Abstract
Purpose
Phenylephrine is used to dilate the iris through α-adrenergic stimulation of the iris dilator muscle. Sympathetic stimulation of the ciliary muscle is believed to be inhibitory, decreasing accommodative amplitude. Investigations in humans have suggested some loss of functional accommodation after phenylephrine. It is unclear whether this loss is due to direct action of phenylephrine on the ciliary muscle or to secondary optical factors associated with mydriasis. The purpose of this study was to determine whether phenylephrine affects Edinger-Westphal (EW)–stimulated accommodation in rhesus monkeys.
Methods
The time course for maximum mydriasis was determined by videographic pupillography after phenylephrine instillation in 10 normal rhesus monkeys. Static and dynamic EW-stimulated accommodative responses were studied in five iridectomized rhesus monkeys before and after phenylephrine instillation. Accommodative amplitude was measured with a Hartinger coincidence refractometer. Dynamic accommodative responses were measured with infrared photorefraction, and functions were fitted to the data to determine peak velocity versus accommodative response relationships.
Results
The maximum dilated pupil diameter of 8.39 ± 0.23 mm occurred 15 minutes after administration of phenylephrine. In iridectomized monkeys, postphenylephrine accommodative amplitudes were similar to prephenylephrine amplitudes. Dynamic analysis of the accommodative responses showed linear peak velocity versus accommodative amplitude relationships that were not statistically different before and after phenylephrine.
Conclusions
α-Adrenergic stimulation causes a strong pupil dilation in noniridectomized monkey eyes but does not affect EW-stimulated accommodative amplitude or dynamics in anesthetized, iridectomized rhesus monkeys.
Phenylephrine is a sympathomimetic agent that is used clinically to dilate the iris without cycloplegia. Phenylephrine (2.5%) is used diagnostically for fundus examination, and 10% phenylephrine is used therapeutically to break posterior synechiae and pupillary block. Phenylephrine is commonly used in studies of accommodative dynamics to dilate the iris, because optometers normally fail to measure through small pupils. The effects of phenylephrine on accommodative amplitude and dynamics have not been unequivocally demonstrated.
Accommodation is the dioptric change in power of the crystalline lens that allows the eye to focus on near objects after contraction of the ciliary muscle. It is controlled by the parasympathetic division of the autonomic nervous system. An accommodative effort results in parasympathetically driven convergence, pupil constriction, and accommodation. The ciliary muscle is dually innervated by parasympathetic and sympathetic neurons.1,2 The role of sympathetic innervation in accommodation is not well understood. It has been suggested that sympathetic stimulation of the ciliary muscle is inhibitory, thereby decreasing accommodative amplitude.3 Patients with unilateral Horner’s syndrome (paralysis of the oculosympathetic pathway causing facial anhydrosis, ptosis, and pupillary constriction) have greater accommodative amplitudes in the affected eye relative to the unaffected eye.1
Phenylephrine is a relatively specific α1-adrenergic receptor agonist. The α1a-receptor subtype has a higher affinity for phenylephrine than the α1b-receptor subtype. Only approximately 1% of the nerve terminals in the monkey ciliary muscle are sympathetic.4 In humans, the ciliary muscle sympathetic receptors have been shown to be of the β2 subtype rather than β1, α1, or α2 subtypes.2,5–8 A small population of α1-adrenergic receptors has been identified in humans.5 Because there are so few α-adrenergic receptors on the ciliary muscle, it is unlikely that phenylephrine has a significant effect on accommodation.
Several aspects of accommodation have been studied in relation to the sympathetic contribution in both humans and monkeys, including effects on tonic accommodation, maximum accommodative amplitude, and dynamic response. It has been proposed that stimulation of the sympathetic system allows focus on objects beyond the tonic position of accommodation,9 and that the sympathetic system inhibits the ciliary muscle to achieve “distance accommodation,”10 later termed the sympathetic range of accommodation.11 It has also been suggested that the β-adrenergic system plays a role in conjunction with the parasympathetic system in maintaining the level of tonic accommodation.12,13 Pharmacological studies with the β-adrenergic antagonists timolol maleate and isoprenaline and the parasympathetic antagonist tropicamide have led to the suggestion that, although the sympathetic system is involved in tonic accommodation, variations in the state of tonic accommodation among individuals are not determined by sympathetic tone, but by parasympathetic tone.14,15 Phenylephrine has been reported both not to change the resting state of accommodation16 and to cause a myopic shift in resting accommodation.17 Gilmartin18 concluded that the sympathetic system does not affect tonic accommodation, but provides a small, slow component of accommodation. The β-adrenergic antagonist timolol maleate is capable of increasing post-task regression to baseline refraction after a sustained reading task,19 which, in agreement with previous studies,8,14,20,21 suggests that the sympathetic contribution to accommodation may be evident after prolonged near work. This allows a build-up of sympathetic inhibitory activity over a background of parasympathetic activity, helping return the accommodative system to its baseline refraction.
Several studies have suggested that phenylephrine affects accommodative amplitude.16,22,23 Studies in humans have reported average decreases up to 3 D in maximum, subjectively measured accommodative amplitude.22,24,25 Many of these studies used the push-up technique to determine the near point. This requires the subject to report when an approaching target is too close to read clearly. Although the push-up test is used clinically to measure accommodation and is a functional subjective measure of near vision, it does not unequivocally determine the dioptric change in power of the eye. There are many confounding factors that influence the results of the push-up test, such as pupil size and ocular aberrations. When measuring accommodative amplitude after phenylephrine, the iris dilates, and the depth of field is greatly reduced. This may explain the perceived reduction in subjectively measured accommodation.26
Even objective measurement of accommodation may not unequivocally prove that phenylephrine has an effect on the accommodative plant when a human subject is required to provide an accommodative response to a visual stimulus. The subject must see the distant and near targets clearly to produce the appropriate accommodative response. The response amplitude may be affected by decreased depth of field and increased ocular aberrations due to mydriasis rather than from a decrease in accommodative ability.27 Therefore, an objectively measured decrease in maximum amplitude28 or change in accommodative dynamics29 may result from the effect that mydriasis has on the subjects’ perception of blur. Investigators who have examined the effects of sympathetic agents on various aspects of stimulus driven dynamic accommodation responses in humans have reported both no change in the step-response times23 and slowed response times29 after phenylephrine administration.
The effect of sympathetic agonists on accommodation has also been studied in monkeys, with contradictory results. Vervet monkeys had a reduced centrally stimulated accommodative amplitude after 5% levarterenol, an α-adrenergic agonist.30 This was attributed to a β-agonist effect of the drug, rather than an α-agonist effect, as the effect was blocked by a β-blocker but not an α-blocker. Others have found that accommodation is “relatively unaffected” by 10% phenylephrine,31 or decreases by up to 7 D with brain-stimulated accommodation after 1 drop of 2.5% phenylephrine.3 This decrease was attributed to vasoconstriction of the ciliary body vasculature, reducing the volume of the ciliary body and depressing accommodative amplitude.
Central stimulation of accommodation in anesthetized monkeys offers an opportunity to establish the direct pharmacological action of a drug on the physiology of the ciliary muscle. Open-loop accommodation can be stimulated in anesthetized monkeys with an electrode implanted in the Edinger-Westphal (EW) nucleus of the midbrain. The accommodative response achieved is not affected by pupil size or visual feedback, can be rigorously controlled by stimulus amplitude, and can be accurately quantified with objective measurements, such as infrared photorefraction.32 Rhesus monkeys represent a unique model for human accommodation because they have high accommodative amplitudes33–35 and an accommodative mechanism36 and anatomy37 similar to that of humans. The intraocular adrenergic receptor characteristics closely resemble those of the human eye.7 Because rhesus monkeys and humans are most frequently used in accommodation studies, it is of interest to understand the effects of phenylephrine on the accommodative plant of rhesus monkeys and humans, rather than in cynomolgus or vervet monkeys. In the present study, the effects of 10% phenylephrine on centrally stimulated accommodation were studied in rhesus monkeys to determine the effects on amplitude, dynamics, and resting state. This study may differentiate effects of phenylephrine on the ciliary muscle versus effects due to secondary optical factors resulting from mydriasis.
Methods
Experiments were performed on 15 rhesus monkeys (Macaca mulatta). Ten monkeys with normal intact irides were used to ascertain the effects of phenylephrine on pupil diameter. Five monkeys that had been surgically iridectomized38 and had stimulating electrodes surgically implanted in the EW nucleus of the brain39 were used to test the effects of phenylephrine on accommodation. All experiments conformed to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research and were conducted under an institutionally approved animal protocol.
Phenylephrine Dosage Experiment
Animal Preparation
Ten monkeys were anesthetized with 10 mg/kg intramuscular ketamine and 0.5 mg/kg intramuscular acepromazine. Anesthesia was supplemented with 6.25 mg/kg ketamine approximately every 30 minutes, as required. Monkeys were placed prone with the head upright and facing forward in a head holder. The eyelids were held open with a speculum. A custom made PMMA contact lens (Metro Optics, Austin, TX) was placed on the cornea to maintain clarity and hydration.
Phenylephrine Dosage Determination
In one noniridectomized monkey (118), four topical phenylephrine concentration and dosage protocols were tested to determine the extent and time course of pupil dilation in four different sessions, each at least 3 days apart. The protocols were (1) one 0.1-mL dose (approximately 2 drops) of 2.5% phenylephrine; (2) two 0.1-mL doses of 2.5% phenylephrine 2 minutes apart; (3) one 0.1-mL dose of 10% phenylephrine; and (4) two 0.1-mL doses of 10% phenylephrine 2 minutes apart.
The effects of phenylephrine were determined by measuring the infrared, retroilluminated pupil diameters. Images of the pupil were captured with a video camera placed 0.3 meters from the eye (Cohu, San Diego, CA). The eye was illuminated with a bank of 20 infrared diodes placed behind a knife-edge aperture shielding the lower half of the camera lens. Ten prephenylephrine images were captured over 2 minutes and saved to a computer by frame grabber card and image analysis software (Optimas; Media Cybernetics, Silver Springs, MD). The contact lens was removed and phenylephrine was topically instilled. The cornea was irrigated with saline 30 seconds after administration of the last dose of phenylephrine, and the contact lens was replaced. Three images were captured every 5 minutes for 65 minutes, except with protocol 4, which was observed for 120 minutes. The calibrated images were later analyzed off-line.
Pupil Diameter Measurements
Once the appropriate phenylephrine protocol was determined to give maximum pupil dilation in a short time, this same protocol was tested in one eye each of 10 noniridectomized rhesus monkeys. The time course and extent of mydriasis were determined by videographic pupillography before and every 5 minutes after topical phenylephrine administration, as described earlier.
Accommodation Experiments
The five monkeys used for accommodation testing had undergone bilateral complete iridectomies38 and had stimulating electrodes surgically implanted in the EW nucleus of the midbrain.36,39 The monkeys are used repeatedly in multiple experimental protocols32,36,40 and the iridectomies,38 justification for it,41 and absence of an effect on centrally stimulated accommodation42 have been described previously. The EW-implanted monkeys were 3 (monkey 111), 4 (monkey 20), 5 (monkeys 38 and 70) and 11 (monkey 4) years old. Accommodative responses were stimulated and measured before and after topical instillation of phenylephrine. As a control, the protocol was repeated on one monkey (monkey 111) with topical instillation of saline instead of phenylephrine.
Monkeys were initially anesthetized with 10 mg/kg intramuscular ketamine and 0.5 mg/kg intramuscular acepromazine. Surgical depth anesthesia was maintained for the duration of the experiment with intravenous propofol (initial bolus of 1.5 mg/kg followed by constant perfusion at 0.5 mg/kg per minute). The contact lens was placed on the cornea, and sutures were tied beneath the lateral and medial rectus muscles to reduce eye movements during accommodation.32,36
Static Accommodation Measurements
Static EW-stimulated accommodative responses were measured before and 30 minutes after instillation of phenylephrine to determine the stimulus response function. The static Hartinger stimulus response function was later used to calibrate the dynamic photorefraction measurements and to compare the accommodative responses for each stimulus amplitude before and after phenylephrine instillation. First, baseline refraction was measured three times with a Hartinger coincidence refractometer (Carl Zeiss Meditec, Jena, Germany).43 An accommodative stimulus response function was then measured. Accommodation was stimulated using 2-second stimulus trains, with 10 current amplitudes ranging from 0 μA up to an amplitude sufficient to produce the maximum accommodative response available to each monkey. For each stimulus amplitude, accommodation was stimulated and measured three times in succession and averaged. Baseline refractions and the stimulus response function were measured again 30 minutes after phenylephrine instillation.
Dynamic Accommodation Measurements
To study the accommodative dynamics, infrared photorefraction was used to determine the relationship between the peak velocity and the amplitude of the accommodative responses (a main sequence relationship).32 Calibrated photorefraction44,45 was performed at a 0.3-m working distance and analyzed over 40% of the iridectomized pupil diameter.32
Before the instillation of phenylephrine, a sequence of dynamic accommodation responses to increasing stimulus currents was recorded with infrared photorefraction, using the same stimulus current amplitudes as those used for the Hartinger stimulus response function. Accommodative responses to eight stimulus amplitudes were recorded spanning the full accommodative range. For each stimulus amplitude, responses to five, 4-second stimulus trains were recorded. The first two of the five responses were recorded but not analyzed.
After the baseline recordings, two doses of 0.1 mL 10% phenylephrine were instilled topically, separated by 2 minutes, as described earlier. Dynamic accommodation was measured every 2 minutes for 20 minutes, at a stimulus amplitude previously determined to elicit maximum accommodation. The stimulation and measurement procedures were the same as those already described.
At 25 minutes after phenylephrine, a dynamic accommodation stimulus response sequence was recorded for amplitudes spanning the full range available to each monkey, using the same stimulus amplitudes as the prephenylephrine sequence.
Dynamic Accommodation Analysis
The effects of phenylephrine on dynamic accommodation were established in terms of peak velocities of accommodation and disaccommodation. Amplitude of accommodation, peak velocity of accommodation, and peak velocity of disaccommodation were determined for each stimulus amplitude of the prephenylephrine sequence, for the repeated single-amplitude stimuli after instillation of phenylephrine, and for the postphenylephrine stimulus amplitude sequence, using methods described previously.32 Maximum accommodative response was determined for each stimulus amplitude by taking the difference between the baseline refraction and the accommodated refraction.
The accommodative response was plotted as a function of time, and exponential curves were fitted to the accommodation phases and the disaccommodation phases.32 The derivative of these functions with respect to time gives the velocity profile of the responses. The Vmax achieved is the peak velocity. The sequence of analysis was as follows.
Pupil Measurements
In 10 monkeys with intact irides, pupil diameters were measured 10 times in 2 minutes before and three times every 5 minutes after administration of phenylephrine, for up to 120 minutes.
Accommodation Measurements
In five iridectomized monkeys with permanent EW electrodes, before administration of phenylephrine, a static Hartinger stimulus response function was measured, and dynamic infrared photorefraction was recorded. Phenylephrine was then administered, and infrared photorefraction was measured for a single maximum stimulus amplitude every 2 minutes for 20 minutes. At 25 minutes after phenylephrine, a dynamic infrared photorefraction stimulus response function was recorded, and at 30 minutes after phenylephrine, a static Hartinger stimulus response function was measured.
RESULTS
Phenylephrine Dosage Experiments
Figure 1 shows pupil diameter as a function of time after instillation of phenylephrine for the four different administration protocols tested in one noniridectomized eye of monkey 118. Two doses of 10% phenylephrine elicited the most dilation in the shortest time. Pupil diameter was measured for 120 minutes, over which period the mydriasis persisted (data not shown). From this experiment, it was determined that two doses of 10% phenylephrine were appropriate to elicit maximum mydriasis, and this protocol was tested in one eye each of 10 noniridectomized rhesus monkeys. Average pupil diameter of 8.39 ± 0.23 mm was recorded within 15 minutes (Fig. 2), and irides remained dilated for at least 65 minutes. A mean increase in pupil diameter of 3.82 ± 1.01 mm from baseline was achieved.
Figure 1.
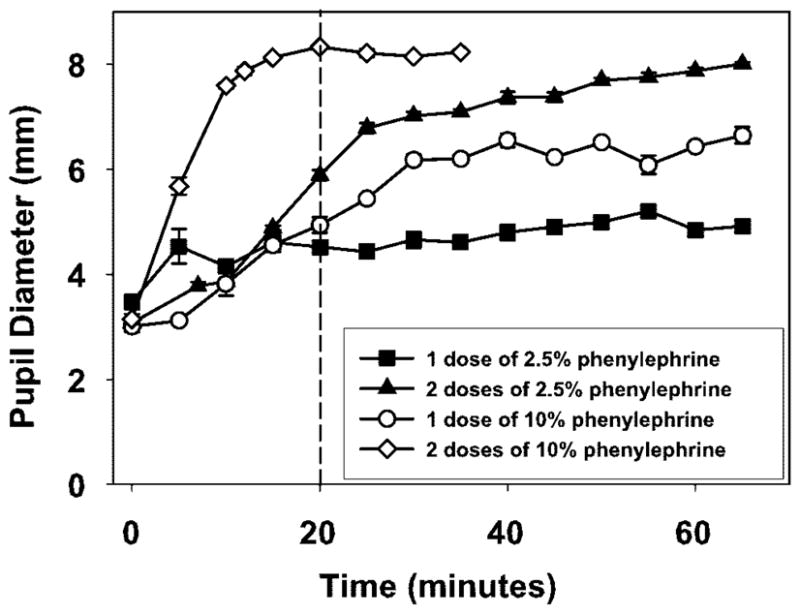
Four different phenylephrine dosage protocols were tested on one noniridectomized monkey eye on different occasions to determine the appropriate dose needed to elicit maximum pupil dilation. Maximum pupil dilation was achieved by 20 minutes with two doses of 10% phenylephrine.
Figure 2.
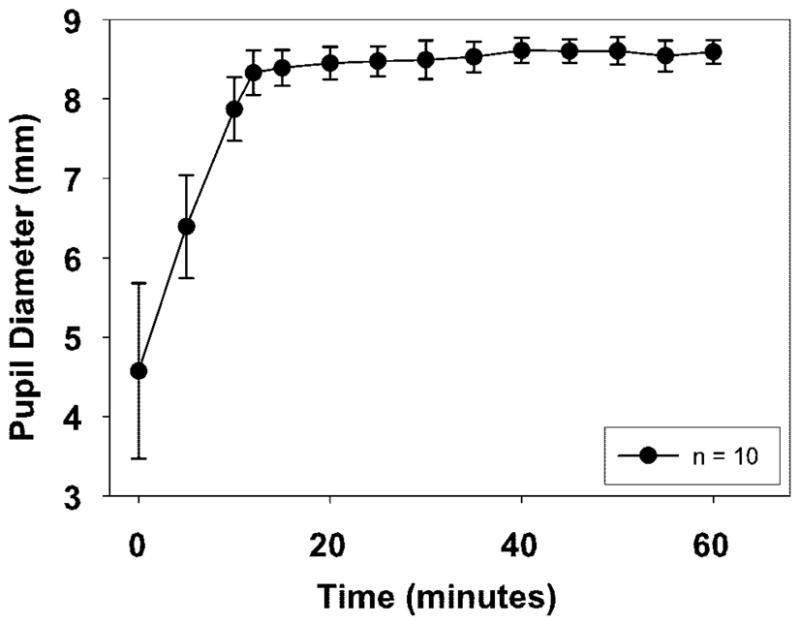
Pupil diameters were measured in one eye each of 10 noniridectomized monkeys after two 0.1-mL doses of 10% phenylephrine. Mean maximum dilation was achieved after 15 minutes.
Accommodation Experiments
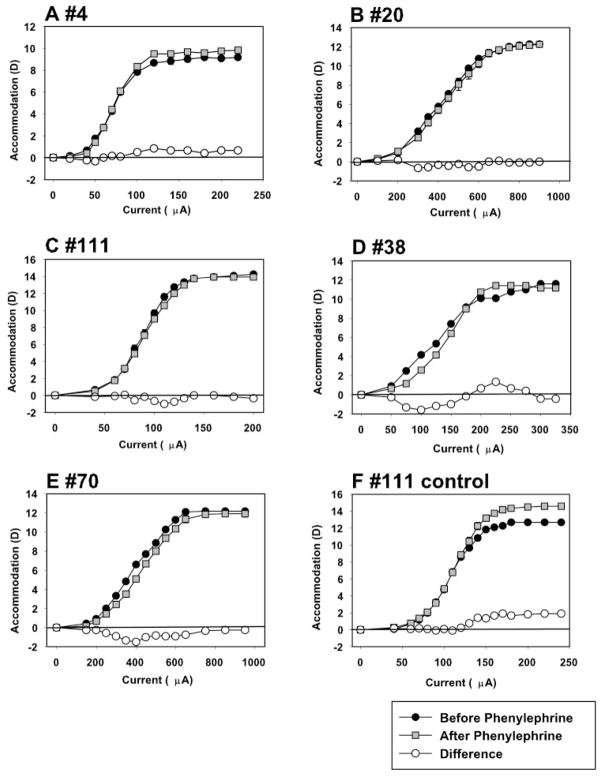
In the five monkeys in which accommodation was measured, dynamic accommodative responses to a single maximum stimulus amplitude were measured with photorefraction every 2 minutes after instillation of phenylephrine. There was no significant difference in amplitude between the average of three stimuli before phenylephrine and the average of the last three stimuli at 16, 18, and 20 minutes after phenylephrine (t-test: t = −0.6685, df = 4, P = 0.54). Although the average results from the five monkeys receiving phenylephrine showed no significant change in amplitude 20 minutes after phenylephrine, individual monkeys showed changes from baseline (Table 1), with a decrease in three monkeys, an increase in two monkeys, and an increase in the saline control monkey.
Table 1.
Accommodative Amplitude before and 20 Minutes after 10% Phenylephrine Measured with Photorefraction
| Monkey | Amplitude Before | Amplitude After | Difference |
|---|---|---|---|
| 4 | 8.30 ± 0 | 7.56 ± 0.04 | −0.74 |
| 20 | 11.40 ± 0.04 | 11.10 ± 0.06 | −0.29 |
| 38 | 10.99 ± 0.16 | 12.30 ± 0.24 | +1.32 |
| 70 | 11.85 ± 0.35 | 15.12 ± 0.25 | +3.27 |
| 111 | 13.02 ± 0.04 | 12.10 ± 0.02 | −0.91 |
| Mean | 11.11 ± 1.75 | 11.64 ± 2.72 | +0.53 ± 1.77 |
| 111 control | 11.57 ± 0.07 | 11.87 ± 0.07 | +0.30 |
For the five monkeys receiving 10% phenylephrine and the saline control, accommodative responses to a single maximum stimulus amplitude measured with infrared photorefraction showed no significant difference in amplitude between the three prephenylephrine stimuli and the last three stimuli at 16, 18, and 20 minutes after phenylephrine (t-test: t = −0.6685, P = 0.54, df = 4), although individual monkeys showed differences, including the saline control monkey. Data are expressed in diopters (mean ± SD).
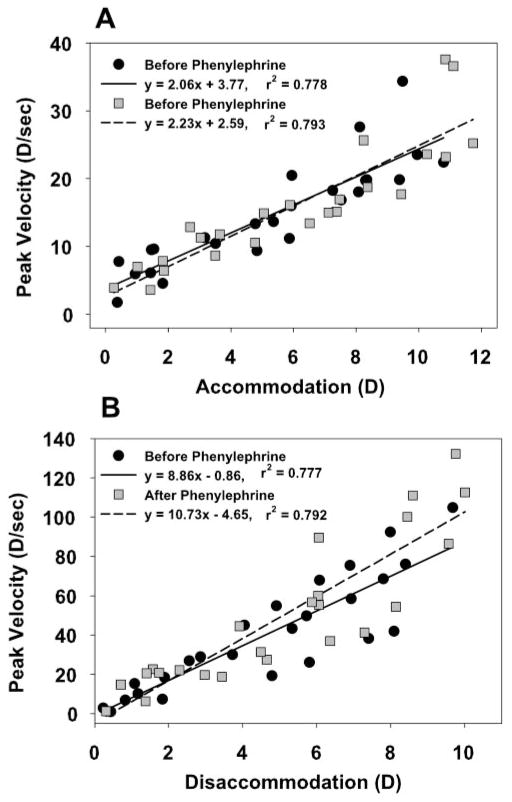
Dynamic analysis of the accommodative responses measured with infrared photorefraction showed linear peak velocity versus amplitude relationships that were not significantly different before and 25 minutes after phenylephrine for accommodation (Fig. 3A, P = 0.52) or disaccommodation (Fig. 3B, P = 0.24). Although peak velocity and amplitude are both independent variables, linear regression analysis, as opposed to orthogonal regression analysis, was performed because the three accommodative responses were averaged before fitting the functions to generate the amplitude and peak velocity data. Therefore, information about variance, which is required for orthogonal regressions, was not available. However, the variance was not likely to differ before and after phenylephrine, and therefore the equation from the linear regression is not likely to differ from the equation that an orthogonal regression would yield.
Figure 3.
The linear relationship (from linear regressions) between peak velocity and accommodative amplitude (main sequence relationship) was not significantly different before and 25 minutes after phenylephrine instillation for (A) accommodation (P = 0.52) or (B) disaccommodation (P = 0.24).
All monkeys had hyperopic unaccommodated refractions (1–7 D) through the contact lens, and, on average, the resting refractions were not significantly different before and 30 minutes after instillation of 10% phenylephrine (Fig. 4, t-test: t = −1.49, df = 4, P = 0.21).
Figure 4.
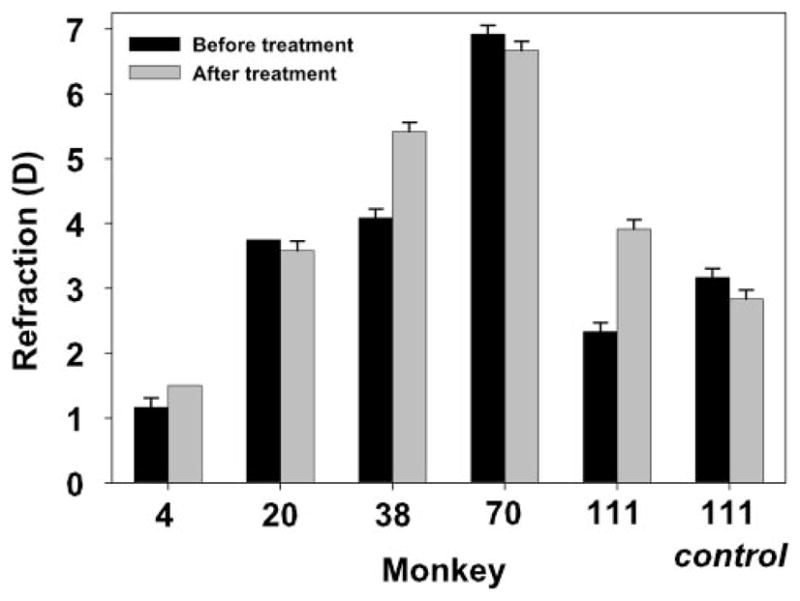
Baseline refraction measurements were similar before and 30 minutes after instillation of 10% phenylephrine (t-test: t = −1.4931, df = 4, P = 0.2097).
Average static accommodation responses from the four highest stimulus current amplitudes, measured with the Hartinger coincidence refractometer, were not significantly different before and 30 minutes after the instillation of phenylephrine (Fig. 5, t-test: t = −0.1050, df = 4, P = 0.9214). Average accommodative amplitude before phenylephrine was 11.73 ± 1.78 D and after phenylephrine was 11.75 ± 1.50 D. Figure 6 shows the maximum accommodative amplitude achieved before phenylephrine versus the amplitude achieved for the same stimulus amplitude after phenylephrine. Orthogonal regression analysis results in a slope of 1.017 and an intercept of −0.476 (r = 0.9898) that are not statistically different from the 1:1 line (95% confidence interval).
Figure 5.
Stimulus response functions measured with a Hartinger coincidence refractometer were similar before and 30 minutes after 10% phenylephrine (A–E). (F) Saline control.
Figure 6.
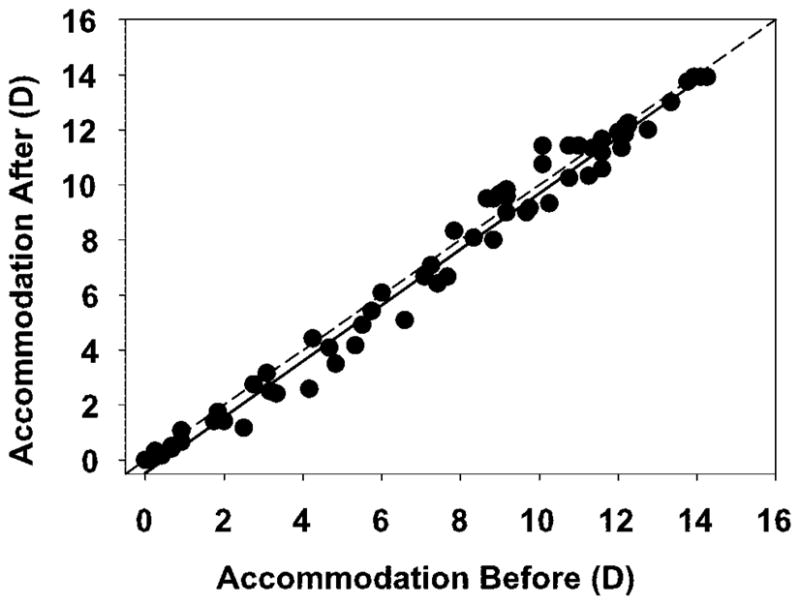
Hartinger-measured accommodative responses to the same stimulus amplitudes before and after phenylephrine administration were not significantly different (orthogonal regression: slope = 1.017, intercept = −0.476, r = 0.9898).
Discussion
The goal of this study was to determine whether phenylephrine, an α1-adrenergic agonist, has a direct effect on the ciliary muscle. It is known that phenylephrine dilates the iris through sympathetic stimulation of the dilator muscle, but its effects on the ciliary muscle are not well understood. Prior studies have reported a large range of differing effects of phenylephrine on accommodation, including decreases of up to 3 D in humans,16,17,22,25,28,46,47 and up to 7 D in monkeys.3,20 Results from the present study indicate that, although there are individual differences before and after phenylephrine, these differences are not systematic and therefore are probably not due to phenylephrine. Within the resolution of the methods, there are no systematic, significant effects of phenylephrine on accommodative amplitude, dynamics, or resting position. Similarly, response amplitude to each stimulus current amplitude, measured with a Hartinger coincidence refractometer, did not change after phenylephrine.
Previous studies measuring the effects of phenylephrine on the accommodative process have been performed in humans with voluntary accommodation. Confounding factors exist, such as effects of increased pupil size, which decrease depth of field and change ocular aberrations. Therefore, it is difficult to determine whether accommodation is altered by a direct action of the drug on the ciliary muscle or by secondary optical factors associated with mydriasis. Electrical stimulation of the EW nucleus allows open-loop, repeatable accommodative responses of a controlled amplitude and duration.32,39 Therefore, the accommodative responses can be studied in anesthetized monkeys without confounds due to the effects of pupil size, visual feedback, or perception on the part of the subject.
Before performing accommodative testing, it was necessary to determine an appropriate dosage of phenylephrine. The time course of action could be observed by a change in pupil size after phenylephrine instillation, although monkeys in which accommodative testing was performed had been previously iridectomized. Therefore, the tests of different phenylephrine dosage protocols were undertaken in noniridectomized monkeys to determine a dose that would elicit maximum mydriasis in the shortest time, with the assumption that any accommodative effects of phenylephrine would follow a time course similar to that of the mydriatic effects. It was determined that two 0.1-mL doses of 10% phenylephrine was appropriate, as pupil dilation was sustained in all monkeys for at least 65 minutes, allowing sufficient time to complete all accommodative testing.
Resting refractions, maximum accommodative amplitude, and accommodative dynamics were unaffected by phenylephrine, and the changes that occurred were within normal physiological variation for anesthetized monkeys.32 This is evident by the fact that the most variability in accommodation before and after treatment in this experiment was in the saline control monkey (Fig. 5). The finding of no significant change in accommodative dynamics over 20 minutes after phenylephrine indicates that with the dosage protocol used, phenylephrine, an α1-adrenergic sympathetic agent, does not affect the ciliary muscle or the centrally stimulated accommodative response in rhesus monkeys. These results differ from those found in other studies of electrically stimulated accommodation in monkeys. Tornqvist20 directly stimulated the cervical sympathetic nerve in cynomolgus monkeys and found a 1.5-D decrease in accommodative amplitude, and Chin et al.,3 found a 7-D decrease in accommodative amplitude after 1 drop of 2.5% phenylephrine in cynomolgus monkeys. It is not clear why such strong effects were found, but in both of these studies cynomolgus monkeys were used, which could have species-dependent differences at the receptor level from rhesus monkeys. Further, Tornqvist20 directly stimulated the cervical sympathetic nerve, which has different effects on both the α- and β-adrenergic systems than topical administration of phenylephrine.
Many studies in humans have also shown a decrease in accommodative amplitude or a change in accommodative dynamics after pharmacological α-adrenergic stimulation. When measured objectively, given a proximal accommodative stimulus, humans are able to achieve the same accommodative amplitude before and after phenylephrine.27 Therefore, if phenylephrine is necessary in accommodation studies to measure refraction through normally small pupils, accommodative amplitude and dynamics may be affected, but the ciliary muscle is not directly affected, and it is still possible to achieve maximum accommodative amplitude, although it is not guaranteed that maximum amplitude will be reached.
In several human studies, researchers have evaluated the effects of pharmacological sympathetic stimulation against a background of parasympathetic activity after a prolonged period of accommodation.18–20 This aspect of the accommodative system was not evaluated in the present study. Therefore, the effects of a slow build-up of sympathetic inhibition of accommodation were not addressed.
The protocol used in the present study to test the effects of phenylephrine on the ciliary muscle–driven accommodative response in anesthetized rhesus monkeys serves as an ideal method to study the effects of pharmaceutical agents on the physiology of the accommodative plant. Although phenylephrine resulted in no significant changes in accommodation, parasympathetic antagonists, such as atropine and cyclopentolate, and sympathetic antagonists, such as timolol maleate and betaxolol, may affect the accommodative response and can be studied in the same way to understand the effects of autonomic agents on the accommodative plant.
Acknowledgments
Supported in part by grants from Pharmacia, National Eye Institute Grant 1 R01-EY014651-01; and Grant 5 T32 EY07024-23 to the University of Texas Health Science Center at Houston. Contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of the awarding agencies.
The authors thank Siddharth Poonja for technical assistance and Ying-Sheng Hu for help with the statistical analyses.
Footnotes
Disclosure: L.A. Ostrin, None; A. Glasser, None
References
- 1.Cogan D. Accommodation and the autonomic nervous system. Arch Ophthalmol. 1937;18:739–766. [Google Scholar]
- 2.Stephens KG. Effect of the sympathetic nervous system on accommodation. Am J Optom Physiol Opt. 1985;62:402–406. doi: 10.1097/00006324-198506000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Chin NB, Ishikawa S, Lappin H, et al. Accommodation in monkeys induced by midbrain stimulation. Invest Ophthalmol. 1968;7:386–396. [PubMed] [Google Scholar]
- 4.Ruskell GL. Sympathetic innervation of the ciliary muscle in monkeys. Exp Eye Res. 1973;16:183–190. doi: 10.1016/0014-4835(73)90212-1. [DOI] [PubMed] [Google Scholar]
- 5.Zetterström C, Hahnenberger R. Pharmacological characterization of human ciliary muscle adrenoreceptors in vitro. Exp Eye Res. 1988;46:421–430. doi: 10.1016/s0014-4835(88)80030-7. [DOI] [PubMed] [Google Scholar]
- 6.Wax MB, Molinoff PB. Distribution and properties of beta adrenergic receptors in the human iris-ciliary body. Invest Ophthalmol Vis Sci. 1987;28:420–430. [PubMed] [Google Scholar]
- 7.van Alphen GW. The adrenergic receptors of the intraocular muscles of the human eye. Invest Ophthalmol Vis Sci. 1976;15:502–505. [PubMed] [Google Scholar]
- 8.Hurwitz B, Davidowitz J, Chin N, Breinin G. The effects of the sympathetic nervous system on accommodation: 1. Beta sympathetic nervous system. Arch Ophthalmol. 1972;87:668–674. doi: 10.1001/archopht.1972.01000020670011. [DOI] [PubMed] [Google Scholar]
- 9.Toates FM. Accommodation function of the human eye. Physiol Rev. 1972;52:828–863. doi: 10.1152/physrev.1972.52.4.828. [DOI] [PubMed] [Google Scholar]
- 10.Morat J, Doyon M. Le grand sympathetique, nerf de l’accommodation pour la vision des objets eloiunges. Ann Ocul. 1891;106:28–30. [Google Scholar]
- 11.Charman WN. The accommodative resting point and refractive error. Ophthalmic Opt. 1982:469–473. [Google Scholar]
- 12.Luckeish M, Moss F. Functional adaptation for near vision. J Exp Psychol. 1940;26:352–356. [Google Scholar]
- 13.Campbell FW, Primrose JAE. The state of accommodation of the human eye in darkness. Trans Ophthalmol Soc U K. 1953;73:353–361. [Google Scholar]
- 14.Gilmartin B, Hogan R, Thompson S. The effect of timolol maleate on tonic accommodation, tonic vergence and pupil diameter. Invest Ophthalmol Vis Sci. 1984;25:763–770. [PubMed] [Google Scholar]
- 15.Gilmartin B, Hogan R. The relationship between tonic accommodation and ciliary muscle innervation. Invest Ophthalmol Vis Sci. 1985;26:1024–1029. [PubMed] [Google Scholar]
- 16.Garner LF, Brown B, Baker R, Colgan M. The effect of phenylephrine hydrochloride on the resting point of accommodation. Invest Ophthalmol Vis Sci. 1983;24:393–395. [PubMed] [Google Scholar]
- 17.Zetterström C. Effects of adrenergic drugs on accommodation and distant refraction in daylight and darkness: a laser optometric study. Acta Ophthalmol. 1988;66:58–64. doi: 10.1111/j.1755-3768.1988.tb08535.x. [DOI] [PubMed] [Google Scholar]
- 18.Gilmartin B. A review of the role of sympathetic innervation of the ciliary muscle in ocular accommodation. Ophthalmic Physiol Opt. 1986;6:23–37. [PubMed] [Google Scholar]
- 19.Winn B, Culhane HM, Gilmartin B, Strang NC. Effect of beta-adrenoceptor antagonists on autonomic control of ciliary smooth muscle. Ophthalmic Physiol Opt. 2002;22:359–365. doi: 10.1046/j.1475-1313.2002.00075.x. [DOI] [PubMed] [Google Scholar]
- 20.Tornqvist G. The relative importance of the parasympathetic and sympathetic nervous systems for accommodation in monkeys. Invest Ophthalmol Vis Sci. 1967;6:612–617. [PubMed] [Google Scholar]
- 21.Gilmartin B, Bullimore MA. Sustained near vision augments inhibitory sympathetic innervation of the ciliary muscle. Clin Vision Sci. 1987;1:197–208. [Google Scholar]
- 22.Gimpel G, Doughty MJ, Lyle WM. Large sample study of the effects of phenylephrine 2.5% eyedrops on the amplitude of accommodation in man. Ophthalmic Physiol Opt. 1994;14:123–128. doi: 10.1111/j.1475-1313.1994.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 23.Culhane HM, Winn B, Gilmartin B. Human dynamic closed-loop accommodation augmented by sympathetic inhibition. Invest Ophthalmol Vis Sci. 1999;40:1137–1143. [PubMed] [Google Scholar]
- 24.Paggiarino DA, Brancato LJ, Newton RE. The effects on pupil size and accommodation of sympathetic and parasympatholytic agents. Ann Ophthalmol. 1993;25:244–253. [PubMed] [Google Scholar]
- 25.Zetterström C. The effect of phenylephrine on the accommodative process in man. Acta Ophthalmol. 1984;62:872–878. doi: 10.1111/j.1755-3768.1984.tb08437.x. [DOI] [PubMed] [Google Scholar]
- 26.Atchison DA, Charman WN, Woods RL. Subjective depth-of-focus of the eye. Optom Vis Sci. 1997;74:511–520. doi: 10.1097/00006324-199707000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Do T, Kasthurirangan S, Ostrin LA, Glasser A. The effects of phenylephrine on accommodation in humans. Optom Vis Sci. 2002;79:174. [Google Scholar]
- 28.Biggs RD, Alpern M, Bennett DR. The effect of sympathomimetic drugs upon the amplitude of accommodation. Am J Ophthalmol. 1959;48:169–172. doi: 10.1016/0002-9394(59)90256-9. [DOI] [PubMed] [Google Scholar]
- 29.Mordi J, Tucker J, Charman WN. Effects of 0.1% cyclopentolate or 10% phenylephrine on pupil diameter and accommodation. Ophthalmic Physiol Opt. 1986;6:221–227. [PubMed] [Google Scholar]
- 30.Hurwitz B, Davidowitz J, Pachter BR, Breinin G. The effects of the sympathetic nervous system on accommodation. II. Alpha sympathetic nervous system. Arch Ophthalmol. 1972;87:675–678. doi: 10.1001/archopht.1972.01000020677012. [DOI] [PubMed] [Google Scholar]
- 31.Jampel RS, Mindel J. The nucleus for accommodation in the midbrain of the macaque. Invest Ophthalmol. 1967;6:40–50. [PubMed] [Google Scholar]
- 32.Vilupuru AS, Glasser A. Dynamic accommodation in rhesus monkeys. Vision Res. 2002;42:125–141. doi: 10.1016/s0042-6989(01)00260-7. [DOI] [PubMed] [Google Scholar]
- 33.Koretz JF, Bertasso AM, Neider MW, et al. Slit-lamp studies of the rhesus monkey eye. II Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res. 1987;45:317–326. doi: 10.1016/s0014-4835(87)80153-7. [DOI] [PubMed] [Google Scholar]
- 34.Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- 35.Tornqvist G. Effect of topical carbachol on the pupil and refraction in young and presbyopic monkeys. Invest Ophthalmol Vis Sci. 1966;5:186–195. [Google Scholar]
- 36.Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- 37.Koretz JF, Neider MW, Kaufman PL, et al. Slit-lamp studies of the rhesus monkey eye. I. Survey of the anterior segment. Exp Eye Res. 1987;44:307–318. doi: 10.1016/s0014-4835(87)80014-3. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman PL, Lütjen-Drecoll E. Total iridectomy in the primate in vivo: surgical technique and postoperative anatomy. Invest Ophthalmol Vis Sci. 1975;14:766–771. [PubMed] [Google Scholar]
- 39.Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- 40.Vilupuru AS, Glasser A. Dynamic accommodative changes in rhesus monkey eyes assessed with A-scan ultrasound biometry. Optom Vis Sci. 2003;80:383–394. doi: 10.1097/00006324-200305000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Bito LZ, Kaufman PL, DeRousseau CJ, Koretz J. Presbyopia: an animal model and experimental approaches for the study of the mechanism of accommodation and ocular aging. Eye. 1987;1:222–230. doi: 10.1038/eye.1987.41. [DOI] [PubMed] [Google Scholar]
- 42.Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of rhesus monkeys. Invest Ophthalmol Vis Sci. 1990;31:2185–2190. [PubMed] [Google Scholar]
- 43.Fincham EF. The coincidence optometer. Proc Phys Soc (Lond) 1937;49:456–468. [Google Scholar]
- 44.Schaeffel F, Farkas L, Howland HC. Infrared photoretinoscope. Appl Opt. 1987;26:1505–1509. doi: 10.1364/AO.26.001505. [DOI] [PubMed] [Google Scholar]
- 45.Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol (Lond) 1993;461:301–320. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mordi JA, Lyle WM, Mousa GY. Effect of phenylephrine on accommodation. Am J Optom. 1986;63:294–297. doi: 10.1097/00006324-198604000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Rosenfield M, Gilmartin B, Cunningham E, Dattani N. The influence of alpha-adrenergic agents on tonic accommodation. Curr Eye Res. 1990;9:267–272. doi: 10.3109/02713689009044522. [DOI] [PubMed] [Google Scholar]