Abstract
How synaptic vesicles (SVs) are localized to the pre-active zone (5-200 nm beneath the active zone) in the nerve terminal, which may represent the slow response SV pool, is not fully understood. Electron microscopy revealed the number of SVs located in the pre-active zone, was significantly decreased in hypothalamic neurons of Carboxypeptidase E knockout (CPE-KO) mice compared to wild type mice. Additionally, we found K+-stimulated glutamate secretion from hypothalamic embryonic neurons was impaired in CPE-KO mice. Biochemical studies indicate that SVs from the hypothalamus of wild type mice and synaptic-like microvesicles (SLMVs) from PC12 cells contain a transmembrane form of CPE, with a cytoplasmic tail (CPEC10), maybe, involved in synaptic function. Yeast two-hybrid and pull-down experiments showed that the CPE cytoplasmic tail interacted with γ-adducin, which binds actin enriched at the nerve terminal. TIRF microscopy using PC12 cells as a model showed that expression of GFP-CPEC15 reduced the steady-state level of synaptophysin-mRFP containing SLMVs accumulated in the area within 200 nm under the sub-plasma membrane (TIRF zone). Our findings identify the CPE cytoplasmic tail, as a new mediator for the localization of SVs in the actin-rich pre-active zone in hypothalamic neurons and the TIRF zone of PC12 cells.
Keywords: Carboxypeptidase E, hypothalamic neuron, synaptic vesicle localization, γ-adducin, synapsin, actin cortex, glutamate secretion, PC12 cells
Introduction
Synaptic vesicle components, including vesicular membrane proteins, are constantly transported by constitutive vesicles from the cell soma to the active zone at the presynaptic membrane. Synaptic vesicles (SVs) are formed by recycling from the presynaptic plasma membrane, filled with neurotransmitters and held in the nerve terminal in an actin-dependent manner (Murthy and De Camilli 2003; Siksou et al. 2007).
In nerve terminals, there are three different synaptic vesicle pools with respect to their sensitivity to stimulation (Rizzoli and Betz 2005): a reserve pool (~80% of the presynaptic vesicles) that responds to stimulation very slowly (~min), a slow response pool (~19%) that secretes neurotransmitters more acutely (within a few seconds), and a readily-releasable pool of SVs that are docked to the presynaptic membrane at the active zone (~1%), and responds immediately to stimulation. Upon extracellular stimulation, the readily releasable pool is fused to the plasma membrane by SNAREs and releases its neurotransmitters to the synaptic cleft. In order to refill the readily-releasable pool depleted after exocytosis during stimulation, sufficient SVs should be available in the slow response and reserve pools within the pre-active zone, a region proximal to the active zone (Murthy and De Camilli 2003). Synapsin, an F-actin-interacting protein has recently been shown to structurally maintain the SVs within the pre-active zone (Bloom et al. 2003; Siksou et al. 2007), and scaffolding proteins, including Rim1, have also been reported to contribute to the maintenance of SVs in these zones (Wang et al. 1997; Dieck et al. 1998; Fenster et al. 2000).
Carboxypeptidase E (CPE) is a major component in large dense core vesicles (LDCVs) in neuroendocrine cells and exists in both soluble and membrane forms (Fricker et al. 1990; Dhanvantari et al. 2002). The soluble form of CPE within the lumen of the LDCV removes basic residues from the C-terminus of endoproteolytically cleaved proneuropeptides to yield biologically active peptides (Fricker and Snyder 1982). A luminal domain of the membrane form of CPE can bind BDNF (Lou et al. 2005) and POMC (Cool et al. 1997) at the trans-Golgi network (TGN) and facilitate sorting of these molecules into vesicles of the regulated secretory pathway in hippocampal neurons and pituitary cells, respectively. Furthermore, a portion of the membrane associated CPE acquires a transmembrane orientation at the TGN (pH = 6.0~6.5) and exposes its C-terminal 10 amino acids to the cytoplasm to form a cytoplasmic tail (Dhanvantari et al. 2002). In hippocampal neurons and anterior pituitary cells, the CPE cytoplasmic tail mediates the transport of BDNF- and POMC-containing vesicles to the secretion sites via its interaction with microtubule motor complex proteins, dynactin and kinesin 2 and 3 (Park et al. 2008a, b). Additionally, CPE knockout mice showed impaired glutamate neurotransmission as evidenced by the lack of glutamate-mediated b-wave in their retinograms (Zhu et al. 2005) and deletion of the C. elegans CPE ortholog, egl-21, impaired the secretion of acetylcholine from the neuromuscular junction (Jacob and Kaplan 2003). Both results strongly indicate that CPE plays some role in neurotransmitter secretion.
Based on these observations, we searched for a physiological link between CPE and neurotransmitter secretion using hypothalamic neurons which we found had SVs enriched in CPE, and PC12 cells which contain acetylcholine synaptic-like microvesicles (SLMVs) and CPE. The current study demonstrates that some of the CPE present in hypothalamic SVs and SLMVs have a transmembrane orientation and a cytoplasmic tail. The cytoplasmic tail interacts directly with γ-adducin, an F actin-interacting protein (Li et al. 1995; Takei et al. 1995). Glutamate release was impaired and the number of synaptic vesicles in the pre-active zone of hypothalamic neurons was significantly reduced in the CPE-KO mice. Finally, competition studies in vivo with the CPE cytoplasmic tail eliminated SLMV accumulation in the TIRF zone within 200 nm under the plasma membrane of PC12 cells, indicating a role of CPE in neurotransmitter release
Materials and Methods
DNA Constructs
To generate the GST-tagged CPEC10 cytoplasmic tail construct, 5′-EcoRI-XhoI-3′ digests of PCR products for CPEC10 were subcloned into pGEX4T-2 vector (Amersham Pharmarcia). For GFP-CPEC15 construct, CPEC15 was cloned into the C-terminal end of GFP in the pEGFP-3C vector (BD Bioscience) at the 5′-EcoRI-KpnI-3′ sites. Synaptophysin-mRFP (Syn-mRFP) was a gift from Dr. Leon Lagnado (Cambridge, UK).
Secretion Analysis of Embryonic Hypothalamic Neuron and Adult Synaptosomes
Embryonic hypothalamic neurons isolated from an entire litter at E16 derived from mating two heterozygote (Cpe+/−) mice as described earlier (Lou et al. 2005) with modification. Briefly, the hypothalamus from each embryo was dissociated individually with digestion buffer (15 mg collagenase, 5 mg DNAse, 40 mg of BSA in 10 ml PBS) at 37°C for 30 min, and grown in a collagen coated 12-well plate. The genotype was identified by PCR of genomic DNA isolated from the embryo as described previously (Cawley et al. 2004). After eight days, the neurons were harvested to assess the total glutamate content from the CPE-KO mice (N=2) and their WT littermate (N=3), and a total of 20 neuron cultures from CPE-KO or WT mice were subjected to an activity-dependent secretion study as described as previously with modification (Lou et al. 2007). The neurons were washed and equilibrated with 500 μl of basal buffer for 1 h, and then incubated with 200μl of fresh basal buffer for 10 min for basal secretion, followed by 10 min incubation with 200μl of 50 mM KCl-containing buffer for stimulated secretion. The samples of total cells and basal or stimulation buffer were lyophilized and analyzed for glutamate quantification.
Synaptosomes were prepared from hypothalami from five CPE-KO and five WT mice littermates (25-35 weeks) by homogenization with 320 mM Sucrose/4 mM HEPES/0.1% BSA buffer (SHB) supplemented with complete Proteinase Inhibitors (Roche). The homogenate underwent differential centrifugations to obtain synaptosomes as described by Huttner (Huttner et al. 1983). The synaptosomes were suspended with basal buffer and divided equally into five tubes. Activity-dependent secretion was preformed by incubating two tubes of synaptosomes with basal buffer for 10 min while the other two were incubated for 10 min in stimulation buffer. Then the buffers were collected by centrifugation at 1000 x g for 5 min, and the supernatants were lyophilized for glutamate quantification. The synaptosomes in the fifth tube were used for total glutamate measurement without the secretion assay. Three independent experiments were carried out for this study.
Glutamate Quantification
Glutamate measurement using HPLC was conducted in the Amino Acid Laboratory of the Department of Pediatrics, Indiana University Medical School, as described previously (Battaglia et al. 1999) with modifications. Briefly, samples were reconstituted with internal standard solution and dried. The sample was then treated with derivative solution, and separated by reverse-phase HPLC, using a Waters PICO Tag column and propriety buffers. The concentration of glutamate in the samples was quantified against the standard curve and expressed as nmole/mL. For comparison between the study groups, the percentage of glutamate secretion was obtained by dividing the amount of glutamate in the buffers with total glutamate obtained from the cultured neurons or synaptosomes and expressed as % ± SE of total. Student’s T test was used to evaluate statistically significant differences. P value < 0.05 was considered significantly different.
Electron microscopy (EM)
Four hypothalami from CPE KO mice and four WT littermates (40-45 weeks) were fixed in 2.5% glutaraldehyde made up in Molling’s solution. Transmission electron microscopy was preformed by JFE Enterprises (Brookeville, MD). The electronic micrograph images for one hundred synaptic junctions from each group were analyzed using ‘Metamorph’ software (Molecular Devices Corp.). First, the total number of the synaptic vesicles in the presynaptic terminals was counted for forty boutons in each group. Next, the docked synaptic vesicles [defined as no space between vesicle and the presynaptic membrane aligned with the PSD] and the number of synaptic vesicles within the pre-active zone [100 nm from the presynaptic membrane (depth) x entire region aligned with the PSD (length)] were analyzed for forty synapses from each group. In addition, the average densities of synaptic vesicles per 100 nm × 100 nm between 0-100, 100-200, 200-300, and 300-400 nm from the presynaptic membrane were calculated from 26 boutons of CPE KO mice and WT littermates using Metamorph.
Immunoprecipitation Assay for Intact Synaptic Vesicles
To examine whether synaptic vesicles have the cytoplasmic tail of CPE on the outside surface, ten hypothalami of adult Sprague Dawley rats (25 weeks) were utilized to obtain crude synaptosomes. The synaptosomes underwent hypotonic lysis in 32 mM sucrose buffer with homogenization, differential centrifugation, and ultracentrifugation to isolate synaptic vesicles (Huttner et al. 1983). Aliquots of the hypothalamic lysate, synaptosomes, and the purified synaptic vesicles were analyzed by Western blotting.
For immunoprecipitation assays, an affinity-purified rabbit antibody against the ten amino acid residues at the extreme CPE C-terminus (anti-CPEC10 or C-CPE) was covalently coupled to the linker bound magnetic Dynabeads M-500 subcellular beads specialized for pulldown of intact vesicles following the manufacturer’s instruction (Invitrogen). Intact synaptic vesicles (100 μl) were incubated with these beads (4×107) overnight at 4°C and gentle rotation. To determine the specificity of CPE binding, the same volume of the synaptic vesicles were incubated with two controls: One with C-CPE coupled Dynabeads that were pre-incubated with 8 μg of the CPEC10 peptide (C-CPE + peptide), and the other with only the linker IgG bound Dynabeads (IgG). Following the incubation, the Dynabeads were washed and the bound proteins were eluted with sample buffer at 95°C for 5 min and analyzed by Western blot.
To examine whether the synaptic vesicles were structurally intact, we coupled Dynabeads with an antibody against an internal sequence of CPE (I-CPE, aa362-379); a sequence that is expected to reside inside the synaptic vesicle. These Dynabeads were incubated with same amount of intact synaptic vesicles or lysed vesicles (with 3 freeze-thaw cycles). The Dynabeads with linker IgG and C-CPE were used in parallel as controls. Three independent experiments were carried out for these studies.
Immunoprecipitation assay for PC12 cell was done with the combined fractions of 5-8, which contains enriched SLMVs after the subcellular fractionation by sucrose density gradient centrifugation.
Co-precipitation study using GST-CPEC10
To determine the CPE-C terminal interactive proteins, CPE Cytoplasmic terminal 10 residues tagged with GST (GST-CPEC10) was made and Co-precipitation studies using GST or GST-CPEC10 with mouse brain and PC12 cell cytosols were performed as described previously (Park et al. 2008a). The interactive proteins in pull down fractions were analyzed by Western blot.
Sucrose Density Gradient Fractionation of Hypothalamic Synaptic Vesicles
Synaptic vesicles isolated from mouse brain were further analyzed by sucrose gradient velocity centrifugation. The synaptic vesicles were layered on the top of a 50-800 mM sucrose gradient generated with a Gradient Master (BIO MP, Canada) and centrifuged for 5 h at 65,000 x g. Sixteen 1 ml fractions were collected from the top of the gradient using the Auto Densi Flow fraction collector (Labconco). In order to determine whether the CPE present in the synaptic vesicle fractions was a contaminant from the homogenization process, exogenous CPE was added to synaptosomes of CPE-KO mice before hypotonic lysis. The exogenous CPE was obtained from conditioned medium of COS7 cells transduced with an adenovirus expressing full-length CPE. Sixteen fractions were collected after centrifugation and proteins in each fraction were precipitated with 10% TCA. The presence of CPE and synaptic vesicle markers were determined by Western blot.
For the analysis of PC12 cell synaptic-like microvesicles, cells grown in four 100 mm dishes were harvested using trypsin, washed, and suspended in 1 ml of 32 mM SHB buffer. The cells were homogenized by passage through 20G and 27G needles ten times each, and centrifuged at 1,000 x g to remove cell debris. The post nuclear supernatant was then fractionated by sucrose gradient centrifugation and 17 fractions were collected and analyzed by Western blot as described above for the hypothalamic synaptic vesicles.
Western blotting and Antibodies
Quantification of proteins in the cell or tissues was preformed by Western blotting. Proteins were separated on 4-12% NuPage (Invitrogen) and transferred onto nitrocellulose or PVDF membrane for immunoblotting. The rabbit antibody against the 10 residues at the CPE carboxyl terminus (Normant and Loh 1998) and the internal control CPE antibody (aa362-379) made in our laboratory were used for immunoprecipitation and immunoblotting. For detection of Rab27A, Rab3A, and Rim1, and DIC, horseradish peroxidase (HRP)-conjugated secondary antibodies and the ECL plus Western Blotting Detection System (GE Healthcare, NJ) were used. To detect CPE, chromogranin A (CgA), synaptophysin, synapsin1, VGLUT 1, VGLUT 2, and vesicle-associated membrane protein 2 (VAMP 2), IRDye™ conjugated secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA) were used and the fluorescent signals were analyzed by the Odyssey Infrared Imager System (LI-COR, Inc. Lincoln, Nebraska). The fluorescence intensities of corresponding bands were recorded as mean ± SE of arbitrary units from at least three separate experiments. Detailed information for antibodies used in the Western blotting is in Appendix S1.
TIRF microscopy
To evaluate the distribution of SLMVs in the TIRF zone (~200 nm below the plasma membrane), 2 × 104 PC12 cells were seeded onto 0.17mm delta TPG dish coated with 0.001% poly-lysine and poly-ornithine, and incubated for 18 hr in DMEM supplemented with 5% horse and 10% fetal bovine sera. The cells were co-transfected with Syn-mRFP and GFP or GFP-tagged CPE cytoplasmic tail (GFP-CPEC15) for 24 hr using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The medium was replaced with basal TIRF buffer (Michael et al. 2007) and the steady state distribution of fluorescent proteins in the TIRF zone were captured with an inverted Olympus IX81 microscope (Olympus). The microscope was equipped with a 60x PlanApo-N 1.45 NA, oil objective and a Hamamatsu Orca C9100 backed-thinned camera (EM-CCD) with 512 × 512 pixels. The CCD detector gain was fixed at 250 for all the images of cells. The temperature of the stage was heated to 37°C before dish mounting. The GFP signals were also observed under epi-fluorescence microscopy, and the average intensity of Syn-mRFP was measured with ‘Metamorph’ software for each group and expressed as arbitrary units. Two independent experiments were performed for this measurement. Details of signal-to-noise ratio determination for TIRF are described in Appendix S1.
Yeast Two-hybrid Library Screen
Mouse brain cDNA library in pACT2-1 (ML4008AH; LEU+) were purchased from Clontech (Clontech, Mountain View, CA). cDNA encoding the C-terminal 25 amino acids of carboxypeptidase E (CPEC25) was subcloned into the EcoRI and PstI sites of the yeast two-hybrid bait plasmid pAS2-1 (Clontech). The construct was confirmed by sequencing. Of cDNAs from 1 × 107 independent clones, 10% of the brain library was screened. Positive cDNAs were purified, sequenced and then subjected to a homology search via NCBI BLAST.
Results
Activity-dependent glutamate secretion is impaired in the hypothalamic neurons of CPE knockout (CPE-KO) mice
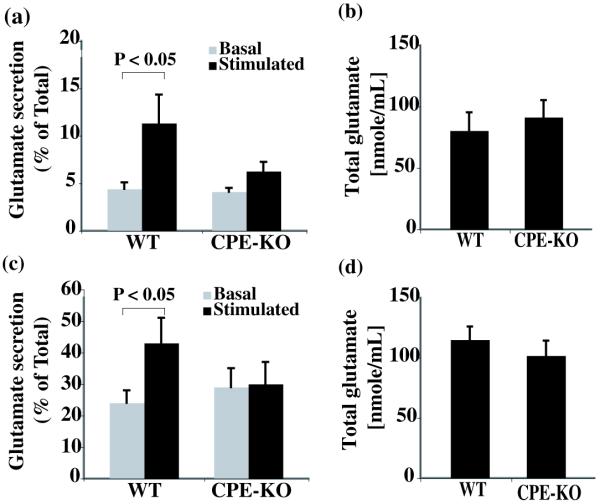
To determine if CPE is involved in neurotransmitter release, we examined the regulated secretion of glutamate, a major neurotransmitter in the hypothalamus, using primary embryonic neurons in culture. Embryonic neurons isolated from WT or CPE-KO mice were incubated with either basal or stimulation buffer and the secretion of glutamate in the buffer was quantified (Fig. 1a). There was no significant K+-induced secretion of glutamate from the neurons of CPE-KO mice (basal secretion [4.1 ± 0.4 %] vs. stimulated secretion [6.3 ± 0.8 %], p > 0.05) while a significant stimulated secretion of glutamate from the WT hypothalamic neurons was observed (basal secretion [4.4 ± 0.7 %] vs. stimulated secretion [11.33 ± 3.0%], p < 0.02). In order to examine whether the reduction of glutamate secretion was due to a decrease in glutamate content in CPE-KO neurons compared to WT, we quantified the total amount of glutamate in these neurons, and found that there was no significant difference (CPE-KO, 99 ± 12 nmole/mL; WT, 115 ± 11 nmole/mL, p > 0.05) (Fig. 1b).
Fig. 1.
Analysis of K+-stimulated glutamate secretion from hypothalamic neurons and synaptosomes. (a) Glutamate secretion (% = glutamate in medium (M) divided by total (T) glutamate ± SEM x 100%) from primary hypothalamic neurons (N=3). Hypothalamic neurons derived from wild type (WT, N=10) or CPE-KO (N=10) E16 mouse hypothalami were treated in basal buffer or 50 mM KCl-containing stimulation buffer for 10 min. The glutamate levels in the basal and stimulation buffers and in the cells were quantified by HPLC against a standard curve and expressed as nmole/mL. The basal level of glutamate secreted were similar in both groups; but stimulated glutamate secretion was significantly higher in WT hypothalamic neurons than that of CPE-KO mice (p<0.05). (c) The percentage of stimulated glutamate secretion by synaptosomes isolated from adult hypothalami of WT and CPE-KO mice (N=3). The total glutamate content was not significantly different between WT and CPE-KO embryonic hypothalamic neurons (b) or adult hypothalamic synaptosomes (d) (N=3).
The glutamate secretion in adult hypothalamic synaptosomes was also examined with synaptosomes isolated from CPE-KO or WT mouse hypothalami. In WT synaptosomes, the percentage of glutamate secretion was significantly higher in the stimulated media (40.2 ± 8.7 %) than that of basal medium (22.2 ± 3.7 %, p < 0.05) while it was not significantly different between basal and stimulated media in CPE-KO mouse synaptosomes (28.6 ± 5.7 % vs. 29.8 ± 7.3 %, respectively, p > 0.05) (Fig. 1c). The total amount of glutamate in the synaptosomes was not significantly different between CPE-KO (91 ± 14 nmole/mL; WT mice (80 ± 15 nmole/mL, p > 0.05) (Fig. 1d). These results suggest that CPE is involved, either directly or indirectly, in activity-dependent glutamate secretion from hypothalamic neurons.
The number of synaptic vesicles in the active and pre-active zones at the presynaptic membrane is reduced in hypothalamic synapses of CPE-KO mice
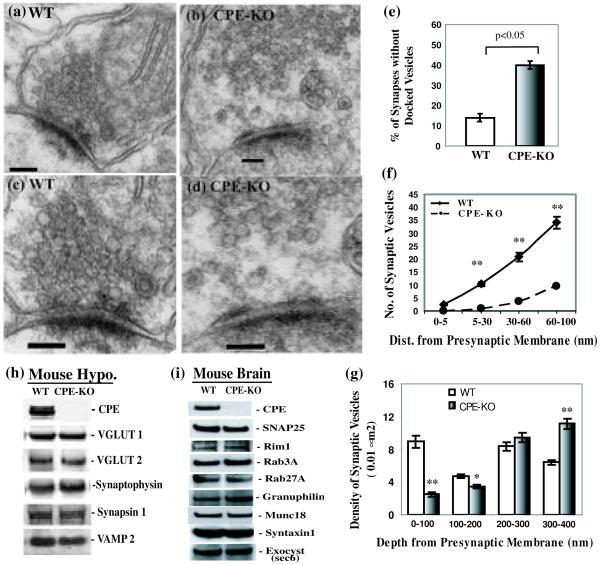
In order to examine whether CPE-KO mouse hypothalami have a normal distribution of synaptic vesicles accumulated at the presynaptic membrane, we examined the hypothalamic synaptic junctions of WT and CPE-KO mice by electron microscopy (Fig. 2a-d). We first analyzed the total number of synaptic vesicles in 40 boutons from each group and found that there was no significant difference between animals (WT; 195.6 ± 8.4/bouton and CPE-KO; 192.5 ± 8.5/bouton, p > 0.2). Next, we found that 39.8 ± 2.1% of the CPE-KO synapses (N = 123) lacked tethered/docked synaptic vesicles at active zone of the presynaptic membrane in contrast to that of 14.2 ± 2.0 % of the WT synapses (N = 134) (Fig. 2e). Finally, by analyzing forty synapses from each group, we found that the number of SVs localized 5, 30, 60 and 100 nm from the presynaptic membrane was significantly reduced in the CPE KO synapses compared to those of WT (all: p < 0.0002, Fig. 2f). These results indicate that the absence of CPE results in the lack of localization of synaptic vesicles in the active and pre-active zone of hypothalamic synapses.
Fig. 2.
Comparison of hypothalamic synapse morphology between wild type (WT) and CPE-KO (KO) mice. Representative electronic micrograph (EM) of hypothalamic synapses from WT (a) and CPE-KO mice (b) (Scale bar = 100 nm). Magnified EM images of hypothalamic synapses from WT (c) and CPE-KO (d) mice (Scale bar = 100 nm). (e) Bar graph showing the percentage of synapses without tethered/docked synaptic vesicles at active zone of the presynaptic membrane in the hypothalami of CPE-KO mice (N=123) was significantly higher than that of their WT littermates (N=134). (f) Line graph showing the number of synaptic vesicles within the zone 5, 30, 60, 100 nm away from the presynaptic membrane (depth) and entire area along side of the post synaptic density (width) of the CPE-KO (N=40) and WT mouse synapses (N=40). (g) The average density of SVs per 100 nm × 100 nm (0.01 μm2) between 0-100, 100-200, 200-300, and 300-400 nm from the presynaptic membrane from 26 boutons of CPE KO mice and WT littermates. * p<0.05; ** p<0.0002. (h) Representative Western blot showing the levels of proteins associated with synaptic vesicle in the hypothalamus (Hypo.) of CPE knock out (KO) mouse or their wild type littermates (WT). (i) Immunoblots demonstrate the levels of proteins related with synaptic vesicle tethering/docking in the brain cytosol of CPE KO mouse or their wild type littermates (WT). All experiments were repeated three times with similar results.
We also determined the density of SVs per 100 nm × 100 nm (0.01 μm2) between 0-100, 100-200, 200-300, and 300-400 nm from the presynaptic membrane from 26 boutons of CPE KO mice and WT littermates. The density of SVs was lower in the hypothalamic boutons of CPE-KO mice compared to WT mice up to 200 nm from the presynaptic membrane (Fig. 2g). Thereafter, the SV density of CPE-KO mice was similar to that of WT mice between 200 and 300 nm, and was slightly higher in CPE-KO versus WT mice between 300 and 400 nm. The data indicate that SVs absent below 200 nm (pre-active zone) were retained in the pool above 300 nm from the presynaptic membrane.
Since it was possible that the knockout of one protein might affect the level of other proteins, we examined the levels of several synaptic vesicle associated proteins from the mouse hypothalamus (Hypo.), such as VGLUT 1 and VGLUT 2, synapsin 1, VAMP 2 (Fig. 2h), as well as other proteins involved in synaptic vesicle tethering or docking, such as Munc18-1, syntaxin1, Rim1, Rab3A, Rab27A, granuphilin, exocyst (Sec6), and SNAP25 (Fig. 2i) in mouse brain. We observed that the levels of these proteins were unchanged in the CPE-KO mouse when compared with their WT littermates. This shows that the lack of SVs in the active zone and in the vicinity of the active zone of the synapses of the CPE-KO mouse is not due to a defect of assembly or integrity of SV, nor lack of known molecules involved in the tethering or docking process.
Hypothalamic synaptic vesicles contain transmembrane CPE
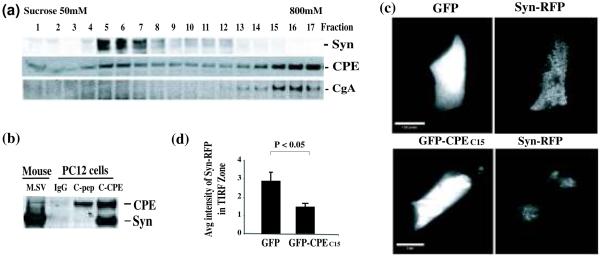
Synaptic vesicle localization in the active zone at the presynaptic membrane is mediated not only by scaffolding proteins but also by vesicle-associated proteins (De Camilli and Jahn 1990; Murthy and De Camilli 2003). We therefore examined synaptic vesicles isolated from rat hypothalamus for the presence of CPE. We found CPE not only in the heavy peptidergic vesicle fractions as expected (data not shown) but also in the synaptosomes and the purified synaptic vesicle fractions, which were marked by synaptophysin (Syn) but lacked chromogranin A (CgA), a peptidergic vesicle marker (Fig. 3a).
Fig. 3.
Proteins in synaptic vesicles (SVs) isolated from rat hypothalamus and mouse brain. (a) Representative Western blots of hypothalamic lysate (Lysate), synaptosomes and purified synaptic vesicles from adult rat hypothalamus. The presence of CPE was evident in all the fractions including synaptic vesicles which were positive for two SV markers, synaptophysin (Syn) and synapsin, while CgA, a marker for dense-core secretory granules, was absent in the SV fraction. (b) Representative Western blot of intact SVs that were immunoprecipitated (IP) by Dynabeads coupled with anti-CPEC10 antibody (C-CPE) indicated by strong activity to anti-VGLUT1, VGLUT2, and synaptophysin (Syn) antibodies. Two controls were used to demonstrate the specificity of anti-CPEC10 antibody: the C-CPE beads preabsorbed with CPEC10 peptide before the IP (C-CPE+peptide), and beads that only had linker IgG (IgG). The immunoreactivity to CPE and all the SV markers were eliminated by the absorption control peptide. (c) IP assay using the anti-CPE internal sequences (I-CPE) antibody with intact or lysed SVs. C-CPE or IgG coupled to Dynabeads was used as positive and negative controls. I-CPE only precipitated the CPE when the vesicles were lysed (Lysed SV) (N=3). (d) SVs isolated from WT mouse brain were analyzed by Western blot after fractionation by sucrose gradient centrifugation. Immunoreactivity of endogenous CPE was observed in fractions containing synaptophysin (fractions 5-8). (e) Western blot of SV fractions after the sucrose gradient for CPE KO mouse brain. The SVs were prepared in the presence of exogenous full-length CPE. This exogenous CPE was present in the first four fractions but not detected in fractions containing synaptophysin (Fractions 5-9).
Given that a subpopulation of CPE in peptidergic vesicles has a transmembrane orientation in endocrine cells (Dhanvantari et al. 2002; Arnaoutova et al. 2003), it is possible that hypothalamic synaptic vesicles may also have a similar transmembrane form of CPE. To test this hypothesis, we used immunoprecipitation experiments with Dynabeads coupled with an antibody against the last 10 residues of the CPE cytoplasmic tail (anti-CPEC10 or C-CPE). The C-CPE did pull down intact synaptic vesicles isolated from adult rat hypothalami; indicated by the presence of CPE and three synaptic vesicles markers, synaptophysin, VGLUT2, and VGLUT1 (Fig. 3b). The two controls, pre-absorbed C-CPE with CPEC10 peptide (C-CPE + peptide) and the Dynabeads cross-linked to a non-specific IgG (IgG), pulled down only background levels of synaptic vesicles (Fig. 3b). These results indicate that hypothalamic synaptic vesicles contain a transmembrane form of CPE that exposes its C-terminus to the cytoplasm.
To rule out the possibility that the presence of CPE in the SVs might be due to adherence of soluble CPE released from lysed peptidergic vesicles during homogenization, we performed two additional control experiments. First, we incubated Dynabeads coupled with an antibody against an internal CPE sequence (I-CPE, aa 362-379: present inside synaptic vesicles) with either intact synaptic vesicles or lysed synaptic vesicles in parallel. We found that the I-CPE antibody pulled down a small amount of CPE from the unlysed synaptic vesicles (Fig. 3c, left panel) with a significant increase in the amount of CPE from lysed synaptic vesicles (2.5 ± 0.3 fold, n=3 p < 0.02) (Fig. 3c, right panel). The intensity of CPE in the lysed samples precipitated by C-CPE also increased significantly compared to unlysed vesicles (2.4 ± 0.02 fold, n=3 p < 0.02). These results strongly suggest that the synaptic vesicles used in the previous experiments (Fig. 3a & b) were for the most part structurally intact and that no significant amount of full length CPE was bound to the outside of the SVs. As a second control experiment, we examined whether exogenous soluble CPE could be co-fractionated with intact synaptic vesicles. We examined the distribution of CPE in the synaptic vesicles isolated from WT and CPE-KO mouse brains by subcellular fractionation and Western blot. In fractions of WT mouse brain, but not the CPE-KO, we found CPE in SV fractions (5-9), which also contained synaptophysin, the synaptic vesicle marker (Fig. 3d). Next we repeated the purification of synaptic vesicles from CPE-KO mice but this time we added conditioned medium containing full-length soluble CPE expressed in COS7 cells, to the synaptosomes before the hypotonic lysis procedure. The conditioned medium from control COS7 cells devoid of CPE was used as control medium added to the synaptosomes in a parallel experiment. We observed that the exogenous CPE did not adhere to the outside of the vesicles, since it was not present in the same fractions as the synaptic vesicles from WT mouse (fractions 5-9; Fig. 3e). These data indicate that CPE detected in the intact WT synaptic vesicles was not due to an artifact of adherence of soluble CPE released from damaged vesicles during the homogenization procedure.
Excessive CPE cytoplasmic tail decreases the steady state accumulation of SLMVs beneath the plasma membrane of PC12 cells
PC12 cells contain synaptic-like microvesicles (SLMVs) as a counterpart of synaptic vesicles and presumably contain a transmembrane form of CPE since chromaffin granules do. Analysis of PC12 cells by subcellular fractionation showed that CPE co-fractionated not only with LDCVs (fractions 14-17, Fig. 4a), but also in some of the synaptophysin-positive SLMV fractions (Fractions 4-8; Fig. 4a). We then examined whether the CPE-containing SLMVs have a CPE cytoplasmic tail by the same method used to immunoprecipitate hypothalamic synaptic vesicles. The anti-CPEC10 antibody coupled Dynabeads pulled down intact SLMVs, as indicated by the presence of CPE and synaptophysin in the eluted fraction (Fig. 4b). The control Dynabeads with linker IgG (IgG) did not pull down SLMVs and CPE tail peptide (C-pep) pre-absorbed C-CPE-Dynabeads significantly reduced binding (Fig. 4b).
Fig. 4.
Analysis of CPE in synaptic-like microvesicles (SLMVs) in PC12 cells. (a) Western blot analysis of PC12 cell cytosol fractions after sucrose gradient centrifugation. CPE was present in SLMV fractions (4-8) marked by synaptophysin (Syn), as well as in the fractions (14-17) containing peptidergic vesicles, marked by CgA (a LDCV marker). (b) Western blot after pulldown experiment for SLMVs from PC12 cell cytosol using Dynabeads coupled C-CPE antibody, control IgG or CPEC10 peptide preabsorbed C-CPE (C-pep). SLMVs were pulled down by the CPEC10 antibody while the CPEC10 peptide absorption control reduced this binding. (c) Analysis of the intensity of Syn-mRFP within 200 nm (TIRF zone) in PC12 cells (Left panels: green epi-fluorescence and right panel: red TIRF). High intensity of Syn-mRFP signal within the TIRF zone showed in cells co-transfected with GFP-vector (upper panel) but not in the GFP-CPEC15-transfected cells (lower panel). Scale bar = 16.6 mm. (d) Bar graph showing the normalized average intensity (arbitrary units ± SEM) of Syn-mRFP in the TIRF zone of the cells expressing either GFP (N=20) or GFP-CPEC15 (N=20) p<0.005.
TIRF microscopy was used for the acquisition of fluorescence signal accumulation within 200 nm underneath the plasma membrane; the focal plane for the TIRF signal or TIRF zone (Steyer et al. 1997). If the presence of excessive CPE tail peptides interferes with the localization of the SLMVs containing Syn-mRFP in the active zone in a dominant negative manner, the intensity of Syn-mRFP within the TIRF zone is expected to be reduced. Using co-transfection and TIRF microscopy, we observed that PC12 cells expressing GFP-CPEC15 showed a significant decrease (by 1.96 fold, n=20 p < 0.005) in the average intensity of the Syn-mRFP signal within the TIRF zone compared to that in the cells expressing the GFP tag alone (Fig. 4c & d). The decrease was not due to an overall reduction in the expression of Syn-mRFP since the intensity of Syn-mRFP observed under epi-fluorescence microscopy was not different between GFP- and GFP-CPEC15-expressing cells (data not shown). This reduced amount of signal within the TIRF zone implies that the excessive CPE tail peptides decreased the accumulation of Syn-mRFP-containing SLMVs within 200 nm underneath the plasma membrane.
CPE cytoplasmic tail interacts with γ-adducin
Based on the findings that the cytoplasmic tail of CPE on peptidergic vesicles in hippocampal neurons and pituitary cells interacts with cytoplasmic machinery for vesicle transport (Park et al. 2008a, b), we hypothesized that the CPE tail might bind to molecule(s) involved in recruiting the vesicle into the pre-active zone at the presynaptic membrane.
To identify a CPE tail-interacting molecule that may be involved in SV localization to the pre-active zone, we conducted a yeast two-hybrid screen of a mouse brain cDNA library using the C-terminal 25 amino acids of CPE (CPEC25) as bait. We found that CPEC25 interacted with γ-adducin (Katagiri et al. 1996), an isoform of the adducins, F actin-interacting proteins present close to the plasma membrane (Kuhlman et al. 1996) (data not shown). Our pulldown experiment using GST-tagged CPEC10 (GST-CPEC10) confirmed the interaction between CPEC10 and γ-adducin in the cytosol prepared from mouse brain or PC12 cells (Fig. 5a). In addition to γ-adducin, actin was also detected in the GST-CPEC10 pulldown fractions from mouse brain (Fig. 5a, left panel), reflecting their interaction with γ-adducin as a complex. In addition, CPE cytoplasmic tail interacted with Rab27A and Rim1, proteins involved in vesicle tethering to the plasma membrane (Wang et al. 1997; Tsuboi and Fukuda 2006) while not with the Rab27A interactor, Munc18-1, and the Rim1 interactor, Rab3A (Fig. 5b). The interaction of CPE tail with Rim1 was also seen in our yeast two-hybrid screening of a rat brain cDNA library, in which the N-terminus of Rim1 (28-226) which contains the Zn finger and its highly charged region, but not the Rab3A-binding region, interacted with the CPE C-terminal domain, starting from amino acids 164-209 of CPE and extending to its C-terminus (12 out of 116 positive colonies).
Fig. 5.
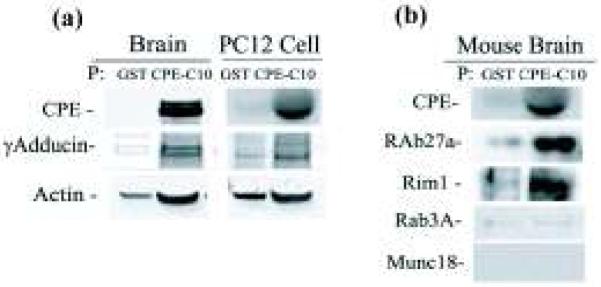
Detection of levels of proteins associated with synaptic vesicles by Western blot. (a) The interactive proteins pulled down by GST-tagged CPE tail peptide (GST-CPEC10) or GST controls in wild type mouse brain or PC12 cell cytosol. In GST-CPEC10 pulled down samples, reactivity were shown with γ-adducin and actin antibodies in mouse brain cytosol (left panel) and in PC12 cell cytosol (right panel). (b) Western blot showing Rim1 and Rab27A, but not Rab3A or Munc18, were pulled down by GST-CPEC10 in mouse brain. All experiments were repeated twice with similar results.
Discussion
The existence of synaptic vesicle (SV) populations in the presynaptic terminal is critical for regulated release of neurotransmitters. In this study the role of CPE in glutamate release and the localization of SVs in the synaptic pre-active zone were examined in mouse hypothalamic neurons. We focused on vesicles in the hypothalamus, an area of the brain highly enriched in glutamate vesicles as well as peptidergic vesicles.
Our observation of defective glutamate secretion in CPE-KO mouse hypothalamic neurons and adult synaptosomes (Fig. 1) and previous findings of glutamate dependent defects in CPE-KO electroretinograms of the photoreceptors (Zhu et al. 2005) indicate a functional role of CPE in neurotransmitter secretion. In addition, deletion of Egl-21, a CPE orthologue in C. elegans, resulted in defective acetylcholine secretion from the neuromuscular junction (Jacob and Kaplan 2003) suggesting that the role of CPE in neurotransmitter secretion is functionally conserved across evolution. However, previous proteomic analyses of SVs isolated from whole brain have not reported the presence of CPE in these organelles (Takamori et al. 2006). Here we demonstrated that SVs from hypothalamic neurons and also SLMVs in PC12 cells contain CPE and that some of these molecules are oriented in a transmembrane manner. When we analyzed SVs from the rest of the brain without the hypothalamus, we did not observe a significant amount of CPE in the SV fractions (data not shown) suggesting that the SVs containing CPE are present primarily in hypothalamic neurons compared to other parts of the brain.
Classical neurotransmitter vesicles coexist with large dense core peptidergic vesicles in neuroendocrine neurons (Hrabovszky and Liposits 2008). The presence of CPE in these classical neurotransmitter vesicles, in CPE-rich peptidergic neurons, is perhaps not so surprising since synaptic vesicles are generated through recycling by endocytosis. This would allow recruitment of CPE from the presynaptic membrane deposited there by exocytosis of peptidergic vesicles. Indeed it has been shown that residual peptidergic cargo from endocrine cells, such as CPE, that remains on the outside of the plasma membrane after exocytosis can be internalized and recycled back to the TGN for possible re-use in another round of granule biogenesis (Arnaoutova et al. 2003; Ferraro et al. 2005). We predict that general classical neurotransmitter release would not be expected to be severely impaired in the CPE-KO mice except in a subpopulation of hypothalamic neurons; although CPE in SVs in minor populations of peptidergic neurons distributed in other parts of the brain may also exist.
As a means to understand the lack of activity-dependent glutamate secretion in the CPE-KO hypothalamic neurons and adult synaptosomes, we analyzed the hypothalamus at the ultrastructural level by electron microscopy (EM). Our analysis showed that there was a significant decrease in the number of synaptic vesicles in the pre-active zone within 200 nm from the presynaptic membrane (Fig. 2). Given the observations that there was 1) no decrease of overall vesicle numbers in the presynaptic boutons, 2) no reduction in total glutamate levels (Fig. 1b & d), and 3) no changes in the protein levels of VGLUT 1 and VGLUT 2, synaptophysin, VAMP 2 and other SV associated molecules in the CPE-KO mouse hypothalamus when compared to their WT littermates (Fig. 2h & i); it is unlikely that the diminished glutamate release is due to a structural defect in these SVs or a failure in the uptake or storage of glutamate. We speculate that the absence of CPE does not affect SV biogenesis and/or degradation, but causes a preferential loss of synaptic vesicles accumulated within the pre-active zone.
If CPE is directly involved in the localization of synaptic vesicles within the pre-active zone, as the static steady state EM micrographs would indicate, it would have to interact with other proteins. Our yeast two-hybrid study demonstrated that the C-terminal 25 amino acids of CPE (CPEC25) interacted with γ-adducin, an interaction that was recapitulated in GST-CPEC10 pulldown experiments using mouse brain and PC12 cell cytosol (Fig. 5a). These results suggest that the CPE cytoplasmic tail on these vesicles can act as a linker to γ-adducin. γ-Adducin is a member of the adducin family (Kuhlman et al. 1996), which has traditionally been regarded as a critical component of the cell cytoskeleton since it binds and stabilizes the barbed end of F actin as well as spectrin, are rich in the presynaptic terminals and structurally stabilizes the terminals (Pielage et al. 2005). Our pulldown experiment also detected actin in the precipitated fraction (Fig. 5a). This was expected since F actins are likely brought down by the GST-CPEC10 through interaction with γ-adducin. Hence we propose that CPE cytoplasmic tail interacts with γ-adducin and actin in the presynaptic terminal to facilitate the localization of SVs to the pre-active zone, although the exact mechanism is unclear. Interestingly, in synapsin I knockout mice, SVs were also dramatically reduced in the region >150 nm from the active zone (Li et al. 1995; Takei et al. 1995) and synapsin IIa was shown to be the primary molecule regulating the reserve pool of glutamatergic synaptic vesicles in hippocampal neurons (Gitler et al. 2008). Mechanistically, synapsin functions in the regulation of SV availability for release by interweaving synaptic vesicles within the F actin network at the presynaptic terminal (Bloom et al. 2003; Siksou et al. 2007). Whether the CPE cytoplasmic tail’s direct interaction with γ-adducin which then interacts with F-actin works in conjunction with synapsin, or independently to localize SVs to the pre-active zone remains to be determined. We also observed the interaction of GST-CPEC10 with Rab27A and Rim1 (Fig. 5b) but not with their binding partners, Munc18-1 and Rab3A respectively, suggesting that the CPE tail also interacts with vesicle tethering machinery.
Our yeast two-hybrid, immunoprecipitation, and GST pull down results all indicate that some CPE in these vesicles have a transmembrane orientation, thus exposing its C-terminal tail in the cytoplasm where it can interact with proteins such as γ-adducin to facilitate vesicle localization within the F-actin rich nerve terminal. To explore this further, we co-expressed the CPEC15 tail in PC12 cells with synaptophysin-mRFP (Syn-mRFP) and used TIRF microscopy to quantify the signal representing SLMVs within 200 nm from the plasma membrane. Our TIRF experiments showed that at steady state, the signal of Syn-mRFP in the CPEC15 expressing cells was significantly reduced within the TIRF zone compared to the control cells, although overall cellular staining was similar. This provides strong physiological evidence that the CPE-tail is involved in the steady-state localization of SLMVs, a PC12 cell counterpart of synaptic vesicles, to the F-actin-rich cortex under the plasma membrane to facilitate neurotransmitter release. Based on PC12 cells as a model system, we can interpret that in the absence of CPE, the loss of the interaction of synaptic vesicles with the F-actin-interacting protein γ-adducin could account for the lack of SVs in the pre-active zone area in the CPE-KO hypothalamic neurons. Such a CPE-tail-based mechanism may be limited to neurons expressing abundant amounts of neuropeptides and CPE, and have peptidergic and classical neurotransmitter CPE-containing vesicles, as in the hypothalamus. While our data favor a physical interaction of the CPE cytoplasmic tail with components of the cytoskeleton at the nerve terminals as a mechanism for lack of localization of SVs in the preactive zone, leading to impaired glutamate secretion, the possibility that the lack of a neuropeptide, as a result of impaired release of peptidergic vesicles, could cause the observed phenotype and cannot be completely ruled out.
In summary, our current findings indicate that glutamatergic and acetylcholine synaptic vesicles in the hypothalamus and chromaffin cell-derived PC12 cells, respectively, employ the transmembrane CPE cytoplasmic tail to interact with γ-adducin for recruiting SVs to the active and/or pre-active zone to facilitate neurotransmitter release. This study provides a new insight into the machinery that mediates localization of synaptic vesicles to the pre-active zone and identifies CPE as a new mediator in this process, hence establishing yet another novel non-enzymatic role of this molecule in the control of classical neurotransmitter release in specific neurons.
Supplementary Material
Acknowledgement
We thank to lab members in SCN, NICHD for technical assistance and helpful discussions. We thank Dr. Ronald W. Holz (U. Michgan) for his scientific advice about TIRF microscopy, Dr. Leon Lagnado (Cambridge, UK) for synaptophysin-mRFP. We thank Dr. Vincent Schram in the NICHD Microscopy Imaging Core for their technical support. Lei Sun was supported by NIH grant RO1 DK050127 to Ronald W. Holz. This research was supported by the Intramural Research Program of the NICHD, NIH. Joshua J. Park has been supported by NICHD K22 and ARRA grants.
Abbreviations used
- CPE
carboxypeptidase E
- LDCV
large dense core vesicle
- SLMV
synaptic-like microvesicles
- TIRF
total internal reflective fluorescence
- VGLUT
vesicular glutamate transporter
- PSD
post synaptic density
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Appendix S1
Experimental details about antibodies for Western blot and signal-to-noise for TIRF
References
- Arnaoutova I, Jackson CL, Al-Awar OS, Donaldson JG, Loh YP. Recycling of Raft-associated prohormone sorting receptor carboxypeptidase E requires interaction with ARF6. Mol Biol Cell. 2003;14:4448–4457. doi: 10.1091/mbc.E02-11-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia A, Bertoluzza A, Calbucci F, Eusebi V, Giorgianni P, Ricci R, Tosi R, Tugnoli V. High-performance liquid chromatographic analysis of physiological amino acids in human brain tumors by pre-column derivatization with phenylisothiocyanate. J Chromatogr B Biomed Sci Appl. 1999;730:81–93. doi: 10.1016/s0378-4347(99)00188-7. [DOI] [PubMed] [Google Scholar]
- Bloom O, Evergren E, Tomilin N, Kjaerulff O, Low P, Brodin L, Pieribone VA, Greengard P, Shupliakov O. Colocalization of synapsin and actin during synaptic vesicle recycling. J Cell Biol. 2003;161:737–747. doi: 10.1083/jcb.200212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Zhou J, Hill JM, Abebe D, Romboz S, Yanik T, Rodriguiz RM, Wetsel WC, Loh YP. The carboxypeptidase E knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology. 2004;145:5807–5819. doi: 10.1210/en.2004-0847. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Jahn R. Pathways to regulated exocytosis in neurons. Annu Rev Physiol. 1990;52:625–645. doi: 10.1146/annurev.ph.52.030190.003205. [DOI] [PubMed] [Google Scholar]
- Dieck S, Sanmarti-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kampf U, Franzer JT, Stumm M, Garner CC, Gundelfinger ED. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanvantari S, Arnaoutova I, Snell CR, Steinbach PJ, Hammond K, Caputo GA, London E, Loh YP. Carboxypeptidase E, a prohormone sorting receptor, is anchored to secretory granules via a C-terminal transmembrane insertion. Biochemistry. 2002;41:52–60. doi: 10.1021/bi015698n. [DOI] [PubMed] [Google Scholar]
- Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron. 2000;25:203–214. doi: 10.1016/s0896-6273(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Ferraro F, Eipper BA, Mains RE. Retrieval and reuse of pituitary secretory granule proteins. J Biol Chem. 2005;280:25424–25435. doi: 10.1074/jbc.M414156200. [DOI] [PubMed] [Google Scholar]
- Fricker LD, Snyder SH. Enkephalin convertase: purification and characterization of a specific enkephalin-synthesizing carboxypeptidase localized to adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1982;79:3886–3890. doi: 10.1073/pnas.79.12.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker LD, Das B, Angeletti RH. Identification of the pH-dependent membrane anchor of carboxypeptidase E (EC 3.4.17.10) J Biol Chem. 1990;265:2476–2482. [PubMed] [Google Scholar]
- Gitler D, Cheng Q, Greengard P, Augustine GJ. Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci. 2008;28:10835–10843. doi: 10.1523/JNEUROSCI.0924-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Liposits Z. Novel aspects of glutamatergic signalling in the neuroendocrine system. J Neuroendocrinol. 2008;20:743–751. doi: 10.1111/j.1365-2826.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Kaplan JM. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J Neurosci. 2003;23:2122–2130. doi: 10.1523/JNEUROSCI.23-06-02122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Ozaki K, Fujiwara T, Shimizu F, Kawai A, Okuno S, Suzuki M, Nakamura Y, Takahashi E, Hirai Y. Cloning, expression and chromosome mapping of adducin-like 70 (ADDL), a human cDNA highly homologous to human erythrocyte adducin. Cytogenet Cell Genet. 1996;74:90–95. doi: 10.1159/000134389. [DOI] [PubMed] [Google Scholar]
- Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271:7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, Zheng D, McNamara JO, Greengard P, et al. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9235–9239. doi: 10.1073/pnas.92.20.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase E. Neuron. 2005;45:245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lou H, Smith AM, Coates LC, Cawley NX, Loh YP, Birch NP. The transmembrane domain of the prohormone convertase PC3: a key motif for targeting to the regulated secretory pathway. Mol Cell Endocrinol. 2007;267:17–25. doi: 10.1016/j.mce.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael DJ, Xiong W, Geng X, Drain P, Chow RH. Human insulin vesicle dynamics during pulsatile secretion. Diabetes. 2007;56:1277–1288. doi: 10.2337/db06-0367. [DOI] [PubMed] [Google Scholar]
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- Normant E, Loh YP. Depletion of carboxypeptidase E, a regulated secretory pathway sorting receptor, causes misrouting and constitutive secretion of proinsulin and proenkephalin, but not chromogranin A. Endocrinology. 1998;139:2137–2145. doi: 10.1210/endo.139.4.5951. [DOI] [PubMed] [Google Scholar]
- Park JJ, Cawley NX, Loh YP. Carboxypeptidase E Cytoplasmic Tail-Driven Vesicle Transport Is Key for Activity-Dependent Secretion of Peptide Hormones. Mol Endocrinol. 2008a;22:989–1004. doi: 10.1210/me.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Cawley NX, Loh YP. A bi-directional carboxypeptidase E-driven transport mechanism controls BDNF vesicle homeostasis in hippocampal neurons. Mol Cell Neurosci. 2008b;39:63–73. doi: 10.1016/j.mcn.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 2005;15:918–928. doi: 10.1016/j.cub.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtova A, Kao HT, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer JA, Horstmann H, Almers W. Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature. 1997;388:474–478. doi: 10.1038/41329. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Takei Y, Harada A, Takeda S, Kobayashi K, Terada S, Noda T, Takahashi T, Hirokawa N. Synapsin I deficiency results in the structural change in the presynaptic terminals in the murine nervous system. J Cell Biol. 1995;131:1789–1800. doi: 10.1083/jcb.131.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wu K, Rife L, Cawley NX, Brown B, Adams T, Teofilo K, Lillo C, Williams DS, Loh YP, Craft CM. Carboxypeptidase E is required for normal synaptic transmission from photoreceptors to the inner retina. J Neurochem. 2005;95:1351–1362. doi: 10.1111/j.1471-4159.2005.03460.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.