Abstract
Uncertainty exists regarding accommodative and age changes in lens diameter and thickness in humans and monkeys. In this study, unaccommodated and accommodated refraction, lens diameter, and lens thickness were measured in rhesus monkeys across a range of ages. Iridectomized eyes were studied in 33 anesthetized monkeys aged 4–23 years. Refraction was measured using a Hartinger coincidence refractometer and lens thickness was measured with A-scan ultrasound. Lens diameters were measured with image analysis from slit-lamp images captured via a video camera while a saline filled, plano perfusion lens was placed on the cornea. Accommodation was pharmacologically stimulated with 2% pilocarpine via the perfusion lens in 21 of the monkeys and lens diameters were measured until a stable minimum was achieved. Refraction and lens thickness were measured again after the eye was accommodated. Unaccommodated lens thickness increased linearly with age by 0.029 mm/year while unaccommodated lens diameter showed no systematic change with age. Accommodative amplitude decreased by 0.462 D/year in response to pilocarpine. The accommodative increase in lens thickness decreased with age by 0.022 mm/year. The accommodative decrease in lens diameter declined linearly with age by 0.021 mm/year. Rhesus monkeys undergo the expected presbyopic changes including increasing lens thickness and a decreasing ability of the lens to undergo changes in thickness and diameter with accommodation, however without an age-related change in unaccommodated lens diameter. As in humans, the age-related decrease in accommodative amplitude in rhesus monkeys cannot be attributed to an age-related increase in lens diameter.
Keywords: presbyopia, accommodation, monkey, lens, aging, growth
1. Introduction
Accommodation of the eye is due to a dioptric change in the power of the lens as the focus of the eye changes from far to near. Humans and rhesus monkeys accommodate by a similar mechanism which was first described by von Helmholtz (1855 von Helmholtz (1909). The main elements of the Helmholtz theory of accommodation have been confirmed by measurements in both humans and monkeys (Glasser and Kaufman, 1999; Strenk et al., 1999; Glasser et al., 2006). As the ciliary muscle contracts, the ciliary body moves anteriorly and centripetally to release tension on the zonular fibers (Bacskulin et al., 1995; Glasser and Kaufman, 1999). The release of zonular tension allows the lens capsule to mold the lens into a more spherical shape (Fincham, 1936, 1937; Glasser and Campbell, 1999). The accommodative change in lens shape can be measured by a decrease in equatorial diameter (Storey and Rabie, 1985; Wilson, 1997; Strenk et al., 1999; Glasser et al., 2006) and an increase in axial lens thickness (Storey and Rabie, 1983; Koretz et al., 1987a; Vilupuru and Glasser, 2005; Ostrin et al., 2006; Bolz et al., 2007). The more spherical lens shape results in an increase in the anterior and posterior lens surface curvatures (Dubbelman et al., 2005; Rosales and Marcos, 2006; Rosales et al., 2006) and an increase in the optical power of the lens.
Both humans and monkeys develop presbyopia, the gradual age-related loss of accommodation. In humans, this gradual loss results in a complete inability to accommodate by about 55 years of age (Duane, 1912; Glasser and Campbell, 1998). Similarly, loss of accommodation is complete in rhesus monkeys by about 25 years of age (Bito et al., 1982). In both humans and monkeys, the unaccommodated axial lens thickness continues to increase with age (Koretz et al., 1987b, 2001; Strenk et al., 1999; Dubbelman et al., 2001). However, the unaccommodated lens equatorial diameter does not change in humans after about 22 years of age (Strenk et al., 1999). Although unaccommodated lens thickness as a function of age has previously been measured in monkey eyes, unaccommodated lens diameter has not. Understanding how both of these lens dimensions change together as the lens grows has important implications for understanding the development of presbyopia. It has been suggested that lens growth and the underlying mechanisms of presbyopia development are very different in humans and monkeys (Strenk et al., 2005, 2006). This study was undertaken to measure lens diameter and thickness before and during pharmacologically stimulated accommodation in rhesus monkeys over a wide range of ages to characterize the age changes in the lens that contribute to the progression of presbyopia and to compare the findings with results from human studies.
2. Methods
All experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were performed in accordance with institutionally approved animal protocols. Experiments were performed on the eyes of 33 rhesus monkeys (Macaca mulatta; 21 at the University of Houston (UH) and 12 at the University of Wisconsin-Madison (UW)) aged 4–23 years that had previously undergone total iridectomy. At UW, all monkeys were anesthetized with intramuscular 15 mg/kg ketamine and 30 μg/kg medetomidine (Pfizer Animal Health, Exton, PA) and experiments were performed under this anesthesia. At UH, all monkeys were initially anesthetized with intramuscular 15 mg/kg ketamine and 0.5 mg/kg acepromazine (Phoenix Pharmaceutical, St. Joseph, MO). All experiments at UH were performed under intravenous propofol (PropoFlo, Abbott Laboratories, North Chicago, IL) anesthesia with an initial bolus of 1.5 mg/kg and a continuous infusion at a rate of 0.5 mg/kg per min. Throughout all experiments, pulse rate, SpO2, and temperature were monitored and the monkey was wrapped in a 37 °C water heated pad to maintain body temperature. The monkey lay prone on a table with its head in a head holder, upright and facing forward.
Following induction of anesthesia, lens thickness was first measured using A-scan ultrasound (Sonomed, A-scan 5500; UH and UW) in which the probe was placed directly on the cornea after irrigation with saline. A clear, plano contact lens (MetroOptics) was then placed on the cornea to prevent dehydration and to maintain optical quality. The unaccommodated refraction of the eye was measured through the contact lens using a Hartinger coincidence refractometer (Carl Zeiss Meditec, Jena, Germany; UH and UW). To measure the crystalline lens diameter, the contact lens was removed and the open base of a custom designed plano perfusion lens was placed on the perilimbal sclera and under the eyelids to create a fluid tight seal (Fig. 1A,B). A plano, clear glass cover slip on the other end of the perfusion lens created a 5 ml volume chamber in front of the cornea. That chamber was filled with fluid via a syringe connected to an inlet tube on the perfusion lens. The fluid interface in front of the cornea kept the cornea hydrated and together with the plano surface on the perfusion lens effectively neutralized the corneal power. A slit-lamp microscope (Carl Zeiss Meditec, Jena, Germany, Model 110; UH and UW) with a video camera attached (COHU, model 4912-2000; UH and UW) was used to image the monkey crystalline lens diameter. The slit-lamp light beam was adjusted so that the perimeter of the monkey crystalline lens was clearly illuminated with a ‘halo’ around a darker lens (Glasser et al., 2006) (Fig. 2A,B). In all monkeys, five initial images of the unaccommodated monkey crystalline lens diameter were captured through the perfusion lens via the slit-lamp and video camera to a computer and saved to disk. Images were analyzed to measure the lens diameter using Optimas image analysis software (Media Cybernetics, Bethesda, MD).
Fig. 1.
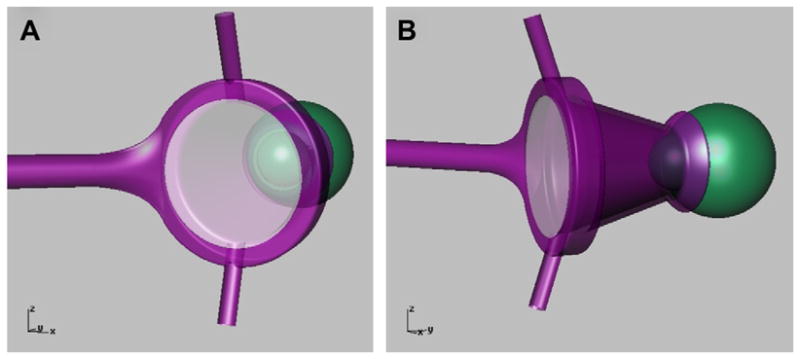
(A,B) Diagram of the perfusion lens on a monkey eye showing the plano, glass cover-slip window creating a chamber that can be filled with fluid via the lower inlet tube.
Fig. 2.
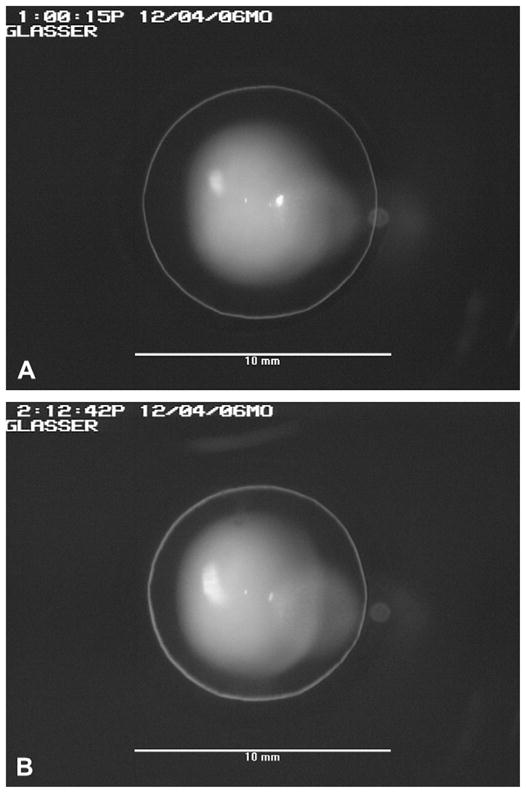
(A) An unaccommodated monkey crystalline lens as viewed through the fluid filled perfusion lens. The slit-lamp illumination was adjusted so the perimeter of the monkey lens was illuminated with a ‘halo’. (B) The accommodated monkey crystalline lens 72 min after the perfusion lens was filled with 2% pilocarpine. Scale bars in (A) and (B) are 10 mm.
Captured images from the UW monkeys were calibrated into millimeters by imaging graph paper inside a fluid filled artificial eye. This artificial eye was created by cutting off the lure loc tip of a 10 cc syringe and adhering a monkey contact lens over the hole. The graph paper was glued on the end of the syringe plunger, facing the contact lens and could be positioned within the syringe at various distances from the contact lens to simulate various anterior chamber depths approximating the range recorded for the monkey eyes. In this way, the graph paper was positioned in the artificial eye at approximately the same plane as the edge of the monkey lens would be in the living monkey eye. The graph paper in the artificial eye was imaged through the plano perfusion lens placed on the artificial eye in the same way as the monkey lens perimeter was imaged. Lens diameters from the UW monkey eyes were calibrated to millimeters with a single standard calibration factor calculated from an in focus image of graph paper in the artificial eye taken at the same slit-lamp magnification as the monkey lens. In the UH experiments, the monkey lens diameter images were calibrated by measuring the inner diameter of the edge of the perfusion lens resting against the limbus of the eye. This edge was rendered visible by minor adjustments to the slit-lamp illumination in the same image and at approximately the same plane of focus as the monkey lens. This same calibration could not be used with the UW images due to slightly different illumination conditions. As a result of these different calibration methods as well as slight differences in equipment, the unaccommodated lens diameter results are represented by different symbols when the UH and UW experiments appear on the same graphs.
Lens diameter was measured in all 33 monkeys in the anesthetized, unaccommodated state. For these measurements, the perfusion lens was filled with balanced salt solution (BSS), pH 7.4 (Alcon, Ft Worth, TX). In addition, the following experiments were performed on all 21 of the UH monkeys. Accommodation was stimulated pharmacologically by replacing the BSS in the perfusion lens with 2% pilocarpine (Sigma-Aldrich, St. Louis, MO) in 0.1% citrate–borate (J.T. Baker, Phillipsburg, NJ; EMD Chemicals, San Diego, CA) solution, pH 7.4 via the inlet and outlet tubes. 2% pilocarpine in citrate–borate buffer was used to ensure the cornea remained clear for the duration of the experiments (Croft et al., 1991). Accommodative changes in lens diameter were measured on-line, automatically once every 10 s using a custom written Optimas macro. Lens diameter measurements were terminated when no further decrease in the lens diameter was observed in the on-line recordings. Refraction and biometry were measured again, as before, after the eye was fully accommodated.
A possible source of error in determining the absolute diameter of the monkey lens is the position and therefore the focus of the slit-lamp microscope. A control experiment was performed to measure the variance due to the slit-lamp position and focus on the monkey lens diameter. The slit-lamp was placed on steel rod rollers and the position of the slit-lamp from the monkey eye (and hence the focus) was controlled by a linear stage with a spindle micrometer. Two different tests were performed on one monkey. In the first test, the distance of the slit-lamp to the eye was adjusted sequentially in 11 positions and an image was captured and lens diameter measured at each distance. In the second test, the lens diameter image was defocused and then refocused 11 times by visual inspection by manual adjustment of the position of the slit-lamp. An image of the monkey lens was captured to disk for each new focus and the images were analyzed to measure the mean lens diameter and to determine the standard deviation of these measurements.
3. Results
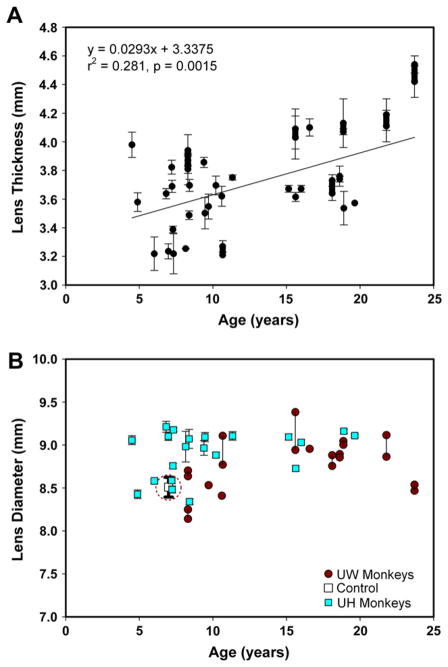
Unaccommodated lens thickness as measured in all 33 UH and UW monkeys increased linearly with age by 0.0293 mm/year (r2 = 0.281, SE = 0.0084, p = 0.0015). In several of the UW monkeys, measurements were made in both eyes, but only the average from the two eyes was used in the regression fit. However, the values from each eye are shown on the graph (Fig. 3A). Unaccommodated lens diameters showed no significant change with age for all monkeys (r2 = 0.049, SE = 0.0093, p = 0.2152). Separately, no significant change was observed for the UH monkeys (r2 = 0.126, SE = 0.0131, p = 0.1139) or for the UW monkeys (r2 = 0.206, SE = 0.0153, p = 0.1384). Measurements from both eyes for several of the UW monkeys are linked with vertical lines on the graph (Fig. 3B). The data obtained from the second control experiment show the mean and standard deviation attributable to image focus and defocus for a single monkey lens (circled symbol with error bar in Fig. 3B). The standard deviation from these 11 repeated measures (0.23 mm) is an indication of the level of precision of the measurements and represents approximately 18.5% of the overall range of lens diameters measured (8.14–9.38 mm).
Fig. 3.
(A) Unaccommodated lens thickness increases with increasing age by 0.029 mm/year (r2 = 0.281, SE = 0.0084, p = 0.0015). Measurements were taken from both eyes of several UW monkeys. The average for each monkey was used to calculate the linear regression reported here, so n = 33. The result from each pair of eyes is shown connected by a thick line. Results are from 42 eyes in 33 monkeys. (B) Unaccommodated lens diameter shows no systematic change with age. Vertical lines connect data from both eyes of the same monkey in several of the UW monkeys. The thicker error bar in this graph (circled) indicates the range of lens diameters measured in the control experiment. Results are from 42 eyes in 33 monkeys.
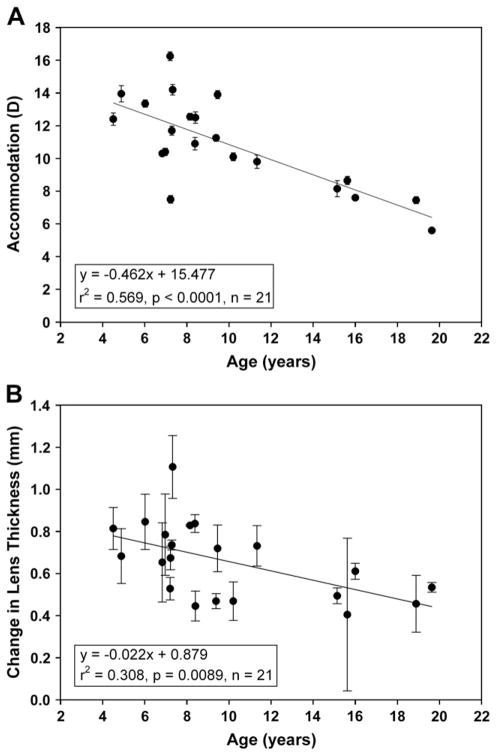
Lens diameters in all 21 UH monkeys were measured before, during and after pilocarpine-stimulated accommodation. The lens diameter measurements for the pilocarpine experiment were calibrated from the internal edge of the perfusion lens on the conjunctiva visible in each image; therefore this image calibration is self-consistent and certain within this experiment. The myopic shift in refraction in response to pilocarpine decreased in an age-dependent manner by 0.462 D/year (r2 = 0.569, SE = 0.0923, p < 0.0001) (Fig. 4A). Lens thickness increased with accommodation with the increase ranging from 0.405 to 1.107 mm. The accommodative increase in lens thickness decreased with age by 0.022 mm/year (r2 = 0.309, SE = 0.0076, p = 0.009) (Fig. 4B).
Fig. 4.
(A) Pilocarpine-stimulated accommodative amplitude decreased by 0.462 D/year (r2 = 0.569, SE = 0.0923, p < 0.0001). (B) The accommodative change in lens thickness declined linearly with age by 0.022 mm/year (r2 = 0.309, SE = 0.0076, p = 0.009).
In all cases, pilocarpine caused a systematic decrease in lens diameter (Fig. 5 and 6A) and the lens diameter reached a stable minimum in all experiments. With the persistent presence of 5 ml of 2% pilocarpine solution in the perfusion lens on the cornea, this suggests that the eyes all achieved a maximum decrease in lens diameter. The time taken for lens diameter to reach a minimum varied from 30 to 70 min. Representative responses from three pilocarpine stimulated monkeys and one control experiment with only BSS and no pilocarpine are shown in Fig. 5. The youngest monkey (age 8) had the greatest decrease and the oldest monkey (age 19) had the smallest decrease in lens diameter. The UH monkeys showed no change in unaccommodated lens diameter with age but accommodated lens diameter increased with age (Fig. 6A). The range of the accommodative decrease in lens diameter was 0.379–0.966 mm. The accommodative decrease in lens diameter declined linearly with age by 0.021 mm/year (r2 = 0.361, SE = 0.0064, p = 0.004) (Fig. 6B).
Fig. 5.
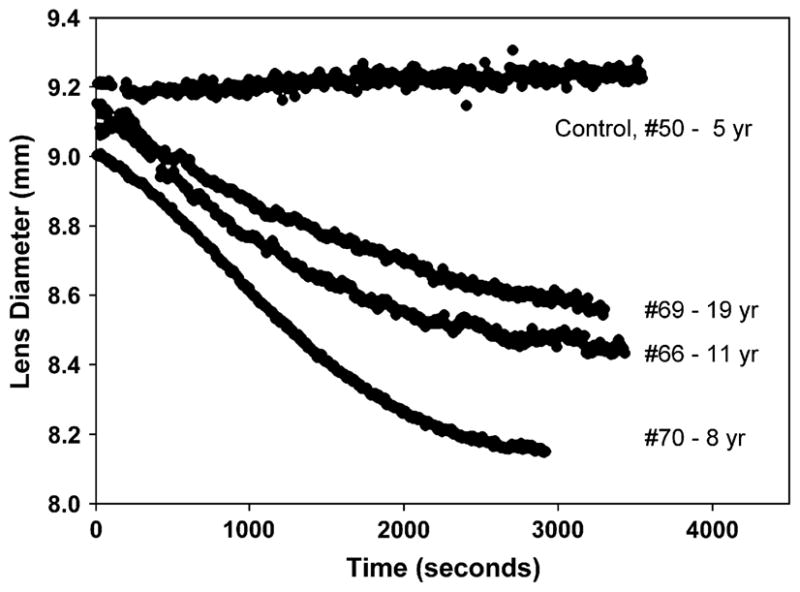
Three sample time courses of the accommodative decrease in lens diameter after 2% pilocarpine topical infusion are shown, along with a BSS control experiment in the absence of pilocarpine. The ages of monkeys #50, #70, #66, and #69 were 5, 8, 11, and 19 years respectively.
Fig. 6.
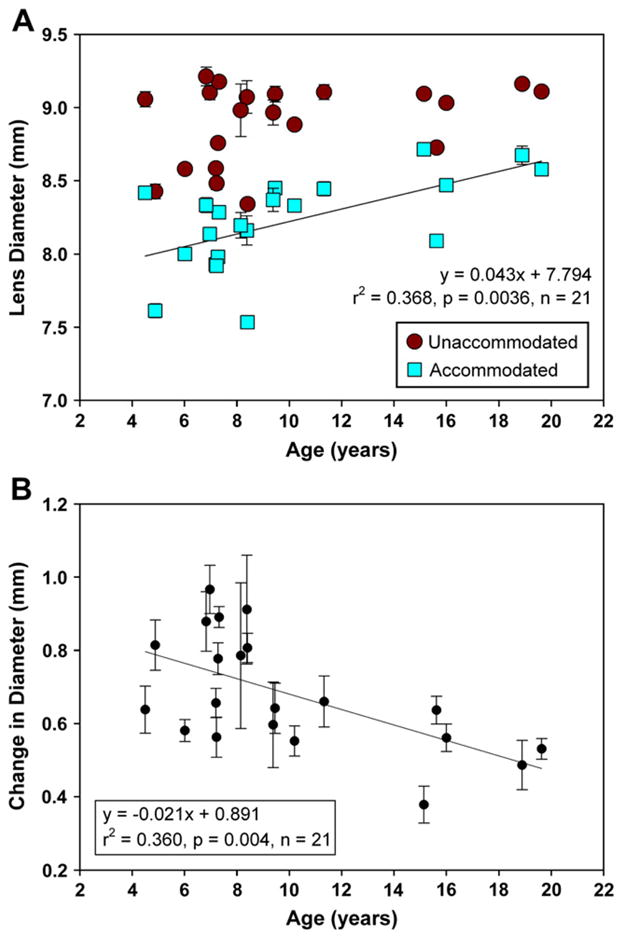
(A) In the UH monkeys, unaccommodated lens diameter shows no significant trend with age (p = 0.1139) while accommodated lens diameters increase with age (r2 = 0.368, SE = 0.0129, p = 0.0036). (B) Accommodative decrease in lens diameter declined linearly with age by 0.021 mm/year (r2 = 0.361, SE = 0.0064, p = 0.004).
4. Discussion
Under propofol anesthesia, the eyes are in an unaccommodated state. In a previous study using the same anesthesia, atropine cycloplegia resulted in a non-significant hyperopic shift in refraction in the eyes of iridectomized monkeys (Ostrin and Glasser, 2007b). This is indicative of an unaccommodated state analogous to the unaccommodated non-cycloplegic refractive state in conscious human subjects. The unaccommodated state under propofol anesthesia is similar to that found with other anesthesia regimes in monkeys (Westheimer and Blair, 1973; Crawford et al., 1990a). Pilocarpine stimulated accommodative decrease in lens diameter was measured until a stable minimum occurred in each of the UH monkeys. As shown previously, lens diameter decreases linearly with the accommodative change in refraction (Glasser et al., 2006), therefore it was assumed that maximal accommodation was achieved in each eye.
The results show a systematic decrease in lens diameter with accommodation. This is similar to that reported previously in rhesus monkeys using central brain stimulation (Glasser and Kaufman, 1999; Glasser et al., 2006) and from several studies in human subjects (Grossmann, 1903, 1904; Storey and Rabie, 1985; Wilson, 1997; Strenk et al., 1999). Lens diameter decreased by as much as 800 μm with pilocarpine stimulation in the 5 year old monkeys, which is more than the approximately 600 μm reported with Edinger–Westphal stimulation in 5–6 year old monkeys. Prior studies show that supramaximal drug stimulated accommodation produces a greater accommodative response than Edinger–Westphal stimulated accommodation (Crawford et al., 1990b; Vilupuru and Glasser, 2002; Ostrin and Glasser, 2005, 2007a). As shown previously (Glasser et al., 2006), the accommodative decrease in lens diameter in rhesus monkeys is more than 5 times greater and opposite in direction to the 50–60 μm increase in lens diameter that Schachar has suggested occurs during accommodation (Schachar et al., 1994, 1995, 1996; Schachar, 1994, 2004). The prior Edinger–Westphal stimulation studies showing an accommodative decrease in lens diameter have been criticized as being confounded by convergent eye movements that accompany the Edinger–Westphal stimulation (Schachar and Kamangar, 2006). Eye movements may occur with pharmacologically stimulated accommodation, but they are not systematic convergent eye movements, nor are they a consequence of the pharmacological stimulation, but rather they are non-systematic and random in nature. Yet, as reported previously (Glasser et al., 2006), lens diameter decreased systematically with accommodation in every case. The presence of eye movements may result in increased variance (compare the data from monkeys #66 and #70 in Fig. 5), but the extent of the noise introduced by eye movements is extremely small relative to the extent of the accommodative decrease in lens diameter observed.
The age range of monkeys used in this study covers approximately 60% of the rhesus lifespan in captivity. Our results show that in rhesus monkeys of this age range, in accordance with prior studies, accommodative amplitude decreases linearly with age (Bito et al., 1982; Neider et al., 1990; Croft et al., 2006) and lens thickness in the unaccommodated eye increases linearly with age (Koretz et al., 1987b). In addition, accommodative changes in lens thickness and diameter show a reduced change with increasing age. These are all clear and distinct indicators of the involvement of the lens in the progression of presbyopia in rhesus monkeys. However, lens diameter in the unaccommodated eye does not show an age-related change. It is well established that the primate lens continues to grow throughout life (Scammon and Hesdorffer, 1937; Rafferty, 1985; Glasser and Campbell, 1999). This is reflected in this study by an increase in lens thickness, but this lens growth is not accompanied by a systematic increase in unaccommodated equatorial diameter. These results are remarkably similar to those seen in human studies (Strenk et al., 1999). In rhesus monkeys, as in humans, there is a systematic decrease in accommodative amplitude with increasing age; accommodative change in lens thickness and diameter decrease with increasing age; in the unaccommodated eye, lens thickness increases but without a change in lens diameter with increasing age.
The age-related growth of the postnatal lens is due to proliferation of lens epithelial cells at the germinative zone just anterior to the lens equator. These epithelial cells begin to differentiate to form lens fiber cells as they migrate posteriorly towards the lens equator (Rafferty, 1985). If fiber cells are progressively laid down at the lens equator, it may appear surprising that lens diameter does not increase with increasing age. However, lens diameter does in fact increase with increasing age—in the isolated lens (Smith, 1883; Rafferty, 1985; Rosen et al., 2006). When the zonular fibers are cut, young lenses become maximally accommodated and undergo a decrease in diameter due to the elastic capsule (Fincham, 1936, 1937; Glasser and Campbell, 1999; Rosen et al., 2006). This is a graded response with the greatest change occurring in young, soft, accommodating lenses and no change occurring in older, stiff, presbyopic lenses, thus resulting in an age-related increase in isolated lens diameter (Smith, 1883; Glasser and Campbell, 1999; Rosen et al., 2006). Dimensions of isolated lenses bear a striking resemblance to dimensions of maximally accommodated lenses (Burd et al., 2002; Dubbelman et al., 2005). As shown in this study, accommodated lens diameter increases with age in these monkeys (Fig. 6A) as it does in vivo in humans (Strenk et al., 1999). The lens is maintained in an unaccommodated state by the ciliary muscle, zonular fibers and lens capsule to maintain resting refraction of the eye, thus dictating unaccommodated lens diameter. The data suggests that there may be an age-related distribution (or redistribution) of lens cells in such a way as to cause an age-related increase in unaccommodated lens thickness but not equatorial diameter. However, to understand lens growth, the accommodated lens conformation may be the most relevant to consider. This rationale can explain the fact that lens growth occurs at the periphery of the lens, yet the unaccommodated lens diameter does not change with increasing age.
The absence of an age-related change in unaccommodated lens diameter is unlikely to be due to an inability of the video-based system to detect a change. The resolution of this video-based system, which was readily able to resolve accommodative changes in lens diameter and age-related changes in accommodated lens diameter, is 31 μm/pixel as determined from imaging the fluid-filled artificial eye. By way of comparison, the age-related change in lens thickness was measured with A-scan ultrasound that has a lower resolution of 40 μm. MRI, which has been used to show age-related changes in lens thickness and accommodative changes in lens diameter in humans, has a still lower resolution of 156 μm/pixel (Strenk et al., 1999). Therefore, it is likely that if unaccommodated lens diameter changed with age, this would have been detected. This did not occur in either of the two populations of monkeys, either independently or considered together.
Although the other age-related findings reported here have previously been documented in rhesus monkeys, those that pertain to lens diameter have not. To construct a clear and complete picture of the role of the lens in the progression of presbyopia, it is necessary to document all these parameters together in the same group of monkeys. From this it can be concluded that although the primate lens grows throughout the age range studied, with an increase in mass and thickness, there is no systematic, growth-related change in unaccommodated lens diameter with increasing age over this age range. As a result, the age-related decrease in accommodation cannot be due to an age-related increase in unaccommodated lens diameter as has been suggested (Schachar, 1992, 2000; Schachar et al., 1995).
Acknowledgments
This study was funded in part by NEI grant RO1 EY014651 to AG; NEI grant P30 EY007551 to UHCO; R01 EY10213, RPB, OPREF, Walter H. Hemerich Chair from RRF to PLK; P30EY016665 NEI-NIH Core Grant for Vision Research to UWDoVS. We also acknowledge the Wisconsin National Primate Research Center, University of Wisconsin-Madison base grant # 5P51 RR 000167. Thanks to Chris Kuether for technical assistance.
Contributor Information
Mark Wendt, Email: mwendt@uh.edu.
Mary Ann Croft, Email: macroft@wisc.edu.
Jared McDonald, Email: jpmcdonald@wisc.edu.
Paul L. Kaufman, Email: kaufmanp@mhub.ophth.wisc.edu.
Adrian Glasser, Email: aglasser@uh.edu.
References
- Bacskulin A, Bergmann U, Horoczi Z, Guthoff R. Continuous ultrasound biomicroscopic imaging of accommodative changes in the human ciliary body. Klin Monatsbl Augenheilkd. 1995;207:247–252. doi: 10.1055/s-2008-1035376. [DOI] [PubMed] [Google Scholar]
- Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- Bolz M, Prinz A, Drexler W, Findl O. Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. Br J Ophthalmol. 2007;91:360–365. doi: 10.1136/bjo.2006.099879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd H, Judge S, Cross J. Numerical modelling of the accommodating lens. Vision Res. 2002;42:2235–2251. doi: 10.1016/s0042-6989(02)00094-9. [DOI] [PubMed] [Google Scholar]
- Crawford K, Gabelt BT, Kaufman PL, Bito LZ. Effects of various anesthetic and autonomic drugs on refraction in monkeys. Curr Eye Res. 1990a;9:525–532. doi: 10.3109/02713689008999592. [DOI] [PubMed] [Google Scholar]
- Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of rhesus monkeys. Invest Ophthalmol Vis Sci. 1990b;31:2185–2190. [PubMed] [Google Scholar]
- Croft MA, Kaufman PL, Erickson-Lamy K, Polansky JR. Accommodation and ciliary muscle muscarinic receptors after echothiophate. Invest Ophthalmol Vis Sci. 1991;32:3288–3297. [PubMed] [Google Scholar]
- Croft MA, Glasser A, Heatley G, McDonald J, Ebbert T, Dahl DB, Nadkarni NV, Kaufman PL. Accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci. 2006;47:1076–1086. doi: 10.1167/iovs.04-1523. [DOI] [PubMed] [Google Scholar]
- Duane A. Normal values of the accommodation at all ages. J Am Med Assoc. 1912;59:1010–1013. [Google Scholar]
- Dubbelman M, van der Heijde GL, Weeber HA. The thickness of the aging human lens obtained from corrected Scheimpflug images. Optom Vis Sci. 2001;78:411–416. doi: 10.1097/00006324-200106000-00013. [DOI] [PubMed] [Google Scholar]
- Dubbelman M, van der Heijde GL, Weeber HA. Change in shape of the aging human crystalline lens with accommodation. Vision Res. 2005;45:117–132. doi: 10.1016/j.visres.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Fincham EF. An experiment on the influence of tension upon the form of the crystalline lens. Trans Opthalmol Soc UK. 1936;56:138–147. [Google Scholar]
- Fincham EF. The mechanism of accommodation. Br J Ophthalmol Mono. 1937;VIII:7–80. [Google Scholar]
- Glasser A, Campbell MCW. Presbyopia and the optical changes in the human crystalline lens with age. Vision Res. 1998;38:209–229. doi: 10.1016/s0042-6989(97)00102-8. [DOI] [PubMed] [Google Scholar]
- Glasser A, Campbell MCW. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999;39:1991–2015. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
- Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- Glasser A, Wendt M, Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Invest Ophthalmol Vis Sci. 2006;47:278–286. doi: 10.1167/iovs.05-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann K. The mechanism of accommodation in man. Br Med J. 1903;2:726–731. [Google Scholar]
- Grossmann K. The mechanism of accommodation in man. Ophthal Rev. 1904;23:1–19. [Google Scholar]
- Koretz JF, Bertasso AM, Neider MW, True-Gabelt B, Kaufman PL. Slit-lamp studies of the rhesus monkey eye. II Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res. 1987a;45:317–326. doi: 10.1016/s0014-4835(87)80153-7. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Neider MW, Kaufman PL, Bertasso AM, DeRousseau CJ, Bito LZ. Slit-lamp studies of the rhesus monkey eye. I Survey of the anterior segment. Exp Eye Res. 1987b;44:307–318. doi: 10.1016/s0014-4835(87)80014-3. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Cook CA, Kaufman PL. Aging of the human lens: changes in lens shape at zero-diopter accommodation. J Opt Soc Am A Opt Image Sci Vis. 2001;18:265–272. doi: 10.1364/josaa.18.000265. [DOI] [PubMed] [Google Scholar]
- Neider MW, Crawford K, Kaufman PL, Bito LZ. In vivo videography of the rhesus monkey accommodative apparatus: age-related loss of ciliary muscle response to central stimulation. Arch Ophthalmol. 1990;108:69–74. doi: 10.1001/archopht.1990.01070030075032. [DOI] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Comparisons between pharmacologically and Edinger–Westphal-stimulated accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2005;46:609–617. doi: 10.1167/iovs.04-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Edinger–Westphal and pharmacologically stimulated accommodative refractive changes and lens and ciliary process movements in rhesus monkeys. Exp Eye Res. 2007a;84:302–313. doi: 10.1016/j.exer.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Effects of pharmacologically manipulated amplitude and starting point on Edinger–Westphal-stimulated accommodative dynamics in rhesus monkeys. Invest Ophthalmol Vis Sci. 2007b;48:313–320. doi: 10.1167/iovs.06-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin L, Kasthurirangan S, Win-Hall D, Glasser A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom Vis Sci. 2006;83:657–665. doi: 10.1097/01.opx.0000232810.61191.02. [DOI] [PubMed] [Google Scholar]
- Rafferty NS. Lens morphology. In: Maisel H, editor. The Ocular Lens: Structure, Function, and Pathology. Marcel Dekker, Inc; New York: 1985. pp. 1–60. [Google Scholar]
- Rosales P, Marcos S. Phakometry and lens tilt and decentration using a custom-developed Purkinje imaging apparatus: validation and measurements. J Opt Soc Am A Opt Image Sci Vis. 2006;23:509–520. doi: 10.1364/josaa.23.000509. [DOI] [PubMed] [Google Scholar]
- Rosales P, Dubbelman M, Marcos S, van der Heijde R. Crystalline lens radii of curvature from Purkinje and Scheimpflug imaging. J Vis. 2006;6:1057–1067. doi: 10.1167/6.10.5. [DOI] [PubMed] [Google Scholar]
- Rosen AM, Denham DB, Fernandez V, Borja D, Ho A, Manns F, Parel JM, Augusteyn RC. In vitro dimensions and curvatures of human lenses. Vision Res. 2006;46:1002–1009. doi: 10.1016/j.visres.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Scammon R, Hesdorffer M. Growth in mass and volume of the human lens in postnatal life. Arch Ophthalmol. 1937;17:104–112. [Google Scholar]
- Schachar RA. Cause and treatment of presbyopia with a method for increasing the amplitude of accommodation. Ann Ophthalmol. 1992;24:445–452. [PubMed] [Google Scholar]
- Schachar RA. Zonular function: a new hypothesis with clinical implications. Arch Ophthalmol. 1994;26:36–38. [PubMed] [Google Scholar]
- Schachar RA. Theoretical basis for the scleral expansion band procedure for surgical reversal of presbyopia (SRP) Ann Ophthalmol. 2000;32:271–278. doi: 10.1007/s12019-001-0006-4. [DOI] [PubMed] [Google Scholar]
- Schachar RA. Qualitative effect of zonular tension on freshly extracted intact human crystalline lenses: implications for the mechanism of accommodation. Invest Ophthalmol Vis Sci. 2004;45:2691–2695. doi: 10.1167/iovs.03-1267. [DOI] [PubMed] [Google Scholar]
- Schachar RA, Kamangar F. Computer image analysis of ultrasound biomicroscopy of primate accommodation. Eye. 2006;20:226–233. doi: 10.1038/sj.eye.6701838. [DOI] [PubMed] [Google Scholar]
- Schachar RA, Cudmore DP, Torti R, Black TD, Huang T. A physical model demonstrating Schachar’s hypothesis of accommodation. Ann Ophthalmol. 1994;26:4–9. [PubMed] [Google Scholar]
- Schachar RA, Black TD, Kash RL, Cudmore DP, Schanzlin DJ. The mechanism of accommodation and presbyopia in the primate. Ann Ophthalmol. 1995;27:58–67. [Google Scholar]
- Schachar RA, Tello C, Cudmore DP, Liebmann JM, Black TD, Ritch R. In vivo increase of the human lens equatorial diameter during accommodation. Am J Physiol. 1996;271:R670–R676. doi: 10.1152/ajpregu.1996.271.3.R670. [DOI] [PubMed] [Google Scholar]
- Smith P. Diseases of the crystalline lens and capsule: on the growth of the crystalline lens. Trans Opthal Soc UK. 1883:79–100. [Google Scholar]
- Storey JK, Rabie EP. Ultrasound - a research tool in the study of accommodation. Ophthalmic Physiol Opt. 1983;3:315–320. [PubMed] [Google Scholar]
- Storey JK, Rabie EP. Ultrasonic measurement of transverse lens diameter during accommodation. Ophthalmic Physiol Opt. 1985;5:145–148. [PubMed] [Google Scholar]
- Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of aging, accommodating, phakic, and pseudophakic ciliary muscle diameters. J Cataract Refract Surg. 2006;32:1792–1798. doi: 10.1016/j.jcrs.2006.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenk SA, Strenk LM, Koretz JF. The mechanism of presbyopia. Prog Retin Eye Res. 2005;24:379–393. doi: 10.1016/j.preteyeres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. Dynamic accommodation in rhesus monkeys. Vision Res. 2002;42:125–141. doi: 10.1016/s0042-6989(01)00260-7. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. The relationship between refractive and biometric changes during Edinger–Westphal stimulated accommodation in rhesus monkeys. Exp Eye Res. 2005;80:349–360. doi: 10.1016/j.exer.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Helmholtz HH. Ueber die Accommodation des Auges. Arch Ophthalmol. 1855;1:1–74. [Google Scholar]
- vonHelmholtz HH. Mechanism of accommodation. In: Southall JPC, editor. Helmholtz’s Treatise on Physiological Optics. Dover; New York: 1909. pp. 143–173. [Google Scholar]
- Westheimer G, Blair SM. Accommodation of the eye during sleep and anesthesia. Vision Res. 1973;13:1035–1040. doi: 10.1016/0042-6989(73)90142-9. [DOI] [PubMed] [Google Scholar]
- Wilson RS. Does the lens diameter increase or decrease during accommodation? Human accommodation studies: a new technique using infrared retro-illumination video photography and pixel unit measurements. Trans Am Ophthalmol Soc. 1997;95:261–267. [PMC free article] [PubMed] [Google Scholar]