Abstract
Allergic asthma is dependent on chemokine mediated Th2 cell migration and Th2 cytokine secretion into the lungs. The tyrosine kinase Itk regulates the production of Th2 cytokines as well as migration in response to chemokine gradients. Mice lacking Itk are resistant to developing allergic asthma, however, the role of kinase activity of Itk in the development of this disease is unclear. In addition, whether distinct Itk derived signals lead to T cell migration and secretion of Th2 cytokines is also unknown. Using transgenic mice specifically lacking Itk kinase activity, we show that active kinase signaling is required for control of Th2 responses and development of allergic asthma. Moreover, dominant suppression of Itk kinase activity led to normal Th2 responses, but significantly reduced chemokine mediated migration, resulting in prevention of allergic asthma. These observations indicate that signals required for Th2 responses and migration are differentially sensitive to Itk kinase activity. Manipulation of Itk’s kinase activity can thus provide a new strategy to treat allergic asthma by differentially affecting migration of T cells into the lungs, leaving Th2 responses intact.
Introduction
Allergic asthma develops due to a complex interplay between different types of immune cells and airway resident cells. Upon exposure to allergen, airway epithelial cells and macrophages secrete chemokines, while Th2 cells secrete cytokines such as IL-4, IL-5 and IL-13 (1, 2). These cytokines, along with chemokine gradients induce the infiltration of inflammatory cells into the lungs (2). These events can lead to pathophysiological dysfunctions including airway hyperresponsiveness, lung inflammation and mucous production (2).
Tec family tyrosine kinases, including Itk which is expressed in T cells, have been shown to be important for proper immune response against a number of microbial insults, including infections caused by T. gondii, L. major, N. brasiliensis and S. mansoni (3–5). We have also shown that Itk is important for the development of allergic asthma (6, 7). This importance stems from the ability of Itk to modulate the development of normal Th2 responses in vivo (4, 5, 8). Indeed, during the induction of allergic asthma, Itk null mice exhibit reduced symptoms including decreased airway hyperresponsiveness, tracheal responses, lung inflammation, eosinophil infiltration, mucous production and Th2 cytokine production (6, 7). In addition, Itk has also been shown to control the ability of T cells to migrate to a chemokine gradient (9, 10).
The kinase domain of tyrosine kinases is responsible for their catalytic activity. The importance of the kinase domain in the function of Tec kinases is illustrated in the recognition that 50% of the mutations that are responsible for X-linked agammaglobulinemia (XLA) are found in the kinase domain of Btk, a Tec kinase predominantly expressed in B cells (11). More directly, we and others have identified signaling pathways that are modulated by Btk or Itk that are kinase independent. These pathways include the rescue of B cell development by a kinase inactive Btk, which may be due to its ability to partially activate the NFκB pathway (12). Btk also has tumor suppressive activity that is independent of its kinase domain (13). Antigen receptor induced actin cytoskeletal rearrangements and activation of the transcription factor SRF have also been reported to be Itk kinase independent (14–16), raising the possibility that other functions may be kinase independent.
Since mice lacking Itk are resistant to developing allergic asthma (6, 7), a number of inhibitors have recently been developed that target the kinase activity of Itk (17–21). However, the role of kinase activity of Itk in the induction of allergic asthma is still unknown. In addition, it is unclear if the function of Itk in controlling Th2 cytokine secretion and chemokine migration is separable during the development of allergic asthma. Using novel transgenic mice specifically carrying a mutant Itk without any kinase activity, we show here that active kinase signaling is required for the control of Th2 responses and the development of allergic asthma. However, reduction of Itk signals allowed normal Th2 responses, while significantly affecting chemokine mediated migration. Our findings thus suggest that signals required for Th2 responses and migration are differentially sensitive to Itk kinase activity.
Materials and Methods
Mice
C57Bl/6 (WT) and Itk null mice (22) were used for these studies. We also generated mice carrying a kinase deleted mutant of Itk either on a WT background (Tg(Lckpr-ItkΔKin)WT) or on an Itk null background (Tg(Lckpr-ItkΔKin)/Itk−/−) which is expressed at about 25–30% of endogenous Itk. The Tg(Lckpr-ItkΔKin) was generated by cloning a mutant Itk with the kinase domain replaced with EGFP (14), into a transgenic expression cassette driven by the Lck proximal promoter and CD2 enhancer (kind gift of Dr. Anuradha Ray, University of Pittsburgh) (23). All mice were backcrossed to C57Bl/6 background at least 10 generations. Experiments used mice between 6–12 weeks of age, and were approved by the Office of Research Protection’s Institutional Animal Care and Use Committee at Pennsylvania State University.
Induction of Allergic Asthma and measurement of AHR
We induced allergic airway disease as previously described using Ovalbumin as a model antigen (6, 7). Twenty four hours after the final OVA exposure, mice were analyzed for AHR using a mechanical ventilator in response to methacholine as described (24). BALF and RNA was also obtained from the lungs as detailed (7).
Real-time RT-PCR analysis
RNA was extracted from the lungs of mice using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA). cDNA was generated with a kit from Amersham Biosciences (Piscataway, NJ) and quantitative RT-PCR performed using Assays on Demand Taqman primer/probe sets for IL-4, IL-13, IFN-γ and GAPDH as a housekeeping gene with an ABI 7300 Sequence Detection System (Applied Biosystems, Foster City, CA). Values are expressed as 2−ΔΔCT as previously described (7).
Histology
After AHR analysis, lungs were fixed with 4% formaldehyde for sectioning with H&E to examine infiltrating cells and periodic acid-Schiff (PAS) for analysis of mucous production.
Analysis of lymphocyte proliferation and cytokine secretion
Purified splenocytes and lymph node cells from the indicated mice were cultured with 10 or 100 µg/ml of OVA (2 × 105 cells/well in 200 µl per well in 96-well round bottom plates). After 72 hours of culture, cells were pulsed with [3H]-thymidine for 18 hours. The cultures were then harvested and incorporated radioactivity determined by scintillation counting. Cytokine analysis was performed by stimulating these cells in a similar fashion for 96 hours, followed by harvesting of supernatants and analysis by cytokine specific ELISA for IFNγ, IL-4, IL-5 and IL-13 following manufacturer’s instruction (R&D Systems, Minneapolis, MN). BALF was concentrated 3 fold using Centricon concentrators (Millipore, Bedford, Mass), then analyzed using the Bioplex System following the manufacturer’s instructions (sensitivity, 1.5 pg/mL, BioRad, Hercules, CA).
Analysis of IgE levels
Following prime and challenge, mice were sacrificed and serum was obtained. Dilutions of sera were analyzed for OVA-specific IgE by coating OVA onto the ELISA wells (20 mg/ml) and testing dilutions of sera ELISA using anti-murine IgE (2 µg/ml, 1/250) as capture Abs and HRP-conjugated anti-murine IgE (1/250) as detection reagents (Southern Biotechnology Associates, Birmingham, AL).
CCL11 mediated actin polymerization assay
CD4+ T cells were purified from splenocytes and lymph nodes of WT and Tg(Lckpr-ItkΔKin)/WT mice using a T-cell negative selection column (Miltenyi Biotec, Auburn, CA). Purified CD4+ T cells were then differentiated to Th2 cells by stimulating with 1 µg/ml anti-CD3 and CD28 (BDBiosciences), 10 ng/ml rIL-4 (Peprotech) and 10 µg/ml anti-IFNγ (clone XMG1.2) for 7 days. Following analysis of these differentiated Th2 cells for the expression of CCR3, Th2 cells were then stimulated with 100 ng/ml CCL11 or PBS as a control for 5 minutes at 37°C, fixed with 4% paraformaldehyde, washed twice with FACS buffer, then stained with Alexa fluor-568 phalloidin (Invitrogen, Carlsbad, CA) for 30 min on ice. The cells were then washed and analyzed for F-actin content by confocal imaging. The mean fluorescence intensity of individual cells was determined using NIH Image software and the fold increase in fluorescence intensity over PBS treated cells was plotted.
CCL11 mediated migration assay
The bottom wells of a 96-well ChemoTx Chamber with 5 µM pore size was loaded with 100 ng/ml of CCL11 or PBS suspended in migration medium (RPMI 1640 medium containing 0.5% BSA and 20 mM HEPES). In vitro differentiated WT or Tg(Lckpr-ItkΔKin)/WT Th2 cells (2 × 105/well) were applied to the upper wells and incubated for 2 hrs at 37°C in 5% CO2. The migrated cells were collected from the bottom chamber after two hours and the number of cells migrated was determined and expressed as fold over those that migrated in response to carrier (PBS).
NFAT nuclear localization analysis
Purified CD4+ T cells from WT and Tg(Lckpr-ItkΔKin)/WT mice were stimulated with anti-CD3 for 45 minutes, fixed, permeabilized and stained for NFAT-c1 using a specific antibody (Santa Cruz Biotechnology). The cells were analyzed for nuclear versus cytoplasmic location using confocal microscopy. Images were analyzed using ImagePro software (Media Cybernetics, Inc, Bethesda, MD).
Data analysis
Statistical evaluation was conducted by using Student’s t test with a probability value of P ≤ 0.05 considered statistically significant.
Results
The kinase domain of Itk is required for development of AHR and airway inflammation
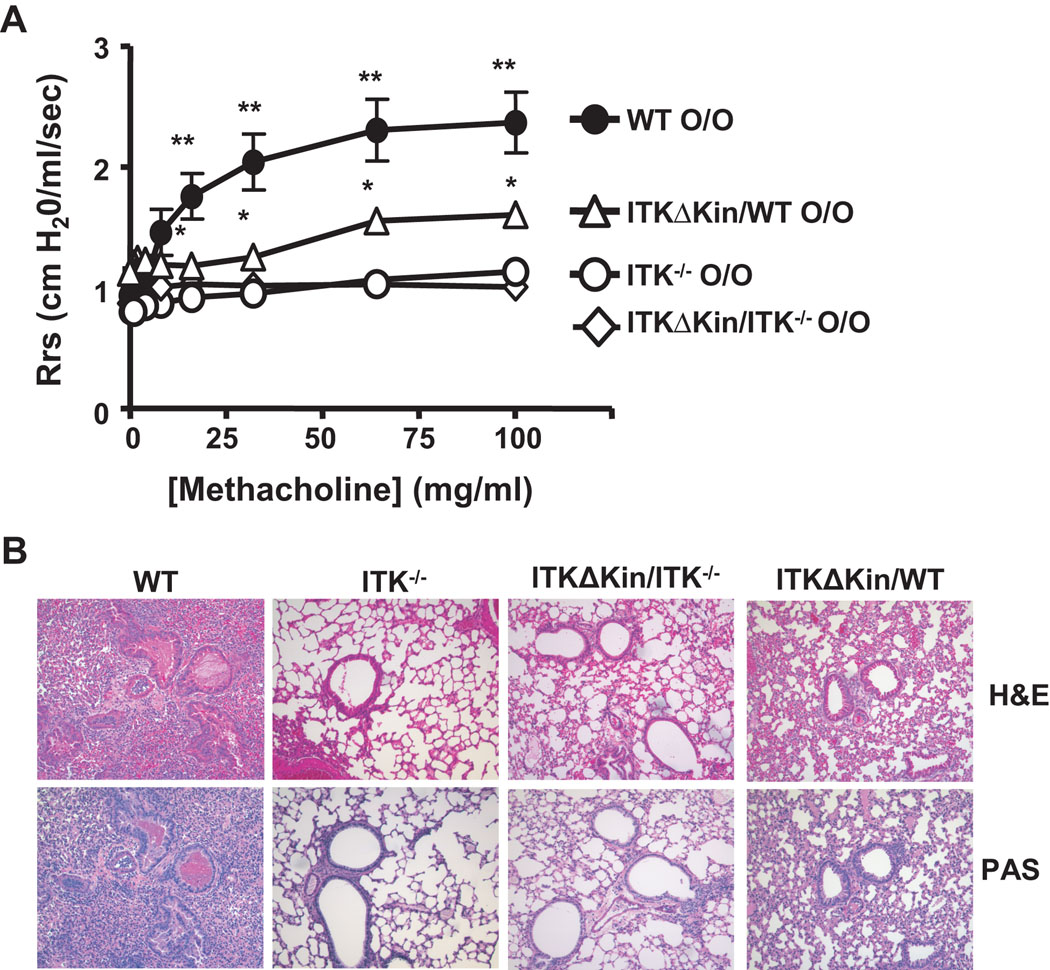
To analyze the role of kinase activity of Itk in allergic asthma, WT, Itk−/−, and transgenic mice carrying a mutant Itk lacking the kinase domain on an Itk−/− background (Tg(Lckpr-ItkΔKin)/Itk−/−) were immunized and challenged with OVA to induce allergic airway responses. Mice were then analyzed for airways resistance in response to methacholine. We found that while WT mice developed significant airways resistance in comparison to Itk−/− mice as previously reported (7), the Tg (Lckpr-ItkΔKin)/Itk−/− mice showed much reduced responses (Fig. 1A). These data indicate that the kinase activity of Itk is essential for the development of airway hyperresponsiveness. We also analyzed mice carrying the mutant Itk on a WT background (i.e. in the presence of endogenous Itk, referred to as Tg(Lckpr-ItkΔKin)/WT) and found that the latter mice also showed significantly reduced airways resistance compared to WT mice, although their responses were higher than Itk−/− mice (Fig. 1A). This decreased airway resistance in Tg(Lckpr-ItkΔKin)/WT mice was an unexpected finding because these mice express endogenous Itk in the periphery at a level approximately 3 fold higher than the transgenic Itk (data not shown). Histological analysis of airway inflammation and mucous production in the lungs of these mice also showed the same pattern, with less inflammation observed in both Tg(Lckpr-ItkΔKin)/Itk−/− and Tg(Lckpr-ItkΔKin)/WT mice compared to the WT mice (Fig. 1B). These data suggest that the kinase domain and thus activity of Itk is required for the induction of airway inflammation, airway constriction and mucous production. More importantly, these data suggest that the kinase domain deleted Itk has the capacity to dominantly suppress the normal function of endogenous Itk in vivo.
Figure 1. The kinase domain of Itk is required for the induction of AHR and allergic inflammation.
(a) WT, Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT mice were immunized and challenged intranasally with OVA, followed by analysis of AHR. ●-●, WT; ◯-◯, Itk−/−; △-△, Tg(Lckpr-ItkΔKin)/WT, ◊-◊, Tg(Lckpr-ItkΔKin)/Itk−/−. **Differences were statistically significant between WT and Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT; *Differences were statistically significant between Tg(Lckpr-ItkΔKin)/WT mice and Itk−/− and Tg(Lckpr-ItkΔKin)/Itk−/−, as well as between WT and Tg(Lckpr-ItkΔKin)/WT mice (p<0.05 in both cases, n=12). (b) WT, Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT mice were immunized and challenged intranasally with OVA, followed by analysis of lung sections by H&E (top panels) or PAS staining (bottom panels).
The kinase domain of Itk is required for the expression of Th2 cytokines in the lung during allergic airway inflammation
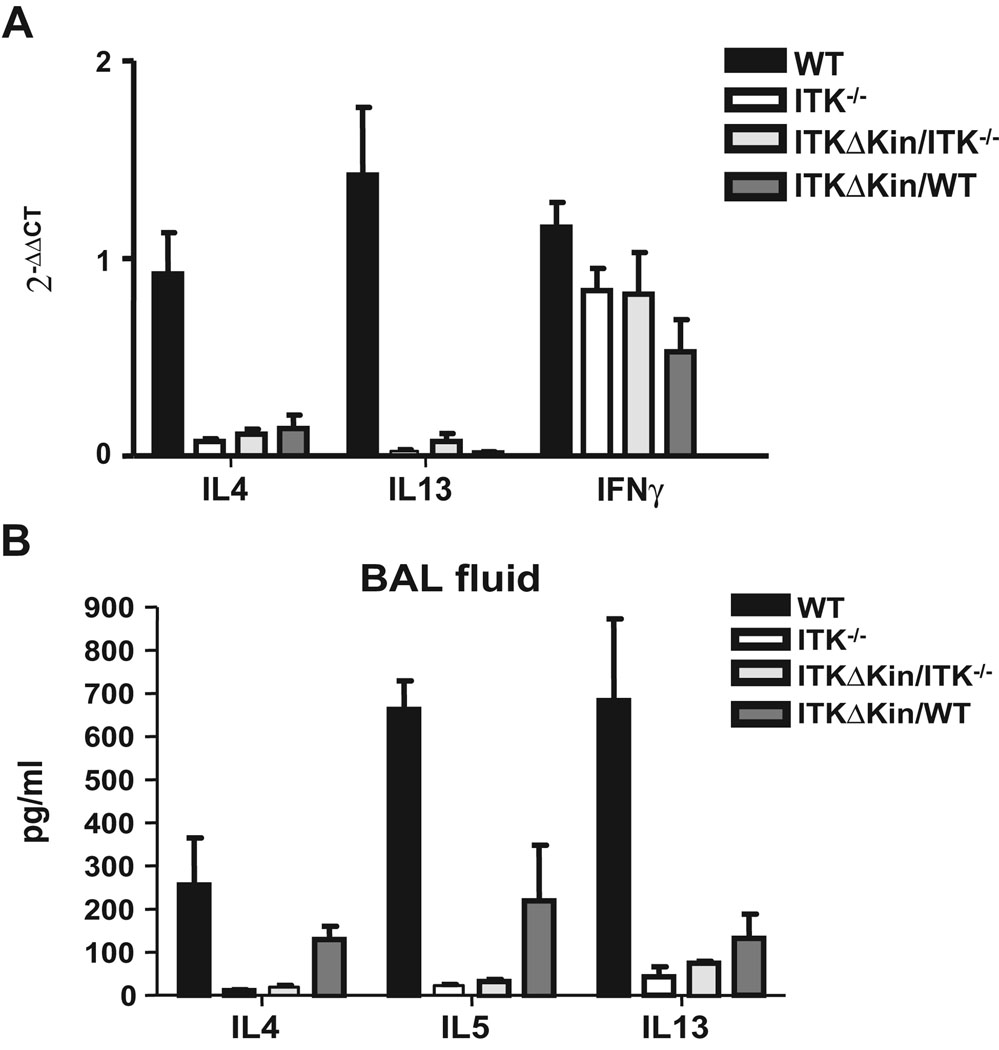
We also analyzed the expression level of Th2 cytokines IL-4 and IL-13 in the lungs of these animals and found that the Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and surprisingly Tg(Lckpr-ItkΔKin)/WT mice, all had reduced levels of message for these cytokines (Fig. 2A). Analysis of protein levels confirmed these results, although the Tg(Lckpr-ItkΔKin)/WT had higher levels of IL-4 and IL-5 than Itk−/− and Tg(Lckpr-ItkΔKin)/Itk−/− mice (Fig. 2B). These results indicate that the kinase domain of Itk is required for the induction of Th2 cytokines in the lungs of mice during the development of allergic asthma. In addition, the kinase domain deleted mutant dominantly affects the production of these cytokines in the lungs of Tg(Lckpr-ItkΔKin)/WT.
Figure 2. The kinase domain of Itk is required for the production of Th2 cytokines in the lung during the development of allergic asthma.
(a) WT, Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT mice were treated as in figure 1, lungs isolated and mRNA for the IL-4, -13 and IFNγ quantified by Q-RT-PCR. (b) BAL fluid from WT, Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT mice treated as in figure 1 were isolated and the amounts of IL-4, -5 and -13 quantified. *p<0.05 vs. WT mice, ND, none detected.
Role of the kinase domain of Itk in modulating the ability of T cells to proliferate and induce Th2 responses to OVA
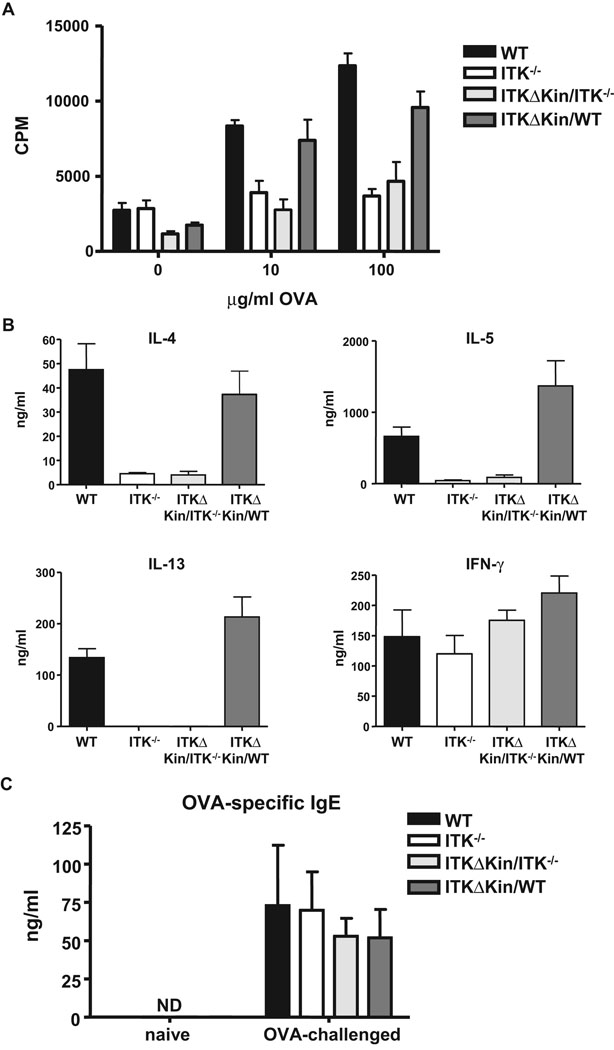
We further analyzed the recall response of splenocytes from OVA exposed mice to subsequent OVA challenge in vitro. T cells from Tg(Lckpr-ItkΔKin)/Itk−/− mice had low proliferative response to OVA similar to those lacking Itk, while T cells from Tg(Lckpr-ItkΔKin)/WT mice behaved similar to WT mice and proliferated normally (Fig. 3A). T cells from Itk−/− and Tg(Lckpr-ItkΔKin)/Itk−/− mice had low levels of Th2 cytokines, IL-4, -5 and IL-13, however those from WT and surprisingly the Tg(Lckpr-ItkΔKin)/WT mice had similar high levels of Th2 cytokines (Fig. 3B). By contrast, cells from all mice secreted similar levels of the Th1 cytokine IFNγ, suggesting that the Itk−/− and Tg(Lckpr-ItkΔKin)/Itk−/− were able to generate a normal Th1 response. In agreement with these findings, we also found that Tg(Lckpr-ItkΔKin)/WT mice were able to generate a normal antigen specific IgE response following OVA exposure (Fig. 3C). Thus the kinase domain of Itk is required for T cell proliferation and Th2 cytokine secretion in vitro in response to antigen restimulation. However, reducing kinase signaling via Itk does not affect antigen specific proliferation or Th2 cytokine secretion in vitro, or the development of IgE responses in vivo.
Figure 3. Role of the kinase domain of Itk in T cell responses and Th2 development in response to OVA immunization.
(a) WT, Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT mice were treated as in figure 1, and spleens and lymph nodes collected, stimulated with the indicated concentration of OVA, and proliferation determined. (b) Supernatants from splenocytes and lymph node cells stimulated with 100 µg/ml OVA in vitro for 96 hours, were collected and assayed for IL-4, -5, -13 and IFNγ. (c) WT, Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT mice were treated as in figure 1, and serum collected and analyzed for OVA specific IgE as described in the materials and methods section. *p<0.05 vs. WT mice; ND, none detected.
The kinase domain of Itk is required for T cell recruitment into the lung and chemokine induced migration in vitro
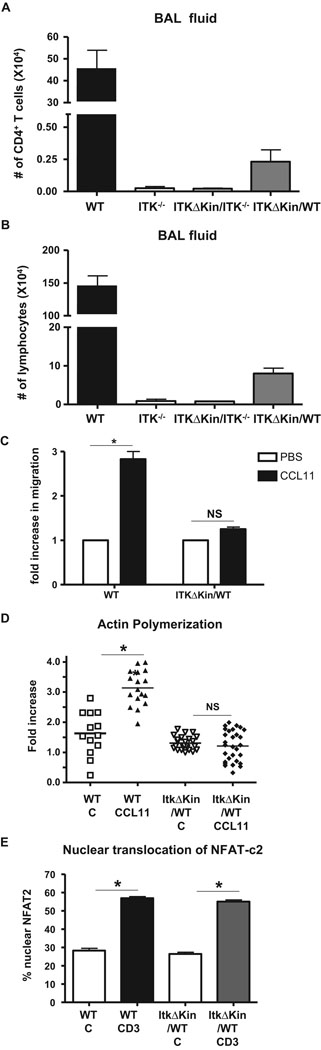
One potential explanation for the observation of reduced Th2 cytokines in the lungs of Tg(Lckpr-ItkΔKin)/WT mice is a reduction in the recruitment of T cells into the lungs as we have previously reported occurs in mice lacking Itk (6, 7). Indeed, Itk is involved in chemokine mediated migration, which requires alterations in the actin cytoskeleton (9, 10), and Itk can modulate chemokine mediated actin cytoskeleton rearrangements, which is suggested to be kinase dependent (9). We therefore determined if CD4+ T cell recruitment into the BALF of mice was affected by the presence of the transgene. We found that while WT mice had significant levels of CD4+ T cells in the BAL fluid (Fig. 4A) and lung (data not shown), Itk−/− or Tg(Lckpr-ItkΔKin)/Itk−/− mice had significantly reduced recruitment of these cells into the lungs. More importantly, Tg(Lckpr-ItkΔKin)/WT also had significantly reduced recruitment of CD4+ T cells in the lung. This was accompanied by an overall reduction in cellular infiltration in BAL (Fig. 4B). Analysis of the ability of in vitro differentiated Th2 cells from WT and Tg(Lckpr-ItkΔKin)/WT to migrate in response to the Th2 chemokine CCL11/eotaxin-1 in vitro confirmed that Tg(Lckpr-ItkΔKin)/WT Th2 cells have defects in chemokine mediated migration (note that these cells differentiated to Th2 cells and expressed similar levels of CCR3, the receptor for CCL11, data not shown) (Fig. 4C). This reduction in migratory response reflected reduced activation and increase in actin polymerization in these cells, which is required for effective migration (Fig. 4D). By contrast, we did not observe any differences in either actin polymerization (data not shown) or nuclear localization of NFAT-c1, events previously shown to be affected by the absence of Itk upon TcR stimulation in T cells from these mice (Fig. 4E)(4, 5). These data thus indicate that chemokine, but not TcR signal is highly dependent on the signaling threshold regulated by the kinase activity of Itk. Together, these data suggest that there is a threshold of Itk signaling dependent on the kinase domain that control the development of symptoms of allergic asthma in vivo, and that this signaling threshold does not affect the development of a Th2 response in the Tg(Lckpr-ItkΔKin)/WT mice, but affects the migratory capacity of the T cells in these mice.
Figure 4. Reduced Itk signals specifically affect chemokine mediated migration in ItkΔKinTg/WT T cells.
(a) WT, Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT mice were treated as in figure 1, and BALF collected and analyzed for the number of CD4+ T cells. (b) WT, Itk−/−, Tg(Lckpr-ItkΔKin)/Itk−/−, and Tg(Lckpr-ItkΔKin)/WT mice were treated as in figure 1, and BALF collected and analyzed for the number of lymphocytes. (c) Migration of WT and Tg(Lckpr-ItkΔKin)/WT Th2 cells in response to 100 ng/ml CCL11 in vitro was determined. (d) Actin polymerization in response to PBS (C) or CCL11 signals (100 ng/ml) were analyzed in WT and Tg(Lckpr-ItkΔKin)/WT Th2 cells. (e) Nuclear translocation of NFAT-c1 in response to PBS (C) or TCR signals (anti-CD3) in WT and Tg(Lckpr-ItkΔKin)/WT CD4+ T cells. *p<0.05 vs. WT mice; NS, not significant.
Discussion
Itk regulates the differentiation and/or cytokine production capacity of Th2 producing cells, as well as the development of allergic asthma (4–8). We and others have also shown that certain functions of Itk are kinase independent, including activation of the transcription factor SRF, modulating the localization of Vav GEF and induction of actin cytoskeleton rearrangements downstream of the TcR (14, 16). However, our data indicate that Itk functions controlling the development of Th2 producing cells in vivo are kinase domain dependent. In addition, our findings show that reducing signaling through Itk can leave the development of Th2 producing cells intact, while selectively affecting recruitment of T cells into the lungs. This indicates that the activity of Itk can be manipulated to separate functions involved in Th2 cytokine production vs. chemokine induced migration. Our data further suggest that T cell activation, differentiation and cytokine secretion is dependent on different levels of kinase activity and subsequent events downstream of Itk, in comparison to signals required for migration.
Itk can regulate signaling pathways induced by the TcR as well as chemokine receptors. Downstream of the TcR, Itk functions to increase intracellular calcium and activate the NFAT transcription factor (25). In addition, Itk is also involved in TcR activation of Ras/MAPK signaling pathways leading to the activation of transcription factors such as AP-1 (26). These pathways eventually influence the secretion of cytokines such as IL-2 and IL-4, both of which have been shown to be dependent on the kinase activity of Itk (8, 27). Similarly, in cell culture models, signals regulated by Itk downstream of chemokine receptors have been shown to be dependent on the kinase activity (9). However, Itk also has been shown to function as a scaffold for the assembly of signaling proteins, such that downstream of antigen receptors, the kinase activity of Itk is not required for the induction of actin polymerization or the activation of the transcription factor SRF (14–16). Using its SH2 and SH3 domains, Itk can interact with the multiprotein complex containing LAT, Slp-76 and Vav, which may explain its ability to regulate SRF activation in a kinase independent fashion since this factor is downstream of actin rearrangements (16, 28). However, our data here indicate that kinase activity of Itk is required for the development of allergic asthma. It is possible that the PH, SH2 and/or SH3 domains of Itk interact with the LAT/Slp-76/Vav multiprotein complex to affect the actin cytoskeleton migration in response to chemokine. This reduction in chemokine induced migration reduces T cell recruitment into the lung, reducing Th2 cytokines, thus leading to the observed reduction in AHR in the Tg(Lckpr-ItkΔKin)/WT. However, the interaction with these effectors may have different functional outcomes downstream of the TCR signaling pathway (16, 28), and thus Th2 cytokine secretion from spleen and lymph nodes is unaffected.
The absence of Itk has also been shown to affect the development of CD4+ as well as CD8+ T cells (29–33). Analysis of our transgenic mice carrying the kinase deleted Itk indicates that further reduction of Tec kinase signal does not significantly affect this process, similar to what has been observed in mice lacking both Itk and Txk/Rlk (data not shown, (29)). Indeed, when expressed on the WT background, T cell development (data not shown), or the development of Th2 cytokine secreting T cells is not affected, suggesting that the reduced signaling that occurs in the presence of the kinase deleted Itk mutant is not sufficient to affect T cell development or T cell differentiation in response to specific antigen. The expression level of Itk has been reported to change during the process of differentiation to Th2 cells (34). However analysis of the expression of the transgene relative to endogenous Itk in naïve as well as differentiated Th2 cells revealed that the transgene is expressed at approximately 30% of endogenous Itk in naïve CD4+ T cells, while in Th2 cells, it is expressed at approximately 60% of endogenous Itk (data not shown). Thus, reduced expression of the transgene relative to endogenous Itk in Th2 cells is unlikely to explain the relative sensitivity of chemokine vs. Th2 differentiation pathways for Itk signals. It is possible that level of transgene expression in our mice may not be sufficient to observe potential roles for the other domains of Itk when expressed on the Itk null background. We do note that Itk heterozygous mice have a WT phenotype, and a WT Itk transgene can rescue all aspects of T cell function when expressed on the Itk null background at similar levels to this transgene (Hu and August, unpublished). However, it is possible that if expressed at higher levels, potential functional rescue may be observed with the ItkΔKin mutant.
In contrast to the Th2 pathway, signals regulated by Itk that control chemokine receptor mediated migration is affected by reducing signals, as observed in the Tg(Lckpr-ItkΔKin)/WT mice. Thus there appears to be a hierarchy of signals that are regulated by this kinase with different processes differentially dependent on these signals. Signals that control development of T cells, and Th2 cytokine production may have a lower threshold, which is reached in our transgenic animals. By contrast, those signals that control migration have a higher threshold and so are preferentially affected with the reduction in signal as in the Tg(Lckpr-ItkΔKin)/WT mice.
A role for migration and recruitment of T cells into the lung during the development of allergic asthma is strongly supported by reports in the literature (2). Chemokine and chemokine receptor modulation of T cell migration has been shown to play an important role in the recruitment of T cells into the lung, leading to disease. Indeed, the compound FTY720 prevents the development of allergic asthma in part by affecting T cell migration out of lymph nodes and into the lung (35). Thus the ability of Itk to control both T cell migration and T cell differentiation implicates Itk as an important player in the development of this disease. In addition, the ability to reduce Itk signaling to preferentially affect migration and not Th2 differentiation suggest that it may be possible to do this to affect the course of disease. Our data suggest that by altering signals coming from this kinase one can manipulate these T cell responses. A number of inhibitors have recently been developed that target the kinase activity of Itk which completely inhibit T cell responses (17–21), but can potentially lead to immunosuppression. Our work here suggest that manipulation of the dose of these inhibitors to selectively alter T cell migration while maintaining normal systemic T cell responses, may provide with a better and more effective strategy to treat allergic asthma.
Acknowledgements
We thank Meg Potter for animal care, Susan Magargee, Nicole Bem, Elaine Kunze and Dr. Deb Grove at Penn State for flow cytometric and Q-RT-PCR analysis. We also thank members of August lab and the Center for Molecular Immunology & Infectious Disease for comments and feedback, and Dr. Cindy McKinney at the Penn State transgenic facility for the generation of transgenic mice.
Abbreviations
- AHR
Airways Hyperresponsiveness
- BALF
Bronchoalveolar Lavage Fluid
- Btk
Bruton’s Tyrosine Kinase
- Itk
Inducible T cell kinase
- ItkΔKin
Itk lacking the kinase domain
- OVA
Ovalbumin
- PH
Pleckstrin Homology
- SRF
Serum Response Factor
- TcR
T cell Receptor
- Txk/Rlk
Resting lymphocyte kinase.
Footnotes
Supported by NIH grants AI051626 and AI065566, and the AHA to AA.
References
- 1.Ferreira M. Inflammation in allergic asthma: Initiating events, immunological response and risk factors. Respirology. 2004;9:16–24. doi: 10.1111/j.1440-1843.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 2.Cohn L, Elias J, Chupp G. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 3.Schaeffer E, Debnath J, Yap G, McVicar D, Liao X, Littman D, Sher A, Varmus H, Lenardo M, Schwartzberg P. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffer E, Yap G, Lewis C, Czar M, McVicar D, Cheever A, Sher A, Schwartzberg P. Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol. 2001;2:1183–1188. doi: 10.1038/ni734. [DOI] [PubMed] [Google Scholar]
- 5.Fowell D, Shinkai K, Liao X, Beebe A, Coffman R, Littman D, Locksley R. Impaired NFATc Translocation and Failure of Th2 Development in Itk Deficient CD4 T Cells. Immunity. 1999;11:399–409. doi: 10.1016/s1074-7613(00)80115-6. [DOI] [PubMed] [Google Scholar]
- 6.Mueller C, August A. Attenuation of Immunological Symptoms of Allergic Asthma in Mice Lacking the Tyrosine Kinase ITK. J Immunol. 2003;170:5056–5063. doi: 10.4049/jimmunol.170.10.5056. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara T, Mueller C, Sahu N, Ben-Jebria A, August A. Reduced airway hyperresponsiveness and tracheal responses during allergic asthma in mice lacking tyrosine kinase inducible T-cell kinase. J Allergy Clin Immunol. 2006;117:780–786. doi: 10.1016/j.jaci.2005.12.1330. [DOI] [PubMed] [Google Scholar]
- 8.Au-Yeung B, Katzman S, Fowell D. Cutting edge: Itk-dependent signals required for CD4+ T cells to exert, but not gain, Th2 effector function. J Immunol. 2006;176:3895–3899. doi: 10.4049/jimmunol.176.7.3895. [DOI] [PubMed] [Google Scholar]
- 9.Takesono A, Horai R, Mandai M, Dombroski D, Schwartzberg P. Requirement for Tec kinases in chemokine-induced migration and activation of Cdc42 and Rac. Curr Biol. 2004:917–922. doi: 10.1016/j.cub.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Fischer A, Mercer J, Iyer A, Ragin M, August A. Regulation of CXC chemokine receptor 4-mediated migration by the Tec family tyrosine kinase ITK. J Biol Chem. 2004;279:29816–29820. doi: 10.1074/jbc.M312848200. [DOI] [PubMed] [Google Scholar]
- 11.Valiaho J, Smith C, Vihinen M. BTKbase: the mutation database for X-linked agammaglobulinemia. Hum Mutat. 2006;27:1209–1217. doi: 10.1002/humu.20410. [DOI] [PubMed] [Google Scholar]
- 12.Middendorp S, Dingjan G, Maas A, Dahlenborg K, Hendriks R. Function of Bruton's tyrosine kinase during B cell development is partially independent of its catalytic activity. J Immunol. 2003;171:5988–5996. doi: 10.4049/jimmunol.171.11.5988. [DOI] [PubMed] [Google Scholar]
- 13.Middendorp S, Zijlstra A, Kersseboom R, Dingjan G, Jumaa H, Hendriks R. Tumor suppressor function of Bruton tyrosine kinase is independent of its catalytic activity. Blood. 2005;105:259–265. doi: 10.1182/blood-2004-07-2708. [DOI] [PubMed] [Google Scholar]
- 14.Hao S, Qi Q, Hu J, August A. A kinase independent function for Tec kinase ITK in regulating antigen receptor induced serum response factor activation. FEBS Lett. 2006;580:2691–2697. doi: 10.1016/j.febslet.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Grasis J, Browne C, Tsoukas C. Inducible T cell tyrosine kinase regulates actin-dependent cytoskeletal events induced by the T cell antigen receptor. J Immunol. 2003;170:3971–3976. doi: 10.4049/jimmunol.170.8.3971. [DOI] [PubMed] [Google Scholar]
- 16.Dombroski D, Houghtling R, Labno C, Precht P, Takesono A, Caplen N, Billadeau D, Wange R, Burkhardt J, Schwartzberg P. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol. 2005;174:1385–1392. doi: 10.4049/jimmunol.174.3.1385. [DOI] [PubMed] [Google Scholar]
- 17.Lin T, McIntyre K, Das J, Liu C, O'Day K, Penhallow B, Hung C, Whitney G, Shuster D, Yang X, Townsend R, Postelnek J, Spergel S, Lin J, Moquin R, Furch J, Kamath A, Zhang H, Marathe P, Perez-Villar J, Doweyko A, Killar L, Dodd J, Barrish J, Wityak J, Kanner S. Selective itk inhibitors block T-cell activation and murine lung inflammation. Biochemistry. 2004;43:11056–11062. doi: 10.1021/bi049428r. [DOI] [PubMed] [Google Scholar]
- 18.Das J, Liu C, Moquin R, Lin J, Furch J, Spergel S, Doweyko A, McIntyre K, Shuster D, O'Day K, Penhallow B, Hung C, Kanner S, Lin T, Dodd J, Barrish J, Wityak J. Discovery and SAR of 2-amino-5-[(thiomethyl)aryl]thiazoles as potent and selective Itk inhibitors. Bioorg Med Chem Lett. 2006;16:2411–2415. doi: 10.1016/j.bmcl.2006.01.115. [DOI] [PubMed] [Google Scholar]
- 19.Das J, Furch J, Liu C, Moquin R, Lin J, Spergel S, McIntyre K, Shuster D, O'Day K, Penhallow B, Hung C, Doweyko A, Kamath A, Zhang H, Marathe P, Kanner S, Lin T, Dodd J, Barrish J, Wityak J. Discovery and SAR of 2-amino-5-(thioaryl)thiazoles as potent and selective Itk inhibitors. Bioorg Med Chem Lett. 2006;16:3706–3712. doi: 10.1016/j.bmcl.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 20.Kashem M, Nelson R, Yingling J, Pullen S, Prokopowicz A, 3rd, Jones J, Wolak J, Rogers G, Morelock M, Snow R, Homon C, Jakes S. Three mechanistically distinct kinase assays compared: Measurement of intrinsic ATPase activity identified the most comprehensive set of ITK inhibitors. J Biomol Screen. 2007;12:70–83. doi: 10.1177/1087057106296047. [DOI] [PubMed] [Google Scholar]
- 21.Snow R, Abeywardane A, Campbell S, Lord J, Kashem M, Khine H, King J, Kowalski J, Pullen S, Roma T, Roth G, Sarko C, Wilson N, Winters M, Wolak J, Cywin C. Hit-to-lead studies on benzimidazole inhibitors of ITK: Discovery of a novel class of kinase inhibitors. Bioorg Med Chem Lett. 2007 doi: 10.1016/j.bmcl.2007.04.045. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Liao X, Littman D. Altered T cell receptor signaling and disrupted T cell development in mice lacking Itk. Immunity. 1995;3:757–759. doi: 10.1016/1074-7613(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Yang L, Cohn L, Parkyn L, Homer R, Ray P, Ray A. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 24.Ewart S, Levitt R, Mitzner W. Respiratory system mechanics in mice measured by end-inflation occlusion. J Appl Physiol. 1995;79:560–566. doi: 10.1152/jappl.1995.79.2.560. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Bunnell S, Gurniak C, Berg L. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg L, Finkelstein L, Lucas J, Schwartzberg P. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox H, Berg L. Itk phosphorylation sites are required for functional activity in primary T cells. J Biol Chem. 2003;278:37112–37121. doi: 10.1074/jbc.M304811200. [DOI] [PubMed] [Google Scholar]
- 28.Hao S, Kurosaki T, August A. Differential regulation of NFAT and SRF by the B cell Receptor via a PLCγ/Ca2+ dependent pathway. Embo J. 2003;22:4166–4177. doi: 10.1093/emboj/cdg401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaeffer E, Broussard C, Debnath J, Anderson S, McVicar D, Schwartzberg P. Tec Family Kinases Modulate Thresholds for Thymocyte Development and Selection. J. Exp. Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broussard C, Fleischecker C, Horai R, Chetana M, Venegas A, Sharp L, Hedrick S, Fowlkes B, Schwartzberg P. Altered development of CD8+ T cell lineages in mice deficient for the tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Atherly L, Brehm M, Welsh R, Berg L. Tec kinases Itk and Rlk are required for CD8+ T cell responses to virus infection independent of their role in CD4+ T cell help. J Immunol. 2006;176:1571–1581. doi: 10.4049/jimmunol.176.3.1571. [DOI] [PubMed] [Google Scholar]
- 32.Lucas J, Atherly L, Berg L. The absence of Itk inhibits positive selection without changing lineage commitment. J Immunol. 2002;168:6142–6151. doi: 10.4049/jimmunol.168.12.6142. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Sahu N, Walsh E, August A. Memory phenotype CD8(+) T cells with innate function selectively develop in the absence of active Itk. Eur J Immunol. 2007;37:2892–2899. doi: 10.1002/eji.200737311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller A, Wilcox H, Lai Z, Berg L. Signaling through Itk Promotes T Helper 2 Differentiation via Negative Regulation of T-bet. Immunity. 2004;21:67–80. doi: 10.1016/j.immuni.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Sawick E, Zuany-Amorim C, Manlius C, Trifilieff A, Brinkmann V, Kemeny D, Walker C. Inhibition of Th1- and Th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J Immunol. 2003;171:6206–6214. doi: 10.4049/jimmunol.171.11.6206. [DOI] [PubMed] [Google Scholar]