To the editor:
Tissue factor (TF) is the cellular trigger of extrinsic coagulation and a signaling coreceptor for protease-activated receptor 2 (PAR2). Procoagulant activity of TF-factor (F) VIIa saturates at subnanomolar concentrations of FVIIa,1 whereas the TF-FVIIa binary complex signals efficiently at approximately 10nM FVIIa, the plasma concentration of zymogen FVII.2,3 Mutation of the TF allosteric Cys186-Cys209 disulfide bond abolishes signaling of the procoagulant TF-FVIIa-FXa coagulation initiation complex, while preserving signaling activity as a binary TF-FVIIa complex.2 Although disulfide-mutated TF is poorly expressed on the cell surface,2 characterization4 of TFC186S/C209S showed (1) severely impaired clotting activity when normalized for TF cell-surface expression, implying a critical role of the disulfide for TF's procoagulant conformation, (2) reduced affinity for FVII, and (3) a complete rescue of impaired substrate X cleavage by supraphysiologic concentrations of FVIIa (≥ 100nM) and FX (≥ 1μM), possibly indicating that disulfide mutated TF on cell surfaces is not misfolded, but rather can adopt a functional conformation by ligand-induced fit.
A recent paper in Blood5 reproduced these key findings but concluded that the allosteric disulfide is not essential for TF's procoagulant activity, taking into consideration only data at supraphysiologic concentrations of TF ligands while ignoring the mutant's severe defect in clotting assays, the “gold standard” for TF procoagulant activity. Experiments performed solely at 1000-fold the plasma concentration (100pM) of FVIIa indicated that disulfide mutated TF undergoes normal conversion from inactive to active TF, termed decryption, induced by cell lysis or other stimuli. The following experiments at physiologically relevant concentrations of FVII/VIIa raise concerns about the general validity of this conclusion.
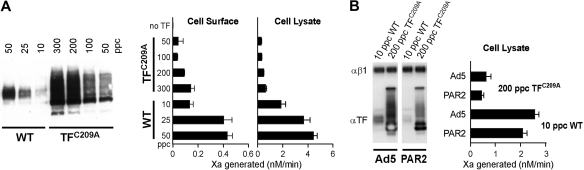
Wild-type TF or TFC209A were expressed to increasing levels by adenoviral cotransduction with PAR2.2 TF procoagulant activity was measured with 10 to 20nM FVIIa, concentrations sufficient for equivalent TF-FVIIa binary complex signaling activity of wild-type or TFC209A.2 Extreme overexpression of TFC209A (300 ppc) yielded cell-surface TF procoagulant activity that was similar to cells transduced with 10 ppc of wild-type TF adenovirus (Figure 1A). Kothari's study5 predicted a comparable increase in activity of wild-type TF and TFC209A, but decryption induced by cell lysis was much more efficient in cells expressing very low levels of wild-type TF. Kothari5 did not observe comparable overexpression of TFC209A, which may be explained by our cotransduction with PAR2. Therefore, cells were transduced with high-dose TFC209A or low-dose wild-type TF virus in the presence of PAR2 or control Ad5 virus (Figure 1B). With both protocols, expression of the mutant was high and the decrypted activity of wild-type TF far exceeded that of TFC209A. Thus, even with extreme overexpression, TFC209A did not achieve decrypted activity comparable with wild-type TF.
Figure 1.
Inefficient decryption of TFC209A procoagulant activity. (A) Human umbilical vein endothelial cells were cotransduced with PAR2 adenovirus and the indicated TF virus dose (calculated as particles per cell [ppc] based on protein concentration of the virus preparation). TF cell-surface proteolytic activity was quantified by measuring the rate of FXa generation after addition of 10nM FVIIa and 100nM FX. Subsequently, cells were lysed in 15mM octylglucoside and, after dilution to 5mM octylglucosides, the rate of FXa generation was measured upon addition of 20nM FVIIa and 100nM FX. For Western blotting, cells were directly lysed in nonreducing sample buffer and transfers were probed with polyclonal antibody to human TF. (B) Cells were transduced with the indicated TF virus doses in the presence of 200 ppc empty Ad5 control or PAR2 virus, followed by FXa generation assay with 10nM FVIIa and 100nM FX in octylglucoside lysates, as described above. Transfers were probed with polyclonal antibody to human TF or, as a loading control, to integrin β1.
These experiments are inconsistent with the broad conclusion that disulfide mutated TF has the same specific procoagulant activity and is decrypted in the same manner as wild-type TF. The functional impairment of disulfide mutated TF and the demonstrated role of protein disulfide isomerase in thrombus formation in vivo6,7 warrant further investigations into thiol/disulfide exchange and S-nitrosylation pathways2,8–10 as regulators of TF prothrombotic activity.
Authorship
Acknowledgments: We thank Jennifer Royce for technical assistance and Cheryl Johnson for manuscript preparation.
This work was supported by National Institutes of Health grant HL-31950.
Contribution: W.R. and H.H.V. interpreted data and wrote the letter.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfram Ruf, MD, Department of Immunology and Microbial Science, SP258, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail: ruf@scripps.edu.
References
- 1.Le DT, Rapaport SI, Rao LVM. Relations between factor VIIa binding and expression of factor VIIa/tissue factor catalytic activity on cell surfaces. J Biol Chem. 1992;267:15447–15454. [PubMed] [Google Scholar]
- 2.Ahamed J, Versteeg HH, Kerver M, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen LC, Albrektsen T, Hjorto GM, et al. Factor VIIa/tissue factor-dependent gene regulation and pro-coagulant activity: effect of factor VIIa concentration. Thromb Haemost. 2007;98:909–911. [PubMed] [Google Scholar]
- 4.Rehemtulla A, Ruf W, Edgington TS. The integrity of the Cys186-Cys209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–10299. [PubMed] [Google Scholar]
- 5.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115(21):4273–4283. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhardt C, von Bruhl ML, Manukyan D, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 9.Liang HP, Hogg PJ. Critical importance of the cell system when studying tissue factor de-encryption. Blood. 2008;112:912–913. doi: 10.1182/blood-2008-05-158766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen VM, Ahamed J, Versteeg HH, et al. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]