Abstract
The lifetime exposure of organisms to oxidative stress influences many aging processes which involve the turnover of the extracellular matrix. In this study, we identify the redox-responsive molecular signals that drive senescence-associated (SA) matrix metalloproteinase-1 (MMP-1) expression. Precise biochemical monitoring revealed that senescent fibroblasts increase steady-state [H2O2] 3.5 fold (13.7→48.6 pM) relative to young cells. Restricting H2O2 production through low O2 exposure or by antioxidant treatments prevented SA increases in MMP-1 expression. The H2O2-dependent control of SA MMP-1 is attributed to sustained JNK activation and c-jun recruitment to the MMP-1 promoter. SA JNK activation corresponds to increases and decreases in the levels of its activating kinase (MKK-4) and inhibitory phosphatase (MKP-1), respectively. Enforced MKP-1 expression negates SA increases in JNK phosphorylation and MMP-1 production. Overall, these studies define redox-sensitive signaling networks regulating SA MMP-1 expression and link the free radical theory of aging to initiation of aberrant matrix turnover.
Keywords: MMP-1, Reactive Oxygen Species, Ageing, Signal Transduction, JNK, MKP
Introduction
Matrix metalloproteinases (MMPs) are a family of zinc-binding, calcium-dependent endopeptidases that degrade components of the extracellular matrix (ECM). MMPs are primarily secreted in their inactive or zymogen form, activated extracellularly, present at low levels and play important roles in many physiologic processes(Brinckerhoff and Matrisian, 2002). MMP-1 is a prototypic interstitial collagenase that degrades collagen type I, II and III in the ECM and acts as the initiator protease generating substrates for other MMPs(Pardo and Selman, 2005). Aberrant MMP-1 expression is commonly linked to many age-associated disease pathologies including: premature ageing syndromes, rheumatoid and osteoarthritis, lung emphysema, atherosclerosis, photo-induced skin wrinkling, tumor progression and metastasis(Brinckerhoff and Matrisian, 2002). MMP expression is tightly controlled and primarily regulated at the level of transcription but also involves controls on translation, secretion, activation in the extracellular milieu and inhibition by endogenous tissue inhibitors of metalloproteinases (TIMPs)(Vincenti and Brinckerhoff, 2007). A number of signaling pathways participate in the transcriptional control of MMPs including the members of the Mitogen Activated Protein Kinase (MAPK), Phosphoinositoly-3-Kinase (PI3K) and Protein Kinase C (PKC) families (Domeij et al, 2004;Vincenti and Brinckerhoff, 2007). The prominent signaling kinase that controls expression of a particular MMP is defined by the initiating signal and encompass a broad array of stimuli including hormones, cytokines, tobacco smoke and thermal, ultraviolet and infrared radiation.
Many of the signals that are propagated by MMP inducing stimuli involve the production of reactive oxygen species (ROS) which play a contributory role in the aging process(Harman, 1956). Aging is also associated with an increase in many degenerative diseases involving ECM breakdown and aberrant MMP expression. Recent work by Fisher et al. has established a redox-component to age-associated increases in MMP-1(Fisher et al, 2009). The involvement of ROS in age-associated MMP expression provides a mechanistic link between free radical theory of aging and many age-associated degenerative diseases and the present study was aimed at defining the molecular triggers that drive this relationship. Our studies analyzed the primary initiating protease MMP-1 and demonstrate that its age-associated expression is ROS-dependent in an in vitro replicative senescence model and regulated by activation of the JNK signaling network.
EXPERIMENTAL PROCEDURES
Cell culture and reagents
IMR-90, primary human fetal lung fibroblasts were ordered from ATCC and cultured in MEM supplemented with 10% FBS, 1000units/ml penicillin, 500g/ml streptomycin and amphotericin B. These cells were either incubated at 21% or 3% oxygen tension at 37°C with 5% CO2. Cells were resuspended in trypsin and serially passaged at a dilution of 1:4.
MMP-1 pulldown and immunoblot analysis
MMP-1 was concentrated from cell media after normalizing to cell number. Samples were incubated overnight at 4 °C with 40 μl of heparin-sepharose beads (Amersham Biosciences) that binds MMP-1. Immunoblot was performed as previously described (Nelson et al, 2006).
Western blot
Protein concentrations were determined using the BCA protein assay kit (Pierce). Unless otherwise indicated 25μg total protein lysate were analyzed. The following antibodies were used: p-MKK-4 (9156), total Erk (9102), total JNK (9258) all from Cell Signaling Technology Inc., Danvers, MA); MMP-1 (MAB901) from R&D Systems Inc., Minneapolis, MN; GAPDH (4300) from Ambion, Austin, TX; p-Erk (sc-7383), MKP-1 (sc-370), p21 (sc-397) and p16 (sc-468) from Santa Cruz biotechnology Inc., Santa Cruz, CA and p-JNK (44-682G) from Biosource, Camarillo, CA. Secondary antibodies anti-mouse (NA931) and anti-rabbit (NA934) were from Amersham.
Analysis of ubiquitinated MKP-1
Cells were treated with 10μM proteasome inhibitor MG132 overnight and then lysed in RIPA lysis buffer with protease inhibitor cocktail (Roche, Nutley, NJ). Protein concentration was determined using the BCA protein assay kit (Pierce). 10μl MKP-1 ab (Santacruz Biotechnology) was added to samples and incubated on a rotator overnight at 4°C. Non-specific IgG was added to one of the samples as a control for non-specific binding. Following incubation, 50μl Protein G Agarose (Thermoscientific) was added to each sample and incubated for 2 hours at 4°C on a rotator. The beads were settled by centrifugation and washed 3 times with RIPA lysis buffer and boiled with 2X sample loading buffer (non-reducing). Samples were electrophoresed on pre-cast 3-8% gradient Tris-Acetate gels (Invitrogen, Carlsbad, CA) and transferred onto nitrocellulose membrane. The membrane was blocked in 5% milk in Tween Tris-Borate Saline, incubated with anti-ubiquitin antibody overnight at 4°C on the rocker washed and incubated with rabbit secondary antibody. This was followed by washing and antibody binding was determined by chemiluminescence. The blot was stripped and reprobed for MKP-1 protein.
RNA isolation and real-time PCR
RNA was isolated from cells using Trizol reagent, quality checked on agarose gel and quantified by measuring the OD260nm. 2μg of RNA was used for cDNA synthesis. Real-time PCR analysis of cDNA was performed using Syber Green (Molecular Probes) and the BioRad IQ cycler. The following primer sets were used: MMP-1, Sense-AGTGACTGGGAAACCAGATGCTGA’, Antisense-GCTCTTGGCAAATCTGGCGTGTAA; and β-Actin, Sense-ACCAACTGGGACGACATGGAGAAA, Antisense-TAGCACAGCCTGGATAGCAACGTA. No template controls were run on each reaction plate.
Chromatin Immunoprecipitation
IMR90 cells were cultured at 3% or 21% O2 for chromatin immunoprecipitation (ChIP) of c-Jun (9162- Cell Signaling Technologies) as described by(Nelson et al, 2006). The following primer set was used to amplify the MMP-1 promoter region encompassing the redox-responsive AP-1/ETS-1 binding site(Ranganathan et al, 2001): Sense-CCCTCTTGAACTCACATGTTATGC, Antisense-CCTTTCCCACTGTATCAGGTTTGC. A no antibody control was used to account for non specific binding and no c-jun binding was observed to random coding regions as previously reported(Nelson et al, 2006). Cycle number was used to calculate relative binding and plotted as relative promoter occupancy. Statistical analysis was performed by one-way ANOVA followed by Tukey’s Multiple Comparison Test.
Analysis of intracellular Redox
The redox-sensitive green fluorescent protein (roGFP1) has been previously described and was designed with two surface exposed cysteine residues that are sensitive to oxidation by ROS (Brejc et al, 1997;Dooley et al, 2004). The oxidation of roGFP1 changes the protonation state of the chromophore shifting the excitation from 470 nm to 400 nm while the emission wavelength remains constant (510nm). Senescent IMR90 cells were transiently transfected with the pEFGP-N1 as an expression vector for roGFP1 in the cytoplasm and maintained either under 21% or 3% O2. The flasks were then moved from 21% to 3% O2 and vice versa and GFP fluorescence was determined after 18hr. GFP fluorescence was determined using the Ziess Axio microscope. At least 10 different fields were marked and photographed. The software was then used to determine excitation at 410nm and 470nm and the ratio was determined. Increases in the 410/470 ratio are indicative of greater oxidation.
Determination of steady-state intracellular [H2O2]
IMR-90 cells were treated with 20mM of 3-amino 1, 2, 4 triazole (ATZ) for 0, 15, and 30mins at 37°C. Following treatment, cells were washed twice and scraped-harvested in PBS. Samples were then centrifuged at 14000rpm for few minutes. The pellet was re-suspended in potassium phosphate buffer (0.05M, pH 7.4) and sonicated. Catalase activity was measured with aid of a spectrophotometeric assay as described by Claiborne et al(Claiborne, 1985). Steady state intracellular [H2O2] was measured based on the determination of the irreversible inactivation of catalase by ATZ, as previously described (Dasgupta et al, 2006).
Adenoviral and Lentiviral JNK and MKP-1 expression constructs
The adenoviral dominant negative JNK and MKP-1 expression constructs were kindly provided by Dr. Harold Singer and Chesi Chen, respectively. Details to their construction can be found in (Liu et al, 2009). IMR90 cells were seeded in a 25 cm2 flask and infected overnight with the adenovirus expressing the dominant-negative JNK conjugated to GFP or a GFP control. Lentivirus was produced according to manufacturers protocol for the packaging cell-line, 293FT from invitrogen was used (Cat. No. R700-07). The virus containing media was added to target cells (IMR-90 fibroblasts) along with polybrene to a final concentration of 10ug/ml. The next day the media was changed to complete media and cells were incubated for another 48hrs before harvesting. Following infection, media was collected and normalized to cell number. MMP-1 levels in the media were determined as described above.
Cytometric bead array
Samples were prepared according to manufacturer’s protocol for adherent cells (BD Biosciences Cat. No.5600006). After treatment, 1X denaturation buffer with protease inhibitor cocktail was added to cells and collected in a tube. Samples were then boiled for 5 min and sonicated. Following this protein concentration was determined by BCA protein assay kit (Pierce) and samples were stored at −70 °C until time for CBA. Phospho JNK1/2 (T183/Y185), total JNK and human GAPDH was measured using beads from Becton Dickinson, Cat. No. 560213, 560214, and 560792. Along with the multiplex flex sets the cell signaling master buffer kit was used from Becton Dickinson, Cat. No. 5600006. The samples were analyzed using BD FACSarray Bioanalyzer System
Effect of antioxidants, Sod-Catalase mimetics and pharmacological inhibitors of various kinases on MMP-1 production
IMR90 cells were treated with 10mM n-acetyl cysteine (NAC) for 8hr, 40μM of the Sod-Catalase mimetic Fe(III) tetrakis (4-benzoic acid) porphyrin (FeTBAP) or its inactive analogue (MesoTBAP) overnight and MMP-1 production was determined and compared to untreated controls. Relative contribution of various kinases in MMP-1 production was similarly determined by treating cells overnight with inhibitors: 50μM LY294002 (PI3K), 25μM of PD98059 (Erk), 10μM each of SB203580 (p38) and SP600125 (JNK) (all from Calbiochem, San Diego, CA). Following treatment, MMP-1 protein levels were determined in the media and RNA extracted from cells for real-time PCR and compared to untreated controls.
Statistical analysis
All analysis was performed using one way ANOVA followed by Tukey’s Student T-test unless otherwise indicated.
RESULTS
Senescence associated MMP-1 production is redox-sensitive
The mechanism of age-dependent MMP-1 production was studied in a well established in vitro aging model of replicative senescence using IMR90 primary human (fetal) lung fibroblasts. Key differences in morphology that reflect the senescence phenotype were observed between the young and aged cells (Fig 1 A). Young cells (passage 12-15) were spindle-shaped with uniform morphology and aligned in parallel, while senescent (passage 25 or >) cells displayed a flattened and enlarged phenotype, developed processes and grew sparsely in culture. In addition to morphological changes, the aged cells also induced the cyclin inhibitors p16 and p21, two classical senescence markers(Stein et al, 1999;Stein and Dulic, 1998) (Fig 1B).
Fig. 1.
Oxygen-dependent control of senescence associated MMP-1 expression. (A) Morphologic differences between young and old cells. (B) Immunoblot analysis of MMP-1 and senescence markers, p21 and p16 in cells cultured at 21% O2 verses 3% O2. (C) MMP-1 protein and mRNA levels from young (passage 12-14) and old (passage 24-26) IMR90 cells. Protein levels are reported as relative densitometric intensity measure by NIH ImageJ and error bars represent ± S.E. of mean (n=7). RNA levels were measured by real-time PCR which were normalized to β-actin and reported as cDNA units ± S.E. of mean (n=4). (D) Immunoblot analysis of MMP-1 from aged cells in 3% O2 or acutely transitioned to 21% O2. Representative blot shown (n=3).
Senescence-associated (SA) increases in MMP-1 expression have previously been reported by a number of investigators(Kumar et al, 1993;Millis et al, 1989;Mawal-Dewan et al, 2002). Analysis of late passage cells also revealed a striking increase in the levels of secreted MMP-1 relative to the young cells both at the level of protein and RNA (Fig 1B and C). Senescence can be delayed by maintaining cells under low oxygen conditions (3%) relative to ambient air (21%) (Chen et al, 1995;Poulios et al, 2007;Poulios et al, 2006). IMR-90 cells maintained at 3% O2 showed a reduced level expression of both p16 and p21 senescent markers (Fig 1B) and also an extended replicative lifespan (data not shown). Similar observations were recently reported by Poulios et al (Yusa et al, 1987). We next evaluated the expression of SA MMP-1 in cells maintained under 3% O2. MMP-1 levels were significantly decreased in late passage cell maintained at 3% O2 as compared to their age-matched counterparts at 21% O2 (Fig 1B). The decrease in immunoreactive MMP-1 in late passage cells cultured at 3% O2 was mirrored by a decrease in MMP-1 transcript (Fig 1C). No significant differences were seen in the MMP-1 levels between the young cells under either O2 conditions. Overnight exposure of late passage fibroblasts cultured at 3% O2 to 21% O2 rescued the SA expression of MMP-1 (Fig 1D).
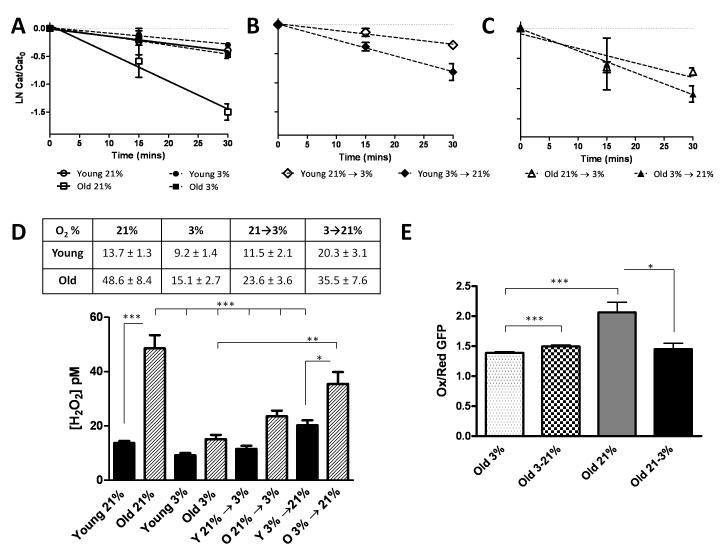
Oxygen tension controls steady state H2O2 concentrations
The O2–dependent regulation of MMP-1 production in the old cells led us to speculate that shifts in steady state ROS levels might impact SA MMP-1 expression. To determine if the differences seen in MMP-1 production between old IMR90 cells at 3% and 21% O2 were associated with changes in ROS we evaluated the redox-state of the cells under both treatment paradigms. Levels of intracellular H2O2 were determined using the 3-Amino 1,2,4-triazole-mediated inhibition of catalase as previously described by Yusa et al.(Dooley et al, 2004). The apparent pseudo-first order rate constant for catalase inhibition by 3-amino1,2,4-triazole was then determined from the slope of the linearizations(Fig 2A-C). The rate constant was then used to determine precise steady state [H2O2] concentrations under the differing treatment conditions and are displayed in both tabular and graphical format in Fig 2D. Late passage cells under 21% O2 showed the highest levels of intracellular H2O2 (48.6 ± 8.4 pM) while late passage cells under 3% O2 (15.1 ± 2.7) had comparable levels to that of early passage cells under 21% O2 (13.7 ± 1.3). The early passage cells under 3% O2 dislayed the lowest [H2O2]. We also observed that increased MMP-1from aged cells maintained at 3% O2 and transitioned overnight to 21% O2 (Fig 1D) was associated with a striking increase in steady state [H2O2] (Fig 2D). Acute transiton from 3→21% or 21→3% O2 increased or decreased steady state [H2O2], respectively (Fig 2D) in both young and old cells. We next evaluated the overall redox-state of the late passage fibroblasts using a redox sensing GFP (roGFP1) construct. The roGFP1 introduces two redox-active cysteines that form a disulfide bridge in response to oxidation that modulates the excitation spectra of GFP and in combination with ratiometric fluorescence microscopy can monitor real time oxidant production. Aged cells under 21% O2 showed a significantly higher proportion of oxidized to reduced roGFP1 relative to 3% O2 (Fig. 2E). Transitioning cells from 3% to 21% O2 brought about a modest but significant increase in the ratio of oxidized to reduced GFP. Interestingly, cells that were transferred from 21% to 3% O2 decreased their oxidation state to that of cells that have been continually cultured at 3% O2 (Fig 2D). These findings indicate that shifts in O2 tension not only modulate the intracellular redox-state of the cell but that aged cells display a more robust steady state production of H2O2 in ambient air.
Fig. 2.
Oxygen tension controls steady-state oxidant production. Intracellular reactive oxygen species (ROS) were determined using an aminotriazole inhibition of catalase assay and a redox-sensitive green fluorescent protein (RoGFP) as described in materials and methods. (A-C) Exponential decline of catalase activity following treatment with ATZ in IMR-90 fibroblasts cultured at 3% and 21% O2. (D) Steady-state intracellular [H2O2] in pM in IMR-90 cells at 3% and 21% O2 and cells that were transitioned from low to high O2 or vice versa. The levels were determined based on the rate of catalase inhibition. Values are reported ± S.E. of mean (n=3). (E) Excitation ratio at λ410/λ470 was determined in senescent cells under 3% and 21% O2 or following transfer of cells from 3% to 21% O2 and vice-versa. Data reported as a ratio of excitation at λ410/λ470 with a higher ratio 410/470 indicating an increased oxidized state and represented as ± S.E. of mean. A minimum of 10 cells were measured for roGFP1 oxidation.
Senescence-associated MMP-1 expression is c-Jun N-terminal kinase-dependent
ROS act as important second messengers that regulate many signaling networks. We have previously described the importance of JNK in the redox-control of MMP-1(Reunanen et al, 2002;Mawal-Dewan et al, 2002). In addition, Phosphoinositol-3-Kinase (PI3K) and extracellular signal-regulated kinases (Erk) are important in regulating MMP expression (Auble et al, 1992;Nelson et al, 2006). To evaluate the contribution of these signaling networks on SA MMP-1 expression, aged cells were treated with pharmacological inhibitors for the indicated pathways. Inhibition of PI3K, Erk, JNK and p38 pathway decreased MMP-1 levels suggesting that all of the networks, in part, contribute to maximal SA MMP-1 expression (Fig 3A), while the only statistically significant reduction was seen with JNK inhibitor, SP600125. JNK-dependent inhibition of MMP-1 was observed at both the protein and mRNA levels (Fig. 3A +B). The JNK inhibitor did not affect phosphorylation of Akt or the other MAP Kinases (data not shown) suggesting that the decrease in MMP-1 was JNK-specific. JNK has been reported to regulate MMP-1 expression at the level of transcription and the observed decrease in MMP-1 mRNA (Fig. 3B) suggests that similar transcriptional control might be involved in SA MMP-1 expression. We next tested the ability of adenoviral dominant negative JNK (Ad-DN-JNK1) to suppress SA expression of MMP-1. Infection of aged IMR-90 cells with either an adenoviral GFP control or Ad-DN-JNK1 showed a similar level of transduction as monitored by immunofluorescent microscopy (Fig. 3C). Infection with Ad-DN-JNK1 abolished the age related increase in MMP-1 expression (Fig 3C). These findings indicate that age-associated increases in MMP-1 expression are primarily JNK-dependent.
Fig. 3.
Inhibition of JNK signaling impairs senescence associated MMP-1 production. Old IMR90 cells at passage 25 under 21% O2 were treated with various pharmacological inhibitors (50μM LY294002 for PI3K, 25μM PD98059 for MEK/Erk, 10μM SB203580 for p38 and 10μM SP600125 for JNK) overnight. MMP-1 protein and RNA levels were analyzed as described above. p<0.05, using one-tailed unpaired t-test of protein (A) and RNA (B). Data represented as ± S.E. of means, n=3 and normalized to cell number for protein and to β-actin for RNA. (C) Senescent IMR90 cells under 21% O2 were infected with adenovirus expressing GFP or a dominant/negative JNK-1-GFP fusion protein and MMP-1 production determined by immunoblotting (n=3). A representative immunoblot and quantification (mean ± S.D.) are shown in inset. Adenoviral infection was confirmed by monitoring GFP as shown in the representative transmitted light and fluorescent images.
Senescence associated MMP-1 expression is associated with increased JNK phosphorylation and c-Jun recruitment
We evaluated whether senescence was also associated with an increase in the active or phosphorylated isoforms of PI3K, p38, Erk and Jnk. The most pronounced increase was observed in JNK phosphorylation in aged cells maintained at 21% O2 relative to their aged matched controls at 3% O2 (Fig 4A). JNK regulates gene expression via its downstream target the transcription factor c-Jun, which plays an important role in MMP-1 transcription (Kamata et al, 2005;Meng et al, 2002). Chromatin immunoprecipitation analysis of c-Jun binding revealed its increased recruitment to the MMP-1 promoter in senescent cells under ambient O2 compared to young cells cultured under similar conditions. Interestingly, promoter occupancy of c-Jun was significantly reduced in aged cells at 3% O2 (Fig 4B). Thus, redox-dependent increases in JNK activation culminate in the enhanced recruitment of c-Jun to the MMP-1 promoter. The PI3K downstream target, Akt, displayed a SA increase in its phosphorylation that was insensitive to alterations in O2 tension (Fig 4C). Total Akt was unaffected by senescence or oxygen tension (Fig 4C). Erk phosphorylation was unaffected by either age or oxygen tension (Fig 4C). Attempts to monitor p38 phosphorylation using immunoblotting failed, using a number of commercially available antibodies.
Fig. 4.
The activation of the c-Jun N-terminal kinase pathway is both age and redox-responsive. (A) Immunblot analysis of JNK-1/2 phosphorylation in young and old cells cultured at 3% or 21% O2. p-JNK levels were normalized to total JNK. Data reported as relative densitometric intensity measured by NIH ImageJ and errors bars represent ± S.E. of mean, n=3. (B) ChIP analysis of c-Jun binding to the MMP-1 promoter (n=6). Data was normalized to input as well as a no antibody control. (C) Immunoblot anlaysis of Akt and ERK phosphorylation in young and old IMR90 cells cultured in low and high oxygen. Representative blot shown, n=3.
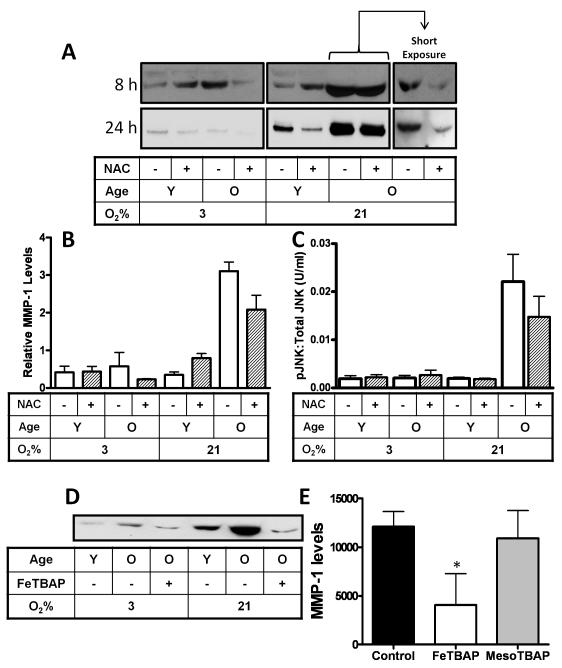
Senescence-associated MMP-1 expression is attenuated by antioxidant treatment
The above observation indicates that shifts in redox-state are associated with parallel changes in MMP-1 expression. To evaluate whether MMP-1 expression is responsive to shifts in ROS levels, aged cells were treated with the glutathione precursor N-acetyl cysteine (NAC) or the sod-catalase mimetic, mimetic Fe(III) tetrakis (4-benzoic acid) porphyrin (FeTBAP). Short (8h) or long (24h) term treatment with NAC restricted SA MMP-1 production from cells maintained at either 3 or 21% O2 (Fig 5A+B). Long term exposure of autoradiograph film was required in most cases to detect MMP-1 expression from young or old IMR-90 cells maintained at 3% O2 using enhanced chemiluminescent substrates. In contrast SA associated MMP-1 required brief exposure to prevent saturation of the chemiluminescent signal (5A, right panels). We also monitored whether the NAC-dependent suppression of SA associated MMP-1 expression was associated with alterations in JNK phosphorylation. Treatment of IMR90 cells with 10mM NAC for 8hr that decreases MMP-1 levels by near 50% in old IMR90 cells (Fig 3A) decreased p-JNK levels in old cells cultured under 21% O2 (Fig 5C). No NAC dependent-shifts in p-JNK levels were observed under any other treatment conditions. These findings indicate that SA increases in Pi-JNK levels respond to shifts in oxygen tension which parallel changes in MMP-1 expression (compare Figs. 5B and C). Treatments with FeTBAP, an efficient scavenger of superoxide, hydrogen peroxide and peroxynitrite, blocked SA MMP-1 production (Fig 5D) while its inactive analogue, MesoTBAP, did not alter SA MMP-1 expression (Fig 5E). Thus, the SA induction of MMP-1 is redox-dependent and can be attenuated by antioxidant treatments.
Fig. 5.
Antioxidants restrict senescence associated MMP-1 expression. (A) MMP-1 immunoblot analysis from IMR-90 cells maintained at 21% and 3% O2 after treatment with 2mM dose of antioxidant, n-Acetyl cysteine (NAC) for 8hrs and 24hrs (B) MMP-1 protein levels determined by densitometry. Data reported as relative densitometric intensity measured by NIH ImageJ and errors bars represent ± S.E. of mean, n=3. MMP-1 levels were normalized to cell number. (C) Phospho-JNK levels in IMR-90 fibroblasts determined by cytometric bead array following treatment with 10mM NAC for 8hrs. Final levels of phospho-JNK were normalized to total JNK and GAPDH. Levels were reported as U/ml ± S.E. of mean, n=5. (D) Analysis of young and old cells cultured at 3 or 21% oxygen following treatment with 20 μM of, the Sod-Catalase mimetic, FeTBAP. A representative immunoblot is shown. (E) MMP-1 analysis of aged cells treated with FeTBAP or its inactive analogue MesoTBAP. Data reported as relative densitometric intensity measure by NIH ImageJ and represented as mean ± S.E, n=3.
Cellular senescence is associated with shifts in the levels of JNK activating kinases and inhibitory phosphatases
MAP kinase kinase-4 (MKK-4) activates JNK by phosphorylation. Analysis of the phospho isoform of MKK-4 revealed that its levels were significantly increase in aged IMR-90 cells maintained at 21% relative to 3% O2, while no differences were observed in the levels of p-MKK4 from young cells maintained at either 3% or 21% O2 (Fig 6A).
Fig. 6.
SA increases in MMP-1 are associated with oxidative-depletion of MKP-1. Immunoblotting for (A) phospho-MKK-4 (n=3), and (B) total MKP-1 in young and old IMR-90 fibroblasts at 21% and 3% O2 (n=2). Human GAPDH used as a loading control. Data reported as relative densitometric intensity measured by NIH ImageJ and error bars represent +/− S.E. of mean. (C) Young and old cells under 21% O2 and (D) senescent cells grown at 21% and 3% O2 were treated with or without 10μM of proteasome inhibitor MG132 overnight followed by immunoprecipitation for MKP-1. Samples were electrophoresed and immunoblotted for anti-ubiquitin using an antibody that detects both mono- and polyubiquitinated forms of the protein.
Dual specificity MAPK phosphatases (MKPs) negatively regulate MAPK signaling and have been reported to be redox-sensitive (Kamata et al, 2005). MKP-1 is a predominant JNK inhibitory phosphatase and is susceptible to redox-inactivation. Thus, we next determined total MKP-1 levels in IMR90 cells by immunoblotting. While no differences were seen in the MKP-1 levels between the young and old cells under 3% O2, aged cells maintained at 21% O2 displayed low level of MKP-1 when compared to their young counterpart (Fig 6B). High level oxidative stress has been reported to bring about oxidative inactivation and degradation of MKPs (Kamata et al, 2005). We hypothesized that SA increases in steady state ROS production (Fig. 2A) may enhance MKP-1 oxidation inactivation thereby sustaining JNK activity. Upon oxidation MKP-1 forms high molecular weight (HMW) aggregates that are ubiquitinated and degraded by the proteasome (Millis et al, 1989;Millis et al, 1992b;Millis et al, 1992a). These ubiquitinated HMW aggregates can be visualized from lysates extracted from cells incubated in the presence of proteasome inhibitor. Cellular lysates from aged cells exposed to 21% O2 displayed a prominent accumulation of ubiquitinated HMW MKP-1 aggregates relative to young cells at 21% O2 (Fig 6C). We also observed that senescent cells maintained in 3% O2 displayed low level MKP-1 ubiquitination relative to cells at 21% O2 (Fig 6D). Overall these findings indicate that senescence is associated with increases in the levels of the JNK-activating kinase, MKK-4 without an accompanying increase in the JNK-inhibitory phosphatase, MKP-1.
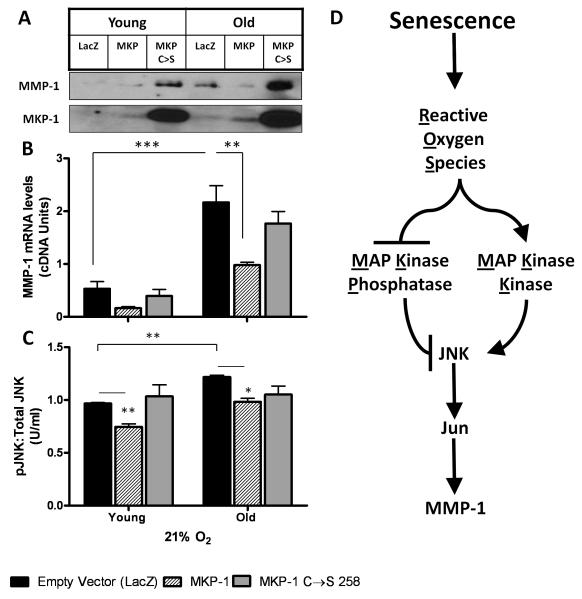
SA MMP-1 expression is prevented by overexpression of JNK-inhibitory phosphatase, MKP-1
Increases in p-MKK4 levels (Fig 6A) along with decreased MKP-1 (Fig 6B) may account for the observed increase in JNK activation (Fig 4A) and SA MMP-1. To test this hypothesis we evaluated whether enforced MKP-1 expression can restrict both SA MMP-1 expression and JNK-activation. Lentiviral-expressed MKP-1 prevented the SA increase in MMP-1 expression and had no effect on the levels of MMP-1 in young cells (Fig. 7A). Enforced expresson of inactive MKP-1 (MKP-1 C248S) did not temper but enhanced MMP-1 expression in both young and old IMR-90 cells. The effect of overexpression of MKP-1 and MKP-1 C248S was also mirrored at the transcript level (Fig 7B). Analysis of JNK-Pi by phospho-bead array indicates MKP-1 attenuates JNK-Pi in both young and old cell cultures. In addition, MKP-1 C248S does not significantly alter JNK-Pi suggesting that its ability to enhance MMP-1 expression may be attributed to some off-target activity that augments MMP-1 expression. Thus, overexpression of the JNK-inhibitory phosphatase MKP-1 restricts JNK signaling and SA MMP-1 exression.
Fig. 7.
Lentiviral mediated over-expression of MKP-1 suppresses senescence associated MMP-1 expression. (A) Immunoblotting for MMP-1 and MKP-1 in young and old IMR-90 fibroblasts at 21% O2 following transient infection with lentiviruses expressing MKP-1. Cells were also infected with lentivirus expressing the phosphatase dead MKP-1 that has a single amino-acid mutation C258S (MKP-1 C→S ). As control, cells were infected with viruses expressing LacZ (empty vector). Human GAPDH was used as loading control for MKP-1. MMP-1 monitored as described in Fig 1. Representative blot shown (n=3). (B) Real Time RT-PCR of MMP-1 in IMR-90 cells following infection with lentiviruses expressing MKP-1 and MKP-1 C→S. Data was normalized to β-actin RNA levels and reported as DNA units ± S.E. of mean (n=3). (C) Phospho-JNK levels determined by cytometric bead array in IMR-90 fibroblasts following infection with lentiviruses expressing MKP-1 and mutant MKP-1. Phospho-JNK levels were normalised to total JNK and GAPDH. Levels were reported as U/ml ± S.E. of mean, n=2. (D) Schematic for the redox and senescence associated regulation of MMP-1 expression.
DISCUSSION
The SA increase in MMP-1 has been reported in a number of cellular aging models as well as in cells isolated from patients with premature ageing syndromes (Chen and Ames, 1994). The current findings indicate that SA MMP-1 is attenuated by conditions that decrease steady state H2O2 production including limiting metabolic O2 flux and antioxidant treatments (Figs. 1 and 3). SA shifts in steady state [H2O2] lead to redox-dependent activation of upstream MAP Kinases and depletion of MKP-1 that maintains JNK signaling and drives the SA increase in MMP-1 expression as outlined in figure 7D.
Replicative senescence can be delayed by low oxygen conditions (3% O2) or by treatment with glutathione-precursor, NAC (Haendeler et al, 2004) (Dooley et al, 2004). Our studies indicate that limiting metabolic O2 consumption by lowering O2 tension restricts intracellular ROS levels as determined both biochemically and using redox-sensing GFP constructs. We observed modest but significant differences in intracellular roGFP1 oxidation between aged cells maintained at 3% O2 relative those at 21% O2 (Fig 2D). This is in contrast to observations by Dooley et al (Takahashi and Zeydel, 1982) where cytosolic roGFP1 oxidation was insensitive to variations in O2 tension. In the current study we have restricted our focus to aged primary cells as compared to tumor cell lines that may possess distinct mechanisms for regulating redox homeostasis. Senescing IMR90 cells are known to decrease levels of the primary H2O2-detoxifying antioxidant, glutathione (Vincenti and Brinckerhoff, 2002). This likely accounts for the ability of the glutathione precursor, NAC, to restrict SA MMP-1 expression (Fig 5C) Decreases in glutathione may limit the ability to scavenge ROS in senescent primary fibroblasts at 21% O2. Our biochemical analysis has revealed that increased steady state H2O2 production is intrinsic to senescent cells. While under low oxygen tension both young and old cells produce similar steady quantities of H2O2. However, upon acute transition of both young and old cells to ambient air, senescent cell cultures show a much more robust increase in oxidant production than presenescent cells. It is likely that shifts in both oxidant-generating and -scavenging systems contribute to enhanced steady state H2O2 generating capacity of aged cells.
The SA redox-dependent induction of MMP-1 was observed at the level of both protein and RNA (Fig 1 C&D). MMP-1 is predominantly regulated at the level of transcription (Ranganathan et al, 2001) and can involve the activation of a myriad of signaling networks. Our findings also indicate that inhibition of JNK, PI-3-K, Erk and p38 individually, can impair SA MMP-1 expression (Fig 3A). Phosphorylation of Akt is age-dependent but not redox-responsive (Fig 4C) suggesting that this pathway might play redox-independent role in regulating SA MMP-1 expression. The redox-insensitive nature of Akt may be related to upstream signals that drive age-related PI3K signaling. MEK/Erk inhibition also attenuated SA MMP-1 (Fig 3A) and has been shown to be contributory to redox-dependent MMP-1 regulation in tumor cell lines (Brauchle et al, 2000) (Brauchle et al, 2000). Our current findings suggest that Erk phosphorylation is unaffected by age or oxygen tension (Fig 4C) though inhibition of the pathway did bring about a decrease in MMP-1 levels suggesting that Erk may also contribute to maximal SA MMP-1 expression. Similarly, p38 inhibition also reduced MMP-1 expression and has been reported to play a role in regulating MMP-1 levels by stabilizing the MMP-1 mRNA via its 3′ untranslated region (Reunanen et al, 2002) (Fleming et al, 2000). Whether MMP-1 mRNA stability is altered with age or is redox-dependent requires examination.
The regulation of MAPK signaling is largely dependent on phosphorylation-dependent activation by upstream MAPK Kinases and negative regulation by phosphatases including MAP Kinase Phosphatases (MKPs). JNK is activated by MKK-4 and MKK-7 that are known to phosphorylate it on Thr 185 and Tyr-183 respectively (Sundarrajan et al, 2003). MKK-4 and MKK-7 form a complex with JNK (Wang et al, 2007) and genetic ablation of either MKK indicates that they are mutually exclusive in their function and that synergy is needed for complete JNK activation (Moriguchi et al, 1997;Foltz et al, 1998). The degree of activation of the MKKs can be dictated by the activating stimulus. MKK-7 is more sensitive to activation by inflammatory cytokines, environmental stress and physiological stimuli (Wada et al, 2001). Wada et al report that while MKK-4 and MKK-7 are both needed for JNK activation, loss of MKK-4 inhibits MKK-7 mediated phosphorylation of JNK (Kondoh and Nishida, 2007;Keyse, 1999). This suggests that MKK-4 mediated phosphorylation of JNK precedes that of MKK-7. We have also established that MKK-4 phosphorylation is increased in a redox-dependent manner in the senescent cells (Fig 6A) and likely contributes to the SA associated expression of MMP-1.
In addition to phosphorylation-dependent activation, MAPK signaling is tempered by a large family of inhibitory phosphatases including the protein phosphatases and the dual specificity phosphatase or MAPK phosphatase (MKP) family members (Kamata et al, 2005). MKPs show cross-reactivity in their dephosphorylating substrates with some degree of specificity and affinity. MKP-1, 2, 5, 7 and M3/6 are the primary JNK-specific phosphatases that are activated in response to oxidative stress. The current work suggest that the SA attenuation of MKP-1 is associated with its enhanced ubiquitination that is tempered by restricting exposure to 21% O2 (Fig 6C+D ). Oxidative stress can drive oxidative inactivation and degradation of active site cysteines leading to the formation of high molecular weight aggregates that are degraded by proteasome (Chen et al, 2001). Thus, increases in steady state ROS production, as observed in senescent IMR90 fibroblast under ambient air (21% O2) (Fig 2D), may drive MKP turnover through oxidation and subsequent ubiquitin-mediated degradation (Fig 6C) leading to the low steady state immunoreactive MKP-1 in these cells (Fig 6B). Moreover, ubiquitinated MKP-1 was only observed in the presence of proteasome inhibitor and was decreased by lowering oxygen tension. These findings suggest that increases in SA steady state [H2O2] may restrict regulatory phosphatases thereby enhancing overall kinase signaling. Overall a decrease in MKP-1 and a concomitant increase in MKK-4 activity as seen in the old cells under 21% O2 would potentially sustain JNK phosphorylation. Restoring MKP-1 levels in the senescent cells under 21% O2 by lentiviral infection impairs SA MMP-1 production (Fig 7A). A similar mechanism has been reported for the phosphatase M3/6 leading to JNK activation by H2O2 (Zhao et al, 2008). It is also possible that other redox-sensitive phosphatases participate in JNK activation by modulating the activity of upstream kinases such as MKK-4 (Fisher et al, 2009). Reconstitution studies with the distinct MKPs will be necessary to evaluate the contribution of any particular MKP in the SA redox-dependent expression of MMP-1.
In summary, these studies demonstrate that cellular aging is associated with an increase in MMP-1 expression that involves alterations in steady state ROS production leading to JNK activation. JNK activation is mediated by redox-alterations in the activation state of regulatory kinases and inhibitory phosphatases. Fisher et al. recently demonstrated that the levels of the JNK target, c-Jun, are increased in human dermal fibroblasts from aged (>80 years) relative to young individuals. Our findings extend those of Fisher et al. and further elaborate the redox-sensitive signaling networks that likely drive age-associated increases in MMP-1 expression. It is intriguing to speculate that other MMPs may also show similar age-associated and oxidant-dependent control as many of their transcriptional regulatory elements are positionally conserved. These findings also further provide a mechanistic link between the free radical theory of aging and degenerative disease processes associated with aberrant MMP production. We are excited by the possibility that antioxidant-based therapies, by their ability to limit MMP expression, may prove useful in the treatment of many age-related disease processes that are associated with excessive matrix turnover.
Acknowledgements
The research described in this article was supported by NIA (AG031067 + AG031067-02S1 ) (J.A.M) and in part from Philip Morris USA, Inc., and Philip Morris International.
Contract grant sponsor: National Institute of Aging; Contract grant number: AG031067 + AG031067-02S1.
Literature Cited
- Auble DT, Sirum-Connolly KL, Brinckerhoff CE. Transcriptional regulation of matrix metalloproteinase genes: role of AP-1 sequences. Matrix Suppl. 1992;1:200. [PubMed] [Google Scholar]
- Brauchle M, Gluck D, Di PF, Han J, Gram H. Independent role of p38 and ERK1/2 mitogen-activated kinases in the upregulation of matrix metalloproteinase-1. Exp Cell Res. 2000;258:135–144. doi: 10.1006/excr.2000.4913. [DOI] [PubMed] [Google Scholar]
- Brejc K, Sixma TK, Kitts PA, Kain SR, Tsien RY, Ormo M, Remington SJ. Structural basis for dual excitation and photoisomerization of the Aequorea victoria green fluorescent protein. Proc Natl Acad Sci U S A. 1997;94:2306–2311. doi: 10.1073/pnas.94.6.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci U S A. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci U S A. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Shrivastava A, Tan TH. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene. 2001;20:367–374. doi: 10.1038/sj.onc.1204105. [DOI] [PubMed] [Google Scholar]
- Claiborne A. Catalase activity. 772. In: Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. CRC Press Inc.; Boca Raton, FL: 1985. pp. 283–284. [Google Scholar]
- Dasgupta J, Subbaram S, Connor KM, Rodriguez AM, Tirosh O, Beckman JS, Jourd’heuil D, Melendez JA. Manganese superoxide dismutase protects from TNF-alpha-induced apoptosis by increasing the steady-state production of H2O2. Antioxid Redox Signal. 2006;8:1295–1305. doi: 10.1089/ars.2006.8.1295. [DOI] [PubMed] [Google Scholar]
- Domeij H, Modeer T, Yucel-Lindberg T. Matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 production in human gingival fibroblasts: the role of protein kinase C. J Periodontal Res. 2004;39:308–314. doi: 10.1111/j.1600-0765.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ. Collagen Fragmentation Promotes Oxidative Stress and Elevates Matrix Metalloproteinase-1 in Fibroblasts in Aged Human Skin. Am J Pathol. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming Y, Armstrong CG, Morrice N, Paterson A, Goedert M, Cohen P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem J. 2000;352(Pt 1):145–154. [PMC free article] [PubMed] [Google Scholar]
- Foltz IN, Gerl RE, Wieler JS, Luckach M, Salmon RA, Schrader JW. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J Biol Chem. 1998;273:9344–9351. doi: 10.1074/jbc.273.15.9344. [DOI] [PubMed] [Google Scholar]
- Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda Si, Maeda S, Chang L, Hirata H, Karin M. Reactive Oxygen Species Promote TNF[alpha]-Induced Death and Sustained JNK Activation by Inhibiting MAP Kinase Phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Keyse SM. The role of protein phosphatases in the regulation of mitogen and stress-activated protein kinases. Free Radic Res. 1999;31:341–349. doi: 10.1080/10715769900300911. [DOI] [PubMed] [Google Scholar]
- Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta. 2007;1773:1227–1237. doi: 10.1016/j.bbamcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Kumar S, Vinci JM, Millis AJ, Baglioni C. Expression of interleukin-1 alpha and beta in early passage fibroblasts from aging individuals. Exp Gerontol. 1993;28:505–513. doi: 10.1016/0531-5565(93)90039-g. [DOI] [PubMed] [Google Scholar]
- Liu R, Zheng HQ, Zhou Z, Dong JT, Chen C. KLF5 promotes breast cell survival partially through fibroblast growth factor-binding protein 1-pERK-mediated dual specificity MKP-1 protein phosphorylation and stabilization. J Biol Chem. 2009;284:16791–16798. doi: 10.1074/jbc.M808919200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawal-Dewan M, Lorenzini A, Frisoni L, Zhang H, Cristofalo VJ, Sell C. Regulation of collagenase expression during replicative senescence in human fibroblasts by Akt-forkhead signaling. J Biol Chem. 2002;277:7857–7864. doi: 10.1074/jbc.M104515200. [DOI] [PubMed] [Google Scholar]
- Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- Millis AJ, Hoyle M, McCue HM, Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp Cell Res. 1992a;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- Millis AJ, McCue HM, Kumar S, Baglioni C. Metalloproteinase and TIMP-1 gene expression during replicative senescence. Exp Gerontol. 1992b;27:425–428. doi: 10.1016/0531-5565(92)90076-c. [DOI] [PubMed] [Google Scholar]
- Millis AJ, Sottile J, Hoyle M, Mann DM, Diemer V. Collagenase production by early and late passage cultures of human fibroblasts. Exp Gerontol. 1989;24:559–575. doi: 10.1016/0531-5565(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFalpha and cellular stresses. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KK, Subbaram S, Connor KM, Dasgupta J, Ha XF, Meng TC, Tonks NK, Melendez JA. Redox-dependent Matrix Metalloproteinase-1 Expression Is Regulated by JNK through Ets and AP-1 Promoter Motifs. J Biol Chem. 2006;281:14100–14110. doi: 10.1074/jbc.M601820200. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37:283–288. doi: 10.1016/j.biocel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Poulios E, Trougakos IP, Chondrogianni N, Gonos ES. Exposure of human diploid fibroblasts to hypoxia extends proliferative life span. Ann N Y Acad Sci. 2007;1119:9–19. doi: 10.1196/annals.1404.025. [DOI] [PubMed] [Google Scholar]
- Poulios E, Trougakos IP, Gonos ES. Comparative effects of hypoxia on normal and immortalized human diploid fibroblasts. Anticancer Res. 2006;26:2165–2168. [PubMed] [Google Scholar]
- Ranganathan AC, Nelson KK, Rodriguez AM, Kim KH, Tower GB, Rutter JL, Brinckerhoff CE, Epstein CJ, Huang TT, Jeffrey JJ, Melendez JA. Manganese superoxide dismutase signals matrix metalloproteinase expression Via H2O2-dependent ERK1,2 activation 1311. J Biol Chem. 2001;276:14264–14270. doi: 10.1074/jbc.M100199200. [DOI] [PubMed] [Google Scholar]
- Reunanen N, Li SP, Ahonen M, Foschi M, Han J, Kahari VM. Activation of p38 alpha MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J Biol Chem. 2002;277:32360–32368. doi: 10.1074/jbc.M204296200. [DOI] [PubMed] [Google Scholar]
- Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GH, Dulic V. Molecular mechanisms for the senescent cell cycle arrest. J Investig Dermatol Symp Proc. 1998;3:14–18. [PubMed] [Google Scholar]
- Sundarrajan M, Boyle DL, Chabaud-Riou M, Hammaker D, Firestein GS. Expression of the MAPK kinases MKK-4 and MKK-7 in rheumatoid arthritis and their role as key regulators of JNK. Arthritis Rheum. 2003;48:2450–2460. doi: 10.1002/art.11228. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Zeydel M. gamma-Glutamyl transpeptidase and glutathione in aging IMR-90 fibroblasts and in differentiating 3T3 L1 preadipocytes. Arch Biochem Biophys. 1982;214:260–267. doi: 10.1016/0003-9861(82)90029-7. [DOI] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Signal transduction and cell-type specific regulation of matrix metalloproteinase gene expression: can MMPs be good for you? J Cell Physiol. 2007;213:355–364. doi: 10.1002/jcp.21208. [DOI] [PubMed] [Google Scholar]
- Wada T, Nakagawa K, Watanabe T, Nishitai G, Seo J, Kishimoto H, Kitagawa D, Sasaki T, Penninger JM, Nishina H, Katada T. Impaired synergistic activation of stress-activated protein kinase SAPK/JNK in mouse embryonic stem cells lacking SEK1/MKK4: different contribution of SEK2/MKK7 isoforms to the synergistic activation. J Biol Chem. 2001;276:30892–30897. doi: 10.1074/jbc.M011780200. [DOI] [PubMed] [Google Scholar]
- Wang X, Nadarajah B, Robinson AC, McColl BW, Jin JW, jas-Bailador F, Boot-Handford RP, Tournier C. Targeted deletion of the mitogen-activated protein kinase kinase 4 gene in the nervous system causes severe brain developmental defects and premature death. Mol Cell Biol. 2007;27:7935–7946. doi: 10.1128/MCB.00226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa T, Beckman JS, Crapo JD, Freeman BA. Hyperoxia increases H2O2 production by brain in vivo. J Appl Physiol. 1987;63:353–358. doi: 10.1152/jappl.1987.63.1.353. [DOI] [PubMed] [Google Scholar]
- Zhao B, Sun L, Haas M, Denenberg AG, Wong HR, Shanley TP. PP2A regulates upstream members of the c-jun N-terminal kinase mitogen-activated protein kinase signaling pathway. Shock. 2008;29:181–188. doi: 10.1097/SHK.0b013e318070c840. [DOI] [PubMed] [Google Scholar]