous FOXP3+ cells surround the nodules. H (×100): Many cells with the follicles are PD1+. K and N (×400): A significant proportion of cells are double stained for FOXP3 and PD1 or ICOS (arrows). AITL: C (×100) and F (×200): FOXP3+ cells are rare and scattered. I (×100): Many cells are PD1+, including AITL neoplastic cells. L and O (×400): Double staining with FOXP3 and PD1 or FOXP3 and ICOS do not show double-positive cells.
Figure 2.
Quantitative and qualitative evaluation of Treg by immunohistochemistry and flow cytometry. Immunohistochemistry: A: Lymph nodes FOXP3+ cells in eight reactive lymph nodes, 19 follicular lymphomas (FL), and 30 AITL. There are significantly fewer Treg in AITL than FL or reactive lymph nodes. Flow cytometry: B: Lymph nodes FOXP3+/TCD4+ Treg in follicular lymphoma (n = 6) and AITL (n = 7). C: Blood FOXP3+/TCD4+ Treg in FL (n = 6) and AITL (n = 7). D: Lymph nodes FOXP3+ CD45RA+/TCD4+ Treg in FL (n = 5), AITL (n = 5), and reactive lymph nodes (n = 7). E: Blood FOXP3+ CD45RA+/TCD4+ Treg in FL (n = 6), AITL (n = 7), and healthy donors (n = 5). (ns indicates nonsignificant; **P < 0.01, ***P < 0.001).
Treg Phenotype in AITL Blood and Lymph Nodes
We then searched for Treg depletion in diagnostic blood samples and assessed the state of activation of the residual lymph node and peripheral blood Treg. We found no significant difference between FL patients’ (n = 6) and AITL patients’ blood Treg (n = 7) by eight-color FC, suggesting a local origin of the Treg depletion observed in the lymph nodes (Figure 2C).
Next, we evaluated levels of CD45RA+ resting Treg (rTreg) in peripheral blood and lymph nodes of AITL, FL, or healthy donors. As shown in Figure 2D, CD45RA+ rTreg are significantly increased in AITL lymph nodes [n = 5, 57% of Treg are CD45RA+ (16–96)] compared with FL [n = 5, 7.4% (1–13)], and also increased compared with healthy controls [n = 7, 18.6% (6–48)]. Peripheral blood Treg (Figure 2E) are mostly naïve and not activated in AITL [n = 7, 41% (4–67)] in contrast to those isolated from FL [n = 6, 11.2% (5–31)] and healthy donors [n = 5, 24.8% (9–39)]. These results demonstrate that Treg depletion in AITL is a local nodal phenomenon and that residual Treg in lymph nodes or peripheral blood are predominantly CD45RA+ rTreg. By contrast, Treg in FL appear to be mostly activated Treg (aTreg).
To confirm these data, we then performed double-immunostaining with PD1 and ICOS. These markers are expressed by normal TFH and neoplastic TFH-cells in AITL20,21 as well as in activated Treg.22,23 In reactive lymph nodes, FOXP3+ cells predominate at the periphery of germinal centers (Figure 1D), and PD1+ cells are mainly found inside the germinal centers (Figure 1G). Rare interfollicular cells coexpress PD1/FOXP3 or ICOS/FOXP3 (arrows in Figure 1, J and M). In FL, FOXP3+ cells preferentially surround neoplastic follicles, with a minority being located within neoplastic follicles (Figure 1E), where numerous PD1+ cells are found (Figure 1H), and some cells have a PD1+/FOXP3+ phenotype (arrows in Figure 1, K and N). In AITL, FOXP3+ cells are scattered and rare (Figure 1F), whereas, as expected, numerous neoplastic cells express PD1 (Figure 1I). In most AITL, PD1+/FOXP3+ or ICOS+/FOXP3+ cells were rare (Figure 1, L and O). These data suggest that in AITL, a minority of Treg express the activation proteins ICOS and PD1 and as such are mostly naïve.
Discussion
Regulatory T-cells (Treg) play an emerging role in the prognosis of non-Hodgkin B-cell lymphoma and mediate significant autoreactive T-cell suppression.12,13,14 To date, few data are available for the nodal Treg infiltration in the T-cell lymphoma. In this report we show that angioimmunoblastic T-cell lymphoma, considered to be an unfavorable and aggressive T-cell lymphoma, is associated with a decreased lymph node Treg infiltrate compared with follicular lymphoma and reactive lymph nodes. Within the FL context, an increased Treg infiltration has been shown to correlate with improved survival,13 whereas decreased Treg lymph node infiltration can predict unfavorable outcome.14
Taken together, we show a reduced infiltration of Treg, which are mostly rTreg in AITL, potentially contributing to the autoimmune symptoms, as described in systemic lupus11 and that could participate in the poor prognosis of AITL. However, the cause of this Treg depletion and the mechanisms of interaction between AITL neoplastic T-cells and Treg remain to be defined. PD1 is known to be expressed by the AITL neoplastic T-cells and also to be involved in the Treg negative regulation. It is possible that the PD1 pathway is involved in down-regulation of the Treg population observed in AITL.18,21
So, it is tempting to hypothesize that Treg depletion in AITL neoplastic lymph nodes could therefore be involved in AITL pathogenesis and be responsible for the poor prognosis and dysimmune state found in this disorder. Restoring Treg level and activation status in this pathology could represent a potential for therapeutic intervention that deserves further investigation. Overall, our data open new perspectives in understanding the dysimmune disorders, tumoral growth, and the poor prognosis observed in AITL.
Acknowledgments
We thank “la tumorothèque” (Necker-Enfants Malades Hospital) for providing materials.
Footnotes
Address reprint requests to Vahid Asnafi, M.D., Ph.D., GH Necker-Enfants Malades, Hématologie biologique, Tour Pasteur 2ème Étage, 149 rue de Sèvres, 75743 Paris Cedex 15, France. E-mail: vahid.asnafi@nck.aphp.fr.
Supported by The “Foundation pour la Recherche Médicale” and from a grant from the Leukemia Research United Kingdom (No. 0382).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Swerdlow HS CE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. Lyon; WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 2008:pp p309–311. [Google Scholar]
- Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Briere J, Haioun C, Cazals-Hatem D, Bosly A, Xerri L, Tilly H, Berger F, Bouhabdallah R, Diebold J. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood. 1998;92:76–82. [PubMed] [Google Scholar]
- Mourad N, Mounier N, Briere J, Raffoux E, Delmer A, Feller A, Meijer CJ, Emile JF, Bouabdallah R, Bosly A, Diebold J, Haioun C, Coiffier B, Gisselbrecht C, Gaulard P. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463–4470. doi: 10.1182/blood-2007-08-105759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipford EH, Smith HR, Pittaluga S, Jaffe ES, Steinberg AD, Cossman J. Clonality of angioimmunoblastic lymphadenopathy and implications for its evolution to malignant lymphoma. J Clin Invest. 1987;79:637–642. doi: 10.1172/JCI112860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attygalle A, Al-Jehani R, Diss TC, Munson P, Liu H, Du MQ, Isaacson PG, Dogan A. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627–633. doi: 10.1182/blood.v99.2.627. [DOI] [PubMed] [Google Scholar]
- Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Expression of CXCL13, a chemokine highly upregulated in germinal center T-helper cells, distinguishes angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified. Mod Pathol. 2006;19:1101–1107. doi: 10.1038/modpathol.3800625. [DOI] [PubMed] [Google Scholar]
- Huang Y, Moreau A, Dupuis J, Streubel B, Petit B, Le Gouill S, Martin-Garcia N, Copie-Bergman C, Gaillard F, Qubaja M, Fabiani B, Roncador G, Haioun C, Delfau-Larue MH, Marafioti T, Chott A, Gaulard P. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol. 2009;33:682–690. doi: 10.1097/PAS.0b013e3181971591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, Lamant L, Leroy K, Briere J, Molina T, Berger F, Gisselbrecht C, Xerri L, Gaulard P. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- Hilchey SP, De A, Rimsza LM, Bankert RB, Bernstein SH. Follicular lymphoma intratumoral CD4+CD25+GITR+ regulatory T cells potently suppress CD3/CD28-costimulated autologous and allogeneic CD8+CD25− and CD4+CD25− T cells. J Immunol. 2007;178:4051–4061. doi: 10.4049/jimmunol.178.7.4051. [DOI] [PubMed] [Google Scholar]
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, Banham AH, Roncador G, Montalban C, Piris MA. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabattini E, Bisgaard K, Ascani S, Poggi S, Piccioli M, Ceccarelli C, Pieri F, Fraternali-Orcioni G, Pileri SA. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506–511. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30:802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol. 2006;176:2808–2816. doi: 10.4049/jimmunol.176.5.2808. [DOI] [PubMed] [Google Scholar]
- Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]