Abstract
Blood-sucking arthropods have evolved a number of inhibitors of platelet aggregation and blood coagulation. In this report we have molecularly and functionally characterized aegyptin, a member of the family of 30-kDa salivary allergens from Aedes aegypti, whose function remained elusive thus far. Aegyptin displays a unique sequence characterized by glycine, glutamic acid, and aspartic acid repeats and was shown to specifically block collagen-induced human platelet aggregation and granule secretion. Plasmon resonance experiments demonstrate that aegyptin binds to collagen types I-V (Kd ≈ 1 nM) but does not interact with vitronectin, fibronectin, laminin, fibrinogen, and von Willebrand factor (vWf). In addition, aegyptin attenuates platelet adhesion to soluble or fibrillar collagen. Furthermore, aegyptin inhibits vWf interaction with collagen type III under static conditions and completely blocks platelet adhesion to collagen under flow conditions at high shear rates. Notably, aegyptin completely prevents collagen but not convulxin binding to recombinant glycoprotein VI. These findings indicate that aegyptin recognizes specific binding sites for glycoprotein VI, integrin α2β1, and vWf, thereby preventing collagen interaction with its three major ligands. Aegyptin is a novel tool to study collagen-platelet interaction and a prototype for development of molecules with antithrombotic properties.
Blood-sucking arthropod saliva is a rich source of molecules that affect hemostasis (1) including vasodilators (2,3) and inhibitors of blood coagulation (4,5) and platelet aggregation (6-8). Among the platelet inhibitors, salivary lipocalins bind to and remove pro-aggregatory amines such as ADP (8), epinephrine and serotonin (9,10), while RGD-containing peptides block integrin αIIβ3 interaction with fibrinogen (7). In addition, enzymes such as apyrases and lipid acethyl hydrolases degrade biologically active molecules such as ADP and PAF, respectively (11,12). Further, specific antagonist of collagen-induced platelet aggregation/adhesion have been found in salivary glands of ticks and other hematophagous animals such as leeches (13-15).
Most salivary components described above have been identified through classical procedures where a given function was used to isolate an active molecule (1). Transcriptome and proteomics analyses of salivary glands also allow investigators to assign functions to arthropod saliva compounds based on sequence similarities (16-19). For example, Ixolaris from Ixodes scapularis was initially identified by its sequence similarity to tissue factor pathway inhibitor and shown to display a potent anticoagulant activity in vitro (5) and antithrombotic effects in vivo (20); however, as a result of this high-throughput approach, a number of salivary gland transcript-encoded products have no similarity to proteins deposited in databases. Accordingly, annotation and functional assignment for these proteins has proven difficult (1). One typical example of this complex picture is the family of 30-kDa salivary allergen found in different blood-sucking arthropods including Aedes sp (17), Culex sp (19), and Anopheles sp (16,18,21,22). While these proteins are major salivary components that display significant sequence similarity, and despite several papers devoted to understanding their functional and physicochemical properties (16-19,21,22), their function has remained elusive thus far. In this report we show that a member of this family, herein named aegyptin, is a specific ligand of collagen.
Collagen is a matrix protein that plays a pivotal role in the process of primary hemostasis: at sites of vascular injury, it initiates recruitment of circulating platelets and triggers platelet activation cascade required to stimulate thrombus growth (23-25). The first step in platelet recruitment to collagen occurs indirectly via binding of platelet glycoprotein (GP) Ib to collagen-bound von Willebrand factor (vWf), which plays a critical role in tethering of platelets at high shear levels (26,27). The rapid off-rate of GPIb–vWf interactions results in platelet translocation at the site of injury, allowing adhesive interactions with slower binding kinetics—such as the platelet collagen receptors glycoprotein VI (GPVI) and α2β1 integrins—to mediate platelet adhesion and activation (23-28). The relative contributions of these two receptors to collagen-mediated platelet responses are under intense investigation (28-34) and different models have been proposed in an attempt to explain how platelets are activated by collagen (23,28,35).
Continuing our studies on antihemostatic components of blood-sucking arthropod saliva, we have assigned a function for aegyptin expressed in salivary glands of the yellow fever vector, Aedes aegypti. Aegyptin binds to collagens (I-V), and interferes with its interaction with major physiological ligands: GPVI, integrin α2β1, and vWf. Notably, aegyptin blocks GPVI interaction with collagen and inhibits platelet aggregation and adhesion. This report is the first to identify a collagen-binding protein in the saliva of a blood-sucking arthropod.
Material and Methods
Horse tendon insoluble fibrillar (quaternary, polymeric structure) Horm collagen (Chrono-Log Corp., Haverstown, PA) composed of collagen I (95%) and III (5%) was used because this microscopically visible collagen is routinely employed in platelet aggregation studies and in shear-controlled perfusion studies. Pepsin-digested, soluble non-fibrillar (tertiaty structure, triple helical) collagen type I-V (BD Biosciences, San José, CA) was used because it is the collagen of choice to study molecular interactions between human collagen and vWf. ADP, phorbol myristate acetate (PMA) was obtained from Sigma Chemical Co. (St. Louis, MO). Ristocetin, arachidonic acid, and Chrono Lumi-reagent were from Chronolog. 9,11-dideoxy-9α,11α- methanoepoxy prostaglandin F2α (U46619) was purchased from Cayman Chemical (Ann Arbor, MI), thrombin receptor activating peptide (TRAP) was from EMD (San Diego, CA), and thrombin from Haematologic Technologies (Essex Junction, VT). Convulxin was purified as described (36), and GPVI was prepared as published (37).
Aedes aegypti collection of saliva and salivary gland dissection
Saliva from female Ae. aegypti mosquitoes was collected by oil-induced salivation (38) and salivary glands dissected as indicated (17).
Cloning and expression of aegyptin
Salivary glands of 20 female mosquitoes (non-blood fed) were dissected. Total RNA was extracted with TRIZOL reagent (Invitrogen, San Diego, CA), and specific cDNA was amplified using OneStep RT-PCR kit (Qiagen, Chatsworth, CA) and the gene-specific primers Aegyptin-For (5′-AGGCCCATGCCCGAAGATGAAGAACCAG-3′) and Aegyptin-Rev (5′-TTAGTGGTGGTGGTGGTGGTGACGTCCTTTGGATGAAA CAC-3′). These two primers were designed based on aegyptin sequence (gi 94468546) to amplify the DNA fragment encoding the mature protein and a 6× His tag before the stop codon. The PCR-amplified product was cloned into VR2001-TOPO vector (modified version of the VR1020 vector; Vical Incorporated, San Diego, CA) and sequence and orientation verified by DNA sequencing. Approximately 1 mg of plasmid DNA (VR2001-aegyptin construct) was obtained using GeneElute™ HP endotoxin-free plasmid MEGA prep kit (Sigma). The plasmid was purified through a 0.22 μm filter and recombinant protein was produced by transfecting FreeStyle™ 293-F cells (Invitrogen) with 240 μg of purified VR2001-aegyptin plasmid, following manufacturer's recommendations (Invitrogen). After 72 hours, transfected cell culture was harvested. Supernatant containing the secreted recombinant protein was centrifuged (100×g, 15 minutes), frozen, and stored at −30°C until use.
Recombinant aegyptin purification
293-F cells supernatant containing the recombinant protein was loaded onto a Ni2+ column (5 ml bed volume; Amersham Biotech, Piscataway, NJ) following the manufacturer's directions. Fractions were eluted with 10, 40, and 300 mM imidazole (in 50 mM Tris, 300 mM NaCl, pH 8.0), and the fraction eluted at 300 mM was pooled and concentrated in an Amicon (10 MWMCO) to 1 mL and then loaded onto a size-exclusion column (Superdex 75 HR10/30; Amersham Biotech) using the AKTA purifier system (Amersham Biotech). Proteins were eluted at a flow rate of 0.5 ml/minute in 50 mM Tris, 150 mM NaCl, pH 7.4. Purified recombinant protein was submitted to automated Edman degradation for N-terminal sequencing. To detect purity of aegyptin, 5 μg of purified protein was loaded in a 4–12% NU-PAGE gel (MES buffer) and the gel stained with Coomassie blue.
Sequence analysis and estimative of aegyptin concentration
Concentration of purified aegyptin (gi 94468546) was estimated by its absorbance at 280 nm using a NanoDrop ND1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and corrected according to the molar extinction coefficient ε280 nm = 5600 M−1.cm−1; A 280 nm/cm (1 mg/mL) = 0.220. Other calculated parameters are: Mr, 27038.09; pI, 3.96. Other protein parameters were obtained at http://ca.expasy.org/tools.
Salivary gland fractionation
One hundred pairs of salivary gland homogenates were loaded onto a size-exclusion column (Superdex 75 HR10/30; Amersham Biotech) using the AKTA purifier system (Amersham Biotech). Proteins were eluted at a flow rate of 0.5 ml/minute in HBS-N (10 mM HEPES, pH 7.4, 150 mM NaCl). The active fraction (detected by surface plasmon resonance, see below) containing the collagen-binding protein was further purified by ion-exchange chromatography using a MonoQ column HR 5/5 (Amersham Biotech). Proteins were eluted with a linear gradient of NaCl (0-1M) over 60 minute at a flow rate of 0.5 ml/minute. Eluted proteins were again tested for collagen binding activity as described below.
Surface plasmon resonance (SPR) analysis
All SPR experiments were carried out in a T100 instrument (Biacore Inc., Uppsala, Sweden) following the manufacturer's instructions. This instrument features an integrated degasser, allowing problem-free kinetic measurements at temperatures up to 45°C, as well as a temperature-controlled flow cell and sample compartment. The Biacore T100 evaluation software was utilized for kinetic and thermodynamic evaluation. Sensor CM5, amine coupling reagents, and buffers were also purchased from Biacore Inc (Piscataway, NJ). HBS-P (10 mM Hepes, pH 7.4, 150 mM NaCl, and 0.005% (v/v) P20 surfactant) was used as the running buffer for all SPR experiments. All SPR experiments were carried out three times.
Immobilization and kinetic analysis
Collagen type I or type III (30 μg/ml) in acetate buffer pH 4.5 was immobilized over a CM5 sensor via amine coupling. The immobilization target was aimed to 1500 resonance units (RU), resulting in a final immobilization of 1737.5 RU for collagen type I and 1613.3 RU for collagen type III. Blank flow cells were used to subtract the buffer effect on sensorgrams. Kinetic experiments were carried out with a contact time of 180 s at a flow rate of 30 μl/min at 25°C. Aegyptin-collagen I/III complex dissociation was monitored for 1800 s, and the sensor surface was regenerated by a pulse of 20 s of 10 mM HCl at 40 μl/minute. Sensorgrams were fitted using the two-state reaction (conformational change) interaction model, and a linked-reactions control experiment was carried out to confirm the multiphase binding kinetics of aegyptin-collagen I interaction.
Solution competition assays
Experiments were performed in an attempt to detect whether aegyptin blocks collagen interaction with GPVI. Recombinant GPVI (25 μg/ml) in acetate pH 4.5 buffer was immobilized on a CM5 sensor with a final surface density of 1753.2 RU. A blank flow cell was used to subtract any effect of buffer in the refractory index change. Then different concentrations (3.175, 6.125, 12.5, 25, and 50 μg/ml) of collagen I alone (control) or previously incubated (15 minutes at room temperature) with 500 nM of aegyptin in HBS-P buffer was injected over immobilized GPVI for 120 s at 20 μl/minute. Complex dissociation was monitored for 400 s. Sensor surface was regenerated between runs with by a 30-second pulse of glycine solution, pH 1.5. To verify that immobilized GPVI was still active after all the injection-regeneration cycles, 50 μg/ml of collagen I was injected for 120 s at a flow rate of 20 μl/minute and the resulting sensorgram compared with the one obtained before. Additionally, a control experiment was carried out using convulxin at different concentrations (2.5, 5, and 10 nM) incubated with buffer or saturating concentrations of aegyptin (500 nM) followed by injection of the mixture over immobilized GPVI, as described above.
Identification of collagen-binding protein from salivary gland homogenate and saliva
An aliquot of 10 μl obtained after size-exclusion or anion-exchange chromatographies were dissolved in 100 μl of HBS-P (10 mM Hepes, pH 7.4, 150 mM NaCl, 0.05% surfactant P-20) and injected over collagen type I and III immobilized on a CM5 sensor chip for 120 s at a flow rate of 20 μl/minute. Complex dissociation was monitored for 400 s, and the sensor chip surface was regenerated with a 10-second pulse of 10 mM HCl at 30 μl/minute. In some experiments, saliva was used as an analyte.
Thermodynamic analysis
Thermodynamic parameters for aegyptin-collagen type I interaction were obtained from independent kinetic experiments using the Thermo Wizard assay program. Briefly, different concentrations of recombinant aegyptin (0.1 to 3 nM) were injected over immobilized collagen type I at 15°C, 20 °C, 25 °C, 30 °C, 35°C, and 40°C. The sample compartment was kept at 25°C. Contact time, dissociation time, and regeneration of the sensor surface were done as described above. Resulting sensorgrams were fitted to the two-state reaction (conformational change) interaction model with local Rmax. The association (Ka) and dissociation (Kd) rate constants, as well as the affinity constant (KD), were obtained and fitted to a linear form of the van't Hoff and Eyring equations to estimate the ΔH and ΔS as well as ΔH°‡ and ΔS°‡, respectively.
Production of aegyptin polyclonal antibodies and western blot
Female Swiss Webster mice, 8—12 weeks old, were purchased from the Division of Cancer Treatment, National Cancer Institute, NIH. Mice were maintained in the NIAID Animal Care Facility under pathogen-free conditions. Three mice were anesthetized with 100 μl of 20 mg/ml ketamine HCl (Fort Dodge [IA] Animal Health) and immunized with DNA plasmids intradermally in the right ear using a 29.5-gauge needle. DNA plasmids (1 μg/μl) were injected in 10 μl volume, 3 times at 2-week intervals. Two weeks after the last DNA immunization, sera were collected and stored at −30°C until use. Western blot was performed using anti-aegyptin antibodies at 1:200 dilution.
Platelet preparation and ATP release
Blood collection, platelet aggregation, and ATP release were performed as described (8).
vWf binding to collagen
Polystyrene plates were coated with 100 μl of collagen type III (3 μg/ml) or a 2% (w/v) solution of bovine serum albumin (BSA) diluted in PBS for 2 hours at 37°C. After washing twice with PBS to remove unbound protein, residual binding sites were blocked by adding 5 mg/ml denatured BSA overnight at 4°C. After washing 3 times with 50 mM Tris-HCl, 150 mM NaCl, and 0.05% (v/v) Tween 20, pH 7.4 (TBS-T), increasing concentrations of recombinant aegyptin (ranging from 0.0015 to 1.5 μM) was added to the well and incubated at 37°C for 1 hour. Wells were washed again and incubated with 3 nM of vWf factor VIII-free (Haematologic Technologies Inc) in TBS-T supplemented with 2% (w/v) BSA. After 1 hour at 37°C, wells were washed 3 times with TBS-T, and a polyclonal rabbit anti-human vWf (DakoCytomation, Glostrup, Denmark) was added (1:500 in TBS-T) and incubated for 1 hour at 37°C. After 3 washes with TBS-T, an alkaline phosphatase conjugate anti-rabbit IgG (whole molecule; Sigma) was added (1:10000) and incubated at 37°C for 45 minutes. Before adding the stabilized p-nitrophenyl phosphate liquid substrate (Sigma), wells were washed 6 times with TBS-T. After 30 minutes of substrate conversion, the reaction was stopped with 3 N NaOH and absorbance read at 405 nm using a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA). Net specific binding was obtained by subtracting optical density values from wells coated only with BSA from the total binding measured as described above. All experiments were performed in triplicate.
Platelet adhesion assay under static conditions
Coverslips (22 × 22 mm, no. 0) were treated with H2SO4: H2O2 (4:1) for 20 minutes to remove contaminants (39) followed by ultrasonic washing with deionized water and ultravioletcleaning. Coverslips were coated with fibrillar (100 μg/ml; Chronolog-Par) or soluble collagen type I (100 μg/ml) for 10 minutes, rinsed in deionized water, and incubated overnight with denaturated BSA (5 mg/ml). Coverslips were treated with 100 μl of aegyptin (0–3 μM) for 15 minutes, and inhibitor was removed by inverting and touching the borders of coverslips with precision wipes (Kimberly-Clark, Ontario, Canada). Platelets (200 μl, 2 × 105/ml) were mixed with aegyptin (0–3 μM) and applied to coverslips for 45 minutes at room temperature followed by washing in Tyrode-BSA, and mounted for imaging. Differential interference contrast images were obtained with a Leica DMI6000 microscope (Leica Microsystems, Inc., Bannockburn, IL) using 100× objective with NA = 1.30, and an ORCA ER digital camera (Hamamatsu Photonic Systems, Bridgewater, NJ). Image acquisition and the digital camera were controlled by ImagePro 5.1 software (Media Cybernetics, Silver Spring, MD). Extent of platelet adhesion was expressed as percent area covered by platelets.
Platelet adhesion under flow conditions
Glass slides were coated with fibrillar collagen (300 μl, 100 μg/ml) for 10 min, washed in TBS and incubated overnight with denatured BSA (5 mg/ml). Coated slides were treated with aegyptin (300 μl in Tyrode-BSA; 0–3 μM) for 15 minutes, and excess removed by inversion. The slides was placed in the bottom of the parallel-plate flow chamber (Glycotech, Rockville, MD), and a silicone rubber gasket determined the flow path height of 254 μm as described (40). Anticoagulated blood (50 μM PPACK) was mixed with aegyptin (0–3 μM) and aspirated using a infusion/withdrawal pump with multi-speed Transmission (Model 940; Harvard Apparatus, Dover, MA) through the flow chamber at a flow rate of 0.65 ml/minute, producing a shear rate of 1,500 s-1 (40). Blood was perfused for 240 s followed by immediate perfusion with Tyrode-BSA (0.65 ml/min) for 120 sec to remove blood, and slides subsequently washed in Tyrode-BSA. Platelet adhesion under flow conditions was recorded using differential interference contrast imaging as described above. Extent of platelet adhesion was expressed as percent area covered by platelets.
Blood coagulation assays
Prothrombin time, activated partial thromboplastin time, prothrombinase and Xnase assembly were performed as described (41).
Statistical analysis
Results are expressed as means ± SEM.
Results
Aegyptin cloning, expression, purification, and identification as a collagen-binding protein
Aegyptin displays sequence similarity to members of 30-kDa salivary allergens found in salivary glands of blood-sucking arthopods whose function has remained elusive so far (16-19,21,22). Extensive sequence comparison and phylogenetic analysis have been reported for this family of proteins (17). Fig. 1A shows a diagram displaying highly acidic N-terminus containing 28 negatively charged amino acids Glu or Asp and 5 Gly-Glu-Glu-Asp-Ala (GEEDA) repeats. The repeats are followed by 19 residues of Glu or Asp and a high content of Gly. The C-terminus is typically basic and display 25 positive amino acids Arg or Lys and 18 negative residues. Overall, Gly, Asp, and Glu content of aegyptin is ≈ 45%, while Arg and Lys represent 11.5% of the protein, which also displays 4 cysteines. In an attempt to identify the function of aegyptin, cDNA was cloned in a VR2001 expression vector subsequently used for transfection of 293-F cells. Medium containing the secreted recombinant protein was centrifuged and supernatant loaded in a Ni2+-column and eluted with a buffer containing increments of imidazole concentration. The protein eluted with 300 mM imidazole was further purified to homogeneity using gel-filtration chromatography (Fig. 1B). Purified aegyptin was analyzed by NU-PAGE as a pure material of ≈ 30 kDa (Fig. 1B, inset), and the amino terminal obtained by Edman degradation yielded the sequence RPMPEDEEVAEG, which is in agreement with the N-terminus predicted for the mature protein, according to the corresponding cDNA.
Fig. 1.
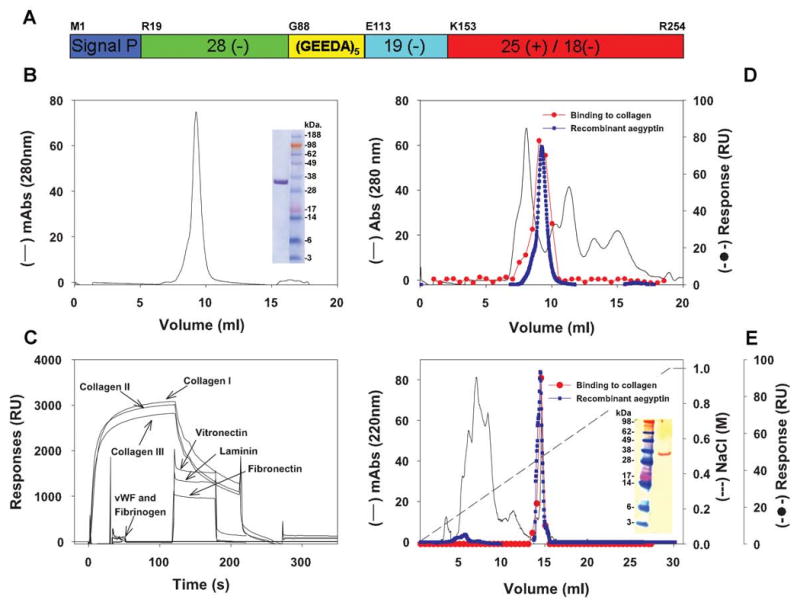
Molecular characterization of aegyptin and identification in the Ae. aegypti salivary gland. (A) Schematic representation of aegyptin with typical acidic N-terminus, (GEEDA) repeats, and basic carboxyterminal depicted. (B) Purification of recombinant aegyptin after Ni2+ agarose and gel-filtration columns. Inset: NU-PAGE of purified aegyptin under denaturating conditions. (C) Plasmon resonance experiments: aegyptin interacts with collagen (I-III) but not with vitronectin, laminin, fibronectin, vWf, and fibrinogen. (D) Gel filtration of salivary gland homogenate (—) and identification of the active fraction that binds to collagen (–•–). The elution pattern of recombinant aegyptin is superimposed (–•–). (E) Anion-exchange chromatography of the active fraction obtained in (D) and identification of collagen-binding activity (–•–). The elution pattern of recombinant aegyptin is superimposed (–•–). Inset: Western blot for detection of aegyptin in the salivary gland using polyclonal antiaegyptin antibodies.
Because aegyptin has no significant matches to proteins with known function in the databases, and considering that this protein is female specific (17) and therefore potentially involved with blood-feeding capabilities of the mosquito, we initially carried out screening using anticoagulant assays. A series of experiments demonstrated that aegyptin does not affect coagulation tests (partial prothrombin time, prothrombin time, and thrombin time), esterolytic activity of purified enzymes (e.g. FXa, FIXa, FXIa, FXIIa, kallikrein), and multimolecular coagulation complex assembly (extrinsic Xase, intrinsic Xnase, and prothrombinase) (data not shown). Preliminary experiments also demonstrated that aegyptin was without effect on platelet aggregation induced by thrombin, ADP, and thromboxane A2 mimetic (U46619); however, inhibition was observed when collagen was tested as a platelet agonist suggesting that aegyptin could operate either as a platelet receptor antagonist or as collagen-binding protein. Therefore, experiments were performed to investigate whether recombinant aegyptin could directly interact with collagen using SPR experiments. Aegyptin was found to bind to soluble collagen I-III, but no interaction was observed with other matrix proteins including laminin, vitronectin, fibronectin, vWf, and fibrinogen (Fig. 1C). It was concluded that recombinant aegyptin inhibits platelet aggregation because it specifically binds to collagen, thus preventing its interaction with platelets.
Identification of aegyptin as a secreted salivary gland protein
To determine whether the salivary gland homogenate of Ae. aegypti contains a collagen-binding activity, 100 pairs were sonicated and centrifuged, and supernatant was loaded in a gel-filtration column. Fractions were tested for collagen-binding activity by SPR and the active fraction found at a retention volume of ≈ 8.5 ml (Fig. 1D). For comparative purposes, recombinant aegyptin was applied to the same column and also eluted at ≈ 8.5 ml retention volume. In an attempt to isolate native aegyptin from salivary glands, the active fractions obtained above were combined, concentrated, and loaded in an anion-exchange column. The active fraction was eluted at approximately 0.4 M NaCl, which was the same salt concentration needed to elute recombinant aegyptin (Fig. 1E). In addition, Edman degradation of native aegyptin present in the active fraction identified three amino acids, Arg, Pro, and Met, which are identical to the N-terminus for the mature protein as predicted by cDNA. Finally, western blot analysis of the salivary gland homogenate using a polyclonal antibody generated by DNA vaccination identified a protein of 30 kDa whose migration pattern is identical to that of recombinant aegyptin. Therefore, we concluded that aegyptin is expressed in salivary glands of Ae. aegypti and behaves as a collagen-binding protein. In addition, both recombinant and native inhibitor display identical chromatographic and functional properties. These results validate use of recombinant aegyptin for further experimentation.
Aegyptin is a potent and specific inhibitor of collagen-induced platelet aggregation
The effect of aegyptin on collagen-induced human platelet aggregation was tested using test-tube stirring conditions. The results show that aegyptin dose-dependently inhibits onset time for shape change and decreases the extent of platelet aggregation (Fig. 2A) and ATP secretion with an IC50 of ≈ 50 nM (Fig. 2B). Additionally, aegyptin (300 nM) does not affect platelet aggregation induced by ADP, PMA, ristocetin, araquidonic acid, U46619, convulxin, TRAP, and thrombin (Fig. 2A).
Fig. 2.

Aegyptin specifically inhibits human platelet aggregation and granule secretion induced by collagen. (A) Human platelet-rich plasma (2×105/ml) was incubated with aegyptin (in nM; a, 0; b, 30; c, 60; d, 120) for 1 minute followed by addition of platelet agonists as indicated. Platelet aggregation was estimated by turbidimetry under test-tube stirring conditions. Washed human platelets were used when thrombin was used as an agonist. (B) Dose-response inhibition of collagen-induced platelet aggregation and ATP release by aegyptin. The tracings represent a typical experiment (n = 6).
Aegyptin displays high-affinity binding to collagens
To investigate binding kinetics of aegyptin-collagen interaction, SPR experiments were performed as described in Materials and methods. Typical sensorgrams obtained for recombinant aegyptin interaction with collagen I and III, respectively, are shown in Fig.s 3A and 3B. In both cases, the best fit was attained using a two-state reaction model (Table 1), suggesting that aegyptin undergoes a conformational change after interaction with collagens. Using this model, Kd of 1.22 nM for collagen type I and Kd of 1.40 nM for collagen III was calculated (Table 1). Aegyptin also binds to soluble collagen types II, IV, and V (data not shown). Next, saliva was collected from Ae. aegypti mosquitoes, and the secretion obtained by salivation was used to verify whether it contains collagen-binding properties. Fig. 3C shows that saliva readily interacts with collagen I (sensorgram a) and III (sensorgram b). Kinetics of interaction were comparable to the pattern obtained with 0.25 nM recombinant aegyptin (Table 2). Based on these results, we confirm that aegyptin is a protein secreted in the saliva of Ae. aegypti with an estimated concentration of at least 0.3 nM in the mosquito, considering that saliva obtained by oil-induced salivation is a diluted secretion which was used for the experiments.
Fig. 3.
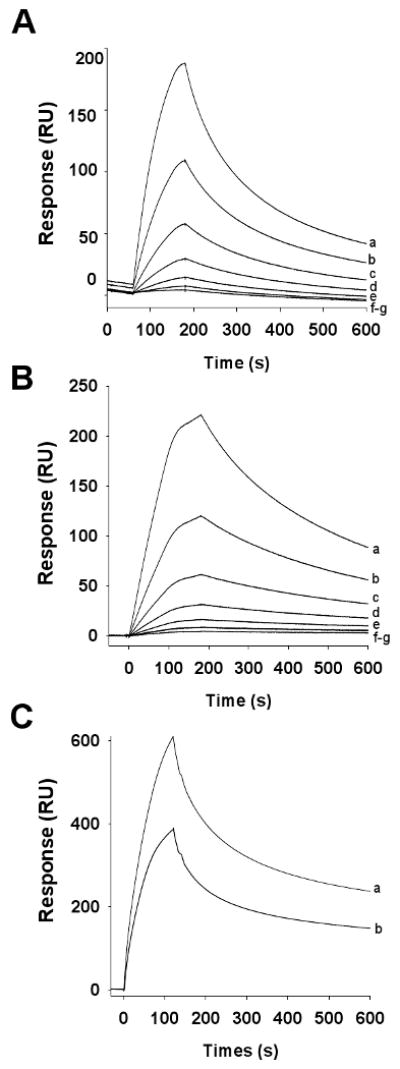
Aegyptin binds to collagen I and III. Typical sensorgrams of aegyptin interaction with collagen type I (A) or type III (B). Different concentrations of recombinant aegyptin (in nM: a, 5; b, 2.5; c, 1.25; d, 0.625; and e, 0.31) were injected over immobilized collagen for 180 s. Dissociation of aegyptin-collagen complex was monitored for 1800 s, and a global two-state binding model was used to calculate kinetic parameters. (C) Ae. aegypti saliva (0.07 μg/ml) was injected over immobilized collage type I (a) and III (b) for 120 s at a flow rate of 20 μl/minute. Sensorgrams are representative of triplicate experiments.
Table 1.
Kinetics of aegyptin collagen interaction. Responses were obtained by injecting recombinant aegyptin over immobilized collagen type I and III for 180 s at a flow rate of 30 μl/min.
| Ka1 (1/Ms) | Kd1 (1/s) | Ka2(1/Ms) | Kd2(1/s) | KD (nM) | χ2 | |
|---|---|---|---|---|---|---|
| Collagen Type I | 4.237E + 6 | 0.015400 | 9.818E−4 | 0.0193 | 1.22 | 1.26 |
| Collagen Type III | 2.490E + 6 | 0.006055 | 4.889E−4 | 9.101E−4 | 1.40 | 2.50 |
Table 2.
Comparison between Ae. aegypti saliva and recombinant aegyptin. Responses were obtained by injecting saliva or recombinant aegyptin over immobilized collagen type I and III for 120 s at a flow rate of 20 μl/min
| Response (RU) | ||
|---|---|---|
| Collagen type I | Collagen type III | |
| Saliva (0.07 μg/ml) | 556.4 | 348.5 |
| Aegyptin (0.25 nM) | 590.3 | 397.9 |
Thermodynamic analysis of aegyptin-collagen I interaction
Affinity and kinetic data (two-state binding model) collected at 5 temperatures (15, 20, 25, 30, 35, and 40°C) were fitted using Biacore evaluation software to obtain equilibrium and transition-state thermodynamic parameters for the aegyptin-collagen interaction. The van't Hoff plot is linear over a temperature range of 15–40°C, and the calculated free energy difference (ΔG°) of −48 ± 0.013 kJ/mol indicates that the binding reaction occurs spontaneously. Both entropic and enthalpic components of the interaction are favorable, as indicated by a positive value for TΔS° (28 ± 0.83 kJ/mol) and a negative value for ΔH° (−20 ± 0.85 kJ/mol). This suggests that both hydrophobic and hydrogen-bonding interactions contribute significantly to the aegyptin-collagen binding reaction. Table 3 summarizes our findings.
Table 3.
Thermodynamic parameters of aegyptin interaction with collagen type I. Aegyptin (0.1 to 3 nM) were injected over immobilized collagen type I at 15°C, 20 °C, 25 °C, 30 °C, 35 °C, and 40°C. Resulting sensorgrams were fitted to the two-state reaction (conformational change) interaction model with local Rmax (*, times).
| Parameter name | Value | (± SE) |
|---|---|---|
| ΔH° [kJ/mol] | -20 | (0.85) |
| ΔS° [J/(K*mol)] | 95 | (2.8) |
| TΔS° [kJ/mol] | 28 | (0.83) |
| ΔG° [kJ/mol] | -48 | (0.013) |
Aegyptin interferes with GPVI interaction with collagen
GPVI plays a crucial role in platelet responses to collagen and directly participates in platelet activation and supports platelet adhesion (23-25) To ascertain whether aegyptin interferes with GPVI-collagen interaction, GPVI was immobilized in a CM5 chip followed by injection of collagen I in the flow cell, previously incubated with or without inhibitor. Fig. 4A shows that increasing concentrations of collagen effectively binds to GPVI (sensorgrams a–e). Fig. 4B shows that when collagen I was incubated with buffer (sensorgram a) or increasing concentrations of aegyptin (sensorgrams c–f), collagen-GPVI interaction was abrogated only in the presence of inhibitor. As a control, sensorgram b shows that aegyptin alone does not interact with GPVI. Additional control experiments depicted in Fig. 4C demonstrate that convulxin displays high-affinity binding to GPVI (sensorgrams a–c) that was not affected by high concentrations (500 nM) of recombinant aegyptin (Fig. 4D, sensorgrams a–c).
Fig. 4.
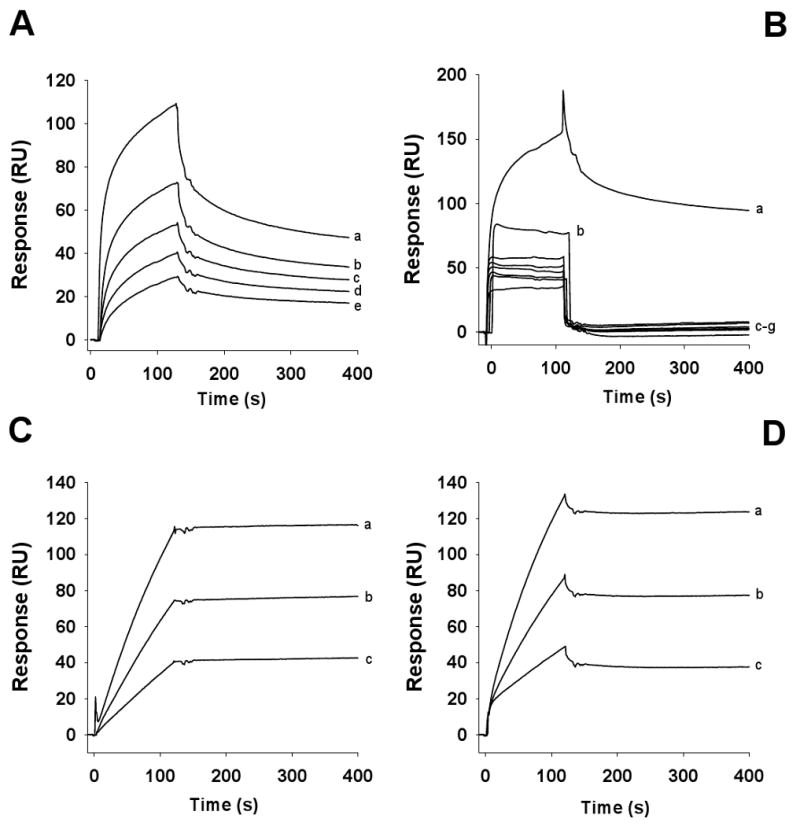
Aegyptin inhibits collagen, but not convulxin, interaction with GPVI. (A) Collagen type I was injected at different concentrations (in μg/ml: a, 50; b, 25, c, 12.5; d, 6.25; and e, 3.175) over immobilized GPVI. (B) Collagen type I was incubated with buffer (sensorgram a) or 500 nM aegyptin (sensorgrams c–g) at the following concentrations (in μg/ml: a and c, 50; d, 25, e, 12.5; f, 6.25; and g, 3.175). The mixtures were injected over immobilized GPVI. Sensorgram b shows that aegyptin at 500 nM does not bind to immobilized GPVI. (C) Convulxin was injected at different concentrations (a, 10 nM; b, 5 nM and c, 2.5 nM) over immobilized GPVI. (D) Convulxin (a, 10 nM; b, 5 nM and c, 2.5 nM) was saturated with 500 nM of aegyptin and the mixture injected over immobilized GPVI (n = 3).
Aegyptin interferes with platelet interaction with collagen I
Integrin α2β1 is known to mediate adhesion in Mg2+-dependent manner. It is now recognized that the type of collagen largely determines the requirement for α2β1. Whereas α2β1 is essential for platelet adhesion and activation on monomeric type I collagen in stasis and flow, it is dispensable for these processes on native fibrillar collagen (42-44). To investigate whether aegyptin blocks integrin α2β1-collagen interaction, platelets were added to coverslips coated with either fibrillar or soluble collagen. Fig. 5A shows that aegyptin dose-dependently inhibits platelet deposition to fibrillar collagen (IC50 ≈ 250 nM) (Fig. 5B), while Fig. 5C demonstrates that it prevents platelet adhesion to soluble collagen with an IC50 ≈ 200 nM (Fig. 5D).
Fig. 5.
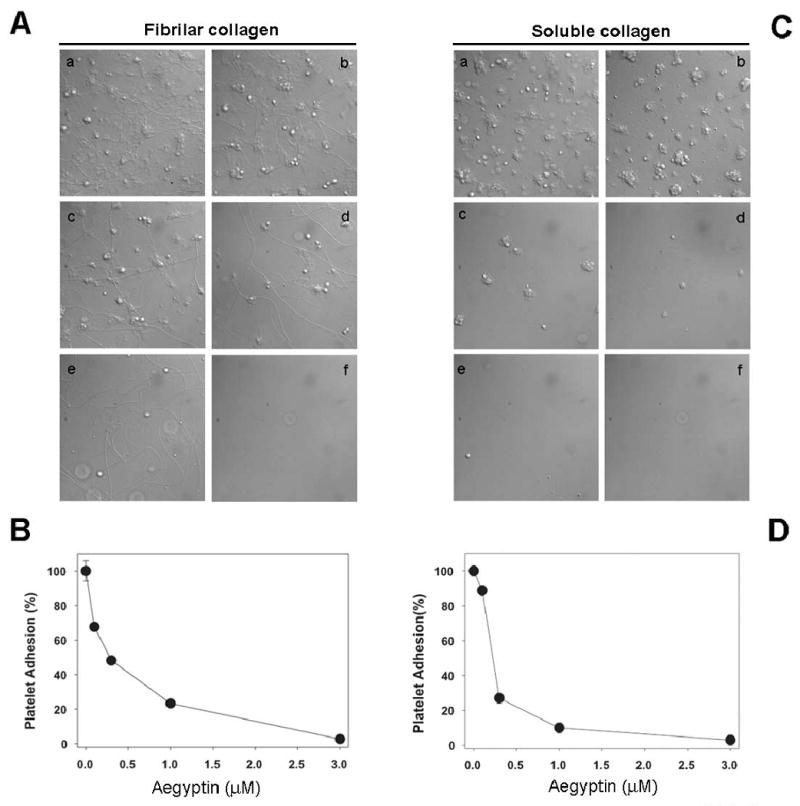
Aegyptin prevents platelet adhesion to fibrillar and soluble collagen under static conditions. Washed human platelets (2 × 105/ml) incubated with (A) fibrillar or (C) soluble collagen for 45 minutes in presence of aegyptin at various concentrations: a, 0 μM; b, 0.1 μM; c, 0.3 μM; d, 1 μM; and e, 3 μM. In f, coverslips were coated with denatured BSA in absence of collagen. (B) and (D) are dose-responses curves for aegyptin-mediated inhibition of platelet adhesion to fibrillar or soluble collagen, respectively. Typical experiments are shown (n = 3).
Aegyptin interferes with vWf interaction with collagen III under static and flow conditions
Platelet-collagen interactions are believed to have the greatest significance at the medium and high shear rates found in arteries. At the very high shear rates found in small arteries and arterioles, the rapid onset of interaction between GPIb-V-IX and vWf immobilized on collagen is crucial for initial tethering (or capture) of flowing platelets (23-26). Interaction between vWf and GPIb-IX-V, however, is rapidly reversible and insufficient for stable adhesion. At low shear rates or static condition, vWf plays a secondary role, but interactions can be detected using in vitro assays. To estimate the effects of aegyptin in vWf-collagen interaction, an ELISA assay was optimized as described in Materials and methods. The results presented in Fig. 6A show that aegyptin dose-dependently inhibits vWf interaction with soluble collagen III with an IC50 of ≈ 50 nM. Next, we evaluated effects of aegyptin in platelet adhesion to collagen under flow conditions. Fig. 6B demonstrates that aegyptin dose-dependently inhibits platelet adhesion at shear rates of 1500 s-1; complete blockade was attained at ≈ 1 μM inhibitor. Fig. 6C shows a dose-response curve with an IC50 ≈ 300 nM.
Fig. 6.
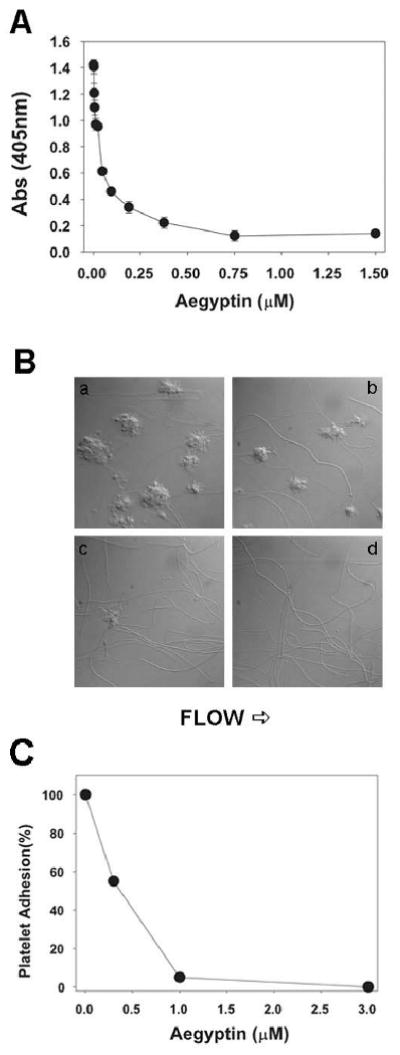
Aegyptin inhibits interaction of vWf with collagen under static and flow conditions. (A) Inhibition of vWf binding to soluble collagen III was estimated by ELISA in the presence of indicated concentrations of aegyptin. (B) Anticoagulated whole blood was perfused over immobilized fibrillar collagen for 240 s at a shear rate of 1500 s-1 in the presence of a, 0; b, 0.1; c, 0.3; and d, 1 μM of aegyptin. (C) Dose-response curve for aegyptin-mediated inhibition of platelet adhesion to collagen under flow conditions. Typical experiments are shown (n = 3).
Discussion
In an attempt to identify a function of aegyptin, a salivary 30-kDa protein of the yellow fever mosquito Ae. aegypti, the corresponding cDNA was cloned into an expression vector that was used to transfect mammalian HEK293 cells. Recombinant aegyptin was shown to specifically bind to collagen and prevent its interaction with physiological ligands GPVI, integrin α2β1, and vWf. The fact that aegyptin affects interaction of collagen with major ligands indicates that it significantly interferes with primary hemostasis. In fact, sub-endothelial collagen plays an important role in initiating hemostatic plug formation at sites of vascular injury, where vWf immobilized on collagen is crucial for initial capture of flowing platelets (23-26). This interaction occurs at the very high shear rates found in small arteries and arterioles but is rapidly reversible and insufficient for stable adhesion in the absence of additional platelet receptors (e.g. GPVI) (29-33) and release of pro-aggregatory mediators (e.g. ADP)(32,45).
Because aegyptin interacts with high affinity with soluble collagen type I to V—all but type II present in the vessel wall (46)—we examined whether aegyptin blocks collagen interaction with vWf. Accordingly, inhibitor dose-dependently prevents binding of vWf to soluble collagen III (47), under static conditions, with an IC50 of ≈ 50 nM. Most important, aegyptin completely inhibits interaction of platelets with fibrillar collagen I under high shear rate (1500 s-1)(26). Accordingly, platelet deposition in collagen coverslips was severely impaired by nanomolar concentrations of inhibitor, as depicted in Fig. 6. These findings indicate that aegyptin recognizes specific or nearby sequences (47) and functionally inhibits GPIb-vWf binding to collagen under flow conditions, thus preventing subsequent events involved in normal hemostasis and/or pathologic thrombus formation in vivo (29-33).
After initial vWf-dependent tethering of platelets, GPVI-collagen interactions initiate cellular activation followed by shifting of integrins to high-affinity state and release of second-wave agonists, most importantly ADP and TxA2 (32,45). Cellular activation and upregulation of integrin affinity is also proposed to be an important prerequisite for adhesion (32,35,45). Therefore, we investigated whether complex formation between aegyptin and collagen could prevent GPVI-mediated platelet responses. As expected, aegyptin is a potent inhibitor of collagen-induced platelet-rich plasma aggregation under test-tube stirring conditions (IC50 ≈ 50 nM). This effect was accompanied by attenuation of shape change and granule secretion, suggesting that platelet-collagen interaction was fully prevented by inhibitor at appropriate concentrations. Further, SPR experiments show that interaction of collagen with immobilized recombinant GPVI was inhibited by aegyptin, while convulxin-GPVI binding was not affected. These results indicate that aegyptin binds to specific (or nearby) sequences that mediate collagen-GPVI interaction (48). This is a notable finding, because GPVI has been regarded as a critical receptor involved in platelet activation through a mechanism involving tyrosine-phosphorylation of FcR γ chain, syk, fyn, lyn, and the participation of adapter proteins such as LAT and SLP-6. This cascade of phosphorylation reactions lead to PLCγ2 activation, which is a major step necessary for collagen-induced platelet activation (49,50).
It is important to recognize that firm adhesion of platelets to collagen through activated α2β1 results in sustained GPVI signaling accompanied by enhanced release and procoagulant activity (51,52). Therefore, it was of interest to ascertain whether aegyptin specifically prevents platelet adhesion to collagen mediated by integrin α2β1 and/or GPVI. Our results unambiguously show that platelet adhesion was prevented by inhibitor under static conditions regardless of whether soluble or fibrillar collagens were tested. Because adhesion to fibrillar collagen is mediated mostly by GPVI, while adhesion to soluble collagen is exclusively dependent on integrin α2β1 (42-44), it is clear that inhibitor recognizes binding sites in the collagen molecule that mediate its interaction with both receptors (48,53). Further, it is evident that aegyptin does not discriminate between fibrillar (insoluble) and non-fibrillar (soluble) collagen and binds with similar affinity to different types of collagens (I-V). It may be that the unique sequence of aegyptin characterized by high content of GDE provides an efficient template for high-affinity binding to collagen through a thermodynamically favorable reaction (Table 3). Therefore, aegyptin appears to recognize the fundamental structure of the collagen molecule, which is characterized by the presence of one or more triple-helical domains formed by three polypeptide α chains. Within these domains, the three α chains wind around one another in a characteristic left-handed triple helix (25). In other words, aegyptin recognizes the three-dimensional structure of collagen crucial for ligand recognition. Actually, all collagens tested to date exhibit the ability to induce platelet aggregation in vitro when in the appropriate polymeric form (46,54,55). This may reflect the presence within the triple helix of GPO triplets that interact with platelet GPVI (48,56) and presumably so with aegyptin.
In addition to aegyptin, two leech-derived proteins, namely LAPP (from Haementeria officinalis), and saratin (from H. medicinalis)(13-15) and a plasma protein C1qTNF-related protein-1 (CTRP-1)(57) have been molecularly cloned and shown to specifically interact with collagen. While LAPP prevents platelet aggregation and vWf binding to collagen, it marginally inhibits platelet adhesion to collagen under low shear rates (58). On the other hand, saratin effectively inhibits vWf binding but only partially affects platelet aggregation at very high concentrations (100 μg/ml)(15). Further, CTRP-1 prevents platelet aggregation primarily because it blocks vWf binding to collagen (57). Therefore, each molecule distinctly recognizes binding sites in the collagen molecule, ultimately resulting in a certain degree of inhibition of platelet function as demonstrated for saratin (59), rLAPP(60) and CTRP-1 (57) when tested in vivo.
Our results indicate that aegyptin displays antihemostatic properties through inhibition of collagen interaction with its three major ligands and therefore mechanistically and functionally distinguishes it from other collagen-binding proteins previously described (13-15,57). These findings are particularly relevant because the adhesive potential of platelets results from the sum of distinct pathways supported by coordinated receptor-ligand interactions specially adapted to respond to different environmental conditions (26). For example, fibrillar collagen is widely recognized as a particularly atherogenic molecule together with tissue factor (61-63). In addition, pepsin-digested collagen—which is readily recognized by aegyptin—displays pro-adhesive properties in vitro and possibly in vivo where matrix metalloprotease activity of infiltrating macrophages and activated smooth muscle cells has been reported (64). Accordingly, α2β1 may substantially contribute to thrombus formation in synergy with other receptors GPVI/FcRγ and GPIb/IX/V, whose interaction is also prevented by aegyptin. Thus, aegyptin may be useful as a tool to study structural features of collagen in designing specific inhibitors targeting collagen interaction with GPVI, integrin α2β1, and/or vWf.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We are grateful to Drs. Thomas Wellems, Robert Gwadz, and Kathryn Zoon (NIAID/NIH) for encouragement and support. We are thankful to the Department of Transfusional Medicine at the NIH Clinical Center for providing fresh platelet-rich plasma. We thank Dr. Mark Garfield and Robert J Hohman (Research Technologies Branch, NIAID) for performing the Edman degradation of aegyptin and Dr. John Andersen (NIAID/NIH) for expertise in the thermodynamic analysis. We also thank NIAID intramural editor Brenda Rae Marshall for assistance.
Abbreviations used
- ADP
adenosine diphosphate
- TXA2
thromboxane A2
- PAF
Platelet-activating factor
- GPVI
glycoprotein VI
- vWf
von Willebrand factor
- SPR
surface plasmon resonance
- BSA
bovine serum albumin
- TBS
Tris-buffered saline
References
- 1.Ribeiro JM, Francischetti IM. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 2.Champagne DE, Ribeiro JM. Proc Natl Acad Sci U S A. 1994;91:138–142. doi: 10.1073/pnas.91.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro JM, Hazzard JM, Nussenzveig RH, Champagne DE, Walker FA. Science. 1993;260:539–541. doi: 10.1126/science.8386393. [DOI] [PubMed] [Google Scholar]
- 4.Stassens P, Bergum PW, Gansemans Y, Jespers L, Laroche Y, Huang S, Maki S, Messens J, Lauwereys M, Cappello M, Hotez PJ, Lasters I, Vlasuk GP. Proc Natl Acad Sci U S A. 1996;93:2149–2154. doi: 10.1073/pnas.93.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francischetti IM, Valenzuela JG, Andersen JF, Mather TN, Ribeiro JM. Blood. 2002;99:3602–3612. doi: 10.1182/blood-2001-12-0237. [DOI] [PubMed] [Google Scholar]
- 6.Keller PM, Waxman L, Arnold BA, Schultz LD, Condra C, Connolly TM. J Biol Chem. 1993;268:5450–5456. [PubMed] [Google Scholar]
- 7.Mans BJ, Louw AI, Neitz AW. J Biol Chem. 2002;277:21371–21378. doi: 10.1074/jbc.M112060200. [DOI] [PubMed] [Google Scholar]
- 8.Francischetti IM, Ribeiro JM, Champagne D, Andersen J. J Biol Chem. 2000;275:12639–12650. doi: 10.1074/jbc.275.17.12639. [DOI] [PubMed] [Google Scholar]
- 9.Andersen JF, Francischetti IM, Valenzuela JG, Schuck P, Ribeiro JM. J Biol Chem. 2003;278:4611–4617. doi: 10.1074/jbc.M211438200. [DOI] [PubMed] [Google Scholar]
- 10.Calvo E, Mans BJ, Andersen JF, Ribeiro JM. J Biol Chem. 2006;281:1935–1942. doi: 10.1074/jbc.M510359200. [DOI] [PubMed] [Google Scholar]
- 11.Valenzuela JG, Charlab R, Galperin MY, Ribeiro JM. J Biol Chem. 1998;273:30583–30590. doi: 10.1074/jbc.273.46.30583. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro JM, Francischetti IM. J Exp Biol. 2001;204:3887–3894. doi: 10.1242/jeb.204.22.3887. [DOI] [PubMed] [Google Scholar]
- 13.Harsfalvi J, Stassen JM, Hoylaerts MF, Van Houtte E, Sawyer RT, Vermylen J, Deckmyn H. Blood. 1995;85:705–711. [PubMed] [Google Scholar]
- 14.Connolly TM, Jacobs JW, Condra C. J Biol Chem. 1992;267:6893–6898. [PubMed] [Google Scholar]
- 15.Barnes CS, Krafft B, Frech M, Hofmann UR, Papendieck A, Dahlems U, Gellissen G, Hoylaerts MF. Semin Thromb Hemost. 2001;27:337–348. doi: 10.1055/s-2001-16887. [DOI] [PubMed] [Google Scholar]
- 16.Francischetti IM, Valenzuela JG, Pham VM, Garfield MK, Ribeiro JM. J Exp Biol. 2002;205:2429–2451. doi: 10.1242/jeb.205.16.2429. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro JM, Arca B, Lombardo F, Calvo E, Phan VM, Chandra PK, Wikel SK. BMC Genomics. 2007;8:6. doi: 10.1186/1471-2164-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvo E, Andersen J, Francischetti IM, de LCM, deBianchi AG, James AA, Ribeiro JM, Marinotti O. Insect Mol Biol. 2004;13:73–88. doi: 10.1111/j.1365-2583.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro JM, Charlab R, Pham VM, Garfield M, Valenzuela JG. Insect Biochem Mol Biol. 2004;34:543–563. doi: 10.1016/j.ibmb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Nazareth RA, Tomaz LS, Ortiz-Costa S, Atella GC, Ribeiro JM, Francischetti IM, Monteiro RQ. Thromb Haemost. 2006;96:7–13. doi: 10.1160/TH06-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jariyapan N, Choochote W, Jitpakdi A, Harnnoi T, Siriyasatein P, Wilkinson MC, Bates PA. J Med Entomol. 2006;43:867–874. doi: 10.1603/0022-2585(2006)43[867:agagpi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Cazares-Raga FE, Gonzalez-Lazaro M, Montero-Solis C, Gonzalez-Ceron L, Zamudio F, Martinez-Barnetche J, Torres-Monzon JA, Ovilla-Munoz M, Aguilar-Fuentes J, Rodriguez MH, de la Cruz Hernandez-Hernandez F. Insect Mol Biol. 2007;16:187–198. doi: 10.1111/j.1365-2583.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 23.Nieswandt B, Watson SP. Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 24.Ruggeri ZM. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 25.Farndale RW, Sixma JJ, Barnes MJ, de Groot PG. J Thromb Haemost. 2004;2:561–573. doi: 10.1111/j.1538-7836.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 26.Savage B, Almus-Jacobs F, Ruggeri ZM. Cell. 1998;94:657–666. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 27.Moroi M, Jung SM, Nomura S, Sekiguchi S, Ordinas A, Diaz-Ricart M. Blood. 1997;90:4413–4424. [PubMed] [Google Scholar]
- 28.Auger JM, Kuijpers MJ, Senis YA, Watson SP, Heemskerk JW. FASEB J. 2005;19:825–827. doi: 10.1096/fj.04-1940fje. [DOI] [PubMed] [Google Scholar]
- 29.Sarratt KL, Chen H, Zutter MM, Santoro SA, Hammer DA, Kahn ML. Blood. 2005;106:1268–1277. doi: 10.1182/blood-2004-11-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. Blood. 2003;102:1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 31.Lecut C, Arocas V, Ulrichts H, Elbaz A, Villeval JL, Lacapere JJ, Deckmyn H, Jandrot-Perrus M. J Biol Chem. 2004;279:52293–52299. doi: 10.1074/jbc.M406342200. [DOI] [PubMed] [Google Scholar]
- 32.Jung SM, Moroi M. J Biol Chem. 2000;275:8016–8026. doi: 10.1074/jbc.275.11.8016. [DOI] [PubMed] [Google Scholar]
- 33.Massberg S, Gawaz M, Gruner S, Schulte V, Konrad I, Zohlnhofer D, Heinzmann U, Nieswandt B. J Exp Med. 2003;197:41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuijpers MJ, Schulte V, Bergmeier W, Lindhout T, Brakebusch C, Offermanns S, Fassler R, Heemskerk JW, Nieswandt B. Faseb J. 2003;17:685–687. doi: 10.1096/fj.02-0381fje. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Kahn ML. Mol Cell Biol. 2003;23:4764–4777. doi: 10.1128/MCB.23.14.4764-4777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francischetti IM, Saliou B, Leduc M, Carlini CR, Hatmi M, Randon J, Faili A, Bon C. Toxicon. 1997;35:1217–1228. doi: 10.1016/s0041-0101(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 37.Jandrot-Perrus M, Busfield S, Lagrue AH, Xiong X, Debili N, Chickering T, Le Couedic JP, Goodearl A, Dussault B, Fraser C, Vainchenker W, Villeval JL. Blood. 2000;96:1798–1807. [PubMed] [Google Scholar]
- 38.Ribeiro JM, Sarkis JJ, Rossignol PA, Spielman A. Comp Biochem Physiol B. 1984;79:81–86. doi: 10.1016/0305-0491(84)90081-6. [DOI] [PubMed] [Google Scholar]
- 39.Tokumasu F, Dvorak J. J Microsc. 2003;211:256–261. doi: 10.1046/j.1365-2818.2003.01219.x. [DOI] [PubMed] [Google Scholar]
- 40.Cruz MA, Chen J, Whitelock JL, Morales LD, Lopez JA. Blood. 2005;105:1986–1991. doi: 10.1182/blood-2004-04-1365. [DOI] [PubMed] [Google Scholar]
- 41.Francischetti IM, Seydel KB, Monteiro RQ, Whitten RO, Erexson CR, Noronha AL, Ostera GR, Kamiza SB, Molyneux ME, Ward JM, Taylor TE. J Thromb Haemost. 2007;5:155–165. doi: 10.1111/j.1538-7836.2006.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura T, Jamieson GA, Okuma M, Kambayashi J, Tandon NN. J Biol Chem. 1998;273:4338–4344. doi: 10.1074/jbc.273.8.4338. [DOI] [PubMed] [Google Scholar]
- 43.Jung SM, Moroi M. J Biol Chem. 1998;273:14827–14837. doi: 10.1074/jbc.273.24.14827. [DOI] [PubMed] [Google Scholar]
- 44.Savage B, Ginsberg MH, Ruggeri ZM. Blood. 1999;94:2704–2715. [PubMed] [Google Scholar]
- 45.Atkinson BT, Jarvis GE, Watson SP. J Thromb Haemost. 2003;1:1278–1287. doi: 10.1046/j.1538-7836.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- 46.Barnes MJ, Farndale RW. Exp Gerontol. 1999;34:513–525. doi: 10.1016/s0531-5565(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 47.Lisman T, Raynal N, Groeneveld D, Maddox B, Peachey AR, Huizinga EG, de Groot PG, Farndale RW. Blood. 2006;108:3753–3756. doi: 10.1182/blood-2006-03-011965. [DOI] [PubMed] [Google Scholar]
- 48.Smethurst PA, Onley DJ, Jarvis GE, O'Connor MN, Knight CG, Herr AB, Ouwehand WH, Farndale RW. J Biol Chem. 2007;282:1296–1304. doi: 10.1074/jbc.M606479200. [DOI] [PubMed] [Google Scholar]
- 49.Watson SP, Auger JM, McCarty OJ, Pearce AC. J Thromb Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 50.Gibbins JM. J Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 51.Munnix IC, Strehl A, Kuijpers MJ, Auger JM, van der Meijden PE, van Zandvoort MA, oude Egbrink MG, Nieswandt B, Heemskerk JW. Arterioscler Thromb Vasc Biol. 2005;25:2673–2678. doi: 10.1161/01.ATV.0000193568.71980.4a. [DOI] [PubMed] [Google Scholar]
- 52.Heemskerk JW, Kuijpers MJ, Munnix IC, Siljander PR. Trends Cardiovasc Med. 2005;15:86–92. doi: 10.1016/j.tcm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Raynal N, Hamaia SW, Siljander PR, Maddox B, Peachey AR, Fernandez R, Foley LJ, Slatter DA, Jarvis GE, Farndale RW. J Biol Chem. 2006;281:3821–3831. doi: 10.1074/jbc.M509818200. [DOI] [PubMed] [Google Scholar]
- 54.Jarvis GE, Atkinson BT, Snell DC, Watson SP. Br J Pharmacol. 2002;137:107–117. doi: 10.1038/sj.bjp.0704834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morton LF, Hargreaves PG, Farndale RW, Young RD, Barnes MJ. Biochem J. 1995;306(Pt 2):337–344. doi: 10.1042/bj3060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knight CG, Morton LF, Onley DJ, Peachey AR, Ichinohe T, Okuma M, Farndale RW, Barnes MJ. Cardiovasc Res. 1999;41:450–457. doi: 10.1016/s0008-6363(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 57.Lasser G, Guchhait P, Ellsworth JL, Sheppard P, Lewis K, Bishop P, Cruz MA, Lopez JA, Fruebis J. Blood. 2006;107:423–430. doi: 10.1182/blood-2005-04-1425. [DOI] [PubMed] [Google Scholar]
- 58.Vilahur G, Duran X, Juan-Babot O, Casani L, Badimon L. Thromb Haemost. 2004;92:191–200. doi: 10.1160/TH03-11-0687. [DOI] [PubMed] [Google Scholar]
- 59.Davis JA, Brown AT, Alshafie T, Poirier LA, Cruz CP, Wang Y, Eidt JF, Moursi MM. Am J Surg. 2004;188:778–785. doi: 10.1016/j.amjsurg.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 60.van Zanten GH, Connolly TM, Schiphorst ME, de Graaf S, Slootweg PJ, Sixma JJ. Arterioscler Thromb Vasc Biol. 1995;15:1424–1431. doi: 10.1161/01.atv.15.9.1424. [DOI] [PubMed] [Google Scholar]
- 61.Penz S, Reininger AJ, Brandl R, Goyal P, Rabie T, Bernlochner I, Rother E, Goetz C, Engelmann B, Smethurst PA, Ouwehand WH, Farndale R, Nieswandt B, Siess W. Faseb J. 2005;19:898–909. doi: 10.1096/fj.04-2748com. [DOI] [PubMed] [Google Scholar]
- 62.Toschi V, Gallo R, Lettino M, Fallon JT, Gertz SD, Fernandez-Ortiz A, Chesebro JH, Badimon L, Nemerson Y, Fuster V, Badimon JJ. Circulation. 1997;95:594–599. doi: 10.1161/01.cir.95.3.594. [DOI] [PubMed] [Google Scholar]
- 63.Furie B, Furie BC. J Clin Invest. 2005;115:3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raines EW, Koyama H, Carragher NO. Ann N Y Acad Sci. 2000;902:39–51. doi: 10.1111/j.1749-6632.2000.tb06299.x. discussion 51-32. [DOI] [PubMed] [Google Scholar]


