Abstract
Introduction
Physician preference has been previously shown to be an important determinant of prescription patterns, independent of patient-specific factors. We evaluated whether physician preference was important in the decision to select anti-TNF therapy rather than non-biologic disease modifying anti-rheumatic drugs (DMARDS) among rheumatoid arthritis (RA) patients initiating a new RA medication.
Methods
Using data from the Consortium of Rheumatology Researchers of North America (CORRONA), we identified biologic-naïve RA patients initiating either anti-TNF therapy or a DMARD in 2001–2008. Physician preference for use of anti-TNF agents was calculated using data from the preceding calendar year for each physician’s other RA patients. Multivariable logistic regression with generalized estimated equations accounted for clustering of patients within physician practice and evaluated the relationship between physician preference and receipt of anti-TNF therapy, controlling for patient-related factors and disease activity using the Clinical Disease Activity Index (CDAI).
Results
We identified 1,532 RA patients initiating anti-TNF therapy or a DMARD. In models adjusting for tender and swollen joint counts and global disease activity, physician preference for use of anti-TNF therapy was an independent predictor of receipt of these agents. Patients of physicians in the highest and middle tertiles of physician preference had a 2.50 (95% CI 1.76 – 3.56) and 1.70 (1.22 – 2.39) greater likelihood to receive anti-TNF medications, respectively.
Conclusion
Physician preference is an important determinant of patients’ receipt of anti-TNF therapy and may be useful to examine in future studies of RA treatment patterns, costs, and medication safety.
Keywords: rheumatoid arthritis, tumor necrosis factor, disease modifying anti-rheumatic drugs (DMARDs), physician preference, instrumental variable, channeling
Introduction
Physicians have many options when deciding which biologic medications to use for the treatment of rheumatoid arthritis (RA) and other inflammatory diseases. Therapies that target tumor necrosis factor alpha (anti-TNF) were the first among these and have substantially altered the management of RA. Anti-TNF therapies, like other biologics, are predominantly indicated as an add-on therapy for patients with an incomplete response to non-biologic disease modifying anti-rheumatic drugs (DMARDs). Traditional DMARDs also can be used in combination to achieve better disease control. While the need for greater efficacy is likely to be a major factor in the decision to add a new RA medication and choose among them, a number of other considerations are likely to impact this decision including economics, access, patient comfort and physician familiarity.
Physician preference may be another important factor affecting receipt of anti-TNF therapies. Indeed, previous rheumatology-related research focused on use of cyclooxygenase-2 (COX-2) non-steroidal anti-inflammatory drugs (NSAIDs) has suggested that a physician’s preference to use COX-2 NSAIDs, rather than older traditional NSAIDs, is important and independent of disease and patient-specific factors (1, 2). Physician preference for COX-2 NSAIDs has been subsequently used in instrumental variable analyses (3), a method that can potentially control for unmeasured confounding (4). In contrast to biologics, however, COX-2 NSAIDs were considered to have equivalent efficacy to traditional NSAIDs, and their benefits were specific to safety (i.e. reduced incidence of gastrointestinal bleeding, a relatively uncommon event). For that reason, it is unclear whether the importance of physician preference as described for use of COX-2 NSAIDs is similarly important for anti-TNF therapy. Moreover, physician preference to use newer medications has been most well-characterized using large administrative claims databases, which typically lack detailed clinical information. For medications that are prescribed to achieve greater clinical efficacy such as anti-TNF therapy, physician preference may be estimated with less validity in data sources that contain no information regarding disease activity.
To test the hypothesis that physician preference is an important determinant for use of anti-TNF therapy, we used data from RA patients enrolled in the Consortium of Rheumatology Researchers of North America (CORRONA). For individuals who are adding an additional agent to their RA treatment regimen, we assessed patient-specific factors that affect prescribing decisions to add anti-TNF therapy, instead of adding a new non-biologic DMARD. We first used a more restricted set of information and included only patient-related data that might be available within large administrative databases. We then added a more replete set of variables that had information regarding clinically disease activity. We subsequently quantified physician preference for use of anti-TNF therapies and assessed whether the effect of physician preference 1) differed depending on whether information regarding clinical disease activity was available; and 2) varied conditional on RA disease activity.
Methods
Study Database
CORRONA is a longitudinal cohort study of RA and psoriatic arthritis (PsA) patients, with information contributed by more than 250 community (2/3) and academic (1/3) rheumatologists practicing across 35 states in the U.S. The cohort is observational and treatments are not mandated; however, a standard evaluation is performed at each visit which includes measurement of tender and swollen joint counts, assessment of disability as measured by a modified Health Assessment Questionnaire, exposure to various medications, etc. Certain laboratory data are also available. Additional details of the cohort, which began recruitment in 2002 and is ongoing, are reported elsewhere (5). CORRONA is governed by central and local institutional review boards.
Eligible population
To evaluate hypotheses of interest, we used CORRONA data from January, 2001 to June, 2008 and identified RA patients who were biologic naïve and had no concomitant diagnosis of PsA. Among the individuals meeting these two criteria, we evaluated persons who initiated a new non-biologic DMARD or anti-TNF therapy. Patients were censored after the first initiation of anti-TNF therapy or a non-biologic DMARD. Because of the requirement to assign patients to a mutually-exclusive anti-TNF or non-biologic outcome category, observations from patients who initiated both a new anti-TNF agent and non-biologic DMARD at the same time were excluded and censored from further analysis. We expected that prior malignancy would be an exceedingly strong contraindication to anti-TNF use and therefore excluded persons with a history of prior malignancy. In contrast, persons with other relative, arguably weaker, contraindications to anti-TNF use (e.g. mild heart failure, infection) were included in the analysis, and these conditions were controlled for in multivariable models.
Physician prescribing preference for anti-TNF therapy
At the time of every drug initiation, we quantified physician preference for use of anti-TNF therapy in two ways. The first method, described as an ‘initiator proportion’, was the proportion of patients treated by that same doctor in the preceding year who initiated an anti-TNF agent divided by the total number of initiators. The second method, described as a ‘prevalence proportion’, represents the proportion of RA patients with no history of cancer treated by that same doctor who were receiving anti-TNF therapy, as assessed on the previous calendar day. In computing both of these proportions, we did not require patients to be biologic naïve (although in sensitivity analyses, we did require this and results were similar). Persons receiving biologic agents other than anti-TNF agents were excluded from the numerator and the denominator of both proportions. Because the initiator and prevalence proportions were re-calculated at the time of each initiation, this method allowed for changes in physician prescribing behavior over the course of the study.
Statistical analysis
Among eligible persons initiating a new DMARD or anti-TNF medication, we evaluated factors associated with anti-TNF therapy using multivariable logistic regression. Generalized estimating equations (GEE) were used to account for the clustering of patients within doctor practice. In our first model, we used only demographic, comorbidity, calendar year of drug initiation, and health services utilization (e.g. physician visits with the rheumatologist, hospitalizations) data to mimic factors commonly available in administrative claims data (the ‘administrative’ model). All variables of clinical interest were included, although only variables that were significant at p < 0.15 were shown for brevity. Model discrimination was assessed using a c-statistic, which is equivalent to the area under a receiver operator curve. We then added in clinical information (e.g. tender/swollen joint counts, Health Assessment Questionnaire, patient-reported global disease activity, patient-reported pain) to re-assess the factors associated with anti-TNF use and evaluated the change in the c statistic for this model (the ‘administrative + clinical’ model).
Missing data were handled by casewise deletion, and all results presented therefore are from complete cases. As part of sensitivity analyses, we did evaluate some additional factors (e.g. education) potentially associated with the outcome. However, since inclusions of these factors did not meaningfully change our main results but did reduce the sample size by 5–15%, these factors were not included in the final models.
To evaluate our physician initiator and prevalence proportions, we used Spearman correlation coefficients to compare them to one another. The two proportions then were each grouped into tertiles to reflect the relative preference of physicians to initiate and prescribe anti-TNF agents to their patients. Multivariable logistic regression was used as described above to adjust for patient-related variables. We stratified our physician preference results by whether subjects were naïve to or were current/past users of methotrexate.
Finally, in order to assess whether the strength of physician prescribing varied by RA disease activity, we stratified the population into low, moderate, and high disease activity using the clinical disease activity index (CDAI) (6). In 3 separate models, we assessed whether the magnitude of the odds ratios associated with each of the two physician prescribing preference variables was different across CDAI categories.
Results
Among biologic naïve RA patients, we identified 1532 initiations of either anti-TNF therapy (n = 717) or non-biologic DMARD therapy (n = 815) during the study period. Using a restricted set of demographic and comorbidity variables (as might be available in administrative claims data), results from the ‘administrative’ model in Table 1 shows the factors independently associated with receipt of anti-TNF therapy. Older age and several comorbidities were associated with a lower likelihood of receipt of anti-TNF therapy. Use of methotrexate, number of prior non-biologic DMARDs, and higher prednisone dose was associated with an increased likelihood to receive anti-TNF therapy. After adding information regarding physician and patient assessment of symptoms and disease activity (e.g. tender/swollen joint count), the results from the ‘administrative + clinical’ model showed that several clinical factors were independently significant. Moreover, most of the factors that were significant in the administrative model remained significant after adjusting for disease activity, and they had similar odds ratios. The c statistic improved from 0.72 for the administrative model to 0.77 for the administrative + clinical model, indicating good discrimination.
Table 1.
Factors associated* with Receipt of Anti-TNF Therapy among Biologic-Naïve RA Patients Initiating Either an Anti-TNF Agent or a Non-Biologic DMARD (n = 1532 total)
| ‘Administrative’ Model | ‘Administrative + Clinical’ Model | |
|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| Demographics | ||
| Age, per 10 years | 0.90 (0.81 – 0.99) | 0.91 (0.83 – 1.01) |
| Comorbidities** | ||
| Coronary Artery Disease | 0.41 (0.19 – 0.85) | 0.37 (0.17 – 0.81) |
| Prior Fracture | 0.73 (0.51 – 1.04) | 0.69 (0.48 – 0.97) |
| Sum of the number of comorbidities | 1.04 (1.02 – 1.06) | 1.02 (1.00 – 1.04) |
| Health Services Utilization | ||
| Number of visits in the past year | NS | 1.14 (1.00 – 1.29) |
| Medications | ||
| Prior/current use of methotrexate | 4.18 (2.81 – 6.20) | 4.66 (3.06 – 7.09) |
| Number of prior non-biologic DMARDs | 1.28 (1.10 – 1.49) | 1.34 (1.12 – 1.60) |
| Number of non-RA medications used | 0.86 (0.76 – 0.97) | 0.86 (0.76 – 0.96) |
| Prednisone dose, mg/day | 1.08 (1.02 – 1.15) | 1.06 (1.00 – 1.14) |
| Insurance | ||
| Private (Commercial) | 1.0 (referent) | 1.0 (referent) |
| Medicare | 0.65 (0.39 – 1.11) | 0.49 (0.28 – 0.85) |
| Medicaid | 0.68 (0.49 – 0.96) | 0.64 (0.46 – 0.88) |
| None (self-pay) | 0.73 (0.50 – 1.08) | 0.57 (0.39 – 0.84) |
| Clinical Factors | ||
| Tender joints (out of 28) | 1.02 (0.99 – 1.05) | |
| Swollen joints (out of 28) | - | 1.02 (1.00 – 1.04) |
| Subcutaneous nodules | 1.32 (0.96 – 1.83) | |
| Physicians global assessment of disease activity (per 10 mm change on 100mm VAS) | 1.19 (1.08 – 1.31) | |
| Patient pain (per 10mm on 100mm VAS) | 1.07 (1.02 – 1.13) | |
| Smoking | 1.48 (1.09 – 2.01) | |
| C statistic | 0.72 | 0.77 |
CI = Confidence Interval; NS = not significant at p < 0.15; VAS = Visual Analog Scale
The ‘Administrative’ model includes only factors commonly available in administrative claims data; the ‘Administrative + Clinical’ model includes these factors and also clinical factors. For the administrative model, the covariates included race/ethnicity, additional comorbidities (diabetes, hypertension, heart failure, prior myocardial infarction, liver disease, elevated creatinine, prior joint surgery, prior infection), prior hospitalization, and calendar year. For the administrative + clinical model, the additional variables included were erosive disease (on x-rays), American Rheumatoid Association (ARA) functional class, history of joint deformity, duration of RA (in years), serum rheumatoid factor status, and the modified Health Assessment Questionnaire (mHAQ) disability index.
Only parameter estimates for factors associated with the outcome at p < 0.15 are shown for brevity and to reduce collinearity; however, the c statistic reflects discrimination for the model with all a-priori specified variables.
see footnote above for additional comorbidities that were adjusted for
The characteristics of the 94 CORRONA physicians who had at least 1 eligible RA patient who contributed to the analysis were as follows: mean +− SD age 52 +− 8 years, 74% male, 75% private practice (vs. academic). Forty-one percent (41%) of these physicians self-reported that formulary restrictions for RA medications were common in their practice environment. After adjustment for the factors shown in Table 1, none of these physician variables were significantly associated with receipt of anti-TNF therapy. We then calculated the physician initiator and prevalence proportions for each physician. The median (Interquartile Range) number of patients per physician used to calculate the initiator and prevalence proportion was 38 (15, 68) and 119 (62, 285), respectively. These two proportions were significantly correlated (r = 0.44, p < 0.0001). As shown in Table 2, both the physician initiator and prevalence proportion were significant factors in patient’s receipt of anti-TNF therapy, regardless of whether information regarding disease activity was available. The magnitude of the odds ratios of the physician preference variables were numerically greater among methotrexate-naïve patients compared to methotrexate-exposed patients. The models’ discrimination increased slightly (approximately 0.02 unit change in the c statistic) after the addition of either of the physician preference variables.
Table 2.
Adjusted Relationship Between Physician Initiator and Prevalence Proportions and the Initiation of Anti-TNF Therapy (vs. non-biologic DMARDs), n = 1532 patients
| Without Disease Activity Variables* Odds Ratio (95% CI) | With Disease Activity Variables** Odds Ratio (95% CI) | |
|---|---|---|
| Methotrexate Naïve | ||
| Physician Prevalence Proportion*** | ||
| Lowest tertile | Referent | Referent |
| Middle tertile | 2.26 (1.10 – 4.62) | 2.44 (1.11 – 5.32) |
| Highest tertile | 5.00 (2.36 – 10.59) | 4.89 (2.10 – 11.39) |
| Physician Initiator Proportion*** | ||
| Lowest tertile | Referent | Referent |
| Middle tertile | 1.32 (0.62 – 2.80) | 1.62 (0.71 – 3.68) |
| Highest tertile | 2.76 (1.34 – 5.67) | 3.18 (1.43 – 7.10) |
| Methotrexate exposed (past or current) | ||
| Physician Prevalence Proportion*** | ||
| Lowest tertile | Referent | Referent |
| Middle tertile | 1.65 (1.21 – 2.26) | 1.70 (1.22 – 2.39) |
| Highest tertile | 2.16 (1.58 – 2.96) | 2.50 (1.76 – 3.56) |
| Physician Initiator Proportion*** | ||
| Lowest tertile | Referent | Referent |
| Middle tertile | 1.26 (0.92 – 1.71) | 1.14 (0.82 – 1.60) |
| Highest tertile | 1.71 (1.26 – 2.32) | 1.61 (1.16 – 2.23) |
adjusted for factors in the ‘administrative’ model described in Table 1
adjusted for factors in the ‘administrative + clinical’ model described in Table 1
the physician initiator and prevalence proportions quantify the preference for use of anti-TNF agents using data from the anti-TNF use patterns of other patients in each physician’s practice. A more complete explanation is provided in the text.
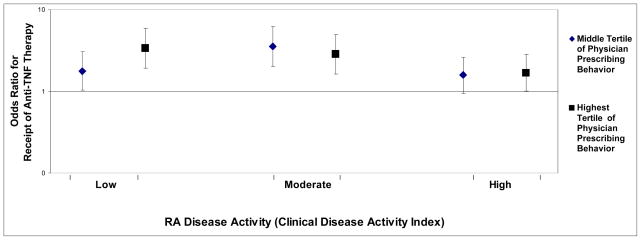
Figure 1 shows the magnitude of the physician prevalence proportion, stratified by disease activity (CDAI). Trends in the magnitude of the odds ratios suggested that the relative effect of physician preference to use anti-TNF agents among physicians in the highest preference tertile was strongest for patients with low or moderate RA disease activity. Among patients of physicians in the middle tertile of physician preference, individuals with moderate RA disease activity seemed to be most affected by physician preference, although confidence intervals were overlapping. The corresponding odds ratios for the physician initiator proportion were similar albeit somewhat smaller in magnitude (data not shown).
Figure 1.
Adjusted* Relationship Between Physician Preference For Use of Anti-TNF Therapy (calculated using data from his/her other RA patients) and the Initiation of Anti-TNF Therapy (vs. non-biologic DMARDs) for the next RA patient, by Disease Activity**
Note: the lowest tertile of physician preference for anti-TNF therapy is referent and corresponds to an Odds Ratio of 1.0
* adjusted for factors in the ‘administrative’ model from Table 1
** RA disease activity was quantified using the Clinical Disease Activity Index (CDAI) and categorized as low (CDAI <= 10), moderate (CDAI > 10 to <= 22), or high (> 22)
Discussion
In a large observational cohort of biologic-naïve RA patients in the U.S., we found that physician preference was a significant determinant of receipt of anti-TNF therapy that was independent of demographic and other clinical factors. Moreover, physician preference was shown to be important regardless of whether information regarding clinical disease activity was available or not. In an analysis where we did use complete information regarding disease activity, physician preference seemed to have a greater influence on treatment decisions for patients at the lower end of the disease activity spectrum. This might be expected since for patients with high disease activity, most physicians would use anti-TNF agents, whereas for those with moderate or low disease activity, physician discretion and prescribing preferences might be expected to be more important factors. Similarly, physician preference was numerically greater among methotrexate-naïve patients than methotrexate-exposed patients. It may be, for example, that this observation is applicable to persons with some relative contraindication to methotrexate (e.g. mild regular alcohol use). Some physicians will choose an anti-TNF agent, whereas other physicians will select a non-biologic DMARD.
If physician preference strongly and independently motivates choice of one treatment compared to another, then the effectiveness and safety of those two treatments can be more validly compared to one another because physician preference may introduce an element of ‘randomness’ into the treatment choice. It implies that for the exact same type of patient, some physicians will prefer treatment A, and other physicians will prefer treatment B. Under this assumption, such ‘randomness’ will therefore reduce (but may not eliminate) concern that results of an observational analysis comparing treatment A to treatment B is subject to residual confounding by disease severity and other patient-related factors that affect outcomes.
Our findings are concordant with data from other studies regarding factors associated with receipt of anti-TNF therapy. A survey of 204 rheumatologists practicing in the US showed that rheumatologists responding to the survey were more likely to prefer aggressive DMARD treatment for younger vs older RA patients (87 vs 71%, P=0.007) (7). A separate survey conducted in 2005 queried U.S. physicians listed in the American College of Rheumatology directory concerning their use of anti-TNF medications for RA patients. These physicians self-reported that the primary determinants of TNF inhibitor use were physician preference (48%), patient preference (20%), and insurance/payor guidelines (21%), in additional to clinical disease activity and formulary and insurance restrictions (8). Our work extends these observations by reporting on actual physician prescribing behavior rather than rheumatologists’ self-reported response to hypothetical prescribing scenarios. Additionally, and as we found, insurance has been shown in previous studies to be a significant factor in selecting patients to receive anti-TNF medications, even after controlling for clinical factors (9).
Our work is consistent with prior literature showing that physician preference was an important determinant of receipt of new Cox-2 NSAIDs and largely independent of patient-related factors (1, 2). Cox-2 NSAIDs are indicated based upon safety concerns to reduce the incidence of gastrointestinal (GI) bleeding (10, 11), a relatively uncommon event that occurs among RA patients at a rate of less than 2 events per 100 person-years (12–14). Thus, reducing a relatively low risk for GI bleeding by selecting Cox-2 NSAIDs may not factor heavily in rheumatologists’ treatment decisions. In contrast, anti-TNF therapy is indicated based upon the frequent need to achieve greater clinical efficacy to control active disease. Our work supports the independent importance of physician preference in the decision to select anti-TNF agents. Our results also support the validity of quantifying physician preference for anti-TNF therapy even in data sources such as administrative claims data that do not have information regarding disease activity. Physician preference can be quantified in a variety of ways that may be dependent on the amount of data available for each physician and the length of the antecedent observation period over which to ‘observe’ physician prescribing behavior. Some past studies have used the prescription choice for the most recent single patient treated by each physician (15); other studies have quantified the proportion of patients receiving newer versus older medications within each physician’s practice (1, 2), similar to what we have used in the current analysis.
As suggested above, in as much as physician preference is independent of patient-related factors, physician preference for the use of anti-TNF therapy might be useful for instrumental variable (IV) analyses (16). IV analyses have the potential to control for unmeasured confounders and have been increasingly used in pharmacoepidemiology (3, 17). By definition, an instrumental variable must be associated with drug exposure, as we have shown it to be with anti-TNF therapy, but must be unrelated to outcome. Both our physician preference variables might satisfy this latter criterion in as much as they are computed using information from unrelated patients in each physician’s practice. Results from observational analyses are always subject to concerns for unmeasured or residual confounding that cannot be fully controlled even using traditional multivariable adjustment or propensity score methods. IV analyses have the potential to overcome this confounding and yield more valid results (4, 18). However, an IV approach requires making some assumptions that may or may not be testable, and it requires caution in understanding which patient group(s) to which the IV results apply (19).
The strengths of our analysis include use of a large data source that allowed us to analyze the treatment selection for a large number of RA patients with a broad range of disease activity. We also analyzed the prescribing patterns of a large number of U.S. rheumatologists scattered throughout a wide geographic region. Moreover, we had information regarding disease activity that is similar to the type of data collected in RA clinical trials.
Despite the above strengths, we recognize that our results may not be as applicable to healthcare settings or insurance plans in which substantial restrictions exist that govern which patients are allowed to receive new medications. However, in as much as those restrictions are based on high disease activity, our results in Figure 1 show that physician preference is still important. Additionally, we recognize that the effect of physician preference may be different for medications that are newly approved by regulatory agencies compared to those that have been available for a long time. However, we accounted for calendar time in our multivariable models, and the extended time frame over which we conducted our study (2001–2008) may attenuate the concern that physician preference is important only for a certain period after medications become commercially available. We also recognize that there are other factors that enter into the treatment decision, such as patient preference. However, this does not compromise the validity of our results showing the impact of physician preference. We also acknowledge that physicians may not enroll all their eligible patients into CORRONA due to lack of patient consent or other factors, despite CORRONA’s intent to have each physician enroll all their consecutive RA patients. This may or may not affect the generalizability of our results. However, CORRONA physicians are asked to self-report what fraction of their RA patients they have or expect to enroll into CORRONA. The median proportion reported by physicians with eligible patients in this analysis was > 75%, and we therefore believe that these patients are representative of the respective practices from which they were drawn. Finally, rheumatologists have an increasing number of treatment choices available for their RA patients. We recognize that the methods to quantify physician preference for one of many treatment choices will need to grow more sophisticated.
In conclusion, we found that physician preference was independently associated with receipt of anti-TNF therapy. This association persisted irrespective of adjustment for RA disease activity and also after simulating using a data source that did not have information on RA disease activity. Studies that examine RA treatment patterns and associated costs, and studies that evaluate medication safety with causal inference methods such as instrumental variables, may benefit from including information on physician preference.
Acknowledgments
This work was supported by the Doris Duke Charitable Foundation. Some of the investigators receive support from the National Institutes of Health (AR 053351: JRC; AR 053856 LRH; AR 047782 and AG 027066: DHS), the Agency for Healthcare Research and Quality (JRC and DHS), and the Arthritis Foundation (JRC and DHS).
Footnotes
Disclosures: JRC: Consulting: Roche, UCB, CORRONA; Speakers bureau: Proctor & Gamble, Eli Lilly, Roche, Novartis; research grants: Merck, Proctor & Gamble, Eli Lilly, Amgen, Novartis
LC: none
LH: none
PN: none
GR: none
DHS: research grants: Amgen and Abbott
References
- 1.Schneeweiss S, Glynn RJ, Avorn J, Solomon DH. A Medicare database review found that physician preferences increasingly outweighed patient characteristics as determinants of first-time prescriptions for COX-2 inhibitors. J Clin Epidemiol. 2005 Jan;58(1):98–102. doi: 10.1016/j.jclinepi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Schneeweiss S, Glynn RJ, Levin R, Avorn J. Determinants of selective cyclooxygenase-2 inhibitor prescribing: are patient or physician characteristics more important? Am J Med. 2003 Dec 15;115(9):715–20. doi: 10.1016/j.amjmed.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Schneeweiss S, Solomon DH, Wang PS, Rassen J, Brookhart MA. Simultaneous assessment of short-term gastrointestinal benefits and cardiovascular risks of selective cyclooxygenase 2 inhibitors and nonselective nonsteroidal antiinflammatory drugs: an instrumental variable analysis. Arthritis Rheum. 2006 Nov;54(11):3390–8. doi: 10.1002/art.22219. [DOI] [PubMed] [Google Scholar]
- 4.Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000 Aug;29(4):722–9. doi: 10.1093/ije/29.4.722. [DOI] [PubMed] [Google Scholar]
- 5.Kremer JM. The CORRONA database. Autoimmun Rev. 2006 Jan;5(1):46–54. doi: 10.1016/j.autrev.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraenkel L, Rabidou N, Dhar R. Are rheumatologists’ treatment decisions influenced by patients’ age? Rheumatology (Oxford) 2006 Dec;45(12):1555–7. doi: 10.1093/rheumatology/kel144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cush JJ. Biological drug use: US perspectives on indications and monitoring. Annals of the rheumatic diseases. 2005 Nov;64(Suppl 4):iv18–23. doi: 10.1136/ard.2005.042549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeWitt EM, Glick HA, Albert DA, Joffe MM, Wolfe F. Medicare coverage of tumor necrosis factor alpha inhibitors as an influence on physicians’ prescribing behavior. Arch Intern Med. 2006 Jan 9;166(1):57–63. doi: 10.1001/archinte.166.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000 Nov 23;343(21):1520–8. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 11.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. Jama. 2000 Sep 13;284(10):1247–55. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Hawley DJ. The comparative risk and predictors of adverse gastrointestinal events in rheumatoid arthritis and osteoarthritis: a prospective 13 year study of 2131 patients. J Rheumatol. 2000 Jul;27(7):1668–73. [PubMed] [Google Scholar]
- 13.Matteson EL, Yachyshyn V, Yachyshyn J, O’Fallon WM. Trends in hospitalizations for gastrointestinal bleeding among patients with rheumatoid arthritis in Rochester, Minnesota, 1950–1991. J Rheumatol. 1995 Aug;22(8):1471–7. [PubMed] [Google Scholar]
- 14.Steen KS, Lems WF, Aertsen J, Bezemer D, Dijkmans BA. Incidence of clinically manifest ulcers and their complications in patients with rheumatoid arthritis. Ann Rheum Dis. 2001 May;60(5):443–7. doi: 10.1136/ard.60.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brookhart MA, Wang PS, Solomon DH, Schneeweiss S. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology. 2006 May;17(3):268–75. doi: 10.1097/01.ede.0000193606.58671.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brookhart MA, Schneeweiss S. Preference-Based Instrumental Variable Methods for the Estimation of Treatment Effects: Assessing Validity and Interpreting Results. The International Journal of Biostatistics. 2007;3(1) doi: 10.2202/1557-4679.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernan MA, Robins JM. Instruments for Causal Inference: An Epidemiologist’s Dream? Epidemiology. 2006 Jul;17(4):360–72. doi: 10.1097/01.ede.0000222409.00878.37. [DOI] [PubMed] [Google Scholar]
- 18.Angrist J, Imbens G, Rubin D. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;81:444–55. [Google Scholar]
- 19.Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH. Instrumental variables: application and limitations. Epidemiology. 2006 May;17(3):260–7. doi: 10.1097/01.ede.0000215160.88317.cb. [DOI] [PubMed] [Google Scholar]