Abstract
The P2Y14 receptor is a relatively broadly expressed G protein-coupled receptor that is prominently associated with immune and inflammatory cells as well as with many epithelia. This receptor historically was thought to be activated selectively by UDP-glucose and other UDP-sugars. However, UDP is also a very potent agonist of this receptor, and may prove to be one of its most important cognate activators.
Keywords: Gi, immunological response, nucleotide, nucleotide sugar, P2Y receptor, UDP
The concept that adenine and uridine nucleotides and nucleotide sugars function as extracellular signalling molecules has expanded markedly in the past decade. At least 15 nucleotide-activated cell surface receptors exist in mammals, and remarkably broad and varied physiological responses occur downstream of nucleotide receptor activation (Ralevic & Burnstock 1998, Leipziger 2003, Burnstock 2007). The significance of extracellular nucleotides/nucleotide sugars as signalling molecules is also underscored by the ubiquitous distribution of several large classes of ectoenzymes that catalyse nucleotide breakdown and interconversion (Zimmermann 2000, Lazarowski et al. 2003a). Purinergic signalling was initially proposed on the basis of smooth muscle responses to autonomic nerve stimulation that were not blocked by adrenergic receptor antagonists (Burnstock 1972). However, observation of responses to nucleotides in the brain and in essentially all peripheral tissues, including those not significantly innervated by the autonomic nervous system, indicates that extracellular nucleotides arising from both neuronal and non-neuronal sources underlie many important physiological processes.
Two distinct families of cell surface receptors mediate the actions of extracellular nucleotides. P2X receptors are ligand-gated ion channels that conduct extracellular cations in response to adenosine triphosphate (ATP) (Khakh & North 2006). Seven receptors (P2X1–P2X7) comprise this family found largely, but not exclusively, on neurones and other excitatory tissues. P2Y receptors (P2Y-R) are a group of eight molecularly defined G protein-coupled receptors (GPCR) that exist both in the central and autonomic nervous systems as well as on most non-excitatory cells (Abbracchio et al. 2006).
The P2Y1 receptor subfamily
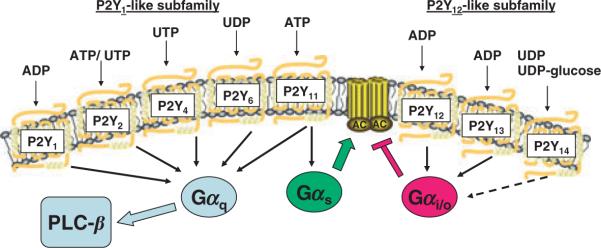
The P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptors (hereafter referred to as P2Y1-R, P2Y2-R, P2Y4-R, P2Y6-R and P2Y11-R) were the first molecularly identified P2Y-R (Harden et al. 1998). Non-consecutive numbering of these receptors occurred due to premature and erroneous designation of six other GPCR that were species homologues of unambiguously identified P2Y-R or proved to be members of other GPCR families. The P2Y1-R, P2Y2-R, P2Y4-R and P2Y6-R activate Gq/phospholipase C-β and can be grouped into a P2Y1 receptor subfamily of P2Y-R that share 35–52% primary sequence identity (Fig. 1). The Gq- (and Gs-) linked P2Y11-R is also considered a member of this subfamily although it exhibits only 28–30% identity to the four other P2Y1 receptor subfamily members and is not present in rodents. In contrast to the P2X receptors, which are all activated by ATP [but not adenosine diphosphate (ADP)], the sparsely expressed P2Y11-R is the only P2Y-R that is activated selectively by ATP (Communi et al. 1997, Qi et al. 2001). The P2Y1-R is selectively activated by ADP (Palmer et al. 1998, Waldo & Harden 2004), and the P2Y2-R is activated equipotently by UTP and ATP (Lustig et al. 1993, Nicholas et al. 1996). The human P2Y4-R is selectively activated by UTP, whereas rodent P2Y4-R are equipotently activated by UTP and ATP (Communi et al. 1995, Nicholas et al. 1996). The P2Y6-R is selectively activated by UDP (Chang et al. 1995, Nicholas et al. 1996). Receptors of the P2Y1 receptor subfamily regulate a broad range of physiological responses (Ralevic & Burnstock 1998, Leipziger 2003, Abbracchio et al. 2006, Burnstock 2007) and are particularly important in brain (P2Y1-R and P2Y2-R), in the autonomic nervous system (most or all of these P2Y-R), in epithelia of the airway, gastrointestinal tract, kidney, eye and other tissues (P2Y2-R, P2Y4-R and P2Y6-R), in vascular tissue (most or all of these P2Y-R), in platelets (P2Y1-R) and in immune and inflammatory cells (P2Y2-R and P2Y6-R).
Figure 1.
The P2Y receptors. The eight mammalian P2Y receptors and their cognate agonists are indicated. The five members of the P2Y1-like subfamily all couple to heterotrimeric G-proteins of the Gαq family and activate phospholipase C-β (PLC-β) isozymes. The ATP-activated P2Y11 receptor also couples to Gαs resulting in activation of adenylyl cyclase (AC). The three members of the P2Y12-like subfamily couple to heterotrimeric G proteins of the Gαi/o family and inhibit adenylyl cyclase (AC) activity.
The P2Y12 receptor subfamily
The P2Y12-R, P2Y13-R and P2Y14-R exhibit 45–50% homology and comprise a second group of P2Y-R, the so-called P2Y12 receptor subfamily of nucleotide/nucleotide sugar-activated receptors (Fig. 1). These GPCR have little sequence identity (20–25%) with members of the P2Y1 receptor subfamily. The P2Y12 receptor (P2Y12-R) accounts for ADP-promoted inhibition of adenylyl cyclase in platelets (Cooper & Rodbell 1979, Hollopeter et al. 2001), and promotion of aggregation by ADP requires simultaneous activation of this Gi-coupled receptor and the Gq-coupled P2Y1-R (Abbracchio et al. 2006, Gachet 2006). The P2Y13 receptor (P2Y13-R) is a third ADP-activated P2Y-R that like the P2Y12-R is Gi-, but not Gq-, coupled (Communi et al. 2001). The P2Y14 receptor (P2Y14-R) was identified initially as a receptor activated by UDP-glucose and other nucleotide sugars (Chambers et al. 2000). As discussed in more detail below, UDP recently was shown to potently activate both rodent and human P2Y14-R (Fricks et al. 2008, Carter et al. 2009).
In addition to its important presence in platelets, the P2Y12-R is widely distributed in brain (Hollopeter et al. 2001) and mediates cell migration responses of microglia during inflammation (Haynes et al. 2006). The ADP-activated P2Y13-R is also expressed in brain and is present in high amounts in spleen (Communi et al. 2001). The P2Y14-R is expressed at relatively high levels in brain and placenta, in neutrophils, lymphocytes and megakaryocytic cells, in epithelial cells from various tissues and in gastrointestinal smooth muscle (Chambers et al. 2000, Abbracchio et al. 2006). Although the platelet P2Y12-R receptor has been broadly studied, much less is known about the signalling properties and physiological roles of the P2Y13-R and P2Y14-R. Recent progress accrued in understanding of the P2Y14-R is the subject of this review.
The P2Y14-R
Chambers and his colleagues (Chambers et al. 2000, Freeman et al. 2001) expressed a number of orphan GPCR in yeast strains engineered to respond to agonist activation with increased signal from a pheromone receptor-induced reporter gene, and tested the potential activation of these GPCR by a library of biologically active small molecules. This screen led to the discovery that the orphan GPCR, KIAA0001 (subsequently referred to as the P2Y14-R), is activated by UDP-glucose. UDP-galactose, UDP-glucuronic acid and UDP-N-acetylglucosamine were also activators of KIAA0001 in the yeast test system whereas a series of nucleoside diphosphates and triphosphates and ADP-, CDP- and GDP-sugars were not.
The P2Y14-R was both transiently and stably expressed with the promiscuous Gα-subunit Gα16 in human embryonic kidney-293 (HEK293) cells, and Ca2+ responses were quantified in response to potential agonists (Chambers et al. 2000). Again, UDP-sugars were potent agonists of the receptor with a relative order of potency of UDP-glucose > UDP-galactose > UDP-glucuronic acid > UDP-N-acetylglucosamine observed. In contrast, CDP-glucose was inactive at concentrations as high as 10 μM.
Membranes were also prepared from HEK293 cells stably expressing the human P2Y14-R, and potential agonist-promoted binding of [35S]GTPγS to endogenous G-proteins was quantified (Chambers et al. 2000). UDP-glucose stimulated [35S]GTPγS binding with an EC50 of 234 nm, and this effect was blocked by pre-treatment of cells with pertussis toxin, suggesting that the human P2Y14-R couples to Gα-subunits of the Gi family of heterotrimeric G proteins.
Fricks et al. (2008) examined the pharmacological selectivity of the human P2Y14-R in COS-7 cells transiently co-expressing a chimeric G protein Gαq/i in which the last five amino acids at the carboxyl terminus of Gαq were substituted with those of Gαi. This chimeric Gα subunit is activated by GPCRs that couple to the Gi family of G-proteins and directs signals through downstream effectors of Gαq such as phospholipase C-β (Coward et al. 1999). The activities of UDP-sugars determined in this test system were similar to those previously reported by Chambers and colleagues. That is, [3H]inositol phosphate accumulation was stimulated with a relative order of potency of UDP-glucose > UDP-galactose > UDP-glucuronic acid > UDP-N-acetylglucosamine.
Release of nucleotide sugars
Whereas expression of the human P2Y14-R or the Gαq/i chimera alone in COS-7 cells had no effect, co-expression of the hP2Y14-R with Gαq/i resulted in increased [3H]inositol phosphate accumulation in the absence of added agonist (Lazarowski et al. 2003b, Fricks et al. 2008). Application of an assay that provides direct quantification of UDP-glucose mass revealed that UDP-glucose is basally released from a broad range of cell types (Lazarowski et al. 2003b) and led to the hypothesis that P2Y14-R-dependent increases in [3H]inositol phosphate accumulation in the transfected cell system occurred as a consequence of autocrine release of an activating agonist(s). Co-expression of the UDP-sugar-inactivating ecto-enzyme, ectonucleotide pyrophosphatase-1, allowed direct testing of this idea. Thus, expression of this ecto-phosphatase with the human P2Y14-R and Gαq/i resulted in a decrease in basal [3H]inositol phosphate levels that corresponded with a decrease in extracellular UDP-glucose levels (Fricks et al. 2008).
Realization that UDP-glucose is a naturally occurring extracellular signalling molecule also prompted studies focused on the properties and potential mechanism of regulated release of nucleotide sugars. The hypothesis that UDP-glucose is released in a regulated manner was first examined using 1321N1 human astrocytoma cells, one of the few cell models exhibiting enhanced release of ATP in response to activation of a GPCR (Joseph et al. 2003). Activation of an endogenous protease-activated receptor (PAR) of 1321N1 cells by thrombin resulted in rapid and robust release of ATP, which was accompanied by enhanced UDP-glucose release (Kreda et al. 2008). As was observed with ATP release, PAR-stimulated release of UDP-glucose was abolished by the intracellular Ca2+ chelator BAPTA and the Golgi-disrupting agent brefeldin A. Release also was attenuated after disruption of the cytoskeleton with cytochalasin D, suggesting that nucleotide release from these cells occurs via a vesicular mechanism. This conclusion was also in agreement with studies illustrating that airway epithelial goblet cells displayed enhanced release of UDP-glucose and ATP concomitantly with Ca2+-regulated secretion of mucins (Kreda et al. 2007).
The hypothesis that extracellular UDP-sugars originate in a vesicular compartment is supported by the fact that these molecules play important roles during glycosylation reactions within the secretory pathway. That is, UDP-sugars are synthesized in the cytosol and transported into the lumen of the endoplasmic reticulum (ER) and Golgi apparatus to serve as sugar donors for glycosyltransferase-catalysed reactions on exportable glycoconjugates. UDP-sugar concentrations in the ER/Golgi are up to 20-fold higher than cytosolic levels (Hirschberg et al. 1998). As ER/Golgi UDP-sugars do not diffuse back to the cytosol, they potentially are cargo molecules released from cells during export of glycoconjugates.
Unambiguous proof of a Golgi contribution to extracellular UDP-sugars was illustrated by a tight correlation between Golgi expression of a Golgi-resident UDP-sugar transporter and the release of the cognate UDP-sugar substrate (Sesma et al. 2009). As the ER/Golgi UDP-glucose transporter has not been identified at the molecular level, Sesma and co-workers chose an alternative system, i.e. the ER/Golgi-resident UDP-N-acetylglucosamine transporter, for their studies. Deletion of Yea4, a gene encoding the ER-resident UDP-N-acetylglucosamine transporter (YEA4) in yeast, resulted in impaired release of this nucleotide sugar. Conversely, overexpression of YEA4 in Yea4−/− cells restored UDP-N-acetylglucosamine release. In addition, overexpression of HFRC1, a human UDP-N-acetylglucosamine transporter, in airway epithelial cells, resulted in enhanced release of UDP-N-acetylglucosamine from these cells. Quantification of mass of UDP-N-acetylglucosamine revealed that this P2Y14-R agonist accumulated with UDP-glucose in submicromolar concentrations in lung secretions from patients with inflammatory lung diseases (Sesma et al. 2009). This observation together with the expression of the P2Y14-R in inflammatory cells strongly suggests a patho-physiological relevance of nucleotide sugar-activated cell signalling responses.
Release of UDP-sugars from cells under various physiological conditions is not restricted to UDP-glucose and UDP-N-acetylglucosamine. Development of an assay that allows quantification of another P2Y14-R agonist, UDP-galactose, with subnanomolar sensitivity (Lazarowski 2010) revealed that UDP-galactose is present in the conditioned medium of various cell models, including physiologically relevant primary cultures of human bronchial epithelial cells. Like UDP-glucose, enhanced release of UDP-galactose was observed in thrombin-stimulated 1321N1 cells. Given the likelihood that release of the activating P2Y14-R ligand may differ according to cell type, UDP-galactose predictably is an important autocrine/paracrine regulator of P2Y14-R activity.
P2Y14-R-dependent activation of native cell signalling pathways
Agonist-promoted activation of Gi and consequential inhibition of adenylyl cyclase is thought to be a primary signalling response of the members of the P2Y12-R subfamily of P2Y receptors. However, initial studies of the P2Y14-R largely relied on overexpression of Gα-subunits, e.g. Gα16 or the Gαq/i chimera, that engineer coupling of Gi-coupled receptors to activation of phospholipase C (Chambers et al. 2000, Freeman et al. 2001, Fricks et al. 2008). Several recent studies of the P2Y14-R have taken advantage of stable cell lines that allow direct quantification of native downstream signalling pathways.
The human P2Y14-R was stably expressed in HEK293 cells using a retrovirus expression system (Fricks et al. 2009). UDP-glucose promoted a concentration-dependent inhibition of forskolin-stimulated accumulation of cyclic AMP in these cells with an EC50 value of 82 nM, and a maximal inhibition of forskolin-stimulated cyclic AMP accumulation of up to 80% was observed. Pre-incubation of P2Y14-HEK293 cells with pertussis toxin resulted in complete loss of UDP-glucose-dependent inhibition of cyclic AMP accumulation, indicating that the P2Y14-R signals through Gα-subunits of the Gi family. UDP-galactose, UDP-glucuronic acid and UDP-N-acetylglucosamine also promoted inhibition of cyclic AMP accumulation in P2Y14-HEK cells, and the maximal inhibition observed with these UDP-sugars was similar to that observed with UDP-glucose. The potencies of UDP-galactose and UDP-glucuronic acid were similar to that of UDP-glucose, while UDP-N-acetylglucosamine exhibited an approx. 10-fold higher EC50.
The capacity of the P2Y14-R to couple to adenylyl cyclase also was examined in other cell backgrounds. For example, C6 rat glioma cells have proved useful for the study of several Gi-coupled receptors (Schachter et al. 1997, Thomas et al. 2000, Castillo et al. 2007), and therefore, the human P2Y14-R was stably expressed in these cells using recombinant pLXSN virus harbouring the human P2Y14-R coding sequence (Fricks et al. 2009). Again, robust inhibitory effects were observed with UDP-glucose in P2Y14-R-expressing C6 cells but not in empty vector-infected cells. An EC50 value of 107 nM was determined and up to 90% inhibition of forskolin-stimulated cyclic AMP accumulation occurred in the presence of a maximally effective concentration of UDP-glucose. The action of UDP-glucose in P2Y14-R-C6 cells was completely blocked by pre-treatment with pertussis toxin, and other UDP-sugars also promoted inhibition of cyclic AMP accumulation with relative potencies similar to that observed in the P2Y14-R-HEK293 cell line. Unambiguous demonstration of coupling of the P2Y14-R to adenylyl cyclase was established by illustrating that GTP-dependent inhibition of adenylyl cyclase activity by UDP-glucose occurred in plasma membrane-enriched fractions prepared from P2Y14-C6 glioma cells. This UDP-glucose-promoted response was not observed in cell-free preparations from P2Y14-R-C6 cells pre-treated with pertussis toxin.
Krzeminski et al. (2008) observed a second messenger response to a high concentration of UDP-glucose in a C6 glioma cell line in the absence of recombinant P2Y14-R expression. No statistically significant effect on cyclic AMP accumulation was observed, but a robust Ca2+ response to 100 μM UDP-glucose was reported that was blocked completely by pre-treatment of cells with pertussis toxin. Neither the agonist selectivity nor the concentration dependence of UDP-glucose for producing this effect was reported.
MAP kinase signalling
Mitogen-activated protein (MAP) kinase signalling pathways often are stimulated by activation of receptors that signal through Gi, and therefore, the capacity of the P2Y14-R to promote phosphorylation of MAP kinases also has been examined. Whereas no effect was observed in mock-infected cells, UDP-glucose-dependent extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation occurred in P2Y14-R-HEK293 cells (Fricks et al. 2009). The effects of UDP-glucose on ERK1/2 phosphorylation were concentration dependent, and the EC50 (30 nM) observed was similar to that obtained in studies of inhibition of cyclic AMP accumulation in these cells. As was observed in studies of adenylyl cyclase, P2Y14-R-regulated ERK1/2 phosphorylation was not observed in cells pre-incubated with pertussis toxin. The time course for UDP-glucose-dependent activation of ERK1/2 differed from that occurring as a consequence of activation of PAR2, which is natively expressed in HEK293 cells (Amadesi et al. 2004, Dai et al. 2007). Thus, incubation of cells with a PAR2 agonist resulted in marked phosphorylation of ERK1/2 within 5 min, and phosphorylation quickly diminished thereafter. In contrast, UDP-glucose-dependent activation of ERK1/2 was not maximal until 15 min of incubation and was retained for at least 30 min.
Signalling responses of P2Y14-R endogenously expressed in differentiated HL-60 cells
Whereas P2Y14-R mRNA was not detected in undifferentiated HL-60 promyeloleukaemia cells, differentiation of these cells with 1.3% DMSO to a neutrophil-like cell resulted in a marked increase in expression of P2Y14-R mRNA (Fricks et al. 2009). Similarly, no effect of UDP-glucose was observed in wild-type cells, but a time-dependent activation of ERK1/2 occurred in the presence of UDP-glucose after differentiation of the cells. This effect was apparent within 5 min and peaked within 30 min. In contrast to the time course of phosphorylation in response to UDP-glucose, cells treated with formyl-Met-Leu-Phe, an agonist of the formyl peptide receptor natively expressed in HL-60 cells (Boulay et al. 1990, Klinker et al. 1996), exhibited a robust ERK1/2 activation at 5 min that quickly diminished thereafter. Neither UDP-glucose- nor formyl-Met-Leu-Phe-dependent ERK1/2 activation was observed in cells pre-incubated with pertussis toxin, consistent with the idea that ERK1/2 activation in differentiated HL-60 cells occurs through a mechanism involving Gi. Consistent with the results observed after stable expression of recombinant P2Y14-R and after up-regulation P2Y14-R in HL-60 cells, Scrivens & Dickenson (2006) reported that UDP-glucose promotes ERK activation in isolated human neutrophils.
Activation of the P2Y14-R or formyl peptide receptor in differentiated HL-60 cells apparently does not result in regulation of adenylyl cyclase activity. That is, incubation of these cells with either UDP-glucose or formyl-Met-Leu-Phe had no effect on cyclic AMP accumulation promoted by forskolin, by amthamine (an agonist of the Gs-coupled H2 histamine receptor) or by forskolin and amthamine in combination (Fricks et al. 2009).
UDP is a cognate agonist of the P2Y14-R
Ault & Broach (2006) used a yeast model system for directed evolution of the P2Y14-R with the goal of identifying mutant P2Y14-R with differential agonist sensitivities. Studies performed using one of these mutant receptors revealed that UDP antagonized UDP-glucose-promoted receptor activation in a concentration-dependent manner. On the basis of these results, Fricks et al. (2008) hypothesized that the P2Y14-R binding pocket may not be restricted to interaction with nucleotide sugars alone, and that UDP and/or other nucleotides potentially are natural ligands at the wild-type P2Y14-R. To test this idea, the human P2Y14-R and Gαq/i were transiently coexpressed in COS-7 cells, and inositol lipid hydrolysis was quantified. Tests of potential activity of UDP revealed a parallel rightward shift of the UDP-glucose concentration–effect curves in the presence of the diphosphate, and Schild analysis confirmed that the antagonism produced by UDP was competitive. Subsequent studies revealed that UDP acts as a partial agonist in this test system with human receptors (Carter et al. 2009). That is, competitive antagonist activity was observed at low cellular levels of the human P2Y14-R, whereas UDP exhibited agonist activity at higher receptor densities. The rat P2Y14-R exhibits approx. 80% amino acid sequence identity to the human P2Y14-R and was shown to exhibit a similar UDP-sugar selectivity to that of the human P2Y14-R. Co-expression of the rat P2Y14-R with Gαq/i in COS-7 cells also revealed that UDP is a cognate ligand of this receptor orthologue (Fricks et al. 2008). However, in contrast to the results observed with the human receptor in this engineered test system, UDP was a potent full agonist at the rat receptor.
More recent studies have focused on comparison of the actions of UDP vs. UDP-sugars in cells in which P2Y14-R-promoted signalling was quantified by measuring natural downstream signalling pathways. For example, UDP caused a concentration-dependent inhibition of forskolin-stimulated cyclic AMP accumulation in P2Y14-HEK293, P2Y14-R-C6 glioma and P2Y14-R-chinese hamster ovary (CHO) cells, but no effect of UDP was observed in wild-type HEK293, C6 or CHO cells (Carter et al. 2009). The maximal inhibition observed with UDP was similar to that seen with UDP-glucose, and the potency of UDP was approximately fivefold greater than that of UDP-glucose in the three different P2Y14-R stable cell lines. As was previously observed with UDP-glucose (Fricks et al. 2009), UDP had no effect on cyclic AMP accumulation in P2Y14-HEK293, P2Y14-R-C6 or P2Y14-R-CHO cells that were pre-incubated with pertussis toxin. UDP apparently is the only naturally occurring nucleoside diphosphate that activates the human P2Y14-R as only modest activities were observed with CDP, GDP and ADP at concentrations greater than 100-fold higher than the concentrations of UDP necessary to produce a maximal response through this receptor (Carter et al. 2009).
The capacity of UDP to function as a P2Y14-R agonist was also examined by quantification of phosphorylation of ERK1/2. No effect of UDP on MAP kinase phosphorylation was observed in wild-type HEK293 cells. In contrast, robust UDP-dependent stimulation of ERK1/2 phosphorylation was observed in P2Y14-HEK293 cells (Carter et al. 2009). The EC50 for this effect was similar to that for UDP-dependent inhibition of adenylyl cyclase activity, and the actions of UDP were blocked by pre-treatment with pertussis toxin. Thus, as was observed in measurements of cyclic AMP accumulation, quantification of MAP kinase signalling also reveals that UDP and UDP-glucose exhibit similar agonist activities at the P2Y14-R.
The UDP-activated P2Y6-R is natively expressed at relatively high levels in HL-60 cells (Carter et al. 2009). Thus, these cells could not be utilized in a simple fashion to determine whether up-regulation of the P2Y14-R after DMSO-induced differentiation results in the appearance of responses to UDP-glucose as well as to UDP. Quantification of inhibition of cyclic AMP accumulation potentially would allow resolution of the effects of UDP on the Gi-coupled P2Y14-R vs. the Gq-coupled P2Y6-R, but as described earlier, no P2Y14-R- or formyl peptide receptor-promoted inhibition of adenylyl cyclase is observed in wild-type or differentiated HL-60 cells (Fricks et al. 2009). However, these limitations were pharmacologically circumvented by identifying analogues of UDP that activate the P2Y14-R at concentrations that exhibit little if any activity at the P2Y6-R. Thus, difluoro-α,β-methylene-UDP (MRS2802) and UDP-β-propylester (MRS2907) caused concentration-dependent inhibition of forskolin-stimulated cyclic AMP accumulation in P2Y14-HEK293 cells over a range of concentrations that caused little or no inositol phosphate response in 1321N1 human astrocytoma cells stably expressing the human P2Y6-R (Carter et al. 2009). Neither difluoro-α,β-methylene-UDP nor UDP-β-propylester promoted ERK1/2 phosphorylation in wild-type HL-60 cells which express the P2Y6-R but do not express the P2Y14-R. However, following differentiation of HL-60 cells with DMSO (and upregulation of the P2Y14-R), both agonists promoted MAP kinase signalling responses that were similar to that observed with UDP-glucose. These results are consistent with the idea that UDP analogues, and by deduction UDP itself, are potent agonists of the P2Y14-R in a native environment.
Drug studies
A broad series of structure activity studies were carried out with the goals of delineating the P2Y14-R pharmacophore, developing highly selective P2Y14-R agonists, and ultimately developing competitive antagonists that selectively block this signalling protein. These studies are only briefly summarized here, and the reader is referred to several published studies (Ko et al. 2007, 2009, Das et al. 2010) for more detailed considerations of the medicinal chemistry and associated molecular modelling directed at the P2Y14-R. Nearly all modifications of the uracil or ribose moieties of UDP-glucose abolished activity, suggesting that the binding pocket of the P2Y14-R is among the least permissive for ligand modification among the P2Y receptors. However, 2-thiouracil modification of UDP-glucose increased potency by sevenfold. Molecular modelling of the human P2Y14-R based on the structure of rhodopsin was applied to hypothesize potential interactions within the ligand binding site, and this model accurately predicted that replacement of the glucose moiety with other sugars, e.g. UDP-fructose, UDP-mannose and UDP-inositol, would result in P2Y14-R molecules exhibiting significant agonist activity (Ko et al. 2009). Activity was also retained after substitution of hydroxyl groups of glucose with fluorine and by chain extension.
The observation that UDP is a potent agonist of the P2Y14-R (Carter et al. 2009) together with structure activity analyses of molecules at the P2Y6 -R (Besada et al. 2006, Ko et al. 2008) led to the synthesis of UDP analogues containing β-phosphate substitution that might favour activation of the P2Y14-R (Carter et al. 2009, Das et al. 2010). Although the glucose moiety of UDP-glucose was suggested to interact with multiple H-bonding and/or charged resides within the putative binding site of the P2Y14-R, its deletion or substitution with smaller phosphoester groups was tolerated at this receptor. Simple alkyl esters of UDP displayed highly potent agonist activity at the P2Y14-R. The effects of these modifications to preserve and enhance potency at the P2Y14-R were additive with the previously identified potency-enhancing effect of 2-thio substitution of the uracil moiety, and several of these molecules achieved low or subnanomolar potency. Moreover, a number of these UDP analogues exhibit high selectivity for activation of the P2Y14-R over the P2Y6-R. For example, 2-thiouracil derivatives displayed selectivity for activation of the human P2Y14-R vs. the P2Y6-R, and a 2-thio-substituted β-methyl ester of UDP is a highly potent (EC50 = 56 nM) and selective P2Y14-R agonist. Interestingly, UDP analogues with phosphate chains containing α,β-methylene or difluoromethylene groups were well tolerated at the P2Y14-R, and α,β-methylene-2-thio-UDP exhibited subnanomolar potency.
Das et al. (2009) synthesized polymeric macromolecular nanostructures incorporating UDP-glucose with the ultimate idea of applying these dendrimers as nanocarriers for drug delivery and other applications. Uridine-5′-diphosphoglucuronic acid and its ethylenediamine adduct proved to be suitable functionalized congeners for coupling to several generations of dendrimers, and for example, a conjugate containing 20 bound nucleotide moieties was 100-fold more potent than UDP-glucose. Molecular modelling suggests that the nucleotide-substituted branches of this conjugate could extend far beyond the dimensions of the receptor and be available for multivalent docking to receptor aggregates. Indeed, larger dendrimer carriers with greater loading resulted in subnanomolar potency at the P2Y14-R.
P2Y14-R in immune/inflammatory responses
Nucleotides and nucleotide sugars are released from cells as a consequence of cell damage, stress, bacterial infection and various other deleterious stimuli. Similarly, message for many if not all of the P2Y-R has been reported to be present in multiple inflammatory cell types, including mast cells, dendritic cells, monocytes, neutrophils and eosinophils. Although P2Y-R-regulated signalling is almost certainly important in a panoply of events associated with immune responses, it has been difficult to associate unambiguously the presence of a given extracellular agonist with the functional presence of its target P2Y-R(s) mediating a physiologically relevant immune or inflammatory response. However, several rigorous studies suggest a role of, for example, the ATP/UTP-activated P2Y2-R and the A3 adenosine receptor in chemotactic responses of human neutrophils (Chen et al. 2006) and the P2Y2-R in `find-me' signalling associated with phagocytic clearance of apoptotic cells (Elliott et al. 2009).
While the physiological role(s) of the P2Y14-R in immunological responses remains to be clearly defined, the high expression levels of this receptor often encountered in leucocytes and other cells involved in immune responses support a potential role for the P2Y14-R in immune system homeostasis. P2Y14-R mRNA was markedly up-regulated in rat brain after immunological challenge with lipopolysaccharide (LPS) (Moore et al. 2003), and P2Y14-R mRNA was detected at high levels in immature human dendritic cells that mature and migrate to sites of inflammation in response to insult or injury (Skelton et al. 2003). The P2Y14-R was selectively expressed on bone marrow haematopoietic cells where it was proposed to mediate chemotaxis (Lee et al. 2003). Non-neuronal spinal cord cells express high levels of the P2Y14-R (Kobayashi et al. 2006), as do synoviocytes from human patients suffering from rheumatoid arthritis (Caporali et al. 2008). Similarly, UDP-glucose has been reported to stimulate Ca2+ accumulation in a variety of cells related to immune or inflammatory responses including astrocytes, monocyte-derived dendritic cells, microglia and several cells of epithelial origin. Scrivens and Dickenson also reported P2Y14-R expression in rodent T-lymphocytes (Scrivens & Dickenson 2005) and human neutrophils (Scrivens & Dickenson 2006). UDP-glucose was reported to cause a modest decrease in cyclic AMP levels, although this effect was not observed with other UDP-sugars (Scrivens & Dickenson 2005, 2006). All four UDP-sugars partially inhibited IL-2- and anti-CD3-induced cell proliferation of T-lymphocytes. A caution has been introduced by Brautigam et al. (2008) for studies of inflammatory and other effects potentially regulated by the P2Y14-R. That is, although commercial sources of UDP-glucose induced inflammatory effects in N9 microglia, this action was only observed with bacterially derived but not synthetic UDP-glucose, and other nucleotide sugars such as UDP-galactose also had no effect.
The P2Y14-R is expressed in RBL-2H3 mast cells (Gao et al. 2010). UDP-glucose promoted a concentration-dependent increase in Ca2+ mobilization in these cells, which was blocked by pre-treatment with pertussis toxin. MAP kinase signalling pathways were also activated by UDP-glucose, and UDP-glucose-promoted phosphorylation of ERK1/2 was pertussis toxin-sensitive. Moreover, both UDP-glucose and the P2Y14-R-selective agonist MRS2690 caused concentration-dependent increases in β-hexosaminidase release from RBL-2H3 cells. This effect was blocked by pertussis toxin and decreased by P2Y14-R-specific siRNA.
P2Y14-R in epithelial cells
The P2Y14-R is broadly expressed in epithelial tissues. For example, high levels are observed in various epithelium of the mouse including endometrium, oviduct, uterine cervix, vagina, bronchiolar epithelial cells, alveoli pneumocytes, mucosal epithelial cells of the convoluted tubules and enterocytes (Arase et al. 2009). The P2Y14-R is expressed in alveolar epithelial type II cells, as well as in several human airway epithelial cell lines (Muller et al. 2005). Consistent with the presence of this receptor, UDP-glucose caused a concentration-dependent increase in Ca2+ accumulation in both A549 and BEAS-2B human airway epithelial cells. This effect was blocked by pre-treatment of cells with pertussis toxin implicating Gi and most likely a Gβγ-dependent activation of a phospholipase C-β isozyme in this signalling response. Respiratory epithelium is known to produce chemokines that are neutrophil chemoattractants, and UDP-glucose caused a concentration-dependent stimulation of IL-8 production by A549, BEAS-2B and AEC-II cells. This stimulatory effect of UDP-glucose also was inhibited by pre-treatment of cells with pertussis toxin. In contrast, no effect of UDP-glucose was observed on other chemokines including IP-10, MIG and I-TAC.
Message for the P2Y14-R is highly expressed in the surface epithelium of the human and mouse female reproductive tract (Arase et al. 2009). Immunohisto-chemical studies with P2Y14-R-specific antisera were also consistent with prominent expression of this receptor in the epithelium and the lack of receptor expression in stromal tissues. Previous studies suggesting a role of the P2Y14-R in immune responses (Lee et al. 2003, Skelton et al. 2003, Muller et al. 2005, Scrivens & Dickenson 2005) prompted Arase et al. (2009) to quantify the expression of the P2Y14-R in endometria from patients with pelvic inflammatory disease. Message for the P2Y14-R as well as for IL-1β, IL-8 and TNF-α all were up-regulated. Moreover, UDP-glucose caused a concentration-dependent increase in IL-8 secretion in primary cultures of human glandular cells but had no effect on cultured stromal cells (Arase et al. 2009). Ishikawa endometrial epithelial cells also express the P2Y14-R, and UDP-glucose caused a concentration-dependent increase in IL-8 message and secretion of IL-8 protein. In contrast, other proinflammatory cytokines including IL-1β, IL-6 and TNF-α were not upregulated by UDP-glucose. A direct role of the P2Y14-R in stimulating IL-8 expression was supported by two lines of investigation. First, pre-treatment of Ishikawa cells with pertussis toxin completely blocked the effect of UDP-glucose. Second, specific knockdown of the P2Y14-R using gene-specific siRNA resulted in marked attenuation in the capacity of UDP-glucose to induce IL-8 release.
As IL-8 induces neutrophil chemotaxis, Arase et al. (2009) studied the capacity of UDP-glucose-activated Ishikawa cells to promote neutrophil chemotaxis in transwell chamber assays. Whereas UDP-glucose had no effect on chemotaxis in the absence of Ishikawa cells, it increased transmigration activity in the presence of these cells. A role of IL-8 in this response was suggested by the observation that a neutralizing anti-IL-8 antibody blocked the Ishikawa cell-dependent effect of UDP-glucose on neutrophil chemotaxis.
UDP-glucose also induced expression of two functional homologues of IL-8, MIP-2 and KC, in cultured mouse uterine tissue (Arase et al. 2009). Surprisingly, the effect of UDP-glucose was observed at 10 nm but not at 1000 nm, and UDP-N-acetylglucosamine, which is an agonist (albeit less potent than UDP-glucose) of recombinant murine and human P2Y14-R, had no effect. Although these results were inconsistent with the pharmacological selectivity expected of a physiologically relevant P2Y14-R, direct injection of UDP-glucose into one horn of the mouse uterus in vivo induced MIP-2 and KC protein but injection of saline in the opposite horn had no effect. Importantly, induction of these two chemokines was restricted to the endometrial luminal and glandular cells as was observed with IL-8 in the human endometrium. Finally, administration of UDP-glucose to mouse uterus resulted in a time-dependent increase in the number of neutrophils present in the endometrium.
To more directly examine the role of the P2Y14-R, expression of this receptor was reduced by sonoporation of P2Y14-R siRNA into one of the horns of the mouse uterus (Arase et al. 2009). Immunoblots indicated that a 30% reduction in receptor protein was effected, and a similar decrease in the capacity of UDP-glucose to promote neutrophil recruitment also occurred. LPS induced up-regulation of P2Y14-R in Ishikawa cells as well as after administration to mouse uterus. Interestingly, LPS also induced leucocytosis in mouse uterus, and this effect was attenuated by P2Y14-R siRNA. These results also are consistent with the observation that the P2Y14-R is up-regulated in the human endometrium during inflammation. Reports of P2Y14-R mRNA upregulation in mouse uterus after estradiol treatment for 7 days (Crabtree et al. 2006, 2008) also suggest that P2Y14-R expression may be regulated by circulating hormone levels.
P2Y14-R in the gastrointestinal tract
Message for the P2Y14-R is widely expressed in the rodent gastrointestinal tract with the highest levels observed in the forestomache (Chambers et al. 2000, Moore et al. 2003, Bassil et al. 2009). Both UDP-glucose and UDP-galactose promoted concentration-dependent increases in baseline muscle tension in isolated stomache forestomache preparations (Bassil et al. 2009). In contrast, the effects of UDP-glucose were lost in tissue isolated from P2Y14-R(−/−) mice. Interestingly, administration of a high concentration of UDP-glucose in vivo resulted in an increase in gastric emptying in wild-type mice, and this effect was retained in P2Y14-R knock-out mice. Whether these results reflect a P2Y14-R-independent effect of UDP-glucose or an effect due to a contaminant of UDP-glucose purified from microbially derived material as has been demonstrated in studies of UDP-glucose-stimulated responses in microglia remains to be established.
Concluding ideas
The P2Y14-R is unique among the P2Y-R extant because of its regulation by nucleotide sugars. Indeed, its rather potent activation by four different naturally occurring nucleotide sugars suggests that the identity of its principal activator may change in a tissue-specific manner. Nucleotide sugars are subject to breakdown by the ecto-nucleotide pyrophosphatases that also inactivate the nucleotide agonists of the other seven known P2Y-R. In contrast, they are not good substrates for the ecto-nucleotidases that inactivate the extracellular nucleotide agonists. Thus, the kinetics of metabolism of, for example, UDP-glucose vs. ATP markedly differ (Lazarowski et al. 2003b), and therefore, the kinetics of activation of the P2Y14-R are almost certainly to differ from that of any co-expressed P2Y-R.
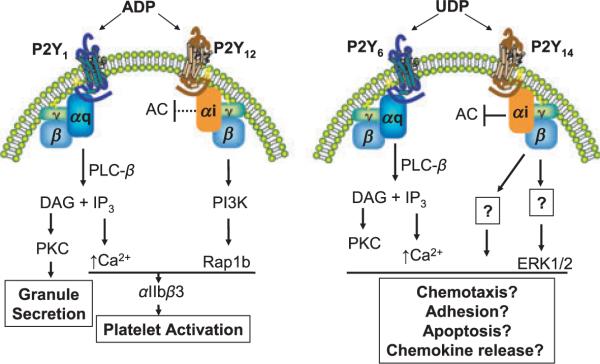
The P2Y14-R has been historically considered a nucleotide sugar-activated receptor, but the recent discovery (Carter et al. 2009) that UDP is a potent agonist adds a new twist to P2Y14-R-dependent signalling. UDP is fivefold more potent than UDP-glucose and other sugars at the P2Y14-R, which suggests but does not prove that it may be the most important agonist of the receptor under certain physiological conditions. Moreover, realization that the P2Y6-R is selectively activated by UDP and is co-expressed with the P2Y14-R in many tissues suggests that these two receptors may be activated by UDP in a coordinated manner to produce certain physiological responses (Fig. 2). Activation of the P2Y6-R results in Gq-dependent activation of phospholipase C and inositol lipid signalling whereas the P2Y14-R activates the Gi-regulated pathways discussed above. Perhaps co-activation of these two receptors results in synergistic responses analogous to that underlying ADP-promoted platelet aggregation, which occurs through P2Y1-R-dependent activation of Gq and P2Y12-R-mediated activation of Gi (Gachet 2006).
Figure 2.
Hypothetical model of synergistic signalling mediated through simultaneous UDP-dependent activation of the P2Y14-R and P2Y6-R. Left panel: The well-established model (Gachet 2006) for ADP-induced aggregation of platelets is illustrated. ADP dually activates the P2Y1-R and P2Y12-R. Activation of the P2Y1-R results in activation of phospholipase C-β (PLC-β) resulting in hydrolysis of PtdIns(4,5)P2 to the second messenger Ins(1,4,5)P3 (IP3), which mobilizes intracellular Ca2+, and diacylglycerol (DAG), which activates protein kinase C. Activation of the P2Y12-R results in activation of Gi. Adenylyl cyclase (AC) is inhibited and phosphinostide-3-kinase (PI3K) is activated. Mobilization of Ca2+ downstream of activation of the P2Y1-R and activation of the GTPase Rap1b downstream of activation of the P2Y12-R result in activation of the integrin αIIbβ3. Right panel: Activation of the P2Y6-R results in activation of PLC-β isozymes and inositol lipid hydrolysis, whereas UDP-dependent activation of the P2Y14-R results in activation of Gi, inhibition of adenylyl cyclase (AC) and activation of MAP kinase isozymes such as ERK1/2. The P2Y6-R and P2Y14-R often are co-expressed, and simultaneous activation of these two P2Y-R by UDP is hypothesized to synergize resulting in promotion of a variety of cellular responses.
The P2Y14-R is relatively broadly expressed and its prominent presence in immune and epithelial cells is consistent with an increasingly broad literature suggesting an important physiological role(s) for the receptor in these and other tissues. Selective and potent P2Y14-R agonists were identified recently (Das et al. 2010). These now can be applied to physiological studies of the receptor, but a P2Y14-R selective competitive antagonist is also greatly needed. A P2Y14-R-knockout mouse is now available (Bassil et al. 2009), and the work of Arase et al. (2009) successfully targeting P2Y14-R expression by introducing siRNA in an epithelial cell line as well as in mouse uterus also introduces useful technology to the field. The purinergic signalling field has travelled a long and exciting road since Geoff Burnstock's ground-breaking work four decades ago. Encouraging results from a rather broad range of recent studies on the P2Y14-R suggest that investigations focused on nucleotide sugar/UDP-dependent signalling will continue to add both new concepts and physiological understanding to the field.
Acknowledgments
Research from the authors' laboratories was supported by USPHS grants GM38213 and HL34322.
Footnotes
Conflict of interest There is no conflict of interest.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase T, Uchida H, Kajitani T, Ono M, Tamaki K, Oda H, Nishikawa S, Kagami M, Nagashima T, Masuda H, Asada H, Yoshimura Y, Maruyama T. The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J Immunol. 2009;182:7074–7084. doi: 10.4049/jimmunol.0900001. [DOI] [PubMed] [Google Scholar]
- Ault AD, Broach JR. Creation of GPCR-based chemical sensors by directed evolution in yeast. Protein Eng Des Sel. 2006;19:1–8. doi: 10.1093/protein/gzi069. [DOI] [PubMed] [Google Scholar]
- Bassil AK, Bourdu S, Townson KA, Wheeldon A, Jarvie EM, Zebda N, Abuin A, Grau E, Livi GP, Punter L, et al. UDP-glucose modulates gastric function through P2Y14 receptor-dependent and -independent mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G923–G930. doi: 10.1152/ajpgi.90363.2008. [DOI] [PubMed] [Google Scholar]
- Besada P, Shin DH, Costanzi S, Ko H, Mathe C, Gagneron J, Gosselin G, Maddileti S, Harden TK, Jacobson KA. Structure–activity relationships of uridine 5′-diphosphate analogues at the human P2Y6 receptor. J Med Chem. 2006;49:5532–5543. doi: 10.1021/jm060485n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay F, Tardif M, Brouchon L, Vignais P. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990;29:11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- Brautigam VM, Dubyak GR, Crain JM, Watters JJ. The inflammatory effects of UDP-glucose in N9 microglia are not mediated by P2Y14 receptor activation. Purinergic Signal. 2008;4:73–78. doi: 10.1007/s11302-008-9095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Caporali F, Capecchi PL, Gamberucci A, Lazzerini PE, Pompella G, Natale M, Lorenzini S, Selvi E, Galeazzi M, Laghi Pasini F. Human rheumatoid synoviocytes express functional P2X7 receptors. J Mol Med. 2008;86:937–949. doi: 10.1007/s00109-008-0365-8. [DOI] [PubMed] [Google Scholar]
- Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Ko H, Das A, Jacobson KA, Lazarowski ER, Harden TK. Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol Pharmacol. 2009;76:1341–1348. doi: 10.1124/mol.109.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo CA, Albasanz JL, Fernandez M, Martin M. Endogenous expression of adenosine A1, A2 and A3 receptors in rat C6 glioma cells. Neurochem Res. 2007;32:1056–1070. doi: 10.1007/s11064-006-9273-x. [DOI] [PubMed] [Google Scholar]
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, et al. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Communi D, Pirotton S, Parmentier M, Boeynaems JM. Cloning and functional expression of a human uridine nucleotide receptor. J Biol Chem. 1995;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- Communi D, Govaerts C, Parmentier M, Boeynaems JM. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems JM. Identification of a novel human ADP receptor coupled to Gi. J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- Cooper DM, Rodbell M. ADP is a potent inhibitor of human platelet plasma membrane adenylate cyclase. Nature. 1979;282:517–518. doi: 10.1038/282517a0. [DOI] [PubMed] [Google Scholar]
- Coward P, Chan SD, Wada HG, Humphries GM, Conklin BR. Chimeric G proteins allow a high-throughput signaling assay of Gi-coupled receptors. Anal Biochem. 1999;270:242–248. doi: 10.1006/abio.1999.4061. [DOI] [PubMed] [Google Scholar]
- Crabtree JS, Zhang X, Peano BJ, Zhang Z, Winneker RC, Harris HA. Development of a mouse model of mammary gland versus uterus tissue selectivity using estrogen- and progesterone-regulated gene markers. J Steroid Biochem Mol Biol. 2006;101:11–21. doi: 10.1016/j.jsbmb.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol. 2008;287:40–46. doi: 10.1016/j.mce.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Zhou Y, Ivanov AA, Carter RL, Harden TK, Jacobson KA. Enhanced potency of nucleotide-dendrimer conjugates as agonists of the P2Y14 receptor: multivalent effect in G protein-coupled receptor recognition. Bioconjug Chem. 2009;20:1650–1659. doi: 10.1021/bc900206g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Ko H, Burianek LE, Barrett MO, Harden TK, Jacobson KA. Human P2Y14 receptor agonists: truncation of the hexose moiety of uridine-5′-diphosphoglucose and its replacement with alkyl and aryl groups. J Med Chem. 2010;53:471–480. doi: 10.1021/jm901432g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K, Tsui P, Moore D, Emson PC, Vawter L, Naheed S, Lane P, Bawagan H, Herrity N, Murphy K, Sarau HM, Ames RS, Wilson S, Livi GP, Chambers JK. Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics. 2001;78:124–128. doi: 10.1006/geno.2001.6662. [DOI] [PubMed] [Google Scholar]
- Fricks IP, Maddileti S, Carter R, Lazarowski ER, Nicholas RA, Jacobson KA, Harden TK. UDP is a competitive antagonist at the human P2Y14 receptor. J Pharmacol Exp Ther. 2008;325:588–594. doi: 10.1124/jpet.108.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks IP, Carter RL, Lazarowski ER, Harden TK. Gi-dependent cell signaling responses of the human P2Y14 receptor in model cell systems. J Pharmacol Exp Ther. 2009;330:162–168. doi: 10.1124/jpet.109.150730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Ding Y, Jacobson KA. UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol. 2010;79:873–879. doi: 10.1016/j.bcp.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden TK, Barnard EA, Boeynaems JM, Burnstock G, Dubyak GR. P2Y receptors. In: Girdlestone D, editor. The IUPHAR Compendium of Receptor Characterization and Classification. IUPHAR Media; London, UK: 1998. pp. 209–217. [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Klinker JF, Wenzel-Seifert K, Seifert R. G-protein-coupled receptors in HL-60 human leukemia cells. Gen Pharmacol. 1996;27:33–54. doi: 10.1016/0306-3623(95)00107-7. [DOI] [PubMed] [Google Scholar]
- Ko H, Fricks I, Ivanov AA, Harden TK, Jacobson KA. Structure–activity relationship of uridine 5′-diphosphoglucose analogues as agonists of the human P2Y14 receptor. J Med Chem. 2007;50:2030–2039. doi: 10.1021/jm061222w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Carter RL, Cosyn L, Petrelli R, de Castro S, Besada P, Zhou Y, Cappellacci L, Franchetti P, Grifantini M, Van Calenbergh S, Harden TK, Jacobson KA. Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists. Bioorg Med Chem. 2008;16:6319–6332. doi: 10.1016/j.bmc.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Das A, Carter RL, Fricks IP, Zhou Y, Ivanov AA, Melman A, Joshi BV, Kovac P, Hajduch J, Kirk KL, Harden TK, Jacobson KA. Molecular recognition in the P2Y14 receptor: probing the structurally permissive terminal sugar moiety of uridine-5′-diphosphoglucose. Bioorg Med Chem. 2009;17:5298–5311. doi: 10.1016/j.bmc.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498:443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol. 2008;153:1528–1537. doi: 10.1038/sj.bjp.0707692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzeminski P, Pomorski P, Baranska J. The P2Y14 receptor activity in glioma C6 cells. Eur J Pharmacol. 2008;594:49–54. doi: 10.1016/j.ejphar.2008.06.092. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER. Quantification of extracellular UDP-galactose. Anal Biochem. 2010;396:23–29. doi: 10.1016/j.ab.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003a;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol. 2003b;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- Lee BC, Cheng T, Adams GB, Attar EC, Miura N, Lee SB, Saito Y, Olszak I, Dombkowski D, Olson DP, Hancock J, Choi PS, Haber DA, Luster AD, Scadden DT. P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev. 2003;17:1592–1604. doi: 10.1101/gad.1071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res. 2003;118:10–23. doi: 10.1016/s0169-328x(03)00330-9. [DOI] [PubMed] [Google Scholar]
- Muller T, Bayer H, Myrtek D, Ferrari D, Sorichter S, Ziegenhagen MW, Zissel G, Virchow JC, Jr, Luttmann W, Norgauer J, Di Virgilio F, Idzko M. The P2Y14 receptor of airway epithelial cells: coupling to intracellular Ca2+ and IL-8 secretion. Am J Respir Cell Mol Biol. 2005;33:601–609. doi: 10.1165/rcmb.2005-0181OC. [DOI] [PubMed] [Google Scholar]
- Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- Qi AD, Kennedy C, Harden TK, Nicholas RA. Differential coupling of the human P2Y11 receptor to phospholipase C and adenylyl cyclase. Br J Pharmacol. 2001;132:318–326. doi: 10.1038/sj.bjp.0703788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Schachter JB, Boyer JL, Li Q, Nicholas RA, Harden TK. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br J Pharmacol. 1997;122:1021–1024. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in murine T-lymphocytes. Br J Pharmacol. 2005;146:435–444. doi: 10.1038/sj.bjp.0706322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in human neutrophils. Eur J Pharmacol. 2006;543:166–173. doi: 10.1016/j.ejphar.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Sesma JI, Esther CR, Jr, Kreda SM, Jones L, O'Neal W, Nishihara S, Nicholas RA, Lazarowski ER. Endoplasmic reticulum/golgi nucleotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem. 2009;284:12572–12583. doi: 10.1074/jbc.M806759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton L, Cooper M, Murphy M, Platt A. Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol. 2003;171:1941–1949. doi: 10.4049/jimmunol.171.4.1941. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Matli JR, Hu JL, Carson MJ, Sutcliffe JG. Pertussis toxin treatment prevents 5-HT(5a) receptor-mediated inhibition of cyclic AMP accumulation in rat C6 glioma cells. J Neurosci Res. 2000;61:75–81. doi: 10.1002/1097-4547(20000701)61:1<75::AID-JNR9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]