Abstract
Previous investigations of somatic hypersensitivity in IBS patients have typically involved only a single stimulus modality, and little information exists regarding whether patterns of somatic pain perception vary across stimulus modalities within a group of patients with IBS. Therefore, the current study was designed to characterize differences in perceptual responses to a battery of noxious somatic stimuli in IBS patients compared to controls. A total of 78 diarrhea-predominant and 57 controls participated in the study. We evaluated pain threshold and tolerance and sensory and affective ratings of contact thermal, mechanical pressure, ischemic stimuli, and cold pressor stimuli. In addition to assessing perceptual responses, we also evaluated differences in neuroendocrine and cardiovascular responses to these experimental somatic pain stimuli. A subset of IBS patients demonstrated the presence of somatic hypersensitivity to thermal, ischemic, and cold pressor nociceptive stimuli. The somatic hypersensitivity in IBS patients was somatotopically organized in that the lower extremities that share viscerosomatic convergence with the colon demonstrate the greatest hypersensitivity. There were also changes in ACTH, cortisol, and systolic blood pressure in response to the ischemic pain testing in IBS patients when compared to controls. The results of this study suggest that a more widespread alteration in central pain processing in a subset of IBS patients may be present as they display hypersensitivity to heat, ischemic, and cold pressor stimuli.
Keywords: Irritable bowel syndrome (IBS), Heat pain threshold and tolerance (HPTh and HPTo), Cold pressor pain threshold and tolerance (CPTh and CPTo), Pressure (mechanical) pain threshold (PPT), Ischemic pain threshold and tolerance (IPTh and IPTo)
1. Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder that is estimated to affect up to 20% of the US population. Even though the pathophysiology of IBS is unclear, visceral and somatic hypersensitivity have been shown to be present in a subset of IBS patients [25]. The pathophysiological mechanisms of pain and hypersensitivity in IBS are not well understood. Some patients with IBS may demonstrate enhanced perception in response to brief phasic distension of the gut lumen, or visceral hypersensitivity [28]. Visceral hypersensitivity may account for symptoms of urgency, bloating, and abdominal pain experienced by patients with IBS and is an accepted biological marker in some IBS patients. Although the exact pathophysiology of gastrointestinal symptoms in IBS is unknown, several mechanisms have been postulated and include triggering events such as post-injury sensitization, inflammation, psychological or environmental stress [9,14,39].
Many patients with IBS also exhibit a wide variety of somatic symptoms including back pain, migraine headaches, heartburn, dyspareunia, and muscle pain in body regions somatotopically distinct from the gut. Collectively, these somatic symptoms suggest that IBS patients may also suffer from central hyperalgesic dysfunction [19,27]. Similar to IBS, patients with other chronic pain disorders such as temporomandibular disorders have been shown to have generalized hyperalgesia [22]. Previously, hypersensitivity was thought to be limited to the gut. Several studies have now shown that some IBS patients demonstrate hypersensitivity to nociceptive stimuli applied to the extremities [2,26,36]. These studies suggest that the processing of visceral and somatic stimuli overlap as a result of viscerosomatic convergence in the lumbosacral distribution. Thus, chronic afferent nociceptive input from the gut may sensitize spinal cord neurons that exhibit somatotopic overlap with the gut. We have previously shown that a subset of IBS patients have greater somatic hypersensitivity than controls and this somatic hypersensitivity is somatotopically organized in that the lower extremities that share viscerosomatic convergence with the colon demonstrate the greatest hypersensitivity [41].
Previous investigations of somatic hypersensitivity have typically involved only a single stimulus modality, and little information exists regarding whether the patterns of somatic pain perception vary across stimulus modalities within a group of patients with IBS. Therefore, the current study was designed to characterize differences in perceptual responses to a battery of noxious somatic stimuli in IBS patients compared to controls. To accomplish this, we evaluated pain threshold and tolerance and sensory and affective ratings of contact thermal, mechanical pressure, cold pressor, and ischemic stimuli. In addition to assessing perceptual responses, we also evaluated differences in neuroendocrine and cardiovascular responses to these experimental somatic pain stimuli. Thus, the aims of the current study were: (1) to compare differences in somatic hypersensitivity between patients with IBS and controls across spatial areas that provide spinal input at differing levels; (2) to test for differences between IBS patients and controls as a function of the characteristics of the pain stimuli; and (3) to examine differences in physiological responses to experimental pain stimuli between IBS patients and controls.
2. Methods
2.1. Participants
The study was approved by the University and Veterans Administration Institutional Review Boards and all subjects signed informed consent prior to the start of the study. All IBS patients that were recruited had gastrointestinal symptoms for at least 5 years and had upper endoscopy and colonoscopy with biopsies which were normal. In addition, all IBS patients had a normal lactulose breath test to exclude bacterial overgrowth; a negative serum tissue transglutaminase to exclude celiac sprue; and negative stool studies. The diagnosis of IBS was made by an experienced gastroenterologist who based the diagnosis on the ROME III criteria and exclusion of organic disease [13]. Fibromyalgia (FM) was excluded in all IBS patients and controls using the 1990 American College of Rheumatology criteria for FM [10]. The controls that were recruited were free of any evidence of acute and/or chronic somatic/abdominal pain based on a complete history and physical exam by an experienced gastroenterologist. Controls were screened for any systemic conditions that could affect sensory responses.
None of the participants were on serotonin antagonists, pain medications, serotonin uptake inhibitors, or tricyclic antidepressants for at least 1 month prior to the study. Subjects were instructed to refrain from the use of any analgesic medication or caffeine for 72 h before their sessions. Subjects with elevated systolic blood pressure (>135 mmHg) and/or elevated diastolic blood pressure (>85 mmHg) were excluded from participating in the study [8]. Female subjects were tested during the follicular phase and not during active menstruation. While previous research suggests that pain responses vary across the menstrual cycle, the effect sizes are generally small to moderate [21].
All study participants completed the Beck Depression Inventory (BDI) and the State Trait Anxiety Inventory (STAI) [1,23]. Depressive and anxiety disorders were exclusions if they were severe enough to interfere with the individual’s ability to participate in the study. Therefore, participants who scored ≥30 on the BDI and/or ≥53 on the STAI were excluded. Such scores are approximately one standard deviation above the mean for general medical patients.
2.2. Experimental pain procedures
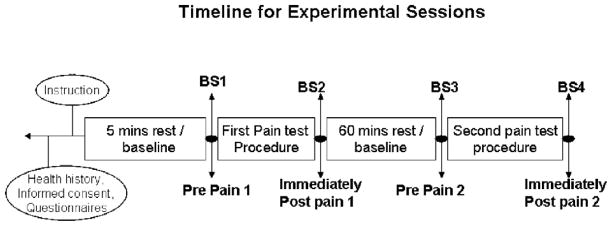
Each subject participated in two experimental sessions lasting approximately for 2 h each, such that two pain tasks were administered during the first sensory testing session, and the other two pain tasks during the second testing session. The assignment of pain tasks to sessions 1 and 2 and the order of the pain tasks within the sessions were randomly determined for each subject. Blood draws were done approximately around 3 and 5 PM to control for circadian rhythm effects of cortisol and corticotrophin (ACTH) secretion. The 2 sensory testing sessions were scheduled <1 week apart and were done on days that the study subjects were free from abdominal pain and/or any extraintestinal symptoms (i.e. migraine headaches, back, or joint pain. Following a 5-min supine rest period, systolic blood pressure and a blood sample were obtained after which pain testing began. Immediately after the first pain procedure, systolic blood pressure was measured and a blood sample was obtained again for the measurement of cortisol and corticotrophin (ACTH). After the 1st pain task, a 60-min rest period in the semi-recumbent position was conducted to allow neuroendocrine and cardiovascular responses to normalize. The timing of blood samples and duration of the recovery period between pain tasks are based on prior research examining cortisol and ACTH responses to stress and pain [12,17]. After the recovery period, systolic blood pressure was then obtained and another blood sample was taken to establish baseline cortisol and ACTH values before the second pain task. The second pain task was then conducted. Recorded instructions were played prior to each pain task. Fig. 1 illustrates a timeline for the two experimental sessions.
Fig. 1.
Timeline for experimental sessions. BS indicated blood sample draw.
Systolic blood pressure was obtained using an automated blood pressure device (Protocol Propaq Encore 104 El Patient Monitor, Welch Allyn, Beaverton, OR). This instrument provides near-continuous measurement of blood pressure with a NIBP cuff that can be automatically inflated at defined time points. Systolic blood pressure was measured at the 4 time points immediately before blood was drawn.
Levels of cortisol and ACTH were determined using the commercially available radioimmunoassay kits. Serum Cortisol determinations were made using the Cortisol Coat-A-Count RIA (Siemens Medical Solutions Diagnostics, 5700 West 9th Street, Los Angeles, CA 90045). Intra-assay coefficient of variation is 4.3% and inter-assay coefficient of variation is 5.2%. Sensitivity is 0.2 μg/dl. This assay was counted on the Packard Cobra II Gamma Counter (Packard Instrument Company, 800 Research Parkway, Meriden, CT 06450) and calculated using Stat Lia™ software (Brendan Scientific). Normal values for serum Cortisol are 5–25 μg/dl in the AM. Cortisol exhibits diurnal variation with the samples collected in PM hours having approximately half the values of AM collections. ACTH was measured using the Immulite 1000 with reagents manufactured specifically for this instrument (Siemens Medical Solutions Diagnostics, 5700 West 9th Street, Los Angeles, CA 90045). Intra-assay coefficient of variation is 5.6% and inter-assay coefficient of variation is 7.8%. Sensitivity is 9 pg/ml. Normal values used for ACTH are 12–46 pg/ml.
2.3. Heat pain threshold (HPTh) and tolerance (HPTo)
All thermal stimuli were delivered using a computer-controlled Medoc Thermal Sensory Analyzer (TSA-2001, Ramat Yishai, Israel), which is a peltier-element-based stimulator. Temperature levels were monitored by a contactor-contained thermistor, and returned to a preset baseline of 32 °C by active cooling at a rate of 10 °C/s. Heat pain thresholds and heat pain tolerances were assessed on the ventral aspect of the right palm of the hand and the right sole of the foot using an ascending method of limits. These sites were chosen because they represent both a remote body site (palm) and a test site that shows spinal segmental convergence with the colon (sole of foot). From a baseline of 32 °C, probe temperature increased at a rate of 0.5 °C/s until the subject responded by pressing a button on a handheld device. This slow rate of rise preferentially activates C-fibers and diminishes artifact associated with reaction time [32]. For heat pain threshold, subjects were instructed to press the button when the sensation “first becomes painful”, and for tolerance the instruction was to press the button when they “no longer feel able to tolerate the pain”.
Three trials of HPTh were presented followed by a 5-min rest period and then three trials of HPTo. The average of the three trials at each site was computed for heat pain threshold and tolerance. Stimulation sites (palm/sole) were alternated to prevent carryover effects due to local sensitization. The position of the thermode was also altered proximal to the site but not overlapping between trials in order to avoid either sensitization or habituation of cutaneous receptors. In addition, interstimulus intervals of at least 30 s were maintained between successive stimuli. The trial was conducted with both a large thermode (3 × 3 cm) and with a small thermode (1 × 1 cm) to determine if spatial summation of thermal pain occurred.
2.4. Pressure (mechanical) pain threshold (PPT)
PPT was performed by applying a constant rate of pressure and recording the pressure in kilograms when the subject responded. An application rate of 1 kg per second was used, as this relatively slow application rate reduced artifact associated with reaction time [11]. Pressure was applied to the right ventral forearm and the right calf using a 0.5 cm2 probe attached to a handheld, clinical scale pressure algometer (Jtech, Heber City, UT). These sites were chosen because they represent both a remote body site (forearm) and a test site that shows spinal segmental convergence with the colon (calf). Subjects were instructed to respond when they first felt pain, at which time the experimenter stopped applying pressure and recorded the PPT. PPT was assessed four times at each site, with the order of site presentation randomized. Mechanical pain tolerance was not used, only pressure pain threshold was used. The reason for this is that the use of prolonged nociceptive mechanical stimuli would activate non-nociceptive mechanoreceptive neurons whose input could be inhibited at higher cortical levels by chronic visceral nociceptive input in IBS patients but not in controls.
2.5. Ischemic pain threshold (IPTh) and tolerance (IPTo)
The ischemic test was conducted using a modified submaximal effort tourniquet test [15]. The right arm was exsanguinated by elevating it above heart level for 60 s after which the arm was occluded with a straight segmental blood pressure cuff inflated to 240 mmHg using a Hokanson cuff inflator and air source (Bellevue, WA). Subjects were then instructed to perform 20 hand grip exercises of 2-s duration at 4-s intervals at 50% of their maximum grip strength. Subjects were instructed to say “pain” when they first felt pain and to continue until the perceived pain became intolerable or for 15-min. The time to IPTh and IPTo was recorded for each subject.
2.6. Cold pressor pain threshold (CPTh) and tolerance (CPTo)
The cold-pressor procedure was conducted as described previously [20]. Subjects immersed both their right hand and foot (during separate sessions) in 5 °C water. These sites were chosen because they represent both a remote body site (hand) and a test site that shows spinal segmental convergence with the colon (foot). The water temperature was maintained (±0.1 °C) by a refrigeration unit (Neslab, Portsmouth, NH), and the water was constantly recirculated to maintain a constant temperature throughout the water bath and to prevent local warming around the submerged hand or foot. Subjects were told that they could remove their hand/foot at any time if the pain became intolerable. CPTh was determined when study subjects first felt pain. CPTo was recorded when the study subjects withdrew their hand/foot from the water. Study subjects were instructed to keep their hand/foot in the cold water for as long as possible for up to 5-min or until they reported intolerable pain, as in previous studies [3,20]. While prior research has used many different cutoff times, a large normative study has used a 5-min cutoff [30], and this maximum will allow greater variability in pain tolerance, which we may be able to predict based on neuroendocrine responses.
2.7. Statistical analysis
The statistical analysis was performed using SAS software, Version 9.1.3 of SAS system Copyright© 2003 SAS institute Inc. and Prism version 6. The analysis involved one between- and two with-in-subject variables, and consequently, the repeated measures command within the General Linear model module of SPSS was used. In the first analysis, differences between controls and IBS patients for HPTh, HPTo, and PPTh at the forearm and calf were tested. In the second analysis, differences between controls and IBS patients for CPTh and CPTo for the hand and foot were tested. The third analysis tested differences between controls and IBS patients for IPTH and IPTo of the arm.
Frequency distribution was used to classify the IBS patients based on pain thresholds on the individual tests. IBS patients were separated into two distinct subsets based on the distribution and range of the threshold and tolerance of reported values. The hypersensitive group of IBS patients was defined as those IBS patients with pain sensitivity ratings that fell out of the total range in normal controls. One way ANOVA followed by Tukey comparison was used to analyze the pain testing data. Values are expressed as means ± standard deviation (SD).
3. Results
A total of 144 participants were recruited for entry into this study. Nine participants (six IBS and three controls) dropped out of the study and did not complete all of the sessions. A total of 135 participants completed the study which included 78 IBS patients and 57 controls (Table 1). There was no difference between the two groups in age, sex or ethnicity. There were no group differences on the Beck Depression Inventory (BDI) and the State Trait Anxiety Inventory (STAI) between the IBS patients and controls (p = 0.8). Covariate analysis was performed did not reveal any effects of scores on the BDI and STAI in IBS patients or controls on pain sensitivity data. All the IBS patients were diarrhea-predominant and met the Rome III criteria for irritable bowel syndrome. Some of the IBS patients included in the study had a history of extraintestinal symptoms, however, they were all free from acute abdominal pain and/or extraintestinal symptoms during the day of the sensory testing.
Table 1.
Demographic variables for IBS patients and controls.
| IBS patients (n = 78) | Controls (n = 57) | |
|---|---|---|
| Age (mean) ± SD | 31.7 ± 7.1 | 29.5 ± 8.3 |
| Sex (%) | 55 Female (71%) 23 Male (29%) |
40 Female (70%) 27 Male (30%) |
| Self-reported race/ethnicity | ||
| White | 56 (72%) | 39 (68%) |
| Black | 8 (10%) | 7 (12%) |
| Hispanic | 6 (8%) | 4 (7%) |
| Asian | 8 (10%) | 7 (12%) |
3.1. Heat pain threshold (HPTh) and tolerance (HPTo)
Overall, IBS patients demonstrated lower HPTh and HPTo in response to 3 × 3 thermal probe stimulation of the foot compared to controls (Fig. 2). There was no difference in HPTh or HPTo measures between IBS patients and controls on the palm of the hand. In the controls, there were no differences in HPTh or HPTo between the hand and foot. In contrast, IBS patients demonstrated lower HPTh and HPTo in the foot compared to the hand (Fig. 3). Comparison of thermal stimulation of the hand and foot yielded a significant main effect for group and site (p < 0.001). This indicated that IBS patients reported significantly higher pain ratings compared to controls, with foot stimulation being more painful than hand stimulation. There were no significant differences using the 1 × 1 cm probe compared to the 3 × 3 cm probe. There were no significant changes in cortisol/ACTH and systolic blood pressure following the thermal pain testing in IBS patients and controls (Figs. 4 and 5 and Table 2).
Fig. 2.
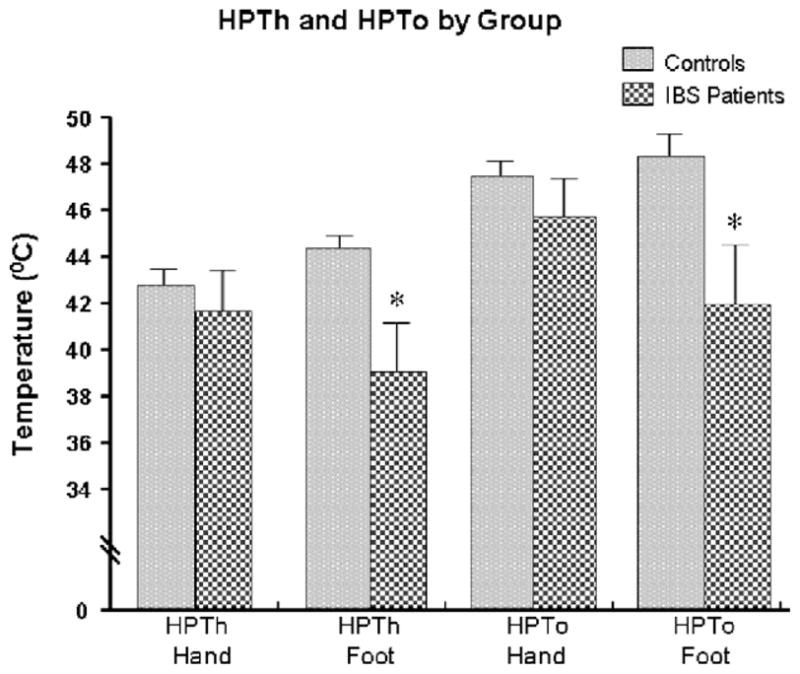
Thermal threshold and tolerance by group.
Fig. 3.
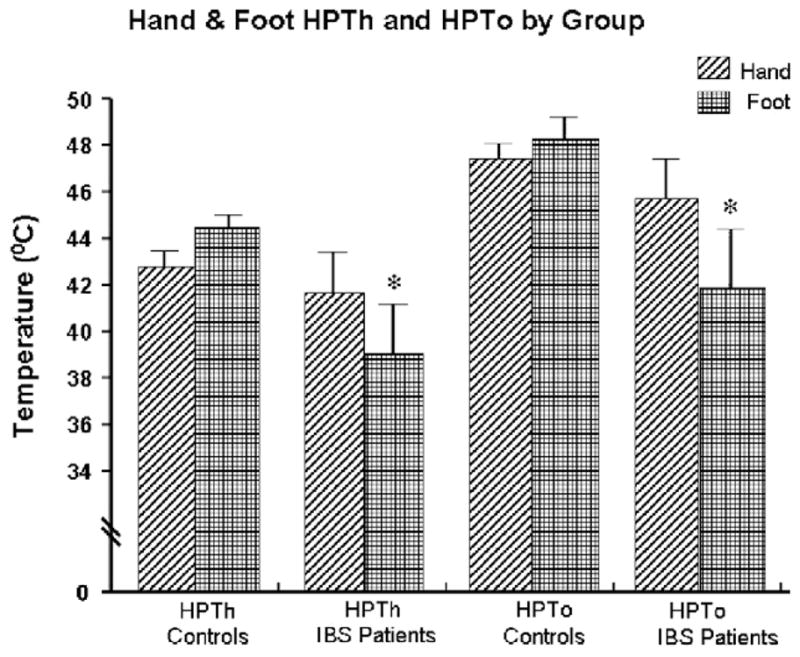
Hand and foot thermal threshold and tolerance comparison by group.
Fig. 4.
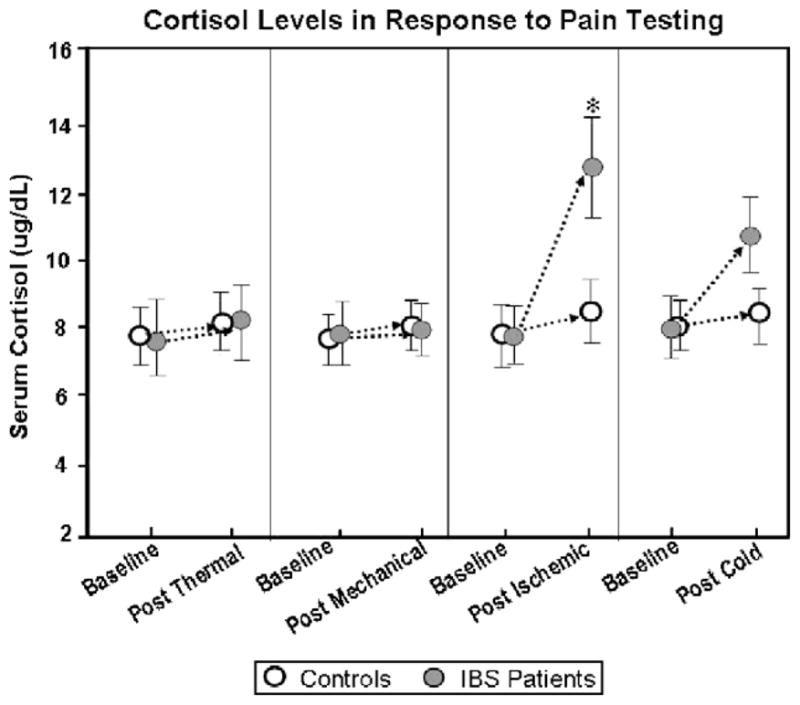
Cortisol levels in response to pain testing.
Fig. 5.
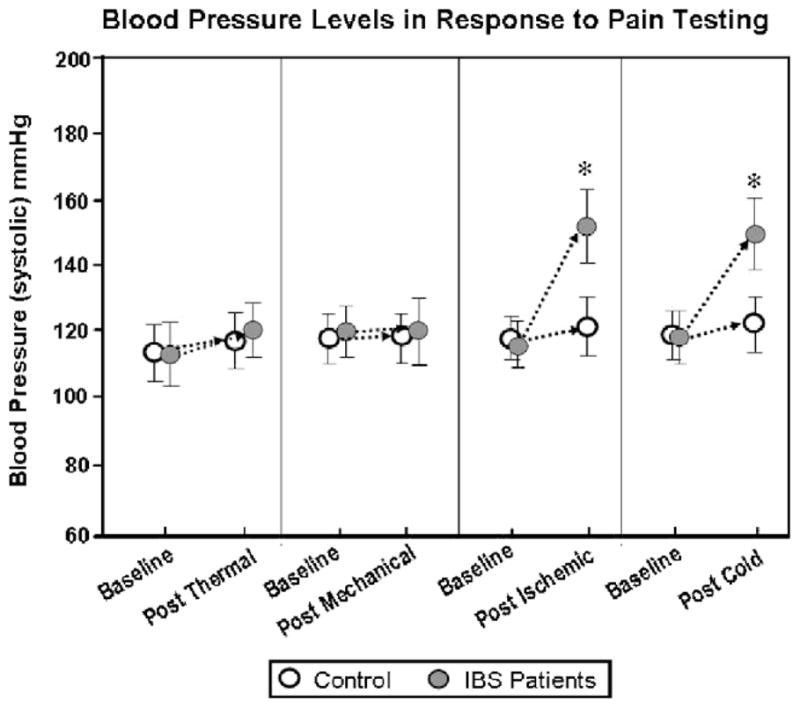
Blood pressure levels in response to pain testing.
Table 2.
ACTH levels in response to pain testing.
| Neuroendocrine and cardio vascular measure | Thermal stimuli (IBS) | Mechanical stimuli (IBS) | Ischemic stimuli (IBS) | Cold 4 °C stimuli (IBS) | Thermal stimuli (controls) | Mechanical stimuli (controls) | Ischemic stimuli (controls) | Cold 4 °C stimuli (controls) |
|---|---|---|---|---|---|---|---|---|
| ACTH (pg mL−1) Baseline (before test) | 16.00 ± 11.4 | 17.34 ± 10.7 | 15.97 ± 9.6 | 16.61 ± 10.3 | 19.03 ± 10.6 | 17.47 ± 8.7 | 18.21 ± 8.0 | 19.10 ± 7.4 |
| ACTH (pg mL−1) Post test | 19.22 ± 11.9 | 19.54 ± 10.3 | 42.03 ± 14.5 | 34.44 ± 13.6 | 20.0 ± 11.9 | 20.36 ± 9.0 | 21.05 ± 11.5 | 20.11 ± 8.5 |
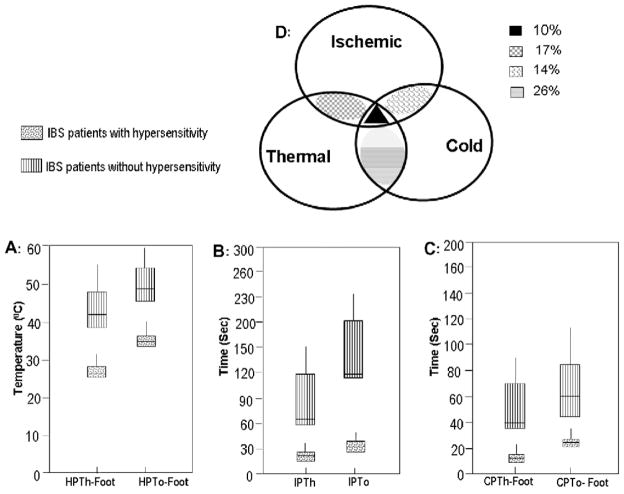
Frequency distribution analysis was then used to cluster the IBS patients into two groups based on the range of all the reported values for HPTh and HPTo for both the hand and the foot (Fig. 6A). One group of IBS patients (50/78, 64%) had a similar range of HPTh and HPTo as the normal control group. The other group of IBS patients (28/78, 36%) demonstrated hypersensitivity to the thermal stimuli and had a significantly lower range of HPTh and HPTo compared to controls.
Fig. 6.
Distribution of subset IBS patients in response to thermal, ischemic and cold pressor stimuli.
3.2. Pressure (mechanical) pain threshold (PPT)
PPTs of the forearm and calf were the same in IBS patients and controls. There was no significant difference in PPTs between the forearm and calf in either controls or IBS patients (data not shown). There were no significant changes in cortisol/ACTH and systolic blood pressure following the pressure (mechanical) pain testing in IBS patients and controls (Figs. 4 and 5 and Table 2).
3.3. Ischemic pain threshold (IPTh) and tolerance (IPTo)
On the ischemic test, IBS patients had a shorter time to both IPTh and IPTo than controls (Fig. 7). Following the ischemic pain testing, there were significant increases in systolic blood pressure, cortisol, and ACTH in IBS patients, but not in controls (Figs. 4 and 5 and Table 2).
Fig. 7.
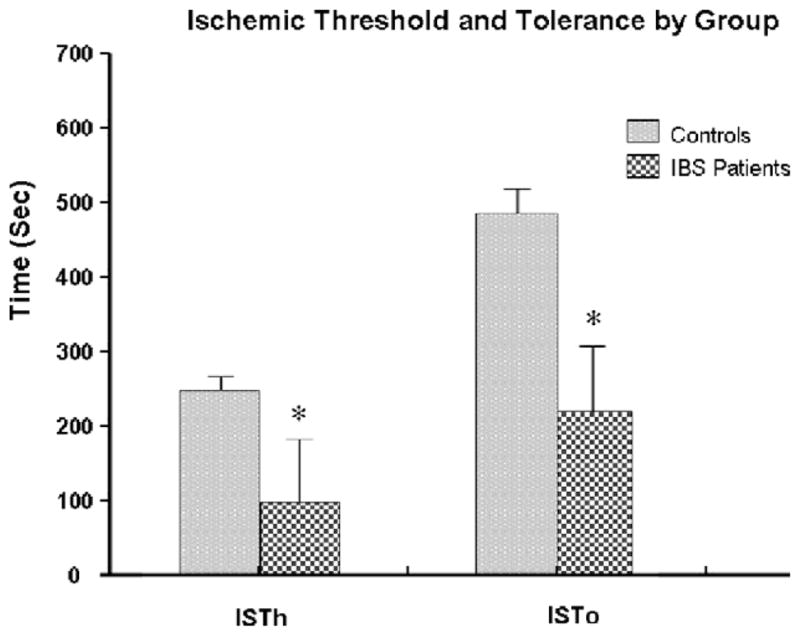
Ischemic threshold and tolerance by group.
Frequency distribution analysis was used to cluster the IBS patients into two groups based on the range of the reported values for IPTh and IPTo. One group of IBS patients (47/78, 60%) had a similar range of IPTh and IPTo as the control group (Fig. 6B). The other group of IBS patients (31/78, 40%) demonstrated hypersensitivity to the ischemic pain stimuli and had a significantly lower range of IPTh and IPTo compared to controls.
3.4. Cold pressor pain threshold (CPTh) and tolerance (CPTo)
In the cold pressor test, IBS patients demonstrated lower CPTh and CPTo in response to cold water immersion of the foot compared to controls (Fig. 8). There was no difference in CPTh or CPTo measures between IBS patients and controls on the hand. Comparison of cold water immersion of the hand and foot yielded a significant main effect for group and site (p < 0.001). This indicated that IBS patients reported significantly higher pain ratings compared to controls, with foot stimulation being more painful than hand stimulation. Interestingly, following the cold pressor test, there was a significant increase in systolic blood pressure in IBS patients, but not in controls. In addition, both cortisol and ACTH also increased in IBS patients following the cold pressor test, however, the increases did not reach statistical significance (Figs. 4 and 5 and Table 2).
Fig. 8.
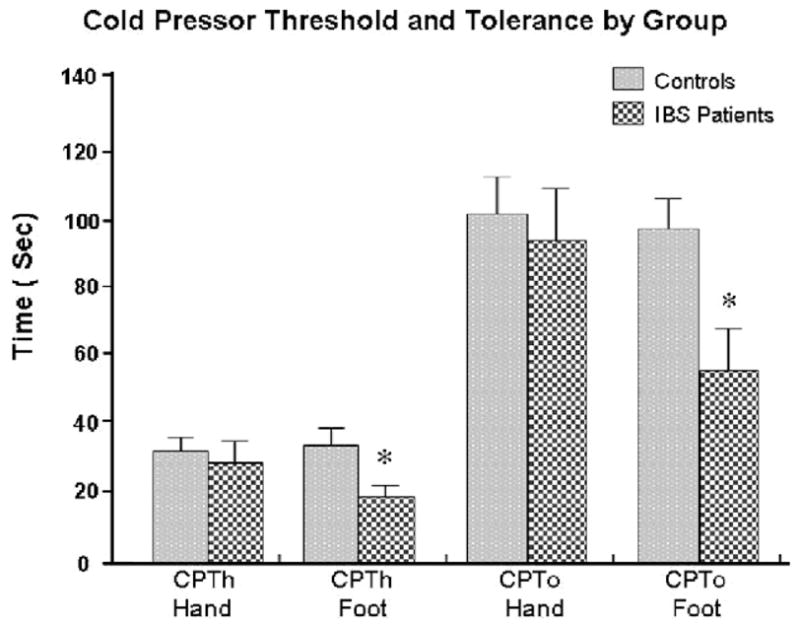
Cold pressor threshold and tolerance by group.
Frequency distribution analysis was then used to cluster the IBS patients into two groups based on the range of all the reported values for CPTh and CPTo for both the hand and the foot. One group of IBS patients (53/78, 68%) had a similar range of CPTh and CPTo as the normal control group (Fig. 6C). The other group of IBS patients (25/78, 32%) demonstrated hypersensitivity to the cold water stimuli and had a significantly lower range of CPTh and CPTo compared to controls.
3.5. Overlap of somatic hypersensitivity in IBS group
Fig. 6D depicts the overlap between IBS patients with thermal, ischemic, and cold pressor hypersensitivity. A total of 10% of IBS patients had hypersensitivity to all three nociceptive stimuli (ischemic, thermal, and cold). Both thermal and ischemic hypersensitivity was present in 17% of IBS patients. In contrast, 14% of IBS patients demonstrated evidence of both cold and ischemic hypersensitivity. Finally, the largest overlap (26%) was between thermal and cold hypersensitivity in IBS patients.
There were no differences in scores on the BDI and STAI between the four groups of IBS patients with overlap. Interestingly, the 10% of IBS patients that had overlap to all three nociceptive stimuli had the history of the most extraintestinal symptoms (75%). Approximately 25% of the IBS patients with both ischemic/thermal and ischemic/cold had baseline evidence of extraintestinal symptoms. Finally, 50% of the patients with thermal and cold hypersensitivity overlap had a history of extraintestinal symptoms.
4. Discussion
Our present study compared differences in somatic hypersensitivity between IBS patients and controls and is unique in several ways. First, to our knowledge, this is the 1st study to examine somatic pain perception in IBS patients using a battery of diverse experimental pain procedures that includes a broad range of stimulus intensities and perceptual qualities. Second, this study tested for differences in somatic hypersensitivity across body sites that provide spinal input at differing levels. We attempted to determine if the somatic hypersensitivity is somatotopically organized in that the lower extremities that share viscerosomatic convergence with the colon demonstrate the greatest hypersensitivity. Finally, the current study examined differences in neuroendocrine and cardiovascular responses to painful stimuli in IBS patients compared to controls using multiple, well-validated psychophysical pain testing procedures.
Overall, our findings indicate that a subset of IBS patients have greater thermal sensitivity than controls as they reported a lower pain threshold and tolerance to heat stimuli, especially in the foot compared to the hand. These findings support our previous studies indicating that some IBS patients are more sensitive to heat stimuli than controls [36,41]. These results also are consistent with our previous work in which the thermal hypersensitivity in IBS patients is somatotopically organized such that the lower dermatomes that share viscerosomatic convergence with the colon demonstrate the greatest hypersensitivity [27]. Interestingly, there was no evidence of spatial summation when HPTh and HPTo with a 3 × 3 cm probe was compared to the 1 × 1 cm probe in the IBS patients. One possible explanation is that the position of the thermode was altered between trials in order to avoid either sensitization or habituation of cutaneous receptors. In addition, our noxious thermal stimuli may not have been robust enough to activate sufficient afferent fibers to produce spatial summation.
This spinal sensitization in IBS patients could be created and/or maintained by tonic impulse input from the colon to the spinal cord. We have previously shown that using a local anesthetic blockade in the colon (i.e. lidocaine jelly), both visceral and somatic hypersensitivity in IBS patients is diminished [29]. Several recent studies by our group using an IBS-like animal model of visceral and somatic hypersensitivity further support the presence of viscerosomatic convergence in IBS patients [35,38]. In this model, NMDA NR1 subunit expression was greater in the L4-S1 in laminae I and II of the spinal cord compared to the T10-L1 cord [33,34,37,40]. Thus, potentially like IBS patients, the somatic hypersensitivity in animals is somatotopically organized such that the lower dermatomes that share viscerosomatic convergence with the colon demonstrate the greatest hypersensitivity (i.e. L4-S1). Thus, our overall findings confirm our previous studies that suggest that viscerosomatic convergence may be an underlying mechanism of thermal hypersensitivity in IBS patients.
Our current results with mechanical testing did not reveal any evidence of somatic hypersensitivity in either the IBS patients or controls. These results compliment and confirm the findings of an earlier study by Chang in which they tested IBS patients with and without fibromyalgia [5]. In their study, IBS patients had similar pain thresholds during an ascending series of pressure stimuli as controls. A possible reason for the common difference in ours and Chang’s results between the mechanical and thermal stimuli may be related to the differences in stimulus modalities (heat vs. mechanical). The use of mechanical stimuli as a nociceptive stimulus may activate non-nociceptive mechanoreceptive neurons whose input could be inhibited at higher cortical levels by chronic visceral nociceptive input in the IBS patients but not in controls [27]. Thus, the total nociceptive input with mechanical stimuli but not thermal stimuli might be reduced in IBS patients compared to controls.
On the ischemic test, IBS patients had a shorter time to both pain threshold and tolerance compared to controls. These findings were expected given the perceptual qualities of the ischemic test, the pain experienced may be more likely to reveal somatic hypersensitivity in IBS patients. The modified submaximal effort tourniquet procedure used in our current study produces a diffuse, deep pain that is associated with the activation of a large number of C-fibers in the muscle, compared to other nociceptive stimuli which include primarily cutaneous stimuli of shorter duration and activate a greater proportion of a-delta fibers [15]. While the ischemic test has not been previously examined in IBS patients, others have reported hypersensitivity to using this pain stimulus in temporomandibular disorders, fibromyalgia, and interstitial cystitis [4,7,16]. Each of these disorders shows significant comorbidity with IBS.
The cold pressor task is a commonly used experimental pain task and cardiovascular challenge. The cold pressor has been previously used in IBS patients by several research groups [24,31]. In our current study, IBS patients showed significantly lower threshold and tolerance to the cold pressor stimuli applied to the foot, but not to the hand, compared to controls. In the hand, there was no significant difference in IBS patients compared to controls for both cold pressure threshold and/or tolerance. While consistent with some other studies [31], our findings contrast with one study which reported hypersensitivity in IBS patients using cold pressor testing applied to the hand [2]. However, considering the distribution of our data, there appears to be a subset of IBS patients with similar cold water tolerance to controls and a smaller group of IBS patients with a much shorter tolerance time. Similar distributions have been observed in prior research [2,31]. Thus, further analysis of these small subsets of sensitive IBS patients may prove to be useful in future studies to help identify the frequency distribution of hypersensitivity in IBS patients.
In addition to differences in perceptual responses, we observed significant increases in cortisol and ACTH in IBS patients, but not in controls following the ischemic pain test. In addition, both cortisol and ACTH also increased in IBS patients following the cold pressor test; however, the increases did not reach statistical significance. The increase in cortisol and ACTH in the latter pain tests suggests that patients experience these stimuli as more stressful [18]. Similarly, IBS patients showed greater systolic blood pressure responses to both the ischemic and cold presser pain tasks. Our current results with increased cortisol and ACTH levels following the ischemic/cold pressor testing support a recent study by Chang in IBS patients [6].
5. Summary
Our current findings support the presence of somatic hypersensitivity to thermal, ischemic, and cold pressor nociceptive stimuli in a subset of IBS patients. The presence of somatic hypersensitivity in IBS patients is somatotopically organized in that the lower extremities that share viscerosomatic convergence with the colon demonstrate the greatest hypersensitivity. Thus, chronic afferent nociceptive input from the gut may sensitize spinal cord neurons that exhibit somatotopic overlap with the gut. The present findings also support a potential role of neuroendocrine and cardiovascular changes in response to painful somatic stimuli in IBS patients. It is interesting that the subsets of IBS patients with thermal, ischemic, and cold pressor hypersensitivity had minimal overlap between groups (Fig. 6D). This suggests that different mechanisms may play a role in the hypersensitivity seen with diverse nociceptive pain stimuli as the IBS patients with a history of the greatest number of extraintestinal symptoms had the highest overlap of sensitivity to nociceptive stimuli. Future studies are needed to: (1) determine if the same findings of somatic hypersensitivity are also present in IBS patients with constipation or alternating bowel patterns; (2) examine the molecular mechanisms that may lead to somatic hypersensitivity in these IBS patients.
Acknowledgments
This study was supported by an NIH Grant (NIH RO1-NS053090) to G.N. Verne and by a VA Merit Review Award to G.N. Verne from the Medical Research Service of the Department of Veterans Affairs.
Footnotes
The authors have no financial or other relationship to report that might lead to a conflict of interest.
References
- 1.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 2.Bouin M, Meunier P, Riberdy-Poitras M, Poitras P. Pain hypersensitivity in patients with functional gastrointestinal disorders: a gastrointestinal-specific defect or a general systemic condition? Dig Dis Sci. 2001;46:2542–8. doi: 10.1023/a:1012356827026. [DOI] [PubMed] [Google Scholar]
- 3.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–6. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Carli G, Suman AL, Biasi G, Marcolongo R. Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. Pain. 2002;100:259–69. doi: 10.1016/S0304-3959(02)00297-X. [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84:297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Sundaresh S, Elliot J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterolol Motil. 2009;21(2):149–59. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris MB. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain. 1996;12:260–9. doi: 10.1097/00002508-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 8.France CR. Decreased pain perception and risk for hypertension: considering a common physiological mechanism. Psychophysiology. 1999;36:683–92. [PubMed] [Google Scholar]
- 9.Gebhart GF. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278:G834–8. doi: 10.1152/ajpgi.2000.278.6.G834. [DOI] [PubMed] [Google Scholar]
- 10.Geel SE. The fibromyalgia syndrome: musculoskeletal pathophysiology. Semin Arthritis Rheum. 1994;23:347–53. doi: 10.1016/0049-0172(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 11.Jensen K, Andersen HO, Olesen J, Lindblom U. Pressure–pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain. 1986;25:313–23. doi: 10.1016/0304-3959(86)90235-6. [DOI] [PubMed] [Google Scholar]
- 12.Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–57. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 14.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–93. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 15.Moore PA, Duncan GH, Scott DS, Gregg JM, Ghina JN. The submaximal effort tourniquet test: its use in evaluating experimental and chronic pain. Pain. 1979;6:375–82. doi: 10.1016/0304-3959(79)90055-1. [DOI] [PubMed] [Google Scholar]
- 16.Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychological evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005;173:1983–7. doi: 10.1097/01.ju.0000158452.15915.e2. [DOI] [PubMed] [Google Scholar]
- 17.Pike JL, Smith TL, Hauger RL, Nicassio PM, Patterson TL, McClintick J, Costlow C, Irwin MR. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med. 1997;59:447–57. doi: 10.1097/00006842-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–8. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price DD, Craggs JG, Zhou Q, Verne GN, Perstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage. 2009;47:995–1001. doi: 10.1016/j.neuroimage.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rainville P, Feine JS, Bushnell MC, Duncan GH. A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res. 1992;9:265–77. doi: 10.3109/08990229209144776. [DOI] [PubMed] [Google Scholar]
- 21.Riley JL, III, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–35. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 22.Sarlani E, Greenspan JD. Evidence for generalized hyperalgesia in temoromandibular disorders patients. Pain. 2003;102:221–6. doi: 10.1016/S0304-3959(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 23.Spielberger CD, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (form Y1) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 24.Thumshirn M, Coulie B, Camilleri M, Zinsmeister AR, Burton DD, Van Dyke C. Effects of alosetron on gastrointestinal transit time and rectal sensation in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2000;14:869–78. doi: 10.1046/j.1365-2036.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- 25.Verne GN, Cerda JJ. Irritable bowel syndrome. Streamlining the diagnosis. Postgrad Med. 1997;102:197–208. doi: 10.3810/pgm.1997.09.322. [DOI] [PubMed] [Google Scholar]
- 26.Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 27.Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002;4:322–8. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- 28.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 29.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–30. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 30.Walsh NE, Schoenfeld L, Ramamurthy S, Hoffman J. Normative model for cold pressor test. Am J Phys Med Rehabil. 1989;68:6–11. doi: 10.1097/00002060-198902000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–92. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 32.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–50. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q, Caudle RM, Price DD, Verne GN. Phosphorylation of NMDA NR1 subunits in the myenteric plexus during TNBS induced colitis. Neurosci Lett. 2006;406:250–5. doi: 10.1016/j.neulet.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Q, Caudle RM, Price DD, Verne GN. Selective up-regulation of NMDA-NR1 receptor expression in myenteric plexus after TNBS induced colitis in rats. Mol Pain. 2006;2:3. doi: 10.1186/1744-8069-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Q, Caudle RM, Price DD, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain. 2008;134:9–15. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Q, Fillingim RB, Riley JR, Verne GN. Thermal hypersensitivity in a subset of irritable bowel syndrome patients. World J Gastroenterol. 2009;15:3254–60. doi: 10.3748/wjg.15.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Q, Price DD, Caudle RM, Verne GN. Spinal NMDA NR1 subunit expression following transient TNBS colitis. Brain Res. 2009;1279:109–20. doi: 10.1016/j.brainres.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain. 2008;139:218–24. doi: 10.1016/j.pain.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2009 doi: 10.1136/gut.2009.181834. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q, Verne GN. NMDA receptor and colitis: basic science and clinical implications. Rev Analgesia. 2008;10:33–43. doi: 10.3727/154296108783994013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–6. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]