Abstract
Purpose
Phase I trial to evaluate the feasibility and toxicity of dose escalated 3DCRT concurrent with chemotherapy in patients with primary supratentorial GBM.
Materials/Methods
209 patients were enrolled. All received 46 Gy in 2 Gy fractions to PTV1, defined as GTV plus 1.8 cm. Subsequent boost was given to PTV2, defined as GTV plus 0.3 cm. Patients were stratified into two groups (gp): (Gp 1: PTV2 < 75 cc, and Gp 2: PTV2≥75 cc). Four RT dose levels were evaluated: 66, 72 ,78 and 84 Gy. BCNU 80 mg/m2 was given during RT, then q 8 weeks for 6 cycles. Pre-treatment characteristics were well balanced.
Results
Acute and late grade (Gr) 3/4 RT-related toxicities were no more frequent at higher RT dose or with larger tumors. There were no DLTs (acute ≥ Gr 3 irreversible CNS toxicities) observed on any dose level in either group. Based on the absence of DLTs, dose was escalated to 84 Gy in both groups. Late RT necrosis was noted at 66 (1 pt), 72 (2), 78 (2) and 84 Gy (3) in Group 1. In Group 2, late RT necrosis was noted at 78 (1 pt) and 84 Gy (2). Median time to RT necrosis was 8.8 months (range: 5.1–12.5). Median survival in Group 1: 11.8–19.3 months. Median survival in Group 2: 8.2–13.9 months.
Conclusions
Our study shows the feasibility of delivering higher than standard (60 Gy) RT dose with concurrent chemotherapy for primary GBM with an acceptable risk of late CNS toxicity.
Keywords: 3DCRT, Dose Escalation, GBM
Introduction
Despite improvements in imaging, surgery, radiation therapy (RT), and chemotherapy, the majority of patients with glioblastoma (GBM) continue to recur at the site of the original tumor location. 1Prior dose escalation studies using radiation alone have failed to improve the median survival despite dose escalation of the enhancing lesion to 90 Gy.2–3 Local tumor progression remains within or adjacent to the contrast-enhancing lesion, in approximately 90% of patients.1–3
RTOG 98–03 is the first prospective multi-institutional study to evaluate the feasibility and toxicity of radiation dose escalation using conformal three-dimensional (3D) radiation therapy delivered concurrently with chemotherapy (CRT) in patients with supra-tentorial GBM. The study hypothesized that the use of highly conformal 3DCRT with smaller treatment margins may permit safe dose escalation without increasing central nervous system (CNS) toxicity, and may potentially improve outcome. Shortly after RTOG 98-03 was completed, a subsequent study demonstrated that an improvement in overall survival in patients with newly diagnosed GBM could be realized when low-dose daily temozolomide is administered concurrently with standard dose 60 Gy RT, for six cycles following RT, as compared to RT alone.4 This combination has now become the standard treatment for patients with GBM. However, long-term survival for all patients remains infrequent, with the majority of patients still developing local failure, underscoring the need for additional treatment advances.
Although the concurrent chemotherapy used in this trial was BCNU, the study provides important data regarding the feasibility, safety and tolerability of conformal 3DCRT dose escalation with concurrent chemotherapy in a multi-institutional setting, which remains applicable in the current era. The primary objective of the study was to test the feasibility of dose escalation of radiation in conjunction with chemotherapy as well as assess the toxicity of conformal radiation dose escalation using 3D treatment planning in patients with GBM. The secondary objectives were to determine the dose and volume characteristics that may influence the rate of radiotherapy-induced CNS toxicity and to evaluate the survival of patients treated with high doses of conformal radiation therapy.
Materials/Methods
Patient Eligibility
Eligible patients were 18 years or older, KPS ≥ 60, and histological diagnosis of a primary supra-tentorial GBM. This study was only open to patients with a unifocal tumor. Further eligibility criteria included a neurological function (NF) of 0−3, no prior chemotherapy or radiotherapy to the head and neck; adequate bone marrow reserve, and acceptable renal and hepatic function as well as a normal chest x-ray. Therapy was required to start within 5 weeks after surgery and 1 week after registration.
Study Design
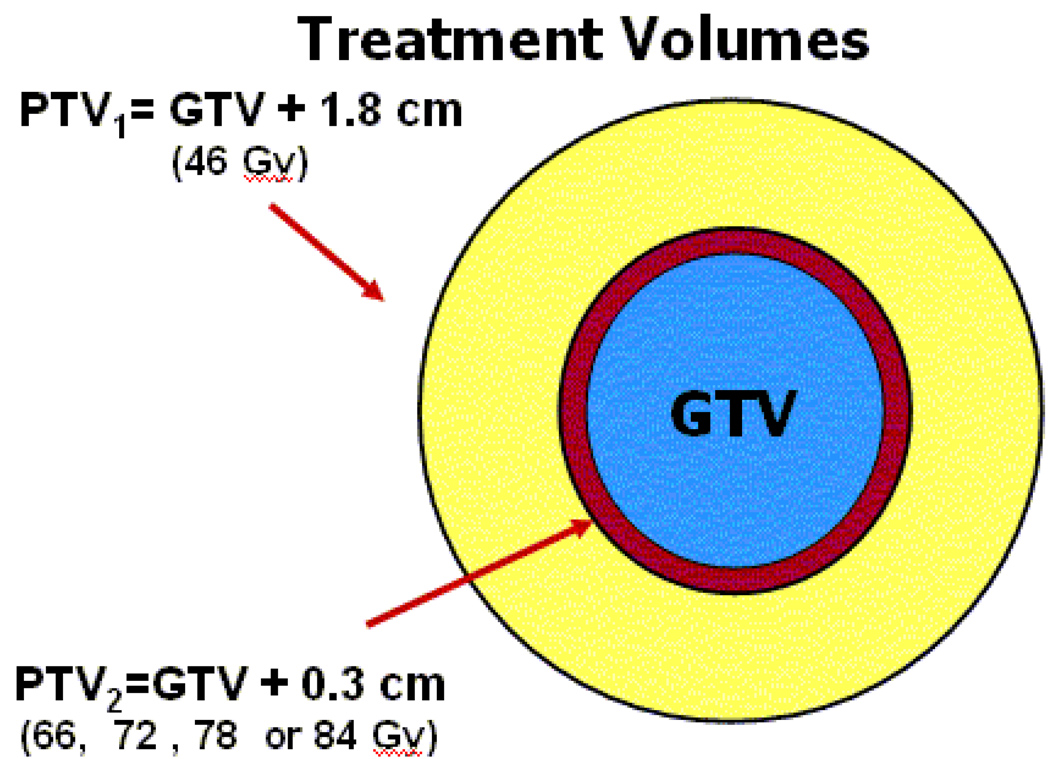
The primary objective of the phase I study was to establish the maximum tolerated dose (MTD) of radiotherapy (RT) delivered using three-dimensional conformal radiation treatment (3D–CRT) in patients with GBM. All patients received carmustine (BCNU) 80 mg/m2 on days 1, 2, 3 and 56, 57, 58 during RT, then every 8 weeks for a total of 6 cycles. All patients received 46 Gy in 2 Gy fractions to the planning target volume (PTV1). PTV1was defined as the gross target volume (GTV) plus 1.8 cm margin. (Figure 1) GTV included the resection cavity and any gross residual tumor as defined from immediate post-operative MR imaging. A further 1.5 cm margin was added to the GTV for microscopic extension, clinical target volume, (CTV1) and an additional 0.3 cm margin was added for set-up uncertainty (PTV1). A subsequent boost was given to PTV2, defined as the GTV plus 0.3 cm margin. Predominant pattern of failure in high grade gliomas remains within or immediately adjacent to the contrast-enhancing tumor.2–3 Elective treatment of peritumoral edema was not included in order to reduce risk of late CNS toxicity. Patients were stratified into two groups: for Group 1, PTV2 was < 75cc and for Group 2, PTV2 was ≥ 75 cc. Group 1 and 2 patients were planned in the same fashion. Four RT doses were evaluated in each group: 66 Gy (dose level 1), 72 Gy (dose level 2), 78 Gy (dose level 3), and 84 Gy (dose level 4).
Figure 1.
PTV1 (shown in yellow) is defined as the GTV (shown in blue) plus 1.8 cm margin treated to 46 Gy. Surrounding edema was not included in the treatment volume. A subsequent boost was delivered to the PTV2 (shown in red), defined as the GTV plus 0.3 cm margin. PTV2 was escalated to four different RT doses in each group: 66, 72, 78, and 84 Gy.
Toxicity was monitored continuously as each patient was accrued and follow-up data were accumulated. Acute RT and chemotherapy toxicities were graded using CTC v 2.0 criteria.5 Late RT toxicities were reported using the RTOG/EORTC Late Toxicity Criteria.6 Twenty patients were required per dose arm to evaluate both acute dose limiting toxicities (DLTs) and late radiation necrosis. Each patient was evaluated for acute DLT during the first 90 days from the start of therapy. DLT was defined as a 30% grade 3 or grade 4 irreversible (not responding to therapy or requiring surgical intervention, i.e. necrosis)CNS toxicity rate. Radiation necrosis was defined in clinically symptomatic patients with diagnostic imaging including PET or MR spectroscopy, and/or surgical pathology consistent with cerebral radiation necrosis.
One dose level per group was open for accrual at a time, starting with 66 Gy for each group. Dose escalation proceeded in a three-step process. The first two criteria for dose escalation were ≤ 2 grade 3 or 4 irreversible acute CNS toxicities and no grade 5 toxicities in the first 14 evaluable patients. If either of these criteria was exceeded then the dose level was deemed unacceptable. The third criterion for dose escalation was whether fewer than 9 of the first 14 progression-free patients required steroids at 3 months from the start of therapy. Furthermore, the dose level was de-escalated if more than 2 patients developed radiation necrosis in the first year of follow-up. Every six months, assessments were made of (1) compliance rate of treatment delivery with respect to protocol prescription; (2) the frequency and severity of the toxicities and (3) the cumulative incidence of radiation necrosis.
Quality Control Assessment
A unique aspect of this protocol was the advanced method of quality control utilized. Each institution was required to be credentialed through a rigorous process that included the submission of a completed 3DCRT Facility Questionnaire and the digital submission of a "Dry-Run" test case demonstrating protocol compliance (unless the institution was previously credentialed for participation in RTOG 3DCRT protocols). Once the institution was credentialed, the treatment planning data used in each protocol case was digitally submitted to the Image Guided Therapy QA Center (ITC) at Washington University in St. Louis, a member of the National Cancer Institute sponsored Advanced Technology QA Consortium (ATC). This required the institution’s treatment planning system to have the capability of digitally submitting CT scans, target volumes and organs at risk contours, 3D dose matrix, and plan geometry details in either RTOG data exchange format or as RT DICOM objects. This capability was assured as a part of the credentialing process.
Each protocol data set underwent QA review of the GTV, CTV, and PTV,, designated organs at risk contours, and dosimetry compliance using advanced 3D display tools provided by the ITC3D. Prescription dose was specified at the ICRU reference point dose within the planning target volume. Minimum dose to the PTV was at least 93% of the International Commission on Radiation Units (ICRU) reference dose. Maximum dose to PTV2 was within 3% of the ICRU reference point dose. Dosimetry compliance was scored as a minor deviation if < 100% but ≥95% of the appropriate PTV was completely encompassed by the 93% isodose line (relative to the prescription dose), and a major deviation if < 95% of the appropriate PTV was encompassed by the 93% isodose line. Dose heterogeneity index, defined by the ratio of the maximal dose divided by the prescription dose (MDPD) was calculated for each case. A minor deviation was defined as a conformality index of >1.03 and ≤1.10 and a major deviation if the conformality index was > 1.10. Doses to designated organs at risk included the cerebral hemispheres, brainstem, optic nerves,chiasm, eyes, and cerebellum were reduced as much as possible using conformal planning. Doses to the optic nerves, and chiasm were limited to < 55 Gy.
Statistical Analysis
Primary endpoint was to assess the early and late treatment-related toxicities. Secondary endpoints were steroid dependency within the first three months of treatment and the incidence of radiation necrosis. Pretreatment patient characteristics, including age, gender, KPS, NF class, Recursive Partitioning Analysis (RPA) RTOG class, extent of surgery and Mini-Mental Status Exam results, were analyzed for both groups.
Overall survival (OS) and progression-free survival (PFS) were evaluated. Actuarial estimates for OS were estimated using Kaplan-Meier. An event in OS is death due to any cause. OS was measured from date of study entry to the date of death or last follow-up. PFS was measured from start of radiotherapy to the date of progression, date of death, or last follow-up. Progression or death to any cause was treated as an event. This study was not designed or powered to make comparisons of efficacy endpoints between dose levels so actuarial estimates with 95% confidence intervals are therefore provided.
Results
Patient Characteristics
Between 1998 and 2003, 209 patients with newly diagnosed GBM were enrolled; 95 in Group 1 and 114 in Group 2. Six patients were deemed ineligible (one in group 1 and 5 in group 2); 4 patients had tumor progression prior to start of treatment, 1 patient in Group 2 had a PTV < 75 cc, and 1 patient had multi-focal tumor. 203 patients were evaluable for acute toxicity and 180 for late toxicity. Pre-treatment patient characteristics for each group are shown in Table 1. Both treatment groups were well balanced including KPS (p=0.6), age (p=0.9), RPA (p=0.6), extent of resection (p=0.4), and neurologic function status (p=0.2). Majority of patients had KPS ≥ 80, partial or gross total resection, and RPA class III or IV. Median follow-up time ranged from 8.2 to 19.3 months.
Table 1.
Pretreatment Characteristics
| Group 1 (PTV2< 75 cc) |
Group 2 (PTV2 ≥ 75 cc) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 66 Gy (n=22) |
72 Gy (n=23) |
78 Gy (n=27) |
84 Gy (n=22) |
66 Gy (n=33) |
72 Gy (n=23) |
78 Gy (n=35) |
84 Gy (n=18) |
|
| Age (%) | ||||||||
| Median | 56 | 56 | 59 | 52 | 54 | 56 | 54 | 50 |
| Range | 20–82 | 37–75 | 24–73 | 24–77 | 20–74 | 28–76 | 26–78 | 23–79 |
| < 50 | 36 | 35 | 30 | 41 | 36 | 22 | 34 | 44 |
| ≥ 50 | 64 | 65 | 70 | 59 | 64 | 78 | 66 | 56 |
| KPS (%) | ||||||||
| 60–70 | 18 | 13 | 19 | 9 | 27 | 9 | 9 | 28 |
| 80–100 | 82 | 87 | 81 | 91 | 73 | 91 | 91 | 72 |
| Gender (%) | ||||||||
| Male | 64 | 52 | 52 | 73 | 82 | 78 | 71 | 72 |
| Female | 36 | 48 | 48 | 27 | 18 | 22 | 29 | 28 |
| Neurological Function (%) | ||||||||
| No symptoms | 36 | 30 | 19 | 18 | 18 | 13 | 37 | 11 |
| Minor symptoms | 36 | 52 | 48 | 50 | 45 | 48 | 54 | 53 |
| Moderate (fully active) | 14 | 13 | 22 | 23 | 21 | 30 | 3 | 17 |
| Moderate (not fully active) |
14 | 4 | 11 | 9 | 15 | 9 | 6 | 22 |
| Extent of Surgery (%) | ||||||||
| Biopsy | 18 | 4 | 11 | 18 | 21 | 17 | 17 | 22 |
| Partial Resection | 59 | 57 | 56 | 18 | 73 | 57 | 60 | 28 |
| Total Resection | 18 | 39 | 26 | 64 | 6 | 26 | 23 | 39 |
| Other | 5 | 0 | 7 | 0 | 0 | 0 | 0 | 11 |
| Mental Status (%) | ||||||||
| Normal function | 68 | 78 | 63 | 77 | 64 | 70 | 63 | 56 |
| Minor confusion | 32 | 22 | 37 | 23 | 30 | 30 | 37 | 44 |
| Gross confusion, but awake |
0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| MMSE at Baseline (%) | ||||||||
| 0–9 | 4 | 0 | 0 | 0 | 3 | 0 | 3 | 0 |
| 10–24 | 14 | 4 | 15 | 5 | 15 | 13 | 8 | 22 |
| 25–30 | 77 | 87 | 85 | 91 | 79 | 87 | 89 | 78 |
| Unknown | 4 | 9 | 0 | 5 | 3 | 0 | 0 | 0 |
| RPA Class (%) | ||||||||
| III | 36 | 30 | 7 | 27 | 18 | 17 | 28 | 22 |
| IV | 27 | 48 | 67 | 55 | 55 | 43 | 48 | 50 |
| V | 27 | 22 | 18 | 14 | 21 | 39 | 17 | 22 |
| VI | 9 | 0 | 4 | 5 | 6 | 0 | 3 | 6 |
| Unknown | 0 | 0 | 4 | 0 | 0 | 0 | 3 | 0 |
Quality Assurance Compliance
Compliance with BCNU guidelines was assessed in 67% of patients. Compliance was per protocol or within acceptable variation for 90% of those reviewed. RT QA review was assessed in 73% patients in Group 1 and 67% patients in Group 2. All complete RT datasets received by the RTOG 3D QA center were reviewed for target volume, organ at risk, and quality of PTV dose coverage. 23 to 30% of cases that were not reviewed represent patients that may not have finished therapy as planned due to disease progression or failure of the QA center to have received complete and validated data sets. As shown in Table 2, the majority of cases in Group 1 and 2 met protocol guidelines or within acceptable variation at 66 and 72 Gy. In comparison, cases at 78 and 84 Gy in Group 2 were less likely to achieve 100% target coverage by the 93% isodose line in both PTV1 and PTV2 coverage. Nonetheless, the vast majority of cases were able to achieve at least 95% target coverage by the 93% isodose line.
Table 2.
RT QA review
| Group 1 (PTV2< 75 cc) | Group 2 (PTV2 ≥ 75 cc) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 66 Gy (n=22) |
72 Gy (n=23) |
78 Gy (n=27) |
84 Gy (n=22) |
66 Gy (n=33) |
72 Gy (n=23) |
78 Gy (n=35) |
84 Gy (n=18) |
||
| Reviewed (%) | |||||||||
| No* | 27 | 26 | 30 | 23 | 36 | 26 | 34 | 33 | |
| Yes | 73 | 74 | 70 | 77 | 64 | 74 | 66 | 67 | |
| PTV1 Coverage Score (%):** | |||||||||
| 1 | 63 | 82 | 89 | 94 | 81 | 94 | 65 | 75 | |
| 2 | 31 | 18 | 11 | 6 | 19 | 6 | 35 | 25 | |
| 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PTV2 Coverage Score (%):** | |||||||||
| 1 | 94 | 88 | 95 | 82 | 90 | 100 | 65 | 58 | |
| 2 | 6 | 12 | 5 | 18 | 5 | 0 | 30 | 42 | |
| 3 | 0 | 0 | 0 | 0 | 5 | 0 | 4 | 0 | |
| Dose Heterogeneity Score (%):*** | |||||||||
| 1 | 63 | 82 | 74 | 65 | 24 | 53 | 39 | 58 | |
| 2 | 37 | 18 | 26 | 35 | 76 | 47 | 57 | 42 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | |
Cases not reviewed due to unavailable/inconsistent data
1 = 0.93RX dose covers 100% of target
2 = 0.93RX dose covers <100% and ≥ 95% of target
3 = 0.93RX dose covers < 95% of target
1 = Dose Heterogeneity (Max Dose/RX dose) is ≤ 1.03
2 = Dose Heterogeneity (Max Dose/RX dose) is > 1.03 and ≤ 1.10
3 = Dose Heterogeneity (Max Dose/RX dose) is > 1.10
Toxicities
Chemotherapy and acute radiation toxicities were graded using Common Toxicity Criteria version 2.0.5 Late radiation toxicities were reported using the RTOG/EORTC Late Toxicity Criteria.6 Acute toxicities (occurring < 90 days from start of therapy) were primarily hematological due to carmustine and are detailed in Table 3. Three deaths occurred within 90 days of start of treatment: in Group 1, a patient experienced a fatal pulmonary toxicity likely due to BCNU at the 66 Gy dose level and in Group 2, a patient died secondary to bilateral pulmonary emboli following acute deep vein thrombosis treated at the 78 Gy level. A second patient in Group 2 died of respiratory failure secondary to pneumonitis/ pulmonary infiltrates possibly related to BCNU at 84 Gy. Acute Grade 3 neurological toxicities were noted in 3/22 (14%) patients at 66 Gy, 1/23 (4%) at 72 Gy, 5/27 (19%) at 78 Gy, and 2/22 (9%) at 84 Gy in Group 1. Acute Grade 3 or higher neurologic toxicities were noted in 4/33 (12%) patients at 66 Gy, 3/23 (13%) at 72 Gy, 3/35 (9%) at 78 Gy, and 3/18 (17%) at 84 Gy in Group 2 (Table 3). The majority of the neurologic symptoms were felt to be tumor-related; 10 patients had motor neuropathy (one also reported confusion, and another speech impairment/confusion), 7 had seizures, 2 patients each had ataxia (incoordination) and confusion, and 1 patient each had speech impairment, depression, and vertigo.
Table 3.
Chemotherapy and Acute Radiation Therapy Toxicities
| Group 1 (PTV2< 75 cc) |
Group 2 (PTV2 ≥ 75 cc) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 66 Gy (n=22) Grade |
72 Gy (n=23) Grade |
78 Gy (n=27) Grade |
84 Gy (n=22) Grade |
66 Gy (n=33) Grade |
72 Gy (n=23) Grade |
78 Gy (n=35) Grade |
84 Gy (n=18) Grade |
||||||||||||
| Category | 3 | 4 | 5 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 5 | 3 | 4 | 5 |
| Blood/bone marrow | 3 | 7 | 0 | 8 | 6 | 9 | 5 | 4 | 5 | 13 | 2 | 5 | 5 | 11 | 3 | 0 | 5 | 2 | 0 |
| Cardiovascular (arrhythmia) |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cardiovascular (general) |
0 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 2 | 1 | 1 | 2 | 0 | 0 |
| Coagulation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Constitutional symptoms |
0 | 0 | 0 | 1 | 0 | 2 | 0 | 3 | 0 | 4 | 0 | 1 | 2 | 2 | 0 | 0 | 1 | 0 | 0 |
| Dermatology/skin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| Hemorrhage | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Infection/febrile neutropenia |
1 | 0 | 0 | 2 | 0 | 3 | 1 | 2 | 0 | 5 | 0 | 3 | 0 | 4 | 0 | 0 | 1 | 1 | 0 |
| Metabolic/laboratory | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 1 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Musculoskeletal | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| Neurology | 3 | 0 | 0 | 1 | 0 | 5 | 0 | 2 | 0 | 3 | 1 | 3 | 0 | 2 | 1 | 0 | 3 | 0 | 0 |
| Ocular/visual | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain | 2 | 0 | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 0 |
| Pulmonary | 1 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 |
| Renal/genitourinary | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Worst non- hematologic |
6 27% |
1 5% |
1 5% |
10 43% |
0 0% |
10 37% |
3 11% |
9 41% |
1 5% |
9 27% |
4 12% |
6 26% |
2 9% |
5 14% |
5 14% |
1 3% |
4 22% |
2 11% |
1 6% |
| Worst overall | 5 23% |
7 32% |
1 5% |
13 57% |
6 26% |
11 41% |
8 30% |
10 45% |
5 23% |
15 45% |
6 18% |
8 35% |
6 26% |
12 34% |
6 17% |
1 3% |
7 39% |
3 17% |
1 6% |
Acute non-hematologic grade 3/4 RT-related toxicities were no more frequent at higher RT dose levels and among larger tumors (Group 2) than at lower RT dose levels and smaller tumors (Group 1). (Group 1: 44% vs. Group 2: 36%, p= 0.26). In Group 1, late radiation toxicity included three patients with late Grade 3 toxicity; one with severe headaches which resolved within a month, and two with radiation necrosis (Table 4). In Group 1, three additional patients presented with clinical late Grade 4 toxicity (radiation necrosis) at the 72, 78, and 84 Gy dose level respectively. In Group 2, two patients presented with late clinical radiation toxicity Grade 3; one with severe somnolence, and one with radiation necrosis at the 78 Gy dose level.
Table 4.
Late RT Toxicities
| Group 1 (PTV2< 75 cc) |
Group 2 (PTV2 ≥ 75 cc) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 66 Gy (n=20) Grade |
72 Gy (n=20) Grade |
78 Gy (n=26) Grade |
84 Gy (n=20) Grade |
66 Gy (n=29) Grade |
72 Gy (n=18) Grade |
78 Gy (n=33) Grade |
84 Gy (n=14) Grade |
|||||||||
| 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |
| Brain | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Skin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eye | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subcutaneous tissue | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Worst overall | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| (5%) | (0%) | (5%) | (5%) | (4%) | (4%) | (0%) | (5%) | (3%) | (0%) | (0%) | (0%) | (3%) | (0%) | (0%) | (0%) | |
Second Resection
The rate of second resection was similar between all dose levels (accounting for a shorter follow-up period in the highest dose levels). (Table 5) In Group 1, approximately 50% of patients in each dose level had a second resection. Far fewer patients per dose level in Group 2 underwent non-protocol surgery. Although information was not available in many cases regarding reasons for second resection; in some centers, patients were routinely offered second resection at time of tumor progression (majority of patients in Group 1). There were also several patients that were symptomatic with increasing mass effect and underwent second resection (majority of patients in Group 2)
Table 5.
Second Resection
| Group 1 (PTV2< 75 cc) | Group 2 (PTV2 ≥ 75 cc) | |||||||
|---|---|---|---|---|---|---|---|---|
| 66 Gy (n=22) |
72 Gy (n=23) |
78 Gy (n=27) |
84 Gy (n=22) |
66 Gy (n=33) |
72 Gy (n=23) |
78 Gy (n=35) |
84 Gy (n=18) |
|
| Surgery (%) | ||||||||
| No | 50 | 43 | 52 | 50 | 58 | 65 | 60 | 61 |
| Yes | 50 | 57 | 48 | 50 | 42 | 35 | 40 | 39 |
| Type of Surgery (%) | ||||||||
| Biopsy | 0 | 15 | 8 | 18 | 0 | 0 | 7 | 29 |
| Partial resection | 55 | 54 | 69 | 27 | 64 | 50 | 29 | 43 |
| Total resection | 27 | 15 | 0 | 36 | 14 | 38 | 21 | 20 |
| Other/no detail | 0 | 8 | 15 | 18 | 14 | 13 | 36 | 20 |
| Unknown | 18 | 8 | 8 | 0 | 7 | 0 | 7 | 0 |
Pathology in the majority of cases showed recurrent or residual GBM. Ten cases demonstrated both recurrent GBM as well as changes consistent with RT effects, including vascular hyalinization, coagulative necrosis, reactive gliosis, and edema. Two cases occurred at 72 Gy and one at 84 Gy in Group 1 and 2 at 66 Gy, 2 at 72 Gy and 3 at 78 Gy in Group 2. Five cases showed no evidence of recurrent glioma, consistent with a pathologic complete response and without evidence of radiation necrosis of normal brain. Those cases were primarily in Group 1, 1 at 66 Gy, 2 at 78 Gy, and 1 case at 84 Gy. In Group 2, there was 1 case at 84 Gy and had a median survival of 24 months.
Corticosteroid Use
The number of progression-free patients that remained on steroids at 3 months following start of therapy is shown in Table 6. The range of steroid use at 3 months for the first 14 randomized progression-free patients in Group 1 was 36 to 64% and for Group 2 it was 29 to 71%. In Group 2, dose level 1 there were 10 patients that were continued on steroids 1–2 months beyond treatment which was either further tapered or stabilized approximately 3 months post-treatment. It was evident that a significant fraction of patients received steroids for what was possibly pseudo-progression therefore this criterion was not used to define dose-limiting toxicity.
Table 6.
Steroid Use at 3 months for the First 14 (if available) Registered Progression-Free Survival Patients
| Group 1 (PTV2< 75 cc) | Group 2 (PTV2 ≥ 75 cc) | |||||||
|---|---|---|---|---|---|---|---|---|
| 66 Gy (n=14/16*) |
72 Gy (n=14/16) |
78 Gy (n=14/22) |
84 Gy (n=14/17) |
66 Gy (n=14/22) |
72 Gy (n=11/11) |
78 Gy (n=14/27) |
84 Gy (n=10/10) |
|
| Yes | 9 (64%) | 5 (36%) | 7 (50%) | 7 (50%) | 10 (71%) | 6 (55%) | 4 (29%) | 5 (50%) |
| No | 5 (36%) | 9 (64%) | 7 (50%) | 7 (50%) | 4 (29%) | 5 (45%) | 10 (71%) | 5 (50%) |
The numerator is the first 14, if available, registered and progression-free patients and the denominator is the total number of progression-free patients at 3 in that RX arm.
Survival
Median survival for Group 1 ranged from 11.6 (66 Gy) to 19.3 months at 84 Gy. Median survival for Group 2 ranged from 8.2 (66 Gy) to 13.9 months (84 Gy). 84 Gy dose level had the highest median survival for both groups and 66 Gy had the lowest. Eighty-seven percent of patients are dead in Group 1 and 97% died in Group 2. (Table 7a and b) Progression-free survival for Group 1 ranged from 5.8 to 6.9 months. The median progression-free survival in Group 2 ranged from 3.0 to 4.4 months. (Table 8a,b)
Table 7.
| Table 7a. Overall Survival - Group 1 (PTV2< 75 cc) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Month | 66 Gy | 72 Gy | 78 Gy | 84 Gy | ||||
| Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
|
| 0 | 100 | 22* | 100 | 23 | 100 | 27 | 100 | 22 |
| 3 | 95 (72,99) |
21 | 87 (65,96) |
20 | 96 (76,99) |
26 | 91 (68,98) |
20 |
| 6 | 86 (63,95) |
19 | 83 (60,93) |
19 | 93 (74,98) |
25 | 82 (59,93) |
18 |
| 9 | 77 (54,90) |
17 | 70 (47,84) |
16 | 63 (42,78) |
17 | 73 (49,87) |
16 |
| 12 | 45 (24,64) |
10 | 48 (27,66) |
11 | 48 (29,65) |
13 | 73 (49,87) |
16 |
| 18 | 27 (11,46) |
6 | 43 (23,62) |
10 | 30 (14,47) |
8 | 55 (32,72) |
12 |
| 24 | 27 (11,46) |
6 | 26 (11,45) |
6 | 11 (3,26) |
3 | 41 (21,60) |
8 |
| 30 | 23 (8,41) |
5 | 22 (8,40) |
5 | 4 (0.3,16) |
1 | 24 (8,44) |
4 |
| 36 | 18 (6,36) |
4 | 17 (5,35) |
4 | -- | 0 | 24 (8,44) |
2 |
| MST (months) | 11.6 | 11.8 | 11.8 | 19.3 | ||||
| Dead/Total | 20/22 | 20/23 | 26/27 | 16/22 | ||||
| Median Follow- up Time (range) |
11.6(1.7–70.2) | 11.8(0.6–71.9) | 11.8(1.5–34.7) | 19.3 (1.8–48.2) | ||||
| Table 7b. Overall Survival - Group 2 (PTV2 ≥ 75 cc) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Month | 66 Gy | 72 Gy | 78 Gy | 84 Gy | ||||
| Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
|
| 0 | 100 | 33 | 100 | 23 | 100 | 35 | 100 | 18 |
| 3 | 94 (78,98) |
31 | 83 (60,93) |
19 | 94 (79,99) |
33 | 78 (51,91) |
14 |
| 6 | 67 (48,80) |
22 | 65 (42,81) |
15 | 83 (66,92) |
29 | 78 (51,91) |
14 |
| 9 | 39 (23,55) |
13 | 61 (38, 77) |
14 | 69 (50,81) |
24 | 61 (35,79) |
11 |
| 12 | 30 (16,46) |
10 | 52 (31,70) |
12 | 46 (29,61) |
16 | 56 (31,75) |
10 |
| 18 | 18 (7,33) |
6 | 13 (3,30) |
3 | 20 (9, 34) |
7 | 39 (17,60) |
7 |
| 24 | 9 (2,22) |
3 | 9 (11,45) |
2 | 11 (4,24) |
4 | 28 (10,49) |
5 |
| 30 | 6 (1,18) |
2 | 4 (0.3,18) |
1 | 6 (1,17) |
2 | 17 (4,37) |
3 |
| 36 | 6 (1,18) |
2 | 4 (0.3,18) |
1 | 6 (1,17) |
2 | 6 (0.4,22) |
1 |
| MST (months) | 8.2 | 12.3 | 10.0 | 13.9 | ||||
| Dead/Total | 31/33 | 23/23 | 34/35 | 18/18 | ||||
| Median Follow- up Time (range) |
8.2 (0.9–79.4) | 12.3 (0.3–71.4) | 10.0 (0.8–49.0) | 13.9 (1.2–43.6) | ||||
One patient died between randomization and RT start after day 1 of the first chemotherapy cycle. C.I., confidence interval
C.I., confidence interval
Table 8.
| Table 8a. Progression-Free Survival – Group 1 (PTV2< 75 cc) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Month | 66 Gy | 72 Gy | 78 Gy | 84 Gy | ||||
| Survival (%) (95% C.I.) |
# at Risk | Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
|
| 0 | 100 | 21* (22) | 100 | 23 | 100 | 27 | 100 | 22 |
| 3 | 76 (52,89) |
16 | 70 (47,84) |
16 | 81 (61,92) |
22 | 77 (54,90) |
17 |
| 6 | 48 (26,67) |
10 | 52 (31,70) |
12 | 59 (39,75) |
16 | 50 (28,68) |
11 |
| 9 | 38 (18,58) |
8 | 30 (14, 49) |
7 | 30 (14, 47) |
8 | 36 (17,56) |
8 |
| 12 | 14 (4,32) |
3 | 26 (11,45) |
6 | 22 (9,39) |
6 | 27 (11,46) |
6 |
| 18 | 14 (4,32) |
3 | 13 (3,30) |
3 | 4 (0.3,16) |
1 | 18 (6,36) |
4 |
| 24 | 10 (2,26) |
2 | 13 (3,30) |
3 | 4 (0.3,16) |
1 | 18 (6,36) |
4 |
| 30 | 5 (0.3,20) |
1 | 13 (3,30) |
3 | -- | 0 | 14 (3,31) |
3 |
| 36 | -- | 0 | 9 (1,24) |
2 | -- | 0 | 9 (2,25) |
1 |
| MST (months) | 5.8 | 6.5 | 6.9 | 6.0 | ||||
| Fail /Total | 21/21 | 21/23 | 27/27 | 21/22 | ||||
| Table 8b. Progression-Free Survival − Group 1 (PTV2 ≥ 75 cc) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Month | 66 Gy | 72 Gy | 78 Gy | 84 Gy | ||||
| Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
Survival (%) (95% C.I.) |
# at Risk |
|
| 0 | 100 | 33 | 100 | 23 | 100 | 35 | 100 | 18 |
| 3 | 67 (48,80) |
22 | 48 (27,66) |
11 | 77 (59,88) |
27 | 56 (31,75) |
10 |
| 6 | 18 (7,33) |
6 | 39 (20,58) |
9 | 23 (11,38) |
8 | 33 (14,55) |
6 |
| 9 | 9 (2,22) |
3 | 22 (8,40) |
5 | 11 (4,24) |
4 | 22 (7,43) |
4 |
| 12 | 6 (1,18) |
2 | 22 (8,40) |
5 | 9 (2,21) |
3 | 6 (0.4,22) |
1 |
| 18 | 3 (0.2,13) |
1 | -- | 0 | 3 (0.2,13) |
1 | -- | 0 |
| 24 | 3 (0.2,13) |
1 | -- | 0 | 3 (0.2,13) |
1 | -- | 0 |
| 30 | 3 (0.2,13) |
1 | -- | 0 | 3 (0.2,13) |
1 | -- | 0 |
| 36 | 3 (0.2,13) |
1 | -- | 0 | 3 (0.2,13) |
1 | -- | 0 |
| MST (months) | 3.8 | 3.0 | 4.4 | 3.2 | ||||
| Fail /Total | 32/33 | 23/23 | 34/35 | 18/18 | ||||
One patient died between randomization and the start of RT – after day 1 of the first chemotherapy cycle C.I., confidence interval
C.I., confidence interval
Dose Limiting Toxicities (DLT)
There were no DLTs (acute grade 3 or 4 irreversible CNS toxicities) observed in dose levels 1 to 3 in Group 1 or Group 2. Therefore, the dose was escalated within each group from 66 to 72 to 78 to 84 Gy. There were no DLTs observed at 84 Gy in either group so the dose was not de-escalated. In Group 1, late radiation necrosis was noted in one patient at 66 Gy, two at 72 and 78 Gy, and three patients at 84 Gy in Group 1 (total RT necrosis = 8). In Group 2, one patient was reported to have late RT necrosis at 78 Gy and two patients at 84 Gy in Group 2. (total RT necrosis = 3) Median time to RT necrosis was 8.8 months (range: 5.1 to 12.5 months)
Discussion
This is the first, large prospective multi-institutional study to analyze the acute and long-term toxicity of conformal 3DCRT of more than 60 Gy with concurrent chemotherapy. Our results show the feasibility and tolerability of delivering higher than standard (60 Gy) radiation dose with concurrent chemotherapy for primary supratentorial GBM with an acceptable risk of late CNS toxicity. No increased acute CNS toxicity has been observed with increasing RT doses or with increasing tumor volume (PTV2). Our results suggest that higher RT doses may be associated with an increase in median survival in Group 1 patients, although this group was also associated with a higher rate of gross total resection. The majority of acute toxicities encountered in this study resulted from BCNU chemotherapy. There was no significant difference in late CNS toxicity in Group 1 and Group 2 patients suggesting that the arbitrary cutoff of a PTV of 75 cc should not be used as stratification for future dose escalation studies. Acceptable late CNS toxicity was considered in this trial as clinically symptomatic radiation necrosis that does not respond to therapy or requires surgical intervention at a rate of ≤10%. Therefore, a MTD of 78 Gy was considered for the entire group.
In this study, we hypothesized that late radiation CNS effects are dose and volume related and therefore the use of conformal radiation therapy with smaller target margins may Conformal 3DCRT minimizes the volume of normal brain receiving high radiation dose with an added incidental benefit that the adjacent normal brain is irradiated at a lower dose per daily fraction than in conventional radiation therapy.7 Several series delivered 3D conformal planned radiation fields to doses greater than 70 Gy and also did not demonstrate a significant increase in morbidity.3,8–9 However, obtaining histopathology confirmation of tumor progression versus radiation necrosis was not routinely performed, therefore some of the presumed tumor progression may be radiation-induced necrosis. This would alter the assessment and estimate of radiation-induced injury.
The rationale for stratification based on PTV2 volume includes that the potential benefit of dose escalation may only be limited to those patients with gross or near total resection and the risk of acute and late CNS toxicity may increase with increasing target volumes.13–14 Although in this study, both groups were able to dose escalate to the maximum dose, there still remains the difficulty in differentiating radiation necrosis from tumor recurrence. Patients require long follow-up to develop late radiation necrosis and there is clearly less likelihood of re-operation in larger tumors to help clarify the diagnosis. Dose escalation trials in other sites including lung and liver were dependent on the volume of normal tissue irradiated.10–11 Irradiated volume appears much more important in dose escalation studies using stereotactic radiosurgery in the CNS.12 We feel that perhaps, fractionation with low fraction size mitigates any volume effect that may be noted or perhaps there is no volume effect noted in this dose range in the CNS. These are potential reasons that it is difficult to clearly show a volume effect as predicted in this study.
Prior RTOG dose escalation studies using standard large RT fields tested the benefit of hyper-fractionation (HF) and accelerated HF.15–17 RT doses were escalated from 1.2 Gy BID to 64.8, 72, 76.8 and 81.6 Gy or 1.6 Gy BID to 48 or 54.5 Gy. A phase III trial comparing the 72Gy arm to standard 60 Gy RT showed no survival benefit for HG (RTOG 90-06)17 RTOG 94-11 used accelerated radio-surgery ineligible GBM. RT dose was 1.6 Gy BID to 64 or 70.4 Gy depending on tumor size.15 A trend towards improved survival in the higher radiation dose group was noted but may be due to smaller tumor size, and not necessarily to higher RT dose. No excessive late RT-related CNS toxicities were observed in all studies.
The strengths of this study are the large number of patients treated in a multi-institutional setting using conformal 3DCRT and chemotherapy, without significant increased risk of late CNS toxicity.
Unfortunately, BCNU has substantial toxicity and has not been proven to be very effective for this disease. A randomized trial in patients newly diagnosed with glioblastoma showed that use of concurrent temozolomide during and after conventional radiotherapy (60 Gy) improved median survival from 12.1 months (11.2–13.0) to 14.6 months (13.2–16.8) compared to RT alone.4 Preclinical data supports temozolomide as an effective radiation sensitizer.18–19 Therefore, our findings suggest that it is reasonable to test if conformal dose escalation with concurrent temozolomide can improve outcome.
This study has some limitations. Most importantly, despite improvements in imaging, it is still difficult to distinguish recurrence from treatment-related necrosis. This may be difficult even when a pathologic sample is obtained as there are no guidelines established to distinguish between residual and recurrent tumor.20 We have made every effort in this study to identify necrosis as the cause for clinical progression, even if there is a small amount of residual tumor, so we feel reasonably confident that we have not underestimated the toxicity of treatment.
Recent technical advances in radiation therapy such as intensity modulated radiation therapy (IMRT) have improved our ability to deliver a higher, and possibly more effective, dose to the most malignant region of the tumor, as well as reduce the dose delivered to the surrounding normal structures.21 Although the current study was performed using 3DCRT, future studies will likely be performed with IMRT. Effective uses of this technology require improved target volume delineation as well as different target volume definitions than have been used traditionally in the past. Our study provides important clinical data that supports the use of ICRU 50 recommendations to define target volumes in the treatment of high grade glioma.
Recent advances in functional imaging including MR spectroscopy, 11 C Methionine [18F] fluorothymidine etc may further improve our ability to define residual tumor from areas of edema, inflammation, or necrosis.22–24 Conventional contrast enhanced T1-weighted MR and T2-weighted magnetic resonance imaging often used for radiation planning, reflect anatomic, rather than molecular or functional, properties of the tumor. The combination of more effective chemotherapy with highly conformal radiotherapy targeted to areas at highest risk for tumor recurrence, may allow us to improve the therapeutic index that previous dose escalation studies using radiotherapy alone may not have allowed us to achieve. Although more effective drugs may be used in combination with radiation in the future, these results confirm the tolerability and safety of delivering higher and possibly more effective, radiation doses using highly conformal radiation techniques in combination with chemotherapy to improve patient outcome.
Acknowledgments
Supported By: NCI CA21661, CA37422, and 32115, NIH U24 CA81647 (ATC Grant)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
REFERENCES
- 1.Mahley MS, Mettlin C, Natarajan N, Laws ER, Peace BB. National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989;71:826–836. doi: 10.3171/jns.1989.71.6.0826. [DOI] [PubMed] [Google Scholar]
- 2.Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncology Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 3.Chan J, Lee S, Fraass B, et al. Survival and Failure Patterns of High-Grade Gliomas after Three-Dimensional Conformal Radiotherapy. JCO. 2002;20(6):1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Arbuck SG, Ivy SP, Setser A;, et al. The Revised Common Toxicity Criteria: Version 2.0. CTEP Website. http://ctep.info.nih.gov.
- 6.Cox JD, Stetz J, Pajak TF, et al. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 7.Thornton AF, Jr, Hegarty TJ, TenHaken RK, Yanke BR, LaVigne ML, Fraass BA, McShan DL, Greenberg HS. Three dimensional treatment planning of astrocytomas: A dosimetry study of cerebral irradiation. Int J Radiat Oncol Biol Phys. 1991;20:1309–1315. doi: 10.1016/0360-3016(91)90243-w. [DOI] [PubMed] [Google Scholar]
- 8.Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: Results of a phase II prospective trial. J Neurosurg. 1999;92:251–260. doi: 10.3171/jns.1999.91.2.0251. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Ino Y, Nakagawa K, Tago M, Todo T. High-dose conformal radiotherapy for supratentorial malignant glioma: a historical comparison. Lancet Oncology. 2005;6(12):953–960. doi: 10.1016/S1470-2045(05)70395-8. [DOI] [PubMed] [Google Scholar]
- 10.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65(4):1075–1086. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 12.Shaw E, Scott C, Souhami L, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05) Int J Radiat Oncol Biol Phys. 1996;34(3):647–654. doi: 10.1016/0360-3016(95)02106-x. [DOI] [PubMed] [Google Scholar]
- 13.Sheline GE, Wara WM, Smith V. Therapeutic irradiation in brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 14.Marks L, Spencer D. The influence of volume on the tolerance of the brain to radiosurgery. J Neurosurg. 1991;75:177–180. doi: 10.3171/jns.1991.75.2.0177. [DOI] [PubMed] [Google Scholar]
- 15.Coughlin C, Scott C, Langer C, et al. Phase II, two-arm RTOG trial (94-11) of bischloroethyl-nitrosourea plus accelerated hyperfractionated radiotherapy (64.0 or 70.4 Gy) based on tumor volume (> 20 or < or = 20 cm(2), respectively) in the treatment of newly-diagnosed radiosurgery-ineligible glioblastoma multiforme patients. Int J Radiat Oncol Biol Phys. 2000;48:1351–1358. doi: 10.1016/s0360-3016(00)01412-7. [DOI] [PubMed] [Google Scholar]
- 16.Werner-Wasik M, Scott CB, Nelson DF, et al. Final report of a phase I/II trial of hyperfractionated and accelerated hyperfractionated radiation therapy with carmustine for adults with supratentorial malignant gliomas. Radiation Therapy Oncology Group Study 83-02. Cancer. 1996;77:1535–1543. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1535::AID-CNCR17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Lustig RA, Seiferheld W, Berkey B, et al. Imaging response in malignant glioma, RTOG 90-06. Am J Clin Oncol. 2007;30:32–37. doi: 10.1097/01.coc.0000236214.13206.38. [DOI] [PubMed] [Google Scholar]
- 18.Van Rijn, Heimans J, van den Berg J, et al. Survival of human glioma cells treated with various combination of temozolomide and x-rays. Int J. Radiat Oncol Biol Phys. 2000;47:779–784. doi: 10.1016/s0360-3016(99)00539-8. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarti A, Erkkinnen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma multiforme:a report on underlying mechanisms. Clin Cancer Res. 2007;12(15):4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 20.Perry A, Schmidt ER. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropath. 2006;111(3):197–212. doi: 10.1007/s00401-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald SM, Ahmad S, Kachris S, et al. Intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for the treatment of high grade glioma: a dosimetric comparison. J Appl Clin Med Phys. 2007;8:47–60. doi: 10.1120/jacmp.v8i2.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson SJ, Graves E, Pirzkall A, et al. In vivo molecular imaging for planning radiation therapy of gliomas: an application of 1H MRSI. J Magn Reson Imaging. 2002;16:464–476. doi: 10.1002/jmri.10183. [DOI] [PubMed] [Google Scholar]
- 23.Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–519. doi: 10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]